Planococcus maritimus ML1206 Isolated from Wild Oysters Enhances the Survival of Caenorhabditis elegans against Vibrio anguillarum
Abstract
1. Introduction
2. Results
2.1. Isolation and Identification of Non-Hemolytic Marine Bacteria from the Intestine of Oysters and Perch
2.2. Detection of Acid and Bile Salt Tolerance by Marine Bacterial Strains
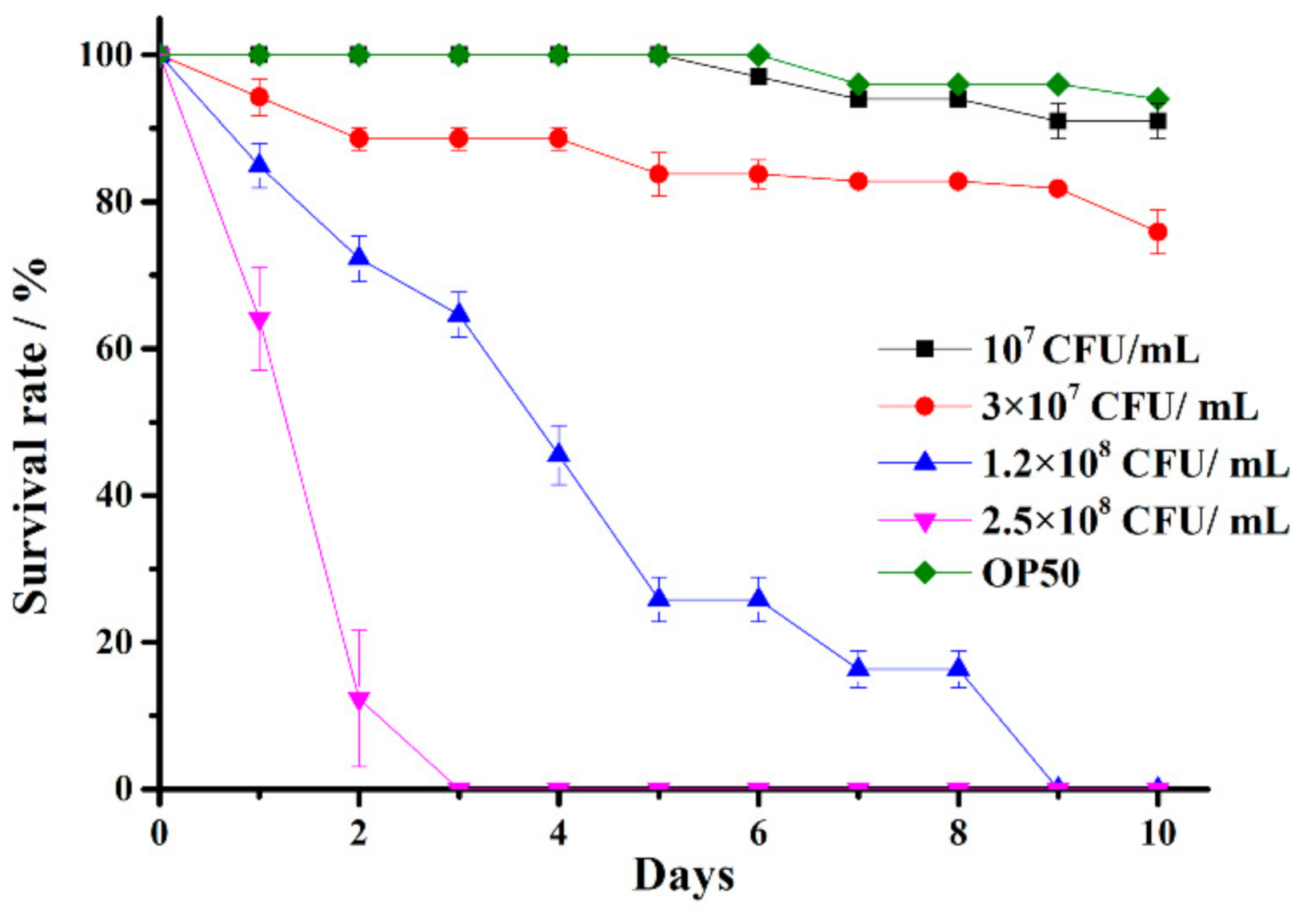
2.3. Establishment of a C. elegans Infected by V. anguillarum Model
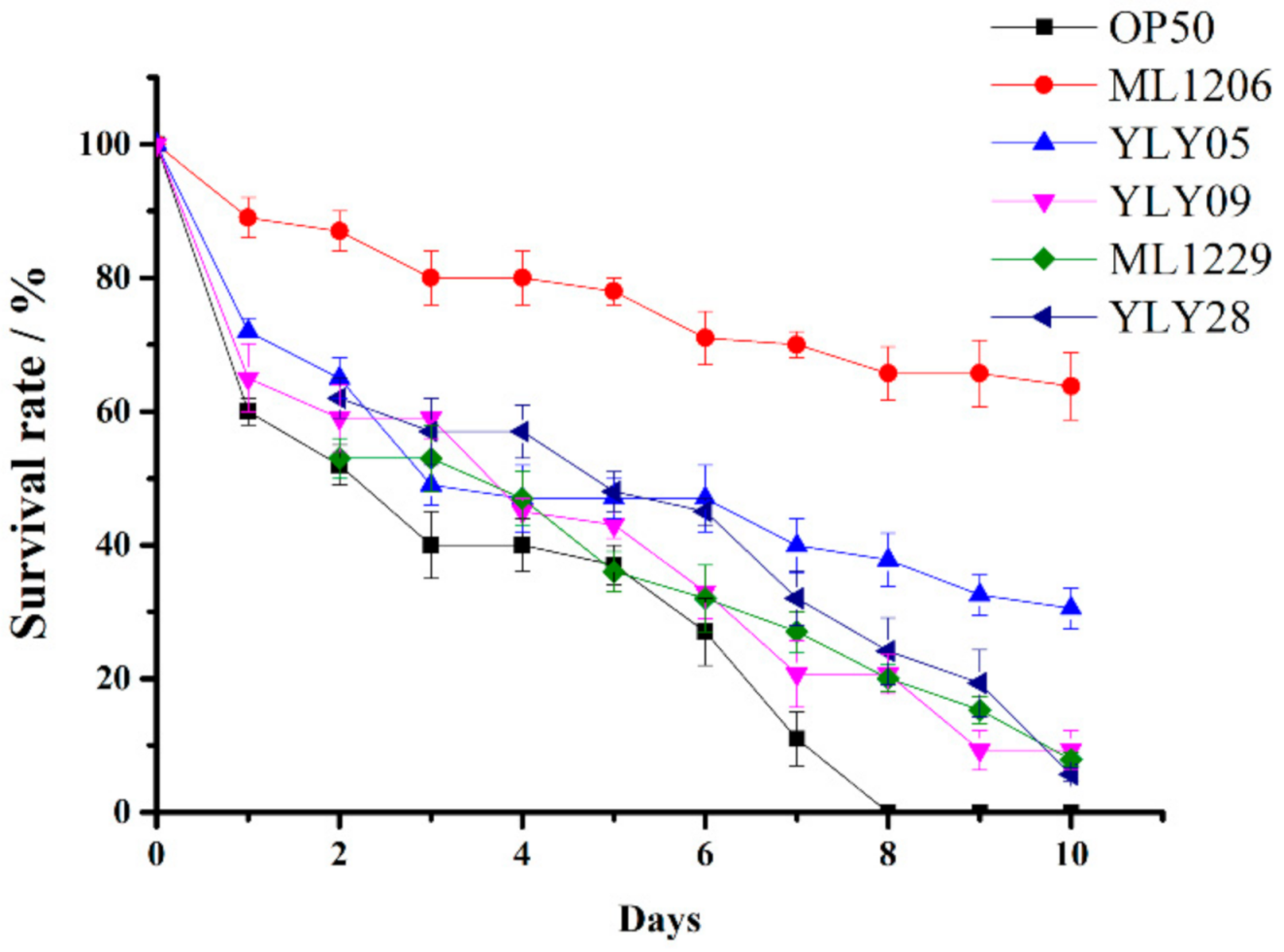
2.4. Screening of Potential Marine Probiotics Able to Protect C. elegans against V. anguillarum Infection
2.5. Safety Evaluation of Strain ML1206
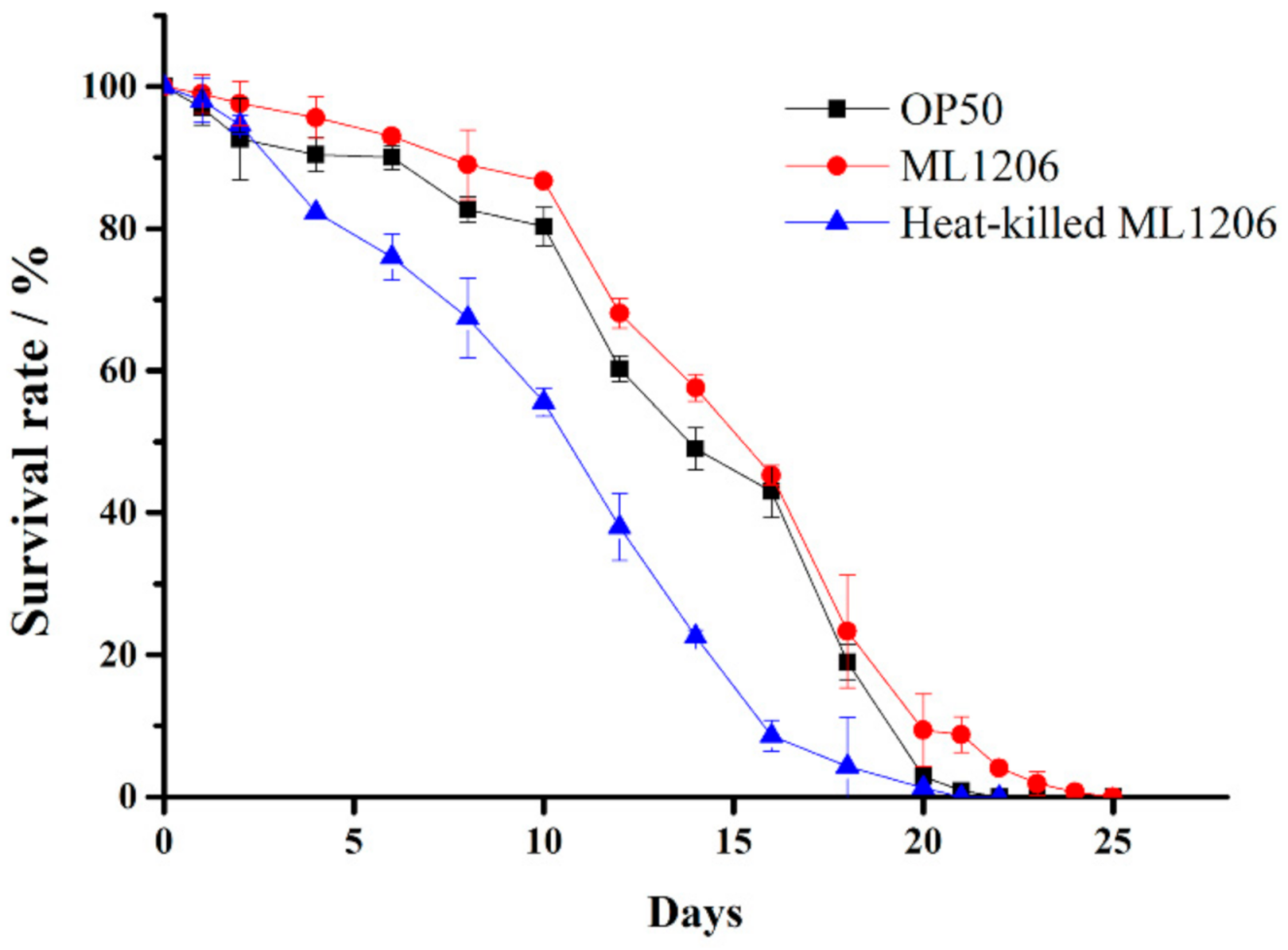
2.6. Evaluation of ML1206 Presence on the Physiological Performance of C. elegans
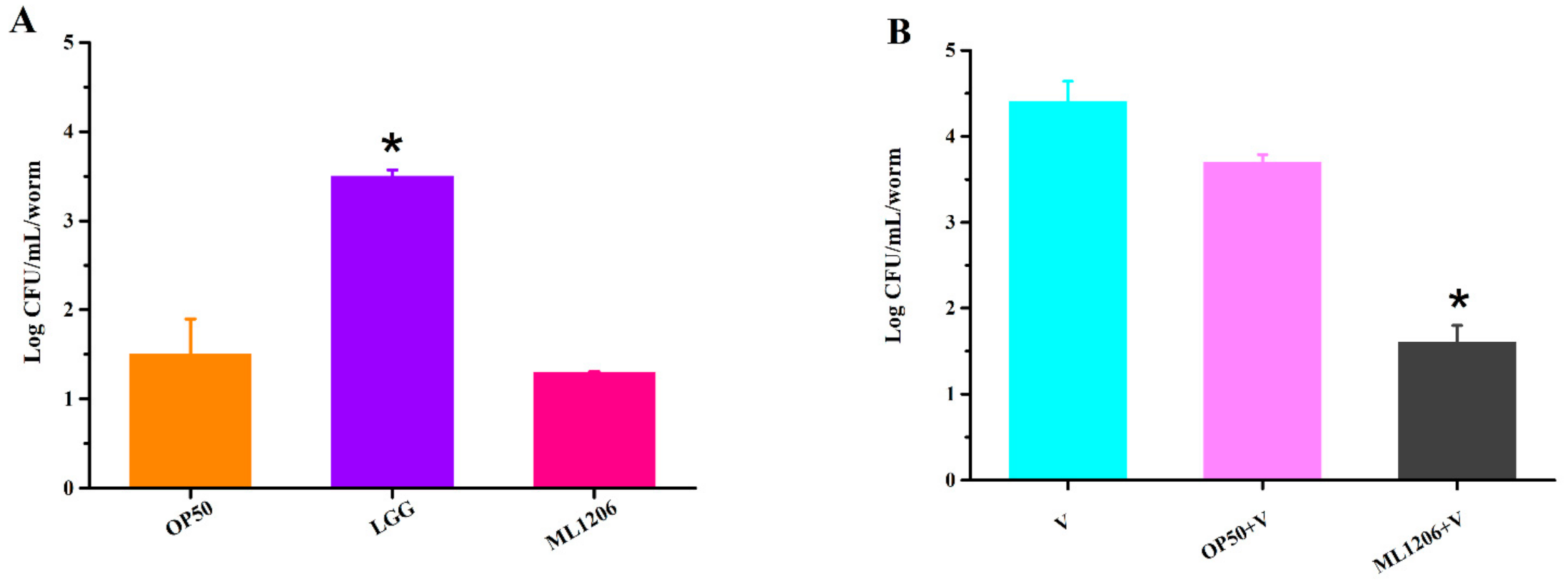
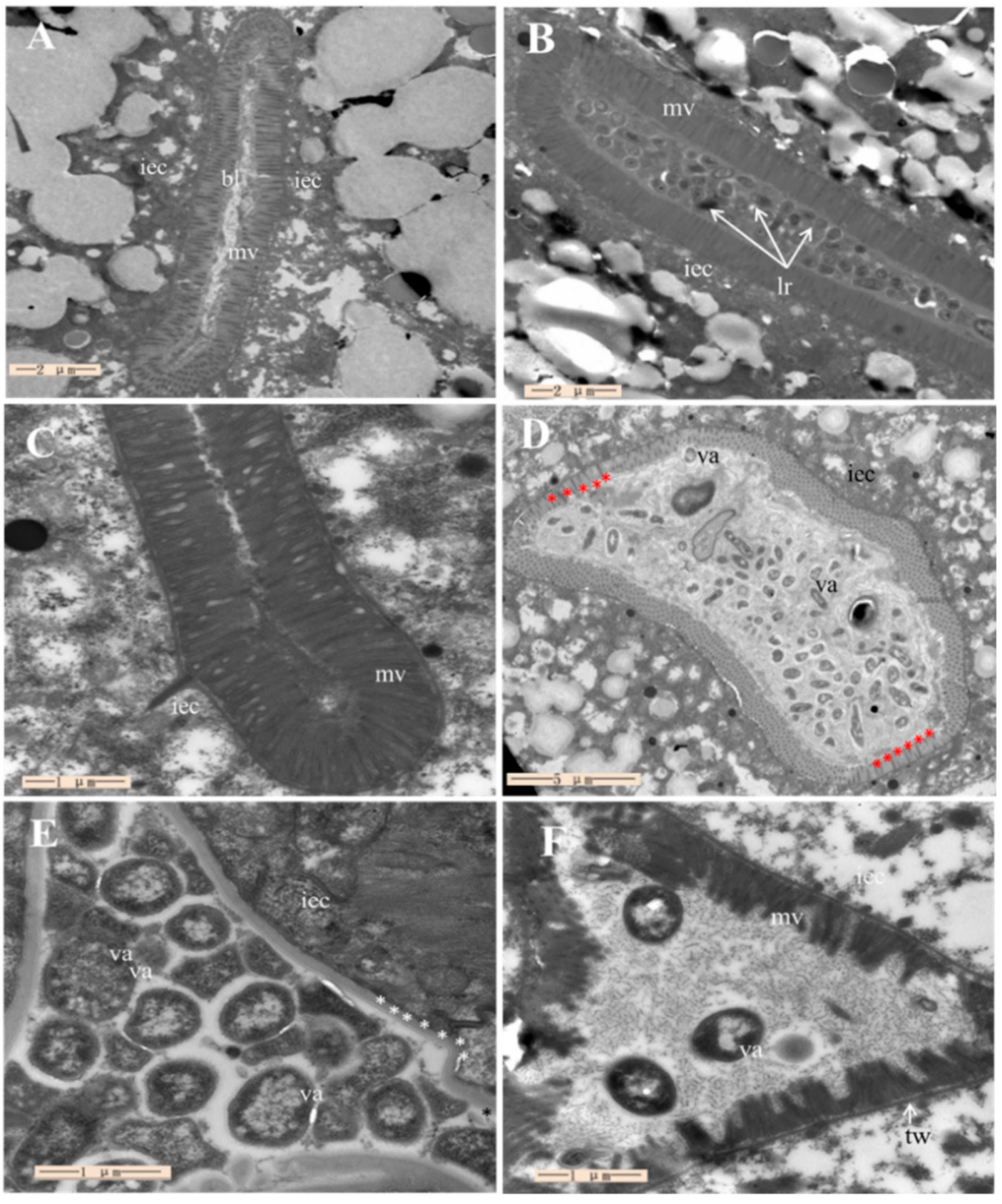
2.7. Assessment of ML1206 Inhibition on Intestinal Colonization of V. anguillarum in C. elegans
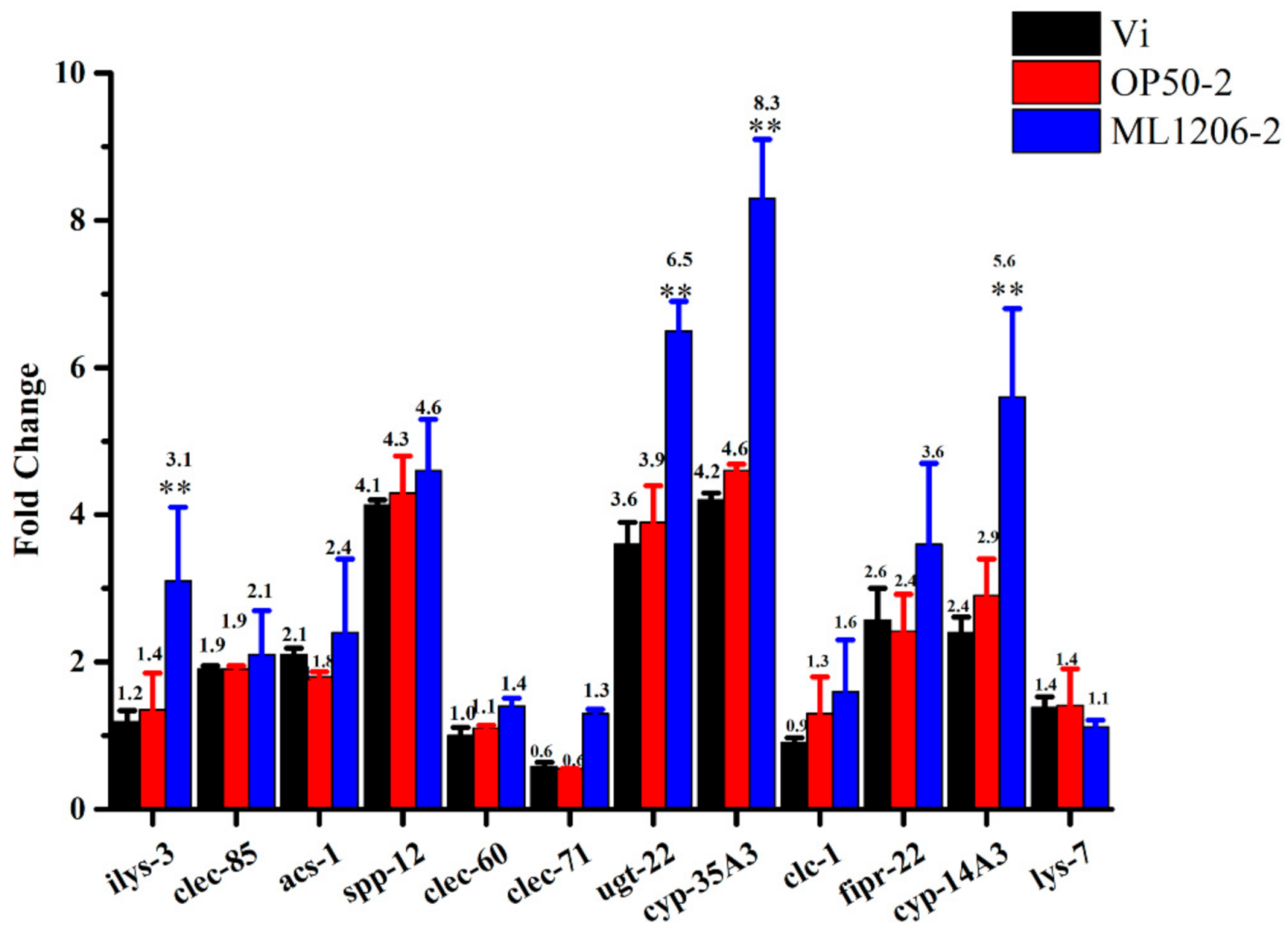
2.8. Result of Real-Time Fluorescence Quantitative PCR
3. Discussion
4. Materials and Methods
4.1. Strains and Growth Conditions
4.2. 16S rRNA Gene Sequencing and Analysis
4.3. Hemolysis Experiment
4.4. Experiment of Acid and Bile Salt Tolerance
4.5. Establishment of the Model of C. elegans Infected with V. anguillarum
4.6. Screening of Potential Marine Probiotics against V. anguillarum Infection Using the C. elegans Model
4.7. Antibiotic Susceptibility Assay
4.8. Safety Test of ML1206
4.9. C. elegans Life Span Assay
4.10. Plate Counting for Intestinal Colonization of V. anguillarum
4.11. Transmission Electron Microscope Observation
4.12. Real-time Fluorescence Quantitative PCR Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringø, E.; Bindelle, J.; Zhou, Z. Use of probiotics in aquaculture of China-a review of the past decade. Fish Shellfish Immunol. 2019, 86, 734–755. [Google Scholar] [CrossRef]
- Ang, C.Y.; Sano, M.; Dan, S.; Leelakriangsak, M.; Lal, T.M. Postbiotics Applications as Infectious Disease Control Agent in Aquaculture. Biocontrol. Sci. 2020, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yanong, R.P. Fungal diseases of fish. Vet. Clin. North. Am. Exot. Anim. Pract. 2003, 6, 377–400. [Google Scholar] [CrossRef]
- Tandel, G.M.; John, K.R.; Rosalind, G.M.; Prince, J.M.J. Current status of viral diseases in Indian shrimp aquaculture. Acta Virol. 2017, 61, 131–137. [Google Scholar] [CrossRef]
- Bach, E.E. Introduction: Shrimp immunity and disease control. Aquaculture 2000, 191, 3–11. [Google Scholar]
- Kuebutornye, F.K.A.; Abarike, E.D.; Lu, Y.A. Review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Aly, S.M.; Abdel-Galil, A.Y.; Abdel-Aziz, G.A.; Mohamed, M.F. Studies on Bacillus subtilis and Lactobacillusacidophilus, as potential probiotics, on the immune response and resistance of tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol. 2008, 25, 128–136. [Google Scholar] [CrossRef]
