Prospects of Marine Sterols against Pathobiology of Alzheimer’s Disease: Pharmacological Insights and Technological Advances
Abstract
1. Introduction
2. Distribution and Pharmacokinetics of Marine Sterols
3. Pathobiology of Alzheimer’s Disease
4. Effects of Marine Sterols against Pathobiology of AD
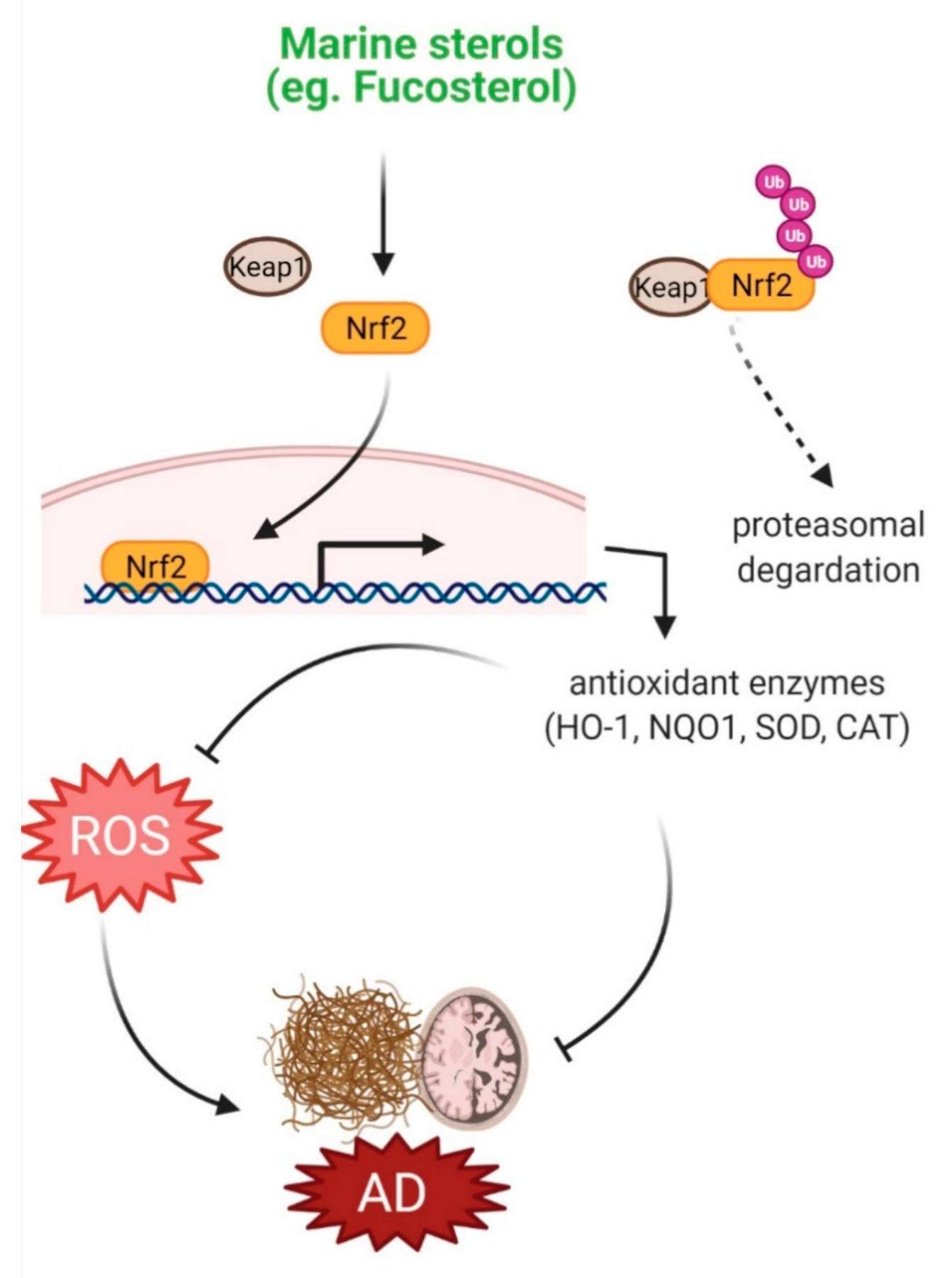
4.1. Protection against Oxidative Stress
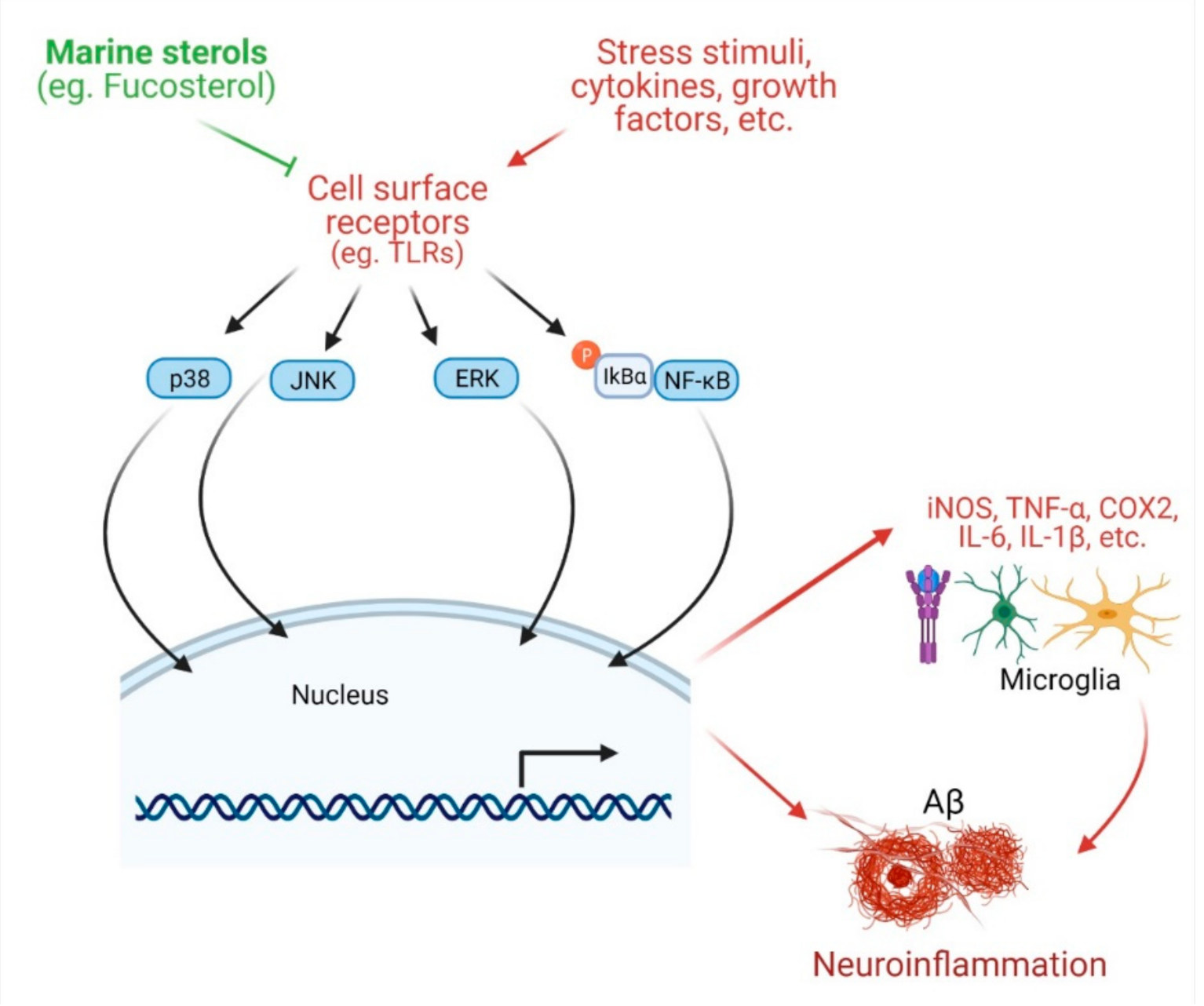
4.2. Protection against Neuroinflammation
4.3. Marine Sterols as Cholinesterase Inhibitors
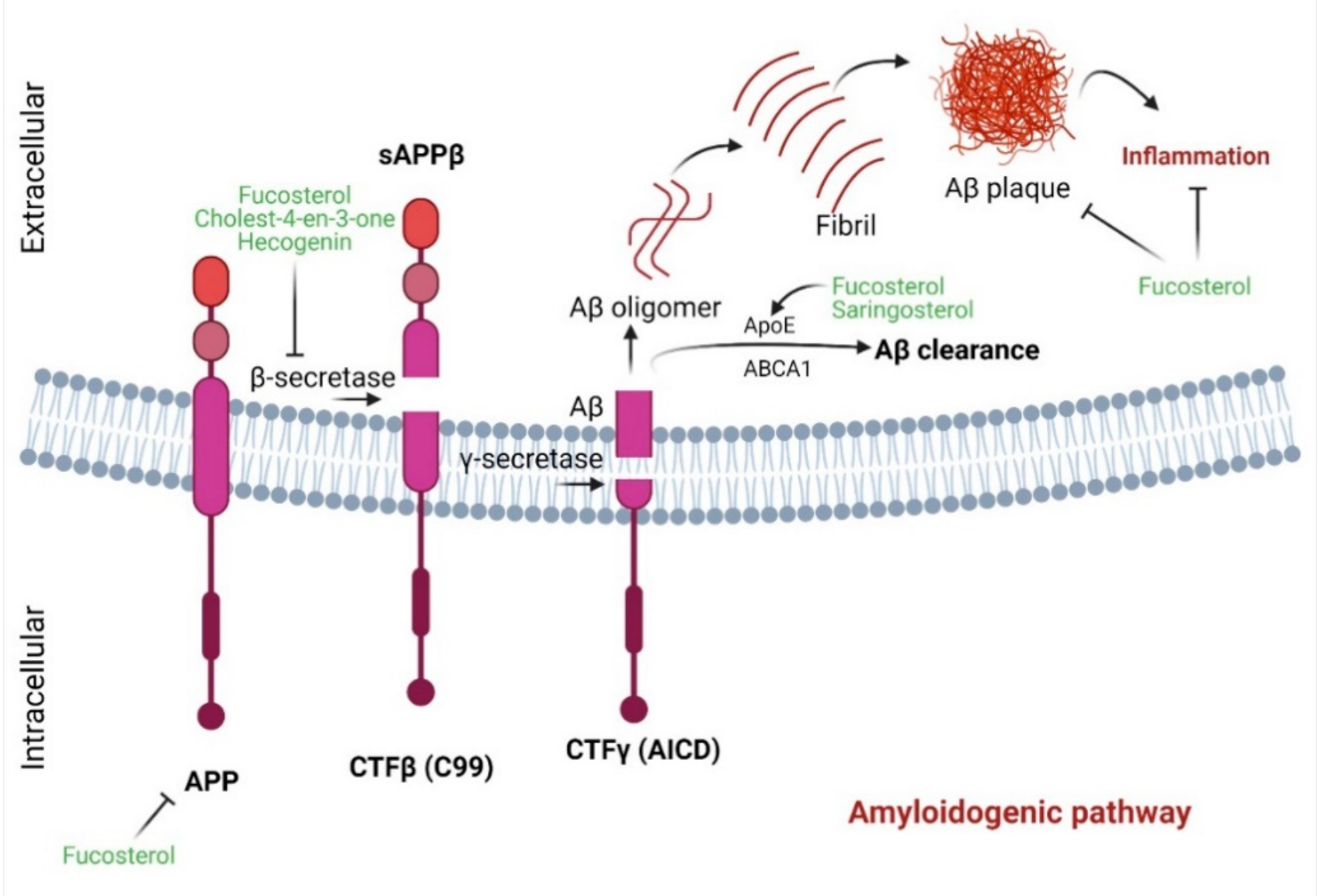
4.4. Marine Sterols as β-Secretase Inhibitors
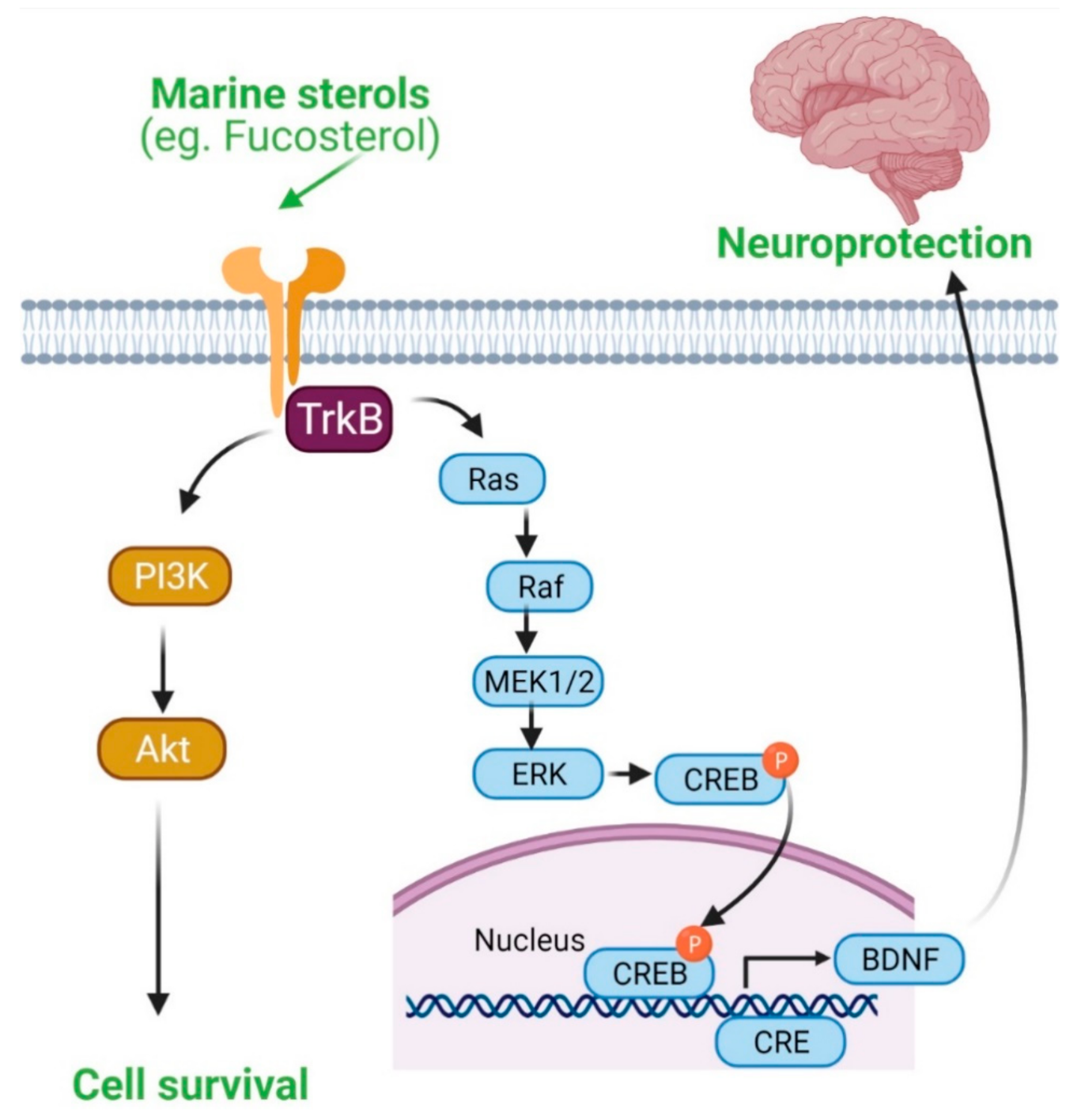
4.5. Marine Sterols as Neuroprotective Agent
4.6. Marine Sterols as Regulators of Cholesterol Homeostasis
5. Pharmacological Mechanism of Protective Actions of Marine Sterols against AD Pathology
6. Technological Advances toward Sterol Therapy
7. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Uddin, M.S.; Al Mamun, A.; Rahman, M.A.; Behl, T.; Perveen, A.; Hafeez, A.; Bin-Jumah, M.N.; Abdel-Daim, M.M.; Ashraf, G.M. Emerging Proof of Protein Misfolding and Interaction in Multifactorial Alzheimer’s Disease. Curr. Top. Med. Chem. 2020, 20, 2380–2390. [Google Scholar] [CrossRef]
- Ballard, C.; Gauthier, S.; Corbett, A.; Brayne, C.; Aarsland, D.; Jones, E. Alzheimer’s disease. Lancet 2011, 377, 1019–1031. [Google Scholar] [CrossRef]
- Ghai, R.; Nagarajan, K.; Arora, M.; Grover, P.; Ali, N.; Kapoor, G. Current Strategies and Novel Drug Approaches for Alzheimer Disease. CNS Neurol. Disord. Drug Targets 2020. [Google Scholar] [CrossRef] [PubMed]
- Bekris, L.M.; Yu, C.E.; Bird, T.D.; Tsuang, D.W. Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213–227. [Google Scholar] [CrossRef]
- Rahman, M.A.; Rahman, M.S.; Rahman, M.H.; Rasheduzzaman, M.; Mamun-Or-Rashid, A.N.M.; Uddin, M.J.; Rahman, M.R.; Hwang, H.; Pang, M.G.; Rhim, H. Modulatory Effects of Autophagy on APP Processing as a Potential Treatment Target for Alzheimer’s Disease. Biomedicines 2021, 9, 5. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Mullard, A. Failure of first anti-tau antibody in Alzheimer disease highlights risks of history repeating. Nat. Rev. Drug Discov. 2021, 20, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.L.; Ma, M.J. Alzheimer Disease: Clues from traditional and complementary medicine. J. Tradit. Complement. Med. 2017, 7, 380–385. [Google Scholar] [CrossRef]
- Wijesekara, I.; Pangestuti, R.; Kim, S.K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohyd. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Rathnayake, A.U.; Abuine, R.; Kim, Y.J.; Byun, H.G. Anti-Alzheimer’s Materials Isolated from Marine Bio-resources: A Review. Curr. Alzheimer Res. 2019, 16, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Menna, M. Steroids from sponges: Recent reports. Steroids 1999, 64, 687–714. [Google Scholar] [CrossRef]
- Sarma, N.S.; Krishna, M.S.; Pasha, S.G.; Rao, T.S.P.; Venkateswarlu, Y.; Parameswaran, P.S. Marine Metabolites: The Sterols of Soft Coral. Chem. Rev. 2009, 109, 2803–2828. [Google Scholar] [CrossRef]
- Carreón-Palau, L.; Özdemir, N.; Parrish, C.C.; Parzanini, C. Sterol Composition of Sponges, Cnidarians, Arthropods, Mollusks, and Echinoderms from the Deep Northwest Atlantic: A Comparison with Shallow Coastal Gulf of Mexico. Mar. Drugs 2020, 18, 598. [Google Scholar] [CrossRef]
