3.1. Chemistry
3.1.1. General Procedure
Chemical reagents and solvents, purchased from commercial sources, were of analytical grade and were used without further purification. All air-sensitive reactions were run under a nitrogen atmosphere. All the reactions were monitored by TLC on recoated silica gel G plates at 254 nm under a UV lamp using ethyl acetate/
n-hexane as the eluent. Column chromatography was performed on a glass column packed with silica gel (200–300 mesh) using ethyl acetate/
n-hexane as the eluent. Melting points were measured on an SGW X-1 microscopic melting point apparatus. Proton nuclear magnetic resonance (
1H NMR) and carbon nuclear magnetic resonance (
13C NMR) spectra were recorded in DMSO-
d6, CD
3OD, or CDCl
3 on a Bruker AV 400 MHz spectrometer. Chemical shifts were reported in
δ (ppm) units relative to the internal standard tetramethylsilane (TMS). Mass spectra and HRMS were obtained on a Waters Quattro Micromass instrument and a Bruker Compact instrument, respectively, using electrospray ionization (ESI) techniques. The purities of the target compounds were ≥95%, measured by HPLC, performed on an Agilent 1260 HPLC system with a UV detector and an Agilent Eclipse Plus C18 column (150 × 4.6 mm, 5 μm), eluting with a mixture of solvents acetonitrile and water. (a) 0–12 min, 50–95% ACN; (b) 12–16 min, 95% ACN; (c) 16–16.01 min, 95–50% ACN; (d) 16.01–20 min, 50% ACN. Peaks were detected at λ 254 nm with a flow rate of 1.0 mL/min. The final structures were fully characterized by
1H NMR,
13C NMR and HRMS (see
Supplementary Materials).
3.1.2. Synthesis of 1-(5-Bromo-1H-indol-3-yl) Ethanone 2
To a solution of 5-bromoindole 1 (5.00 g, 25.5 mmol, 1.00 eq) in 25 mL of anhydrous toluene was added (4.00 g, 51.0 mmol, 2.00 eq) acetyl chloride at 0 °C. The resulting mixture was stirred for 15 min at 0 °C, and a solution of SnCl4 (13.3 g, 51.0 mmol, 2.00 eq) in 24 mL anhydrous toluene was added. The resulting solution was stirred for 2 h at 0 °C, and 75 mL of 8% NaHCO3 was added dropwise. The resulting slurry was diluted with 150 mL of ethyl acetate, dried (Na2SO4), and filtered. The solvent was removed with a rotary evaporator. The crude product was purified by column chromatography to afford the compound 2. Yield: 87%, brick red solid, mp 250.4–251.3 °C. 1H NMR (400 MHz, CD3OD) δ 8.38 (d, J = 2.0 Hz, 1H), 8.17 (s, 1H), 7.45–7.23 (m, 2H), 2.51 (s, 3H).
3.1.3. Synthesis of 1-(5-Bromo-1-tosyl-1H-indol-3-yl) Ethanone 3
To the solution of 5-bromo-3-acetyl indole 2 (5.00 g, 21.0 mmol, 1.00 eq) in dichloromethane was added DMAP (0.128 g, 1.05 mmol, 0.0500 eq), p-toluenesulfonyl choride (TsCl, 4.40 g, 23.1 mmol, 1.10 eq), and N,N-diisopropylethylamine (DIPEA, 4.07 g, 31.5 mmol, 1.50 eq). The mixture was stirred at room temperature for 20 h. The reaction was then quenched by the addition of 10% HCl. Dichloromethane was added to the reaction mixture. The organic layer was separated, dried over anhydrous sodium sulphate, and concentrated to get a crude compound. The crude product was purified by column chromatography to afford the compound 3. Yield: 70%, white solid, mp 140.6–141.5 °C. 1H NMR (400 MHz, CDCl3) δ 8.47 (d, J = 2.0 Hz, 1H), 8.15 (s, 1H), 7.79 (s, 1H), 7.76 (d, J = 7.3 Hz, 2H), 7.44 (d, J = 8.9 Hz, 1H), 7.27 (d, J = 8.1 Hz, 2H), 2.53 (s, 3H), 2.36 (s, 3H).
3.1.4. Synthesis of 1-(5-Bromo-1-tosyl-1H-indol-3-yl)-3-(dimethylamino) prop-2-en-1-one 4
1-(5-bromo-1-tosyl-1H-indol-3-yl) ethanone 3 (4.00 g, 10.2 mmol, 1.00 eq) was taken in anhydrous N,N-dimethylformamide (DMF, 40 mL), and a solution of dimethyl formamide-dimethylacetal (DMF-DMA, 1.82 g, 15.3 mmol, 1.50 eq) was added to the same solvent (4 mL). The resultant solution was heated at 110 °C for 4 h under a N2 atmosphere. After cooling, the solution was poured into water and then extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulphate and concentrated to dryness under reduced pressure. The crude product was purified by column chromatography to afford the compound 4. Yield: 52%, pale yellow solid, mp 176.7–178.5 °C. 1H NMR (400 MHz, CDCl3) δ 8.44 (s, 1H), 7.96 (s, 1H), 7.73–7.64 (m, 4H), 7.37–7.29 (m, 1H), 7.16 (d, J = 8.1 Hz, 2H), 5.46 (d, J = 12.3 Hz, 1H), 3.07 (s, 3H), 2.87 (s, 3H), 2.27 (s, 3H).
3.1.5. Synthesis of Meridianin C 5
A mixture of 1-(5-bromo-1-tosyl-1H-indol-3-yl)-3-(dimethylamino) prop-2-en-1-one 4 (2.00 g, 4.47 mmol, 1.00 eq), guanidine hydrochloride (0.64 g, 6.71 mmol, 1.50 eq), anhydrous K2CO3 (1.24 g, 8.94 mmol, 2.00 eq), and 2-methoxyethanol (20 mL) was heated at reflux temperature for 24 h under a N2 atmosphere. After cooling, the solution was poured into water and then extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulphate and concentrated to dryness under reduced pressure. The crude product was purified by column chromatography to afford the compound 5. Yield: 51%, pale yellow solid, mp 105.4–106.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.86 (s, 1H), 8.75 (s, 1H), 8.25 (s, 1H), 8.11 (s, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.29 (d, J = 8.8 Hz, 1H), 7.00 (s, 1H), 6.49 (s, 2H).
3.1.6. Synthesis of 4-(5-bromo-1H-indol-3-yl)-5-iodopyrimidin-2-amine 6
N-Iodosuccinimide (NIS, 0.490 g, 2.17 mmol, 1.10 eq) was added to a solution of meridianin C 5 (0.570 g, 1.97 mmol, 1.00 eq) in anhydrous N,N-dimethylformamide (DMF, 6 mL). The reaction was stirred at room temperature for 1.5 h before concentrating to remove the solvent. The residue was partitioned between ethyl acetate (100 mL) and Na2S2O3 (100 mL). The organics were washed with NaHCO3 (80 mL) and brine (10 mL), dried over anhydrous sodium sulphate, and concentrated under reduced pressure. The crude product was purified by column chromatography to afford the compound 6. Yield: 87%, pale yellow solid, mp 203.5–204.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.85 (s, 1H), 8.51 (s, 1H), 8.46 (s, 1H), 8.39 (d, J = 2.0 Hz, 1H), 7.45 (d, J = 8.7 Hz, 1H), 7.30 (dd, J = 8.7, 2.1 Hz, 1H), 6.79 (s, 2H).