- Kesarcodi-Watson, A.; Miner, P.; Nicolas, J.L.; Robert, R. Protective effect of four potential probiotics against pathogen-challenge of the larvae of three bivalves: Pacific oyster (Crassostrea gigas), flat oyster (Ostrea edulis) and scallop (Pecten maximus). Aquaculture 2012, 344, 29–33. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Xia, H.Q.; Yang, H.L.; Wang, Y.L.; Zou, W.C. TLR2 signaling may play a key role in the probiotic modulation of intestinal microbiota in grouper Epinephelus coioides. Aquaculture 2014, 430, 50–56. [Google Scholar] [CrossRef]
- Angulo, M.; Reyes-Becerril, M.; Cepeda-Palacios, R.; Tovar-Ramirez, D.; Esteban, M.A.; Angulo, C. Probiotic effects of marine Debaryomyces hansenii CBS 8339 on innate immune and antioxidant parameters in newborn goats. Appl. Microbiol. Biotechnol. 2019, 103, 2339–2352. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, W.; Liu, W.; Gatlin, D.M.; Zhang, Y.; Yao, B.; Ringo, E. Identification of highly-adhesive gut Lactobacillus strains in zebrafish (Danio rerio) by partial rpoB gene sequence analysis. Aquaculture 2012, 370–371, 150–157. [Google Scholar] [CrossRef]
- Ren, P.; Xu, L.; Yang, Y.; He, S.; Liu, W.; Ringo, E.; Zhou, Z. Lactobacillus planarum subsp. plantarum JCM 1149 vs. Aeromonas hydrophila NJ-1 in the anterior intestine and posterior intestine of hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus ♂: An ex vivo study. Fish Shellfish Immunol. 2013, 35, 146–153. [Google Scholar] [CrossRef]
- Midhun, S.J.; Neethu, S.; Vysakh, A.; Radhakrishnan, E.K.; Jyothis, M. Antagonism Against Fish Pathogens by Cellular Components/Preparations of Bacillus coagulans (MTCC-9872) and It’s In Vitro Probiotic Characterisation. Curr. Microbiol. 2018, 75, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y.; Liu, Y.; Miao, L.L.; Li, E.W.; Hou, T.T.; Liu, Z.P. Mechanism of anti-Vibrio activity of marine probiotic strain Bacillus pumilus H2, and characterization of the active substance. AMB Express 2017, 7, 23. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Tang, K.; Yu, M.; Coenye, T.; Zhang, X. Genome analysis of Flaviramulus ichthyoenteri Th78 (T) in the family Flavobacteriaceae: Insights into its quorum quenching property and potential roles in fish intestine. BMC Genom. 2015, 16, 38. [Google Scholar]
- Phillipe, M.R.; Deborah, C.A.L.; Gustavo, A.S.D.; Ricardo, M.C.; Guillaume, J.; Ulisses, N.d.R.; Joao, P.S.; Francisco, D.A.; Jonathan, A.E.; David, G.B.; et al. Marine probiotics: Increasing coral resistance to bleaching through microbiome manipulation. ISME J. 2019, 13, 921–936. [Google Scholar]