- Wu, J.; Xi, Y.; Huang, L.; Li, G.; Mao, Q.; Fang, C.; Shan, T.; Jiang, W.; Zhao, M.; He, W.; et al. A Steroid-Type Antioxidant Targeting the Keap1/Nrf2/ARE Signaling Pathway from the Soft Coral Dendronephthya gigantea. J. Nat. Prod. 2018, 81, 2567–2575. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.H.; Chen, P.C.; Yang, S.N.; Lin, F.Y.; Su, T.P.; Chen, L.Y.; Peng, B.R.; Hu, C.C.; Chen, Y.Y.; Wen, Z.H.; et al. New 1,4-Dienonesteroids from the Octocoral Dendronephthya sp. Mar. Drugs 2019, 17, 530. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, N.T.; Hanh, T.T.H.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Ivanchina, N.V.; Dang, N.H.; Kicha, A.A.; Kiem, P.V.; Minh, C.V. Steroids from Dendronephthya mucronata and Their Inhibitory Effects on Lipopolysaccharide-Induced No Formation in RAW264.7 Cells. Chem. Nat. Compd. 2019, 55, 1090–1093. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, E.; Shin, Y.K.; Lee, D.G.; Yang, J.E.; Park, J.H.; Yi, T.H. Immunostimulatory Effect of Enzyme-Modified Hizikia fusiformein a Mouse Model In Vitro and Ex Vivo. Mar. Biotechnol. 2017, 19, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Kang, S.S.; Shin, K.H. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharmacal Res. 2003, 26, 719–722. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Han, Y.R.; Byeon, J.S.; Choung, S.Y.; Sohn, H.S.; Jung, H.A. Protective effect of fucosterol isolated from the edible brown algae, Ecklonia stolonifera and Eisenia bicyclis, on tert-butyl hydroperoxide- and tacrine-induced HepG2 cell injury. J. Pharm. Pharmacol. 2015, 67, 1170–1178. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.-S.; Lee, W.W.; Vaas, A.P.J.P.; De Silva, H.I.C.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.-S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef]
- Wong, C.H.; Gan, S.Y.; Tan, S.C.; Gany, S.A.; Ying, T.; Gray, A.I.; Igoli, J.; Chan, E.W.L.; Phang, S.M. Fucosterol inhibits the cholinesterase activities and reduces the release of pro-inflammatory mediators in lipopolysaccharide and amyloid-induced microglial cells. J. Appl. Phycol. 2018, 30, 3261–3270. [Google Scholar] [CrossRef]
- Yoo, M.S.; Shin, J.S.; Choi, H.E.; Cho, Y.W.; Bang, M.H.; Baek, N.I.; Lee, K.T. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-kappaB and p38 mitogen-activated protein kinase in RAW264.7 macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef]
- Sun, Z.; Mohamed, M.A.A.; Park, S.Y.; Yi, T.H. Fucosterol protects cobalt chloride induced inflammation by the inhibition of hypoxia-inducible factor through PI3K/Akt pathway. Int. Immunopharmacol. 2015, 29, 642–647. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Chung, H.Y.; Kim, H.R.; Choi, J.S. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish. Sci. 2008, 74, 200–207. [Google Scholar] [CrossRef]
- Harms, H.; Kehraus, S.; Nesaei-Mosaferan, D.; Hufendieck, P.; Meijer, L.; König, G.M. Aβ-42 lowering agents from the marine-derived fungus Dichotomomyces cejpii. Steroids 2015, 104, 182–188. [Google Scholar] [CrossRef]