3.1.7. Synthesis of 5-(4-aminophenyl)-4-(5-bromo-1H-indol-3-yl) pyrimidin-2-amine 8
A mixture of compound 6 (0.500 g, 1.20 mmol, 1.00 eq), 4-aminophenylboronic acid pinacol ester 7 (0.317 g, 1.45 mmol, 1.20 eq), and Na2CO3 (0.319 g, 3.01 mmol, 2.50 eq) under a nitrogen atmosphere was treated with DME (9 mL), water (3 mL), and Pd (dppf) Cl2CH2Cl2 (0.0500 g, 0.0600 mmol, 0.0500 eq). The mixture was purged with bubbling nitrogen for 2 min and then stirred at 85 °C for 16 h, cooled to room temperature, and partitioned between ethyl acetate and water. The aqueous layer was extracted with ethyl acetate twice, and the combined organic layers were washed with brine, dried over anhydrous sodium sulphate, filtered, and concentrated. The crude product was purified by column chromatography to afford the compound 8. Yield: 43%, pale yellow solid, mp 217.7–218.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.49 (s, 1H), 8.65 (s, 1H), 7.94 (s, 1H), 7.32 (d, J = 8.5 Hz, 1H), 7.24 (d, J = 8.5 Hz, 1H), 6.89 (d, J = 7.9 Hz, 2H), 6.74 (s, 1H), 6.61 (d, J = 8.0 Hz, 2H), 6.55 (s, 2H), 5.33 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 161.55, 160.62, 156.92, 148.17, 134.70, 130.90, 130.22, 128.23, 125.40, 125.19, 124.80, 121.82, 114.52, 113.68, 113.45, 112.81. HRMS calcd for C18H15BrN5+ [M + H] + 380.0505, found 380.0504.
3.1.8. Synthesis of 5-bromo-3-(2-chloropyrimidin-4-yl)-1H-indole 10
To the solution of 2,4-dichloropyrimidine (3.04 g, 20.4 mmol, 1.00 eq) in dichloroethane (20 mL) was added aluminum chloride (AlCl3, 3.26 g, 24.5 mmol, 1.20 eq) under a N2 atmosphere, and the mixture was stirred for 5 min. 5-Bromoindole 1 (4.00 g, 20.4 mmol, 1.00 eq) was added, and the mixture was heated at 85 °C for 3 h. After completion of the reaction, the reaction mixture was cooled at room temperature and poured into ice-cold water (30 mL) with continuous stirring for 20 min. The crude products were isolated by extracting with ethyl acetate. The organic phase was separated and washed with brine, dried over sodium sulphate, and evaporated under vacuum. The crude products obtained were further purified by silica gel column chromatography using an ethyl acetate-hexane mixture. Yield: 63%, pale yellow solid, mp 260.5–261.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.25 (s, 1H), 8.57 (d, J = 2.6 Hz, 2H), 8.55 (d, J = 5.6 Hz, 1H), 7.92 (d, J = 5.5 Hz, 1H), 7.47 (d, J = 8.7 Hz, 1H), 7.36 (dd, J = 8.6, 1.9 Hz, 1H).
General Procedure for the Preparation of the Final Compounds A1–29
To a solution of 5-(4-aminophenyl)-4-(5-bromo-1H-indol-3-yl) pyrimidin-2-amine 8 (200 mg, 0.530 mmol, 1.00 eq) in pyridine (5 mL), the appropriate substituted benzoyl chloride derivative (0.680 mmol, 1.30 eq) was added and the mixture stirred for 1 h. The solution was poured into ice-cold water and then extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulphate and concentrated to dryness under reduced pressure. The crude product was purified by column chromatography to afford the target compounds.
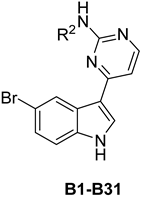
General Procedure for the Preparation of the Final Compounds B1–31
p-Toluene sulfonic acid hydrate (203 mg, 1.07 mmol, 1.10 eq) was added in one portion to a mixture of substituted aromatic amine (0.972 mmol, 1.00 eq) and compound 10 (300 mg, 0.972 mmol, 1.00 eq) in 2-pentanol (10 mL). The resulting mixture was then stirred at 125 °C for 18 h. the solution was poured into ice-cold water and then extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulphate and concentrated to dryness under reduced pressure. The crude product was purified by column chromatography to afford the target compounds.
3.1.9. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) Propionamide A1
Yield: 30%, pale yellow solid, mp 193.6–194.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.36 (s, 1H), 9.86 (s, 1H), 8.44 (s, 1H), 7.87 (s, 1H), 7.50 (d, J = 8.2 Hz, 2H), 7.19 (d, J = 8.6 Hz, 1H), 7.11 (d, J = 8.6 Hz, 1H), 7.05 (d, J = 8.1 Hz, 2H), 6.53 (d, J = 17.5 Hz, 3H), 2.21 (q, J = 7.5 Hz, 2H), 0.96 (t, J = 7.5 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 172.51, 162.91, 159.91, 158.66, 139.03, 134.97, 133.45, 130.61, 130.24, 128.37, 125.20, 124.99, 121.22, 119.67, 113.98, 113.55, 112.93, 30.01, 10.16. HRMS calcd for C21H19BrN5O+ [M + H] + 436.0767, found 437.0765. HPLC purity 98.27% (tR = 10.3 min).
3.1.10. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) Butyramide A2
Yield: 33%, pale yellow solid, mp 187.6–188.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.44 (s, 1H), 9.94 (s, 1H), 8.55 (s, 1H), 7.99 (s, 1H), 7.63 (d, J = 8.1 Hz, 2H), 7.32 (d, J = 8.6 Hz, 1H), 7.23 (d, J = 8.1 Hz, 1H), 7.17 (d, J = 8.1 Hz, 2H), 6.69 (s, 1H), 6.60 (s, 2H), 2.30 (t, J = 7.3 Hz, 2H), 1.62 (m, 2H), 0.92 (t, J = 7.3 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 170.76, 162.02, 159.01, 157.80, 138.08, 134.09, 132.63, 129.72, 129.34, 127.49, 124.30, 124.10, 120.38, 118.84, 113.06, 112.66, 112.09, 37.95, 18.15, 13.23. HRMS calcd for C22H21BrN5O+ [M + H] + 450.0924, found 450.0920. HPLC purity 95.59% (tR = 11.0 min).
3.1.11. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) Isobutyramide A3
Yield: 33%, pale yellow solid, mp 172.6–173.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.44 (s, 1H), 9.90 (s, 1H), 8.57 (s, 1H), 8.00 (s, 1H), 7.65 (d, J = 8.1 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.25 (d, J = 6.6 Hz, 1H), 7.18 (d, J = 8.2 Hz, 2H), 6.71 (s, 1H), 6.61 (s, 2H), 2.61 (m, 1H), 1.12 (d, J = 6.8 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 174.83, 162.03, 159.01, 157.81, 138.19, 134.09, 132.64, 129.74, 129.32, 127.50, 124.31, 124.11, 120.38, 118.94, 113.08, 112.67, 112.08, 34.54, 19.11. HRMS calcd for C22H21BrN5O+ [M + H] + 450.0924, found 450.0917. HPLC purity 95.64% (tR = 11.0 min).