- Das, S.; Ward, L.R.; Burke, C. Prospects of using marine actinobacteria as probiotics in aquaculture. Appl. Microbiol. Biotechnol. 2008, 81, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Sheridan, C.; Osinga, R.; Dionisio, G.; Rocha, R.J.M.; Silva, B.; Rosa, R.; Caladao, R. Marine microorganism-invertebrate assemblages: Perspectives to solve the “supply problem” in the initial steps of drug discovery. Mar. Drugs 2014, 12, 3929–3952. [Google Scholar] [CrossRef]
- Alonso, S.; Castro, M.C.; Berdasco, M.; De la Banda, I.G.; Moreno-Ventas, X.; De Rojas, A.H. Isolation and Partial Characterization of Lactic Acid Bacteria from the Gut Microbiota of Marine Fishes for Potential Application as Probiotics in Aquaculture. Probiotics Antimicrob. Proteins 2019, 11, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Preetha, R.; Jayaprakash, N.; Bright, S.I. Synechocystis MCCB 114 and 115 as putative probionts for Penaeus monodon post-larvae. Dis. Aquat. Org. 2007, 74, 243. [Google Scholar] [CrossRef]
- Ina-Salwany, M.Y.; Al-Saari, N.; Mohamad, A.; Mursidi, F.A.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef]
- Miccoli, A.; Saraceni, P.R.; Scapigliati, G. Vaccines and immune protection of principal Mediterranean marine fish species. Fish Shellfish Immunol. 2019, 94, 800–809. [Google Scholar] [CrossRef] [PubMed]
- De Schryver, P.; Defoirdt, T.; Sorgeloos, P. Early mortality syndrome outbreaks: A microbial management issue in shrimp farming? PLoS Pathog. 2014, 10, e1003919. [Google Scholar] [CrossRef] [PubMed]
- Ewbank, J.J.; Zugasti, O.C. elegans: Model host and tool for antimicrobial drug discovery. Dis. Model Mech. 2011, 4, 300–304. [Google Scholar] [CrossRef]
- Yun, H.S.; Heo, J.H.; Son, S.J.; Park, M.R.; Oh, S.; Song, M.H.; Kim, J.N.; Go, G.W.; Cho, H.S.; Choi, N.J.; et al. Bacillus licheniformis Isolated from Korean Traditional Food Sources Enhances the Resistance of Caenorhabditis elegans to Infection by Staphylococcus aureus. J. Microbiol. Biotechnol. 2014, 24, 1105–1108. [Google Scholar] [CrossRef]
- Moy, T.I.; Mylonakis, E.; Calderwood, S.B.; Ausubel, F.M. Cytotoxicity of Hydrogen Peroxide Produced by Enterococcus faecium. Infect. Immun. 2004, 72, 4512. [Google Scholar] [CrossRef]
- Yang, K.H.; Yun, B.; Choi, H.J.; Ryu, S.; Lee, W.J.; Oh, M.H.; Song, M.H.; Kim, J.N.; Oh, S.; Kim, Y.; et al. Simple Evaluation of Listeria monocytogenes Pathogenesis Using Caenorhabditis elegans Animal Model. Food Sci. Anim. Resour. 2019, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.N.; Lewis, J.; Patel, I.; Bozdag, S.; Lee, J.H.; LeClerc, J.E.; Cinar, H.N. Genomic Analysis of Immune Response against Vibrio cholerae Hemolysin in Caenorhabditis elegans. PLoS ONE 2012, 7, e38200. [Google Scholar] [CrossRef] [PubMed]