- Zhu, Y.-Z.; Liu, J.-W.; Wang, X.; Jeong, I.-H.; Ahn, Y.-J.; Zhang, C.-J. Anti-BACE1 and Antimicrobial Activities of Steroidal Compounds Isolated from Marine Urechis unicinctus. Mar. Drugs 2018, 16, 94. [Google Scholar] [CrossRef]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24(S)-Saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective LXRbeta agonist. J. Agric. Food Chem. 2014, 62, 6130–6137. [Google Scholar] [CrossRef]
- Sheng, L.; Lu, B.; Chen, H.; Du, Y.; Chen, C.; Cai, W.; Yang, Y.; Tian, X.; Huang, Z.; Chi, W.; et al. Marine-Steroid Derivative 5α-Androst-3β, 5α, 6β-triol Protects Retinal Ganglion Cells from Ischemia—Reperfusion Injury by Activating Nrf2 Pathway. Mar. Drugs 2019, 17, 267. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Ali, M.C.; Jahan, I.; Munni, Y.A.; Mitra, S.; Hannan, M.A.; Timalsina, B.; Oktaviani, D.F.; Choi, H.J.; Moon, I.S. Emerging potential of cannabidiol in reversing proteinopathies. Ageing Res. Rev. 2021, 65, 101209. [Google Scholar] [CrossRef]
- Dash, R.; Jahan, I.; Ali, M.C.; Mitra, S.; Munni, Y.A.; Timalsina, B.; Hannan, M.A.; Moon, I.S. Potential roles of natural products in the targeting of proteinopathic neurodegenerative diseases. Neurochem. Int. 2021, 105011. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharm. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Yanuck, S.F. Microglial Phagocytosis of Neurons: Diminishing Neuronal Loss in Traumatic, Infectious, Inflammatory, and Autoimmune CNS Disorders. Front. Psychiatry 2019, 10, 712. [Google Scholar] [CrossRef]
- Hernandes, M.S.; D’Avila, J.C.; Trevelin, S.C.; Reis, P.A.; Kinjo, E.R.; Lopes, L.R.; Castro-Faria-Neto, H.C.; Cunha, F.Q.; Britto, L.R.; Bozza, F.A. The role of Nox2-derived ROS in the development of cognitive impairment after sepsis. J. Neuroinflamm. 2014, 11, 36. [Google Scholar] [CrossRef]
- Mouzat, K.; Chudinova, A.; Polge, A.; Kantar, J.; Camu, W.; Raoul, C.; Lumbroso, S. Regulation of Brain Cholesterol: What Role Do Liver X Receptors Play in Neurodegenerative Diseases? Int. J. Mol. Sci. 2019, 20, 3858. [Google Scholar] [CrossRef]
- Ito, A.; Hong, C.; Rong, X.; Zhu, X.; Tarling, E.J.; Hedde, P.N.; Gratton, E.; Parks, J.; Tontonoz, P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. eLife 2015, 4, e08009. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Li, D.; Tang, X.; Bao, X.; Huang, J.; Tang, Y.; Yang, Y.; Xu, H.; Fan, X. LXR agonists: New potential therapeutic drug for neurodegenerative diseases. Mol. Neurobiol. 2013, 48, 715–728. [Google Scholar] [CrossRef]
- Wolf, A.; Bauer, B.; Hartz, A.M. ABC Transporters and the Alzheimer’s Disease Enigma. Front. Psychiatry 2012, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jo, D.G.; Park, D.; Chung, H.Y.; Mattson, M.P. Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: Focus on the nervous system. Pharmacol. Rev. 2014, 66, 815–868. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Iranshahi, M.; Hasheminezhad, S.H.; Hayes, A.W.; Karimi, G. The neuroprotective activities of natural products through the Nrf2 upregulation. Phytother. Res. 2019, 33, 2256–2273. [Google Scholar] [CrossRef] [PubMed]