3.1.12. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) Pivalamide A4
Yield: 35%, pale yellow solid, mp 139.6–140.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.43 (s, 1H), 9.26 (s, 1H), 8.57 (s, 1H), 7.97 (s, 1H), 7.67 (d, J = 8.3 Hz, 2H), 7.30 (d, J = 8.6 Hz, 1H), 7.25–7.20 (m, 1H), 7.15 (d, J = 8.2 Hz, 2H), 6.67 (s, 1H), 6.61 (s, 2H), 1.21 (s, 9H). 13C NMR (100 MHz, DMSO-d6) δ 176.73, 162.69, 159.66, 158.48, 138.81, 134.75, 133.48, 130.41, 129.76, 128.17, 125.00, 124.79, 121.01, 120.57, 113.75, 113.35, 112.72, 31.39, 27.46. HRMS calcd for C23H23BrN5O+ [M + H] + 464.1080, found 464.1079. HPLC purity 96.11% (tR = 11.1 min).
3.1.13. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-phenylacetamide A5
Yield: 35%, pale yellow solid, mp 207.6–208.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.42 (s, 1H), 10.26 (s, 1H), 8.57 (s, 1H), 8.00 (s, 1H), 7.64 (d, J = 8.3 Hz, 2H), 7.36 (d, J = 2.0 Hz, 2H), 7.33 (d, J = 3.6 Hz, 2H), 7.31 (d, J = 4.5 Hz, 1H), 7.30–7.20 (m, 2H), 7.20 (d, J = 8.2 Hz, 2H), 6.68 (s, 1H), 6.62 (s, 2H), 3.66 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 169.13, 162.46, 159.44, 158.19, 138.38, 135.98, 134.49, 133.36, 130.16, 129.84, 129.11, 128.32, 127.90, 126.56, 124.72, 124.53, 120.73, 119.35, 113.49, 113.09, 112.47, 43.40. HRMS calcd for C26H21BrN5O+ [M + H] + 498.0924, found 498.0924. HPLC purity 95.64% (tR = 11.8 min).
3.1.14. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) Benzamide A6
Yield: 43%, pale yellow solid, mp 187.9–188.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.10 (s, 1H), 10.13 (s, 1H), 9.21 (d, J = 2.0 Hz, 1H), 8.52 (s, 1H), 8.47 (d, J = 7.5 Hz, 2H), 8.37 (d, J = 8.5 Hz, 2H), 8.03 (d, J = 7.3 Hz, 1H), 7.97 (t, J = 7.5 Hz, 2H), 7.82 (d, J = 8.6 Hz, 1H), 7.76 (d, J = 8.5 Hz, 2H), 7.71 (dd, J = 8.6, 2.0 Hz, 1H), 7.32 (s, 1H), 6.55 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 175.94, 173.23, 170.46, 168.93, 149.17, 145.75, 145.40, 144.73, 141.90, 140.83, 140.34, 138.92, 138.81, 137.84, 135.79, 135.21, 132.19, 130.81. HRMS calcd for C25H19BrN5O+ [M + H] + 484.0767, found 484.0770. HPLC purity 95.56% (tR = 12.6 min).
3.1.15. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-fluorobenzamide A7
Yield: 37%, pale yellow solid, mp 159.6–160.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.51 (s, 1H), 8.57 (s, 1H), 8.01 (s, 1H), 7.77 (d, J = 8.1 Hz, 2H), 7.69 (s, 2H), 7.59 (d, J = 6.5 Hz, 1H), 7.36 (q, J = 8.3, 7.2 Hz, 3H), 7.24 (t, J = 8.1 Hz, 3H), 6.77 (s, 1H), 6.61 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 175.41, 163.29, 162.95, 160.57, 159.97, 158.71, 158.10, 138.52, 135.13, 134.52, 132.97 (d, J = 9.2 Hz), 130.81, 130.34, 128.41, 125.51 (d, J = 15.3 Hz), 125.10 (d, J = 8.8 Hz), 124.97 (d, J = 11.1 Hz), 121.11, 120.43, 116.63 (d, J = 21.7 Hz), 114.13, 113.53, 112.81. HRMS calcd for C25H18BrFN5O+, [M + H] + 502.0673, found 502.0668. HPLC purity 95.43% (tR = 12.0 min).
3.1.16. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidiN-5-yl) phenyl)-3-fluorobenzamide A8
Yield: 41%, pale yellow solid, mp 183.6–184.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 1H), 10.40 (s, 1H), 8.57 (d, J = 2.0 Hz, 1H), 8.04 (s, 1H), 7.85–7.76 (m, 4H), 7.64–7.56 (m, 1H), 7.49–7.43 (m, 1H), 7.33 (d, J = 8.6 Hz, 1H), 7.29–7.24 (m, 3H), 6.75 (d, J = 2.9 Hz, 1H), 6.63 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.23, 163.18, 162.51, 160.74, 159.46, 158.31, 138.07, 134.57, 134.10, 130.65 (d, J = 7.9 Hz), 130.16, 129.79, 127.93, 124.73, 124.59, 123.92 (d, J = 2.6 Hz), 120.75, 120.64 (d, J = 3.1 Hz), 114.63, 114.40, 113.55, 113.14, 112.54. HRMS calcd for C25H18BrFN5O+, [M + H] + 502.0673, found 502.0673. HPLC purity 98.18% (tR = 12.2 min).
3.1.17. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-fluorobenzamide A9
Yield: 38%, pale yellow solid, mp 178.6–179.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.35 (s, 1H), 8.58 (d, J = 2.0 Hz, 1H), 8.07–8.03 (m, 3H), 7.82 (d, J = 8.4 Hz, 2H), 7.38 (t, J = 8.8 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.26 (d, J = 8.5 Hz, 3H), 6.74 (d, J = 2.9 Hz, 1H), 6.63 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.76, 164.94, 163.29, 162.92, 159.87, 158.72, 138.68, 134.98, 134.32, 131.81 (d, J = 3.0 Hz), 130.85 (d, J = 9.2 Hz), 130.57, 130.18, 128.35, 125.07 (d, J = 14.9 Hz), 121.20, 120.98, 115.80 (d, J = 22.0 Hz), 113.96, 113.55, 112.96. HRMS calcd for C25H18BrFN5O+ [M + H] + 502.0673, found 502.0669. HPLC purity 95.97% (tR = 12.1 min).
3.1.18. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-chlorobenzamide A10
Yield: 43%, pale yellow solid, mp 211.5–212.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 1H), 10.59 (s, 1H), 8.57 (s, 1H), 8.03 (s, 1H), 7.76 (d, J = 8.2 Hz, 2H), 7.62–7.57 (m, 2H), 7.53 (d, J = 7.9 Hz, 1H), 7.49 (d, J = 5.8 Hz, 1H), 7.46 (d, J = 7.3 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.26 (d, J = 8.2 Hz, 2H), 6.77 (d, J = 2.9 Hz, 1H), 6.64 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.55, 162.09, 159.02, 157.94, 137.64, 136.55, 134.15, 133.64, 130.73, 129.80, 129.52, 129.49, 129.28, 128.54, 127.52, 126.89, 124.30, 124.17, 120.32, 119.45, 113.14, 112.72, 112.09. HRMS calcd for C25H18BrFN5O+ [M + H] + 518.0378, found 518.0381. HPLC purity 96.64% (tR = 12.1 min).