- Paulander, W.; Pennhag, A.; Andersson, D.I.; Maisnier-Patin, S. Caenorhabditis elegans as a model to determine fitness of antibiotic-resistant Salmonella enterica serovar typhimurium. Antimicrob. Agents Chemother. 2007, 51, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yu, H.; Yin, X.; Sabour, P.M.; Chen, W.; Gong, J. Lactobacillus zeae protects Caenorhabditis elegans from enterotoxigenic Escherichia coli-caused death by inhibiting enterotoxin gene expression of the pathogen. PLoS ONE 2014, 9, e89004. [Google Scholar] [CrossRef] [PubMed]
- King, C.D.; Singh, D.; Holden, K.; Govan, B.; Keith, S.; Ghazi, A.; Robinson, R.A. Dataset of proteomics analysis of aging C. elegans exposed to Pseudomonas aeruginosa strain PA01. Data Brief 2017, 11, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rock, J.L.; Nelson, D.R. Identification and characterization of a repeat-in-toxin gene cluster in Vibrio anguillarum. Infect. Immun. 2008, 76, 2620–2632. [Google Scholar] [CrossRef] [PubMed]
- Guantario, B.; Zinno, P.; Schifano, E.; Roselli, M.; Perozzi, G.; Palleschi, C.; Uccelletti, D.; Devirgiliis, C. In Vitro and in Vivo Selection of Potentially Probiotic Lactobacilli from Nocellara del Belice Table Olives. Front. Microbiol. 2018, 9, 595. [Google Scholar] [CrossRef]
- Prasad, J.; Gill, H.; Smart, J.; Gopal, P.K. Selection and Characterisation of Lactobacillus and Bifidobacterium Strains for Use as Probiotics. Int. Dairy J. 1998, 8, 993–1002. [Google Scholar] [CrossRef]
- National Food Safety Standard—Acute Oral Toxicity Test. GB15193.3-2014, National Standard of the People’s Republic of China. Available online: http://down.foodmate.net/standard/sort/3/42553.html (accessed on 21 February 2021).
- Park, M.R.; Yun, H.S.; Son, S.J.; Oh, S.; Kim, Y. Short communication: Development of a direct in vivo screening model to identify potential probiotic bacteria using Caenorhabditis elegans. J. Dairy Sci. 2014, 97, 6828–6834. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.H.; Shang, B.J.; Shao, Y.C.; Li, W.Y.; Sun, J.S. Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 86, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Castex, M.; Lemaire, P.; Wabete, N.; Chim, L. Effect of dietary probiotic Pediococcus acidilactici on antioxidant defences and oxidative stress status of shrimp Litopenaeus stylirostris. Aquaculture 2009, 294, 306–313. [Google Scholar] [CrossRef]
- Truong Thy, H.T.; Tri, N.N.; Quy, O.M.; Fotedar, R.; Kannika, K.; Unajak, S.; Areechon, N. Effects of the dietary supplementation of mixed probiotic spores of Bacillus amyloliquefaciens 54A, and Bacillus pumilus 47B on growth, innate immunity and stress responses of striped catfish (Pangasianodon hypophthalmus). Fish Shellfish Immunol. 2017, 60, 391–399. [Google Scholar] [CrossRef]
- Dodoo, C.C.; Wang, J.; Basit, A.W.; Stapleton, P.; Gaisford, S. Targeted delivery of probiotics to enhance gastrointestinal stability and intestinal colonization. Int. J. Pharm. 2017, 530, 224–229. [Google Scholar] [CrossRef]