- Castro-Silva, E.S.; Bello, M.; Hernandez-Rodriguez, M.; Correa-Basurto, J.; Murillo-Alvarez, J.I.; Rosales-Hernandez, M.C.; Munoz-Ochoa, M. In vitro and in silico evaluation of fucosterol from Sargassum horridum as potential human acetylcholinesterase inhibitor. J. Biomol. Struct. Dyn. 2019, 37, 3259–3268. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Ali, M.Y.; Choi, R.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Kinetics and molecular docking studies of fucosterol and fucoxanthin, BACE1 inhibitors from brown algae Undaria pinnatifida and Ecklonia stolonifera. Food Chem. Toxicol. 2016, 89, 104–111. [Google Scholar] [CrossRef]
- Oh, J.H.; Choi, J.S.; Nam, T.J. Fucosterol from an Edible Brown Alga Ecklonia stolonifera Prevents Soluble Amyloid Beta-Induced Cognitive Dysfunction in Aging Rats. Mar. Drugs 2018, 16, 368. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.Y.; Wong, L.Z.; Wong, J.W.; Tan, E.L. Fucosterol exerts protection against amyloid β-induced neurotoxicity, reduces intracellular levels of amyloid β and enhances the mRNA expression of neuroglobin in amyloid β-induced SH-SY5Y cells. Int. J. Biol. Macromol. 2019, 121, 207–213. [Google Scholar] [CrossRef]
- Leng, T.; Liu, A.; Wang, Y.; Chen, X.; Zhou, S.; Li, Q.; Zhu, W.; Zhou, Y.; Su, X.; Huang, Y.; et al. Naturally occurring marine steroid 24-methylenecholestane-3β,5α,6β,19-tetraol functions as a novel neuroprotectant. Steroids 2016, 105, 96–105. [Google Scholar] [CrossRef]
- Hoang, M.-H.; Jia, Y.; Jun, H.-J.; Lee, J.H.; Lee, B.Y.; Lee, S.-J. Fucosterol Is a Selective Liver X Receptor Modulator That Regulates the Expression of Key Genes in Cholesterol Homeostasis in Macrophages, Hepatocytes, and Intestinal Cells. J. Agric. Food Chem. 2012, 60, 11567–11575. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Qi, Z.; Wang, S.; Feng, H.; Deng, X.; Ci, X. Asiatic Acid Exhibits Anti-inflammatory and Antioxidant Activities against Lipopolysaccharide and d-Galactosamine-Induced Fulminant Hepatic Failure. Front. Immunol. 2017, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xiang, Z.; Wang, Y.; Li, X.; Fang, C.; Song, S.; Li, C.; Yu, H.; Wang, H.; Yan, L.; et al. (−)-Epigallocatechin Gallate Targets Notch to Attenuate the Inflammatory Response in the Immediate Early Stage in Human Macrophages. Front. Immunol. 2017, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Mitra, S.; Ali, M.C.; Oktaviani, D.F.; Hannan, M.A.; Choi, S.M.; Moon, I.S. Phytosterols: Targeting Neuroinflammation in Neurodegeneration. Curr. Pharm. Des. 2021, 27, 383–401. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Liu, G.; Sun, R.; Wang, L.; Wang, J.; Wang, H. Fucosterol attenuates lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2015, 195, 515–521. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M.; Tang, J. Developing beta-secretase inhibitors for treatment of Alzheimer’s disease. J. Neurochem. 2012, 120 (Suppl. 1), 71–83. [Google Scholar] [CrossRef]
- Koelsch, G. BACE1 Function and Inhibition: Implications of Intervention in the Amyloid Pathway of Alzheimer’s Disease Pathology. Molecules 2017, 22, 1723. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Moon, I.S. Deciphering Molecular Mechanism of the Neuropharmacological Action of Fucosterol through Integrated System Pharmacology and In Silico Analysis. Mar. Drugs 2019, 17, 639. [Google Scholar] [CrossRef]
- Morinaga, T.; Yamaguchi, N.; Nakayama, Y.; Tagawa, M.; Yamaguchi, N. Role of Membrane Cholesterol Levels in Activation of Lyn upon Cell Detachment. Int. J. Mol. Sci. 2018, 19, 1811. [Google Scholar] [CrossRef]