3.1.19. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3-chlorobenzamide A11
Yield: 36%, pale yellow solid, mp 204.8–205.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.46 (s, 1H), 10.42 (s, 1H), 8.55 (s, 1H), 8.01 (d, J = 7.0 Hz, 2H), 7.92 (d, J = 7.7 Hz, 1H), 7.80 (d, J = 8.1 Hz, 2H), 7.66 (d, J = 7.3 Hz, 1H), 7.57 (t, J = 7.8 Hz, 1H), 7.32 (d, J = 8.6 Hz, 1H), 7.28–7.23 (m, 3H), 6.73 (d, J = 2.8 Hz, 1H), 6.62 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.13, 162.51, 159.44, 158.35, 158.31, 138.06, 136.91, 134.57, 134.11, 133.25, 131.44, 130.46, 130.15, 129.78, 127.43, 126.52, 124.72, 124.58, 120.74, 120.60, 113.54, 113.13, 112.53. HRMS calcd for C₂₅H₁₈BrClN₅O+, [M + H] + 518.0378, found 518.0371. HPLC purity 95.15% (tR = 13.1 min).
3.1.20. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-chlorobenzamide A12
Yield: 42%, pale yellow solid, mp 261.7–262.5 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.40 (s, 1H), 8.58 (d, J = 2.2 Hz, 1H), 8.04 (s, 1H), 8.01 (d, J = 8.6 Hz, 2H), 7.82 (d, J = 8.3 Hz, 2H), 7.62 (d, J = 8.7 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.27 (d, J = 8.4 Hz, 3H), 6.74 (s, 1H), 6.64 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.70, 162.71, 159.65, 158.49, 138.36, 136.65, 134.76, 134.21, 133.85, 130.35, 129.97, 129.86, 128.70, 128.13, 124.93, 124.77, 120.96, 120.78, 113.73, 113.33, 112.74. HRMS calcd for C₂₅H₁₈BrClN₅O+ [M + H] + 518.0378, found 518.0385. HPLC purity 96.20% (tR = 12.9 min).
3.1.21. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-bromobenzamide A13
Yield: 46%, pale yellow solid, mp 147.4–148.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.49 (s, 1H), 10.58 (s, 1H), 8.59 (s, 1H), 8.04 (s, 1H), 7.80–7.71 (m, 3H), 7.61–7.57 (m, 1H), 7.52 (t, J = 7.5 Hz, 1H), 7.44 (td, J = 7.7, 1.9 Hz, 1H), 7.35 (d, J = 8.7 Hz, 1H), 7.29–7.25 (m, 3H), 6.78 (d, J = 2.8 Hz, 1H), 6.65 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.38, 162.07, 158.96, 157.92, 138.68, 137.64, 134.11, 133.60, 132.31, 130.78, 129.75, 129.45, 128.44, 127.50, 127.32, 124.28, 124.13, 120.28, 119.42, 118.57, 113.09, 112.68, 112.06. HRMS calcd for C₂₅H₁₈Br₂N₅O+, [M + H] + 561.9873, found 561.9870. HPLC purity 96.11% (tR = 12.1min).
3.1.22. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3-bromobenzamide A14
Yield: 38%, pale yellow solid, mp 176.6–177.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 1H), 10.44 (s, 1H), 8.58 (d, J = 2.1 Hz, 1H), 8.20–8.13 (m, 1H), 8.04 (s, 1H), 7.98 (d, J = 7.8 Hz, 1H), 7.86–7.77 (m, 3H), 7.52 (t, J = 7.8 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.31–7.23 (m, 3H), 6.75 (d, J = 2.9 Hz, 1H), 6.64 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.40, 162.91, 159.82, 158.70, 138.45, 137.50, 134.95, 134.73, 134.51, 131.08, 130.66, 130.54, 130.17, 128.32, 127.29, 125.12, 124.96, 122.11, 121.12, 120.98, 113.92, 113.51, 112.93. HRMS calcd for C₂₅H₁₈Br₂N₅O+, [M + H] + 561.9873, found 561.9870. HPLC purity 97.54% (tR = 13.3 min).
3.1.23. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-bromobenzamide A15
Yield: 48%, pale yellow solid, mp 276.4–277.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.40 (s, 1H), 8.57 (d, J = 2.0 Hz, 1H), 8.03 (s, 1H), 7.93 (d, J = 8.5 Hz, 2H), 7.82 (d, J = 8.3 Hz, 2H), 7.76 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.5 Hz, 1H), 7.26 (d, J = 8.5 Hz, 3H), 6.74 (d, J = 2.9 Hz, 1H), 6.63 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.02, 162.91, 159.84, 158.69, 138.55, 134.96, 134.41, 132.53, 131.85, 130.55, 130.24, 130.17, 128.33, 125.78, 125.13, 124.97, 121.15, 120.97, 113.93, 113.52, 112.94. HRMS calcd for C₂₅H₁₈Br₂N₅O+ [M + H] + 561.9873, found 561.9878. HPLC purity 95.23% (tR = 13.1 min).
3.1.24. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-(trifluoromethyl) Benzamide A16
Yield: 20%, pale yellow solid, mp 163.6–164.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.60 (s, 1H), 10.70 (s, 1H), 8.58 (d, J = 2.0 Hz, 1H), 8.04 (s, 1H), 7.86 (d, J = 7.8 Hz, 1H), 7.81 (t, J = 7.5 Hz, 1H), 7.77–7.69 (m, 4H), 7.35 (d, J = 8.6 Hz, 1H), 7.29–7.25 (m, 3H), 6.79 (s, 1H), 6.68 (s, 2H).13C NMR (100 MHz, DMSO-d6) δ 165.69, 162.57, 159.50, 158.45, 138.10, 136.24, 134.65, 134.19, 132.74, 130.30, 130.17, 129.98, 128.62, 126.45 (q, J = 4.9 Hz), 125.24(q, J = 274.0 Hz),126.05, 125.74, 124.78, 124.64, 120.75, 119.99, 113.65, 113.21, 112.53. HRMS calcd for C₂₆H₁₈BrF₃N₅O+ [M + H] +552.0641, found 552.0628. HPLC purity 96.07% (tR = 12.1 min).