- Gobi, N.; Vaseeharan, B.; Chen, J.C.; Rekha, R.; Vijayakumar, S.; Anjugam, M.; Iswarya, A. Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol. 2018, 74, 501–508. [Google Scholar] [CrossRef]
- Anastassopoulou, C.G.; Fuchs, B.B.; Mylonakis, E. Caenorhabditis elegans-based model systems for antifungal drug discovery. Curr. Pharm. Des. 2011, 17, 1225–1233. [Google Scholar] [CrossRef]
- Zhou, S.; Xia, Y.; Zhu, C.; Chu, W. Isolation of Marine Bacillus sp. with Antagonistic and Organic-Substanc es-Degrading Activities and Its Potential Application as a Fish Probiotic. Mar. Drugs 2018, 16, 196. [Google Scholar] [CrossRef] [PubMed]
- Park, M.R.; Oh, S.; Son, S.; Park, D.J.; Oh, S.; Kim, S.H.; Jeong, D.Y.; Oh, N.S.; Lee, Y.; Song, M. Bacillus licheniformis isolated from traditional Korean food resources enhances the longevity of Caenorhabditis elegans through serotonin signaling. J. Agric. Food Chem. 2015, 63, 10227–10233. [Google Scholar] [CrossRef] [PubMed]
- Schulenburg, H.; Hoeppner, M.P.; Weiner, J.; Bornberg-Bauer, E. Specificity of the innate immune system and diversity of C-type lectin domain (CTLD) proteins in the nematode Caenorhabditis elegans. Immunobiol. Stuttg. 2008, 213, 237–250. [Google Scholar] [CrossRef]
- Jensen, V.L.; Simonsen, K.T.; Lee, Y.H.; Park, D.; Riddle, D.L. RNAi Screen of DAF-16/FOXO Target Genes in C. elegans Links Pathogenesis and Dauer Formation. PLoS ONE 2010, 5, e15902. [Google Scholar] [CrossRef]
- Abbas, A.; Valek, L.; Schneider, I.; Bollmann, A.; Knop, G.; Seitz, W.; Schulte-Oehlmann, U.; Oehlmann, J.; Wagner, M. Ecotoxicological impacts of surface water and wastewater from conventional and advanced treatment technologies on brood size, larval length, and cytochrome P450 (35A3) expression in Caenorhabditis elegans. Environ. Sci. Pollut. Res. 2018, 25, 13868–13880. [Google Scholar] [CrossRef] [PubMed]
- Ladage, M.L.; King, S.D.; Burks, D.J.; Quan, D.L.; Garcia, A.M.; Azad, R.K.; Padilla, P.A. Glucose or Altered Ceramide Biosynthesis Mediate Oxygen Deprivation Sensitivity Through Novel Pathways Revealed by Transcriptome Analysis in Caenorhabditis elegans. G3 Genes Genomes Genet. 2016, 6, 3149–3160. [Google Scholar]
- Kniazeva, M.; Shen, H.; Euler, T.; Wang, C.; Han, M. Regulation of maternal phospholipid composition and IP3-dependent embryonic membrane dynamics by a specific fatty acid metabolic event in C. elegans. Genes Dev. 2012, 26, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bakheet, R.; Parhar, R.S.; Huang, C.H.; Hussain, M.M.; Pan, X.Y.; Siddiqui, S.S.; Hashmi, S. Regulation of fat storage and reproduction by Krüppel-like transcription factor KLF3 and fat-associated genes in Caenorhabditis elegans. J. Mol. Biol. 2011, 411, 537–553. [Google Scholar] [CrossRef]
- Kage-Nakadai, E.; Kobuna, H.; Kimura, M.; Gengyo-Ando, K.; Inoue, T.; Arai, H.; Mitani, S. Two very long chain fatty acid acyl-CoA synthetase genes, acs-20 and acs-22, have roles in the cuticle surface barrier in Caenorhabditis elegans. PLoS ONE 2010, 5, e8857. [Google Scholar] [CrossRef]