- Martin, M.G.; Pfrieger, F.; Dotti, C.G. Cholesterol in brain disease: Sometimes determinant and frequently implicated. EMBO Rep. 2014, 15, 1036–1052. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.B.; Tan, X.J.; Wu, W.F.; Warner, M.; Gustafsson, J.A. Liver X receptor beta protects dopaminergic neurons in a mouse model of Parkinson disease. Proc. Natl. Acad. Sci. USA 2012, 109, 13112–13117. [Google Scholar] [CrossRef]
- Futter, M.; Diekmann, H.; Schoenmakers, E.; Sadiq, O.; Chatterjee, K.; Rubinsztein, D.C. Wild-type but not mutant huntingtin modulates the transcriptional activity of liver X receptors. J. Med. Genet. 2009, 46, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- Abumweis, S.S.; Barake, R.; Jones, P.J. Plant sterols/stanols as cholesterol lowering agents: A meta-analysis of randomized controlled trials. Food Nutr. Res. 2008, 52, 1811. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.; AbuMweis, S.S. Phytosterols as functional food ingredients: Linkages to cardiovascular disease and cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, R.E., Jr. Phytosterols, cholesterol absorption and healthy diets. Lipids 2007, 42, 41–45. [Google Scholar] [CrossRef]
- Jones, P.J. Ingestion of phytosterols is not potentially hazardous. J. Nutr. 2007, 137, 2485–2486. [Google Scholar] [CrossRef][Green Version]
- Leong, W.F.; Che Man, Y.B.; Lai, O.M.; Long, K.; Nakajima, M.; Tan, C.P. Effect of sucrose fatty acid esters on the particle characteristics and flow properties of phytosterol nanodispersions. J. Food Eng. 2011, 104, 63–69. [Google Scholar] [CrossRef]
- Keller, S.; Helbig, D.; Härtl, A.; Jahreis, G. Nanoscale and customary non-esterified sitosterols are equally enriched in different body compartments of the guinea pig. Mol. Nutr. Food Res. 2007, 51, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.-F.; Lai, O.-M.; Long, K.; Che Man, Y.B.; Misran, M.; Tan, C.-P. Preparation and characterisation of water-soluble phytosterol nanodispersions. Food Chem. 2011, 129, 77–83. [Google Scholar] [CrossRef]
- Engel, R.; Schubert, H. Formulation of phytosterols in emulsions for increased dose response in functional foods. Innov. Food Sci. Emerg. Technol. 2005, 6, 233–237. [Google Scholar] [CrossRef]
- Ostlund, R.E., Jr.; Spilburg, C.A.; Stenson, W.F. Sitostanol administered in lecithin micelles potently reduces cholesterol absorption in humans. Am. J. Clin. Nutr. 1999, 70, 826–831. [Google Scholar] [CrossRef]
- Türk, M.; Lietzow, R. Stabilized nanoparticles of phytosterol by rapid expansion from supercritical solution into aqueous solution. AAPS Pharmscitech 2004, 5, e56. [Google Scholar] [CrossRef]
- Leong, W.F.; Che Man, Y.B.; Lai, O.M.; Long, K.; Misran, M.; Tan, C.P. Optimization of processing parameters for the preparation of phytosterol microemulsions by the solvent displacement method. J. Agric. Food Chem. 2009, 57, 8426–8433. [Google Scholar] [CrossRef]
- Ling, H.W.; Lin, N.K. In vitro release study of freeze-dried and oven-dried microencapsulated kenaf seed oil. Malays. J. Nutr. 2017, 23, 139–149. [Google Scholar]
- Ubeyitogullari, A.; Moreau, R.; Rose, D.J.; Zhang, J.; Ciftci, O.N. Enhancing the Bioaccessibility of Phytosterols Using Nanoporous Corn and Wheat Starch Bioaerogels. Eur. J. Lipid Sci. Technol. 2019, 121, 1700229. [Google Scholar] [CrossRef]