3.1.25. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3-(trifluoromethyl) Benzamide A17
Yield: 43%, pale yellow solid, mp 172.6–173.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.49 (s, 1H), 10.57 (s, 1H), 8.58 (s, 1H), 8.37–8.23 (m, 2H), 8.06 (s, 1H), 7.97 (d, J = 7.9 Hz, 1H), 7.84 (d, J = 8.5 Hz, 2H), 7.79 (d, J = 7.9 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.29 (d, J = 8.3 Hz, 2H), 7.26 (d, J = 9.0 Hz, 1H), 6.77 (s, 1H), 6.65 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.09, 162.53, 159.46, 158.32, 137.98, 135.81, 134.58, 134.23, 131.86, 130.15, 129.78 (d, J = 4.8 Hz), 129.39, 129.07, 128.75, 128.18 (d, J = 3.6 Hz), 127.93, 126.49, 125.35(q, J = 273.7 Hz),124.65 (d, J = 14.9 Hz), 124.28 (q, J = 3.6 Hz), 120.73, 113.53, 113.13, 112.56. HRMS calcd for C₂₆H₁₈BrF₃N₅O+ [M + H] + 552.0641, found 552.0640. HPLC purity 97.48% (tR = 13.2min).
3.1.26. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-(trifluoromethyl) Benzamide A18
Yield: 43%, pale yellow solid, mp 312.7–313.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 1H), 10.56 (s, 1H), 8.56 (s, 1H), 8.17 (d, J = 8.1 Hz, 2H), 8.04 (s, 1H), 7.93 (d, J = 8.2 Hz, 2H), 7.83 (d, J = 8.5 Hz, 2H), 7.34 (d, J = 8.6 Hz, 1H), 7.29 (s, 1H), 7.27–7.24 (m, 2H), 6.75 (d, J = 2.9 Hz, 1H), 6.64 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 164.89, 162.93, 159.87, 158.72, 139.20, 138.42, 134.99, 134.65, 132.35, 131.97, 131.66, 130.40 (d, J = 32.8 Hz), 129.05, 128.34, 125.86 (q, J = 3.5 Hz), 125.80 (d, J = 279.0 Hz), 123.01(q, J = 3.5 Hz), 121.15, 121.04, 113.96, 113.55, 112.96. HRMS calcd for C₂₆H₁₈BrF₃N₅O+, [M + H] + 552.0641, found 552.0636. HPLC purity 97.91% (tR = 12.9 min).
3.1.27. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-methylbenzamide A19
Yield: 33%, pale yellow solid, mp 170.7–171.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.34 (s, 1H), 8.48 (s, 1 H), 7.93 (s, 1H), 7.70 (d, J = 8.2 Hz, 2H), 7.38 (d, J = 7.1 Hz, 1H), 7.31 (d, J = 7.8 Hz, 1H), 7.28 (d, J = 5.3 Hz, 1H), 7.24 (d, J = 7.2 Hz, 2H), 7.20 (d, J = 7.1 Hz, 1H), 7.18–7.13 (m, 3H), 6.69 (s, 1H), 6.56 (s, 2H), 2.31 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 167.57, 162.12, 159.12, 157.98, 138.08, 136.86, 134.87, 134.31, 133.38, 130.20, 130.00, 129.44, 129.32, 127.59, 126.88, 125.34, 124.35, 124.16, 120.40, 119.54, 113.29, 112.75, 112.09, 19.00. HRMS calcd for C₂₆H₂₁BrN₅O+ [M + H] +498.0924, found 498.0921. HPLC purity 96.94% (tR = 12.2 min).
3.1.28. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3-methylbenzamide A20
Yield: 42%, pale yellow solid, mp 211.8–212.9 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.45 (s, 1H), 10.23 (s, 1H), 8.49 (s, 1H), 7.93 (s, 1H), 7.74 (d, J = 8.2 Hz, 2H), 7.67 (d, J = 12.5 Hz, 2H), 7.32 (d, J = 5.8 Hz, 2H), 7.24 (d, J = 8.6 Hz, 1H), 7.15 (d, J = 8.3 Hz, 3H), 6.64 (s, 1H), 6.56 (s, 2H), 2.30 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 166.44, 163.17, 160.15, 158.97, 139.10, 138.43, 135.65, 135.24, 134.44, 132.88, 130.88, 130.41, 129.02, 128.85, 128.61, 125.54, 125.42, 125.26, 121.45, 121.16, 114.25, 113.82, 113.18, 21.68. HRMS calcd for C₂₆H₂₁BrN₅O+ [M + H] +498.0924, found 498.0923. HPLC purity 95.11% (tR = 12.4 min).
3.1.29. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-methylbenzamide A21
Yield: 42%, pale yellow solid, mp 291.7–292.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.24 (s, 1H), 8.58 (s, 1H), 8.04 (s, 1H), 7.89 (d, J = 7.9 Hz, 2H), 7.83 (d, J = 8.6 Hz, 2H), 7.37–7.31 (m, 3H), 7.25 (d, J = 8.5 Hz, 3H), 6.74 (s, 1H), 6.63 (s, 2H), 2.39 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 165.82, 162.90, 159.86, 158.67, 142.03, 138.85, 134.95, 134.09, 132.48, 130.56, 130.11, 129.34, 128.34, 128.12, 125.15, 124.97, 121.21, 120.89, 113.93, 113.52, 112.94, 21.43. HRMS calcd for C₂₆H₂₁BrN₅O+, [M + H] + 498.0924, found 498.0930. HPLC purity 96.26% (tR = 12.9 min).
3.1.30. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-methoxybenzamide A22
Yield: 22%, pale yellow solid, mp 154.9–155.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.21 (s, 1H), 8.60 (s, 1H), 8.03 (s, 1H), 7.79 (d, J = 8.3 Hz, 2H), 7.65 (d, J = 5.8 Hz, 1H), 7.51 (t, J = 7.8 Hz, 1H), 7.34 (d, J = 8.6 Hz, 1H), 7.27–7.23 (m, 3H), 7.19 (d, J = 8.4 Hz, 1H), 7.08 (t, J = 7.5 Hz, 1H), 6.75 (d, J = 2.9 Hz, 1H), 6.63 (s, 2H), 3.91 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 164.37, 162.24, 159.30, 158.01, 156.28, 138.04, 134.35, 133.47, 131.85, 130.03, 129.63, 129.46, 127.75, 124.79, 124.54, 124.39, 120.59, 120.33, 119.75, 113.34, 112.95, 112.31, 111.82, 55.72. HRMS calcd for C₂₆H₂₁BrN₅O₂+ [M + H] + 514.0873, found 514.0869. HPLC purity 97.46% (tR = 13.1 min).
3.1.31. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3-methoxybenzamide A23
Yield: 43%, pale yellow solid, mp 172.6–173.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.61 (s, 1H), 10.36 (s, 1H), 8.57 (s, 1H), 8.03 (s, 1H), 7.83 (d, J = 8.2 Hz, 2H), 7.56 (d, J = 7.7 Hz, 1H), 7.50 (s, 1H), 7.45 (t, J = 7.9 Hz, 1H), 7.34 (d, J = 8.5 Hz, 1H), 7.25 (d, J = 8.3 Hz, 3H), 7.19–7.13 (m, 1H), 6.75 (s, 1H), 6.65 (s, 2H), 3.84 (s, 3H).13C NMR (100 MHz, DMSO-d6) δ 165.05, 162.15, 159.16, 158.86, 157.95, 137.96, 136.01, 134.26, 133.54, 129.89, 129.40, 129.30, 127.59, 124.39, 124.24, 120.44, 120.30, 119.58, 117.04, 113.27, 112.81, 112.59, 112.15, 55.03. HRMS calcd for C₂₆H₂₁BrN₅O₂+ [M + H] + 514.0873, found 514.0871. HPLC purity 98.12% (tR = 12.1 min).