- Gravato-Nobre, M.J.; Vaz, F.; Filipe, S.; Chalmers, R.; Hodgki, J. The Invertebrate Lysozyme Effector ILYS-3 Is Systemically Activated in Response to Danger Signals and Confers Antimicrobial Protection in C. elegans. PLoS Pathog. 2016, 12, e1005826. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Balamurugan, K. Physiological and Immunological Regulations in Caenorhabditis elegans Infected with Salmonella enterica serovar. Typhi. Indian J. Microbiol. 2014, 54, 52–58. [Google Scholar] [CrossRef]
- Fontaine, P.; Choe, K. The transcription factor SKN-1 and detoxification gene ugt-22 alter albendazole efficacy in Caenorhabditis elegans. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Muroga, K.; Higashi, M.; Keitoku, H. The isolation of intestinal microflora of farmed red seabream (Pagrus major) and black seabream (Acanthopagrus schlegeli) at larval and juvenile stages. Aquaculture 1987, 65, 79–88. [Google Scholar] [CrossRef]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef]
- Englen, M.D.; Kelley, L.C. A rapid DNA isolation procedure for the identification of Campylobacter jejuni by the polymerase chain reaction. Lett. Appl. Microbiol. 2000, 31, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Gong, J.; Yu, H.; Jin, Y.; Zhu, J.; Han, Y.M. Identification of changes in the composition of ileal bacterial microbiota of broiler chickens infected with Clostridium perfringens. Vet. Microbiol. 2010, 140, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Wang, Y.; Li, J.; Du, Z.J.; Chen, G.J. Saccharicrinis carchari sp. nov., isolated from a shark, and emended descriptions of the genus Saccharicrinis and Saccharicrinis fermentans. Int. J. Syst. Evol. Microbiol. 2014, 64, 2204–2209. [Google Scholar] [CrossRef]
- Wang, N.N.; Li, C.M.; Li, Y.X.; Du, Z.J. Aquimarina celericrescens sp. nov., isolated from seawater. Int. J. Syst. Evol. Microbiol. 2018, 68, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Terceti, M.S.; Vences, A.; Matanza, X.M.; Dalsgaard, I.; Pedersen, K.; Osorio, C.R. Molecular Epidemiology of Photobacterium damselae subsp. damselae Outbreaks in Marine Rainbow Trout Farms Reveals Extensive Horizontal Gene Transfer and High Genetic Diversity. Front. Microbiol. 2018, 9, 2155. [Google Scholar] [CrossRef] [PubMed]
- Tulumoğlu, Ş.; Kaya, H.; Şimşek, Ö. Probiotic characteristics of Lactobacillus fermentum strains isolated from tulum cheese. Anaerobe 2014, 30, 120–125. [Google Scholar] [CrossRef]
- Arihara, K.; Ota, H.; Itoh, M.; Kondo, Y.; Sameshima, T.; Yamanaka, H.; Akimoto, M.; Kanai, S.; Miki, T. Lactobacillus acidophilus Group Lactic Acid Bacteria Applied to Meat Fermentation. J. Food Sci. 2010, 63, 544–547. [Google Scholar] [CrossRef]