- Meng, X.; Pan, Q.; Liu, Y. Preparation and properties of phytosterols with hydroxypropyl β-cyclodextrin inclusion complexes. Eur. Food Res. Technol. 2012, 235, 1039–1047. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioactive-loaded nanocarriers for functional foods: From designing to bioavailability. Curr. Opin. Food Sci. 2020, 33, 21–29. [Google Scholar] [CrossRef]
- Tolve, R.; Cela, N.; Condelli, N.; Di Cairano, M.; Caruso, M.C.; Galgano, F. Microencapsulation as a Tool for the Formulation of Functional Foods: The Phytosterols’ Case Study. Foods 2020, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- MacKay, D.S.; Jones, P.J.H. Phytosterols in human nutrition: Type, formulation, delivery, and physiological function. Eur. J. Lipid Sci. Technol. 2011, 113, 1427–1432. [Google Scholar] [CrossRef]
- Mohammadi, M.; Jafari, S.M.; Hamishehkar, H.; Ghanbarzadeh, B. Phytosterols as the core or stabilizing agent in different nanocarriers. Trends Food Sci. Technol. 2020, 101, 73–88. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2021, 11, 599959. [Google Scholar] [CrossRef]
| Sterol | Distribution | Structure | ADME/T Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipinski’s Rule of Five | Jorgensen’s Rule of Three | Blood–Brain Barrier Permeability | Percent Human Oral Absorption | |||||||||
| mol_MW | donorHB | accptHB | QPlogPo/w | QPlogS | QPPCaco | #metabolites | QPlogBB | CNS | ||||
| 7-dehydroerectasteroid-F | Soft coral Dendronephthya gigantea [16] | 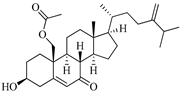 | 470.691 | 1 | 5.7 | 5.766 | −7.264 | 494.218 | 5 | −1.288 | −2 | 95.962 |
| Dendronesterones-D | Octocoral Dendronephthya sp. [17] | 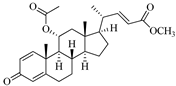 | 440.578 | 0 | 6 | 4.652 | −6.582 | 308.75 | 2 | −1.253 | −2 | 100 |
| 5α-cholestan-3,6-dione | Octocoral Dendronephthya mucronate [18] | 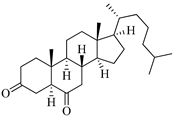 | 400.643 | 0 | 4 | 5.731 | −7.143 | 1210.653 | 4 | −0.683 | 0 | 100 |
| Fucosterol | Brown algae [19,20,21,22,23,24,25,26] | 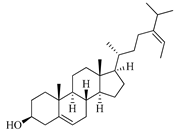 | 412.698 | 1 | 1.7 | 7.577 | −8.812 | 3376.384 | 6 | −0.299 | 0 | 100 |
| 24-hydroperoxy-24-vinylcholesterol | E. stolonifera [27] | 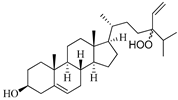 | 444.696 | 2 | 4.15 | 6.183 | −7.195 | 1183.894 | 3 | −0.947 | −1 | 100 |
| 16-O-desmethylasporyergosterol-β-d-mannoside | Fungus Dichotomomyces cejpii [28] | 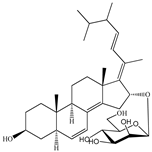 | 572.781 | 5 | 11.9 | 3.639 | −6.171 | 149.465 | 11 | −2.149 | −2 | 74.215 |
| 5α-pregn-20-en-3β-ol | Octocoral Dendronephthya mucronate [18] | 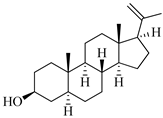 | 316.526 | 1 | 1.7 | 5.097 | −5.957 | 3378.51 | 3 | 0.019 | 1 | 100 |
| Cholest-4-en-3-one | Fatworm Urechis unicinctus [29] | 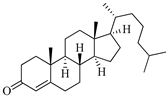 | 384.644 | 0 | 2 | 6.923 | −8.177 | 2769.384 | 2 | −0.316 | 0 | 100 |
| Saringosterol | Brwon algae [30,31] | 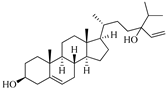 | 428.697 | 2 | 2.45 | 6.912 | −7.854 | 1981.099 | 4 | −0.655 | 0 | 100 |
| 24-methylenecholestane-3β,5α,6β,19-tetraol | Soft coral Nephthea brassica [32] | 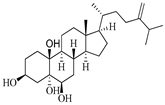 | 434.658 | 4 | 4.9 | 5.105 | −6.979 | 665.416 | 6 | −1.315 | −2 | 94.407 |