3.1.32. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-4-methoxybenzamide A24
Yield: 51%, pale yellow solid, mp 184.6–185.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.47 (s, 1H), 10.18 (s, 1H), 8.58 (d, J = 2.0 Hz, 1H), 8.04 (s, 1H), 7.98 (d, J = 8.8 Hz, 2H), 7.82 (d, J = 8.6 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.24 (d, J = 8.5 Hz, 3H), 7.07 (d, J = 8.8 Hz, 2H), 6.74 (d, J = 2.9 Hz, 1H), 6.62 (s, 2H), 3.84 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 164.77, 162.26, 161.73, 159.27, 158.03, 138.35, 134.34, 133.34, 129.96, 129.47, 129.42, 127.73, 126.76, 124.54, 124.36, 120.63, 120.27, 113.43, 113.32, 112.92, 112.33, 55.25. HRMS calcd for C₂₆H₂₁BrN₅O₂+, [M + H] + 514.0873, found 514.0871. HPLC purity 96.66% (tR = 12.1 min).
3.1.33. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2, 5-difluorobenzamide A25
Yield: 36%, pale yellow solid, mp 163.5–164.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.48 (s, 1H), 10.59 (s, 1H), 8.58 (d, J = 2.0 Hz, 1H), 8.03 (s, 1H), 7.76 (d, J = 8.2 Hz, 2H), 7.58–7.53 (m, 1H), 7.47–7.40 (m, 2H), 7.34 (d, J = 8.6 Hz, 1H), 7.29–7.24 (m, 3H), 6.75 (d, J = 2.9 Hz,1H), 6.64 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.94, 161.91, 159.86, 159.44, 158.72, 157.03, 156.71, 154.27, 138.21, 134.97, 134.71, 130.48 (d, J = 24.3 Hz), 128.35, 126.68 (dd, J = 18.6, 7.4 Hz), 125.07 (d, J = 12.5 Hz), 121.10, 120.46, 119.41 (dd, J = 24.2, 9.0 Hz), 118.47 (dd, J = 25.0, 8.5 Hz), 116.65 (dd, J = 25.8, 3.6 Hz), 113.97, 113.56, 112.91. HRMS calcd for C₂₅H₁₇BrF₂N₅O+, [M + H] + 520.0579, found 520.0586. HPLC purity 95.42% (tR = 12.4 min).
3.1.34. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-3, 5-dichlorobenzamide A26
Yield: 33%, pale yellow solid, mp 177.7–178.5 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.49 (s, 1H), 10.51 (s, 1H), 8.57 (s, 1H), 8.04 (s, 1H), 8.00 (d, J = 2.0 Hz, 2H), 7.87 (s, 1H), 7.81 (d, J = 8.5 Hz, 2H), 7.34 (d, J = 8.6 Hz, 1H), 7.27 (t, J = 10.2 Hz, 3H), 6.75 (d, J = 3.0 Hz, 1H), 6.65 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.28, 162.09, 158.99, 157.86, 137.66, 137.35, 134.13, 133.90, 130.53, 129.68, 129.38, 127.48, 126.59, 126.09, 124.27, 124.13, 120.25, 120.20, 113.09, 112.68, 112.09. HRMS calcd for C₂₅H₁₇BrCl₂N₅O+, [M + H] + 551.9988, found 551.9988. HPLC purity 97.16% (tR = 13.2 min).
3.1.35. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) thiophene-2-carboxamide A27
Yield: 15%, pale yellow solid, mp 303.8–304.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.63 (s, 1H), 10.39 (s, 1H), 8.57 (s, 1H), 8.06 (d, J = 15.0 Hz, 2H), 7.87 (d, J = 5.0 Hz, 1H), 7.79 (d, J = 8.1 Hz, 2H), 7.34 (d, J = 8.6 Hz, 1H), 7.26 (d, J = 8.4 Hz, 4H), 6.70 (d, J = 27.3 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 162.93, 160.37, 159.90, 158.72, 140.49, 138.38, 135.03, 134.33, 132.40, 130.66, 130.23, 129.67, 128.57, 128.36, 125.15, 124.97, 121.13, 120.96, 114.03, 113.54, 112.92. HRMS calcd for C₂₃H₁₇BrN₅OS+ [M + H] +490.0332, found 490.0332. HPLC purity 95.22 % (tR = 11.7 min).
3.1.36. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl) isoquinoline-3-carboxamide A28
Yield: 43%, pale yellow solid, mp 189.3–190.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.46 (s, 1H), 10.81 (s, 1H), 8.59 (d, J = 8.5 Hz, 1H), 8.52 (d, J = 2.0 Hz, 1H), 8.25–8.20 (m, 2H), 8.10–8.05 (m, 1H), 8.02 (s, 1H), 7.99–7.95 (m, 2H), 7.90–7.85 (m, 1H), 7.74–7.69 (m, 1H), 7.30–7.27 (m, 2H), 7.25 (d, J = 2.0 Hz, 1H), 7.22–7.18 (m, 1H), 6.71 (d, J = 2.8 Hz, 1H), 6.63 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.46, 162.14, 159.10, 157.92, 149.73, 145.54, 137.88, 137.12, 134.19, 133.83, 130.35, 129.81, 129.51, 129.01, 128.58, 128.05, 127.79, 127.53, 124.34, 124.20, 120.34, 120.13, 118.45, 113.19, 112.75, 112.16. HRMS calcd for C₂₈H₂₀BrN₆O+ [M + H] +535.0876, found 535.0873. HPLC purity 95.84% (tR = 14.6 min).
3.1.37. N-(4-(2-amino-4-(5-bromo-1H-indol-3-yl) pyrimidin-5-yl) phenyl)-2-naphthamide A29
Yield: 43%, pale yellow solid, mp 189.3–190.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.51 (s, 1H), 10.59 (s, 1H), 8.60 (d, J = 7.1 Hz, 2H), 8.04 (q, J = 11.1, 7.4 Hz, 5H), 7.90 (d, J = 8.0 Hz, 2H), 7.75–7.58 (m, 2H), 7.35 (d, J = 8.7 Hz, 1H), 7.28 (d, J = 9.0 Hz, 3H), 6.72 (d, J = 43.7 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 166.10, 162.82, 159.89, 158.64, 138.78, 134.95, 134.65, 134.19, 132.57, 132.44, 130.58, 130.14, 129.36, 128.44, 128.39, 128.27, 128.05, 127.27, 125.07, 124.96, 124.84, 121.17, 120.95, 113.97, 113.52, 112.86. HRMS calcd for C₂₉H₂₁BrN₅O+ [M + H] +534.0924, found 534.0922. HPLC purity 98.46% (tR = 13.4 min).