- Durai, S.; Pandian, S.K.; Balamurugan, K. Establishment of a Caenorhabditis elegans infection model for Vibrio alginolyticus. J. Basic Microbiol. 2011, 51, 243–252. [Google Scholar] [CrossRef]
- Du, Z.J.; Wang, Y.; Dunlap, C.; Rooney, A.P.; Chen, G.J. Draconibacteriumorientale gen. nov., sp. nov., isolated from two distinct marineenvironments, and proposal of Draconibacteriaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Song, S.L.; Tao, C.C.; Fang, S.G. Safety and toxicology evaluation on probiotics powder Mix-G200. Chin. J. Microecol. 2011, 23, 417–422. [Google Scholar]
- Grompone, G.; Martorell, P.; Llopis, S.; González, N.; Genovés, S.; Mulet, A.P.; Fernández-Calero, T.; Tiscornia, I.; Bollati-Fogolín, M.; Chambaud, I.; et al. Anti-inflammatory Lactobacillus rhamnosus CNCM I-3690 strain protects against oxidative stress and increases lifespan in Caenorhabditis elegans. PLoS ONE 2012, 7, e52493. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Gong, J.; Yu, H.; Pacan, J.C.; Niu, Z.X.; Si, W.D.; Sabour, P.M. Use of Caenorhabditis elegans for preselecting Lactobacillus isolates to control Salmonella Typhimurium. J. Food Prot. 2011, 74, 86–93. [Google Scholar] [CrossRef]
- Gumienny, T.L.; Lambie, E.; Hartwieg, E.; Horvitz, H.R.; Hengartner, M.O. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 1999, 126, 1011–1022. [Google Scholar] [PubMed]
- Li, Y.X.; Na, K.; Lee, H.J.; Lee, E.Y.; Paik, Y.K. Contribution of sams-1 and pmt-1 to lipid homoeostasis in adult Caenorhabditis elegan. J. Biochem. 2011, 149, 529–538. [Google Scholar] [CrossRef] [PubMed]
| Strain Number | Source # | Putative Identity | Survival Rate (%) |
|---|---|---|---|
| M0102 | C | Marinobacter hydrocarbonoclasticus 98.8% | 0.00 k |
| ML1206 | C | Planococcus maritimus 99% | 63.57 ± 0.79 a |
| ML1210 | C | Sulfitobacter pontiacus 98.3% | 11.32 ± 0.35 g |
| ML1229 | C | Ruegeria atlantica 99.3% | 20.02 ± 0.35 d |
| YLY02 | D | Vibrio alfacsensis 99.5% | 0.00 k |
| YLY03 | D | Sunxiuqinia elliptica 99.1% | 4.35 ± 0.29 h |
| YLY04 | D | Maribius pontilimi 95.33% | 12.37 ± 0.33 f |
| YLY05 | D | Idiomarina sediminum 98.9% | 30.75 ± 0.34 b |
| YLY06 | D | Vibrio orientalis 100% | 1.56 ± 0.11 j |
| YLY07 | D | Brumimicrobium mesophilum 95.2% | 0.00 k |
| YLY08 | D | Oceaniglobus indicus 93.4% | 0.00 k |
| YLY09 | D | Nitratireductor aquimarinus 98.1% | 20.18 ± 0.25 d |
| YLY10 | D | Microbacterium esteraromaticum 98.1% | 1.47 ± 0.23 j |
| YLY20 | D | Ornithinimicrobium kibberense 95.7% | 0.00 k |
| YLY21 | D | Litoreibacter arenae 99.8% | 1.98 ± 0.32 j |
| YLY25 | D | Marinobacter pelagius 98.9% | 0.00 k |
| YLY26 | D | Stappia stellulata 99.1% | 0.00 k |
| YLY27 | D | Idiomarina aestuarii 99.4% | 3.54 ± 0.27 i |
| YLY28 | D | Roseovarius halotolerans 98.9% | 22.45 ± 0.72 c |
| YLY32 | D | Halomonas aestuarii 96.8% | 0.00 k |
| E. coli OP50 | 0.00 k |