| Anti-AD Effects | Name of Sterol | Marine Source | Dose Regimen | Experimental Model | Major Findings | Reference |
|---|---|---|---|---|---|---|
| Protection against oxidative stress | Fucosterol, 3,6,17-trihydroxy-stigmasta-4,7,24(28)-triene and 14,15,18,20-diepoxyturbinarin | Pelvetia siliquosa | 30 mg/kg/day for 7 days prior to CCl4 challenge | CCl4-stimualted Rat model | ↑SOD, CAT, and GPx | [20] |
| Fucosterol | Edible brown alga Eisenia bicyclis | 25–400 μM | tert-BHP-induced RAW 264.7 macrophage cells | ↓ROS generation | [21] | |
| Ecklonia stolonifera and Eisenia bicyclis | 25–100 μM | tert-BHP- and tacrine-induced HepG2cell injury model | ↓ROS generation ↑GSH level | [22] | ||
| Brown alga Sargassum Binderi | 3.125–100 μg mL−1 | CPM-stimulated injury and inflammation in A549 epithelial cells | ↓ROS level ↑SOD, CAT, and HO-1 in cytosol, and Nrf2 in nucleus | [23] | ||
| 7-dehydroerectasteroid F | Soft coral Dendronephthya gigantea | 10 μM | H2O2-induced oxidative damage in PC12 cells | Nuclear translocation of Nrf2 and ↑HO-1 | [16] | |
| Protection against inflammation | Fucosterol | Panida australis | 0.004, 0.2, and 10 μM | LPS- and Aβ-induced BV2 (microglial) cells | Attenuates LPS- or Aβ-induced inflammation ↓IL-6, IL-1β, TNF-α, NO, and PGE2 | [24] |
| Eisenia bicyclis | 5–20 μM | LPS-stimulated RAW 264.7 murine macrophages | ↓NO production ↓iNOS and COX-2 ↓NF-κB pathway | [21] | ||
| Brown seaweed Undaria pinnatifida | 10, 25, or 50 μM | LPS-induced RAW 264.7 macrophage and THP-1 human monocyte cell line | ↓iNOS, TNF-α, and IL-6 ↓DNA binding ↓phosphorylation of NF-κB, MKK3/6 and MK2 | [25] | ||
| Hizikia fusiformis | 1–10 μM | CoCl2-induced hypoxia in keratinocytes | ↓IL-6, IL-1β and TNF-α ↓pPI3K and pAkt and HIF1-α accumulation | [26] | ||
| Sargassum binderi | 3.125, 6.25, 12.5, 25, 50, 100 μg mL−1 | CPM-stimulated injury and inflammation in A549 epithelial cells | ↓COX-2, PGE2, TNF-α and IL-6 ↓nuclear translocation of NF-κB and phosphorylation of MAPK, ERK1/2 and JNK | [23] | ||
| 5α-pregn-20-en-3β-ol and 5α-cholestan-3,6-dione | Octocoral Dendronephthya mucronate (Cnidaria) | IC50 of 30.15 ± 1.05 and 35.97 ± 2.06 μM, respectively | LPS-induced RAW264.7 murine macrophage cells | ↓NO formation | [18] | |
| Dendronesterones D | Octocoral Dendronephthya sp. | 10 μM | LPS-induced RAW264.7 macrophage cells | ↓iNOS and COX-2 | [17] | |
| Anticholinesterase activity | Fucosterol and 24-hydroperoxy 24-vinylcholesterol | E. stolonifera | IC50 values of 421.72 ± 1.43, 176.46 ± 2.51 µM, respectively | Enzymatic assay | Selective inhibition of BChE | [27] |
| Fucosterol | Panida australis | Anti-AChE (10.99–20.71%) and anti-BChE (4.53–17.53%) activities with concentrations ≤ 56 μM | Enzymatic assay | Nonselective cholinesterase inhibition | [24] | |
| Sargassum horridum | - | In vitro enzymatic assay | ↓AChE activity (Non-competitive inhibition) | [47] | ||
| β-Secretase inhibitory activity | Fucosterol | Eckloniastolonifera and Undaria pinnatifida | 10-100 μM (IC50 64.12 ± 1.0 μM) | In vitro enzymatic assay and In silico analysis | ↓β-secretase activity (Noncompetitive inhibition) | [48] |