3.1.38. 4-(5-Bromo-1H-indol-3-yl)-N-(2-fluorophenyl) pyrimidin-2-amine B1
Yield: 25%, pale yellow solid, mp 206.6–207.1 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.88 (s, 1H), 8.96 (s, 1H), 8.43 (s, 1H), 8.29 (s, 1H), 8.20 (d, J = 5.3 Hz, 1H), 7.70–7.63 (m, 1H), 7.32 (d, J = 8.5 Hz, 1H), 7.25–7.08 (m, 5H). 13C NMR (100 MHz, DMSO-d6) δ 162.71, 161.10, 157.53, 157.22, 154.78, 136.21, 130.77, 128.25 (d, J = 11.4 Hz), 127.32, 126.46, 125.47 (d, J = 7.3 Hz), 125.25, 124.87, 124.78 (d, J = 4.0 Hz), 116.29 (d, J = 19.8 Hz), 114.13 (d, J = 29.7 Hz), 113.58, 107.68. HRMS calcd for C₁₈H₁₃BrFN₄+ [M + H] +383.0302, found 383.0301. HPLC purity 99.29% (tR = 14.1 min).
3.1.39. 4-(5-Bromo-1H-indol-3-yl)-N-(3-fluorophenyl) pyrimidin-2-amine B2
Yield: 48%, pale yellow solid, mp 211.8–212.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.93 (s, 1H), 9.64 (s, 1H), 8.67 (s, 1H), 8.29 (s, 2H), 7.72 (d, J = 12.4 Hz, 1H), 7.49 (d, J = 8.2 Hz, 1H), 7.35 (d, J = 8.6 Hz, 1H), 7.29–7.19 (m, 3H), 6.66 (t, J = 8.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 164.12, 162.67, 161.73, 160.28, 157.42, 143.20 (d, J = 11.4 Hz), 136.35, 130.99, 130.44 (d, J = 9.7 Hz), 127.26, 125.39, 124.75, 115.27, 114.33 (d, J = 29.6 Hz), 113.62, 108.46, 107.85 (d, J = 21.1 Hz), 106.01 (d, J = 26.4 Hz). HRMS calcd for C₁₈H₁₃BrFN₄+ [M + H] +383.0302, found 383.0295. HPLC purity 98.07% (tR = 14.2 min).
3.1.40. 4-(5-Bromo-1H-indol-3-yl)-N-(4-fluorophenyl) pyrimidin-2-amine B3
Yield: 41%, pale yellow solid, mp 246.8–247.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.02 (s, 1H), 9.44 (s, 1H), 8.65 (s, 1H), 8.34 (s, 1H), 8.27 (d, J = 5.3 Hz, 1H), 7.75–7.70 (m, 2H), 7.39 (d, J = 8.6 Hz, 1H), 7.26 (d, J = 9.0 Hz, 1H), 7.23 (d, J = 5.3 Hz, 1H), 7.13 (t, J = 8.7 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.53, 161.51, 159.55, 157.77, 156.42, 155.41, 136.45, 135.25, 129.84, 126.20, 123.96 (d, J = 46.4 Hz), 120.75 (d, J = 7.5 Hz), 114.47 (d, J = 22.2 Hz), 113.39, 112.76 (d, J = 37.2 Hz), 106.71. HRMS calcd for C₁₈H₁₃BrFN₄+ [M + H] +383.0302, found 383.0296 HPLC purity 96.24% (tR = 14.1 min).
3.1.41. 4-(5-Bromo-1H-indol-3-yl)-N-(2-chlorophenyl) pyrimidin-2-amine B4
Yield: 41%, pale yellow solid, mp 173.8–174.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.94 (s, 1H), 8.74 (s, 1H), 8.41 (s, 1H), 8.36 (d, J = 2.7 Hz, 1H), 8.29 (d, J = 5.4 Hz, 1H), 7.83 (d, J = 8.3 Hz, 1H), 7.55 (d, J = 8.0 Hz, 1H), 7.43 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 8.3 Hz, 1H), 7.26 (t, J = 6.8 Hz, 2H), 7.21 (d, J = 7.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.65, 161.04, 157.64, 137.31, 136.19, 130.82, 130.15, 128.30, 128.06, 127.30, 126.98, 125.99, 125.24, 124.85, 114.29, 113.96, 113.52, 107.76. HRMS calcd for C₁₈H₁₃BrFN₄+ [M + H] +399.0007, found 399. 0002.HPLC purity 98.97% (tR = 15.7 min).
3.1.42. 4-(5-Bromo-1H-indol-3-yl)-N-(3-chlorophenyl) pyrimidin-2-amine B5
Yield: 38%, pale yellow solid, mp 210.3–211.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.23 (s, 1H), 9.69 (s, 1H), 8.73 (s, 1H), 8.40 (s, 1H), 8.38 (d, J = 5.4 Hz, 1H), 8.10 (s, 1H), 7.83 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 8.6 Hz, 1H), 7.35–7.30 (m, 2H), 7.28 (d, J = 8.1 Hz, 1H), 7.13 (d, J = 7.9 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 161.83, 159.32, 156.51, 142.18, 135.47, 130.14, 130.03, 126.40, 124.53, 123.87, 123.14, 121.21, 120.66, 117.35, 113.59, 113.33, 112.70, 107.64. HRMS calcd for C₁₈H₁₃BrClN₄+ [M + H] +399.0007, found 399.0000. HPLC purity 96.15% (tR = 14.4 min).
3.1.43. 4-(5-Bromo-1H-indol-3-yl)-N-(4-chlorophenyl) pyrimidin-2-amine B6
Yield: 43%, pale yellow solid, mp 273.5–274.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.28 (s, 1H), 9.58 (s, 1H), 8.66 (s, 1H), 8.36 (s, 1H), 8.28 (d, J = 5.3 Hz, 1H), 7.77 (d, J = 8.8 Hz, 2H), 7.40 (d, J = 8.6 Hz, 1H), 7.31 (d, J = 8.8 Hz, 2H), 7.28–7.23 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.15, 159.93, 157.03, 139.74, 135.88, 130.59, 128.42, 126.81, 124.87, 124.80, 124.33, 120.94, 114.03, 113.62, 113.15, 107.69. HRMS calcd for C₁₈H₁₃BrClN₄+, [M + H] +399.0007, found 399.0005. HPLC purity 95.20% (tR = 15.4 min).
3.1.44. 4-(5-Bromo-1H-indol-3-yl)-N-(2-bromophenyl) pyrimidin-2-amine B7
Yield: 37%, pale yellow solid, mp 177.8–178.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.96 (s, 1H), 8.71 (s, 1H), 8.39–8.36 (m, 2H), 8.28 (d, J = 5.3 Hz, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 7.39 (d, J = 8.6 Hz, 1H), 7.29–7.23 (m, 2H), 7.15 (t, J = 7.7 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.63, 161.06, 157.65, 138.64, 136.18, 133.31, 130.88, 130.80, 128.71, 127.30, 126.53, 125.22, 124.86, 119.55, 114.27, 113.94, 113.52, 107.70. HRMS calcd for C₁₈H₁₃Br₂N₄+ [M + H] +442.9501, found 442.9504. HPLC purity 98.68% (tR = 15.9 min).