| Gene Name | Accession Number | Size (bp) | Description | Forward_Seq (5′ to 3′) | Reverse_Seq (5′ to 3′) | Efficiency (%) |
|---|---|---|---|---|---|---|
| act-1 * | NM_073418.9 | 121 | Actin-1 | CCCCACTCAATCCAAAGGCT | GTACGTCCGGAAGCGTAGAG | 100.0 |
| ilys-3 | NM_067805.5 | 194 | Invertebrate-type lysozyme 3 | CCGGAGAAACAACTGAAGCC | TGTGGTTACGAGCCATCACT | 101.0 |
| clec-85 | NM_001383060.2 | 121 | C-type LECtin | CCAATGGGATGACGGAACCA | CTTCTGTCCAGCCAACGTCT | 103.4 |
| acs-1 | NM_001392534.1 | 186 | Fatty Acid CoA Synthetase family | CTTATTCGCAAGTCGCCACA | CAAGAGCACTGGCAAACTGT | 100.2 |
| spp-12 | NM_074042.3 | 123 | SaPosin-like Protein family | AGGAAGCTGGAGATGTTGCT | AGATGTCATGCTCAGCCACT | 104 |
| clec-60 | NM_063858.4 | 199 | C-type LECtin | TGGTGGACAACTCGATTGGA | CCGCAGCTTTGTTGTAGGTT | 99.8 |
| clec-71 | NM_068039.4 | 227 | C-type LECtin | TTGGCTGTTGTAGGCAATCAA | TCACTGGGAATCCGTTATCC | 99.9 |
| ugt-22 | NM_070232.6 | 168 | UDP-glucuronosyltransferase | ACATGTTGAAACGGCATGGT | TGTCCTTTGGATTCGTTGGC | 102.5 |
| cyp-35A3 | NM_071720.6 | 185 | Cytochrome P450 family | GGGCCTATTTCCTTCCCACT | CGGAACTGGACCAACCCATA | 100.9 |
| clc-1 | NM_077446.6 | 175 | CLaudin-like in Caenorhabditis | TGAAATGTGTCGTCGTCTGC | TGCAAAGAGGGTGAGTGGAT | 98.1 |
| cyp-14A3 | NM_077804.4 | 133 | Chrome P450 family | TTGCGTTGGTGAAGGATTGG | TGGCTTTGTTGTCAGAACCG | 101.6 |
| lys-7 | NM_071571.9 | 153 | Lysozyme-like protein 7 | GTACAGCGGTGGAGTCACTG | GCCTTGAGCACATTTCCAGC | 96.6 |
| fipr-22 | NM_061069.5 | 184 | Fungus-Induced Protein Related Cytochrome P450 family | ATCGGAGCATTCTGTGCAAC | CATACTGGATTGGGCTTCCG | 105.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-X.; Wang, N.-N.; Zhou, Y.-X.; Lin, C.-G.; Wu, J.-S.; Chen, X.-Q.; Chen, G.-J.; Du, Z.-J. Planococcus maritimus ML1206 Isolated from Wild Oysters Enhances the Survival of Caenorhabditis elegans against Vibrio anguillarum. Mar. Drugs 2021, 19, 150. https://doi.org/10.3390/md19030150
Li Y-X, Wang N-N, Zhou Y-X, Lin C-G, Wu J-S, Chen X-Q, Chen G-J, Du Z-J. Planococcus maritimus ML1206 Isolated from Wild Oysters Enhances the Survival of Caenorhabditis elegans against Vibrio anguillarum. Marine Drugs. 2021; 19(3):150. https://doi.org/10.3390/md19030150
Chicago/Turabian StyleLi, Ying-Xiu, Nan-Nan Wang, Yan-Xia Zhou, Chun-Guo Lin, Jing-Shan Wu, Xin-Qi Chen, Guan-Jun Chen, and Zong-Jun Du. 2021. "Planococcus maritimus ML1206 Isolated from Wild Oysters Enhances the Survival of Caenorhabditis elegans against Vibrio anguillarum" Marine Drugs 19, no. 3: 150. https://doi.org/10.3390/md19030150
APA StyleLi, Y.-X., Wang, N.-N., Zhou, Y.-X., Lin, C.-G., Wu, J.-S., Chen, X.-Q., Chen, G.-J., & Du, Z.-J. (2021). Planococcus maritimus ML1206 Isolated from Wild Oysters Enhances the Survival of Caenorhabditis elegans against Vibrio anguillarum. Marine Drugs, 19(3), 150. https://doi.org/10.3390/md19030150