| Cholest-4-en-3-oneand hecogenin | Urechis unicinctus(fat innkeeper worm or marine spoon worm or penis fish) | EC50 390.6 µM and 116.3 µM, respectively | Fluorescence Resonance Energy Transfer (FRET)-based enzyme assay | Anti-BACE1 activity was comparable to curcuminoids, terpenoids, and tannins | [29] | |
| Neuroprotectiveactivity | Fucosterol | Ecklonia stolonifera | 1–10 µM at 24 h before sAβ1-42 challenge (effective fucosterol conc. 5–10 µM) | sAβ1–42 (10 µM) -induced ER stress model of primary neurons and sAβ1–42-induced memory dysfunction in aging rats | Reduces apoptosis in Aβ1–42-stimulated cytotoxicity and ameliorates Aβ1–42-induced cognitive decline ↑TrkB-mediated ERK1/2 signaling ↓GRP78 expression ↑BDNF expression | [49] |
| - | 0.0032 to 20 μM | Aβ-stimulated cytotoxicity in SH-SY5Y cells | Attenuates apoptosis in Aβ-induced SH-SY5Y cells ↑Ngb mRNA ↓APP mRNA and Aβ levels | [50] | ||
| 24(S)-Saringosterol | Sargassum fusiforme | 10 µM | Microglia-treated conditioned medium of 24(S)-Saringosterol-treated astrocytes; Mouse neuroblastoma (N2a)-APP695 cells | Aβ1−42 clearance; ↓Aβ-42 secretion; LXRβ activation | [30] | |
| 16-O-desmethylasporyergosterol-β-d-mannoside | Fungus Dichotomomyces cejpii | 10 μM | Aftin-5 treated N2a-APP695 cells | Moderate Aβ-42 lowering activity | [28] | |
| 24-methylenecholestane-3β,5α,6β,19-tetraol | Soft coral Nephthea brassica | 10 μM | Glutamate-induced neuronal injury | Promote cell survival; Negative modulation of NMDA receptor | [51] | |
| Cholesterol homeostasis | Fucosterol | - | 100 or 200 μM | HEK293 cell cultures (Reporter system); THP-1-derived macrophages, Caco-2 cells and HepG2 cells | Reverses cholesterol transport; Nonselective LXR agonist ↑ABCA1, ABCG1, and ApoE ↑Intestinal NPC1L1 and ABCA1 ↑Insig-2a, that delays nuclear translocation of SREBP-1c | [52] |
| Saringosterol | Sargassum fusiforme | 30 μM | Luciferase reporter assay system; HEK293T, THP-1 monocytes, HepG2, RAW264.7, THP-1 macrophages and Caco-2 cells | Selective LXRβ agonist. ↑ABCA1, ABCG1, and SREBP-1c | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A.; Dash, R.; Sohag, A.A.M.; Alam, M.; Rhim, H.; Ha, H.; Moon, I.S.; Uddin, M.J.; Hannan, M.A. Prospects of Marine Sterols against Pathobiology of Alzheimer’s Disease: Pharmacological Insights and Technological Advances. Mar. Drugs 2021, 19, 167. https://doi.org/10.3390/md19030167
Rahman MA, Dash R, Sohag AAM, Alam M, Rhim H, Ha H, Moon IS, Uddin MJ, Hannan MA. Prospects of Marine Sterols against Pathobiology of Alzheimer’s Disease: Pharmacological Insights and Technological Advances. Marine Drugs. 2021; 19(3):167. https://doi.org/10.3390/md19030167
Chicago/Turabian StyleRahman, Md. Ataur, Raju Dash, Abdullah Al Mamun Sohag, Mahboob Alam, Hyewhon Rhim, Hunjoo Ha, Il Soo Moon, Md Jamal Uddin, and Md. Abdul Hannan. 2021. "Prospects of Marine Sterols against Pathobiology of Alzheimer’s Disease: Pharmacological Insights and Technological Advances" Marine Drugs 19, no. 3: 167. https://doi.org/10.3390/md19030167
APA StyleRahman, M. A., Dash, R., Sohag, A. A. M., Alam, M., Rhim, H., Ha, H., Moon, I. S., Uddin, M. J., & Hannan, M. A. (2021). Prospects of Marine Sterols against Pathobiology of Alzheimer’s Disease: Pharmacological Insights and Technological Advances. Marine Drugs, 19(3), 167. https://doi.org/10.3390/md19030167