3.1.45. 4-(5-Bromo-1H-indol-3-yl)-N-(3-bromophenyl) pyrimidin-2-amine B8
Yield: 48%, pale yellow solid, mp 214.8–215.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.04 (s, 1H), 9.71 (s, 1H), 8.73 (d, J = 2.0 Hz, 1H), 8.40 (d, J = 3.9 Hz, 1H), 8.38 (s, 1H), 7.99 (s, 1H), 7.79–7.71 (m, 1H), 7.45 (d, J = 8.6 Hz, 1H), 7.36 (d, J = 8.4 Hz, 1H), 7.34–7.30 (m, 2H), 7.02–6.96 (m, 1H).13C NMR (100 MHz, DMSO-d6) δ 161.82, 159.34, 156.53, 142.01, 135.46, 132.64, 130.15, 129.71, 126.38, 124.52, 123.85, 120.24, 117.80, 116.97, 113.59, 113.30, 112.70, 107.64. HRMS calcd for C₁₈H₁₃Br₂N₄+ [M + H] +442.9501, found 442.9498. HPLC purity 98.57% (tR = 15.1 min).
3.1.46. 4-(5-Bromo-1H-indol-3-yl)-N-(4-bromophenyl) pyrimidin-2-amine B9
Yield: 36%, pale yellow solid, mp 276.7–277.5 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.22 (s, 1H), 9.55 (s, 1H), 8.63 (s, 1H), 8.32 (s, 1H), 8.25 (d, J = 5.4 Hz, 1H), 7.69 (d, J = 8.5 Hz, 2H), 7.44–7.33 (m, 3H), 7.24–7.20 (m, 2H).13C NMR (100 MHz, DMSO-d6) δ 161.57, 159.28, 156.39, 139.56, 135.31, 130.68, 129.99, 126.18, 124.18, 123.70, 120.78, 113.48, 112.98, 112.42, 112.07, 107.12. HRMS calcd for C₁₈H₁₃Br₂N₄+, [M + H] + 442.9501, found 442.9502. HPLC purity 95.11% (tR = 15.7 min).
3.1.47. 4-(5-Bromo-1H-indol-3-yl)-N-(2-(trifluoromethyl) phenyl) pyrimidin-2-amine B10
Yield: 40%, pale yellow solid, mp 150.5–151.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.10 (s, 1H), 8.68 (s, 1H), 8.34 (s, 1H), 8.26 (d, J = 5.3 Hz, 1H), 8.21 (s, 1H), 7.78 (d, J = 8.2 Hz, 3H), 7.47 (d, J = 7.6 Hz, 1H), 7.39 (d, J = 8.6 Hz, 1H), 7.28–7.21 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.51, 161.89, 157.49, 138.26, 136.06, 133.55, 130.58, 130.00, 127.18, 126.75 (q, J = 5.3 Hz), 126.07, 125.74 (q, J = 272 Hz), 125.04, 124.70 (d, J = 5.7 Hz), 124.44, 114.15, 113.76, 113.29, 107.41. HRMS calcd for C₁₉H₁₃BrF₃N₄+, [M + H] +433.0270, found 433.0271. HPLC purity 95.40% (tR = 14.7 min).
3.1.48. 4-(5-Bromo-1H-indol-3-yl)-N-(3-(trifluoromethyl) phenyl) pyrimidin-2-amine B11
Yield: 45%, pale yellow solid, mp 171.8–172.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.02 (s, 1H), 9.82 (s, 1H), 8.72 (s, 1H), 8.41 (d, J = 5.2 Hz, 2H), 8.22 (s, 1H), 8.15 (d, J = 8.4 Hz, 1H), 7.56 (t, J = 8.0 Hz, 1H), 7.45 (d, J = 8.9 Hz, 1H), 7.35 (t, J = 4.9 Hz, 2H), 7.32-7.27 (m, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.31, 159.80, 156.97, 141.74, 135.93, 130.58, 129.93 (q, J = 29.3 Hz), 129.00, 126.83, 125.79 (q, J = 272.7 Hz) 124.97, 124.27, 122.45, 117.22, 114.90, 114.04, 113.76, 113.12, 108.24. HRMS calcd for C₁₉H₁₃BrF₃N₄+, [M + H] +433.0270, found 433.0278. HPLC purity 99.39% (tR = 15.1 min).
3.1.49. 4-(5-Bromo-1H-indol-3-yl)-N-(4-(trifluoromethyl) phenyl) pyrimidin-2-amine B12
Yield: 34%, pale yellow solid, mp 279.5–280.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.02 (s, 1H), 9.91 (s, 1H), 8.76 (s, 1H), 8.43 (d, J = 3.0 Hz, 1H), 8.41 (d, J = 5.4 Hz, 1H), 8.02 (d, J = 8.5 Hz, 2H), 7.68 (d, J = 8.5 Hz, 2H), 7.45 (d, J = 8.6 Hz, 1H), 7.38 (d, J = 5.4 Hz, 1H), 7.35–7.31 (m, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.25, 159.72, 157.06, 144.51, 135.94, 130.76, 126.81, 125.87 (d, J = 4.1 Hz), 124.92, 124.32, 123.47 (q, J = 272.7 Hz), 120.95 (q, J = 31.1 Hz), 118.75, 114.08, 113.66, 113.09, 108.36. HRMS calcd for C₁₉H₁₃BrF₃N₄+ [M + H] +433.0270, found 433.0273. HPLC purity 97.41% (tR = 15.3 min).
3.1.50. 4-(5-Bromo-1H-indol-3-yl)-N-(o-tolyl) pyrimidin-2-amine B13
Yield: 39%, pale yellow solid, mp 187.6–188.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.86 (s, 1H), 8.65 (s, 1H), 8.39–8.06 (m, 3H), 7.38 (d, J = 57.8 Hz, 2H), 7.12 (d, J = 59.2 Hz, 5H), 2.20 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 162.58, 161.66, 157.61, 138.73, 136.16, 134.54, 132.91, 131.01, 130.56, 127.34, 126.65, 125.85, 125.21, 124.93, 114.22, 113.94, 113.72, 106.80, 18.80. HRMS calcd for C₁₉H₁₆BrN₄+ [M + H] +379.0553, found 379.0561. HPLC purity 95.08% (tR = 14.4 min).
3.1.51. 4-(5-Bromo-1H-indol-3-yl)-N-(m-tolyl) pyrimidin-2-amine B14
Yield: 52%, pale yellow solid, mp 141.5–142.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.00 (s, 1H), 9.42 (s, 1H), 8.76 (s, 1H), 8.39 (s, 1H), 8.34 (d, J = 5.3 Hz, 1H), 7.63 (d, J = 7.1 Hz, 2H), 7.44 (d, J = 8.6 Hz, 1H), 7.34–7.30 (m, 1H), 7.27 (d, J = 5.4 Hz, 1H), 7.25–7.19 (m, 1H), 6.80 (d, J = 7.5 Hz, 1H), 2.31 (s, 3H).13C NMR (100 MHz, DMSO-d6) δ 162.47, 160.58, 157.42, 141.10, 138.02, 136.28, 130.76, 128.84, 127.27, 125.28, 124.77, 122.49, 120.16, 116.97, 114.38, 114.00, 113.76, 107.69, 21.90. HRMS calcd for C₁₉H₁₆BrN₄+ [M + H] + 379.0553, found 379.0547. HPLC purity 97.81% (tR = 14.7 min).
3.1.52. 4-(5-Bromo-1H-indol-3-yl)-N-(p-tolyl) pyrimidin-2-amine B15
Yield: 41%, pale yellow solid, mp 227.8–228.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 1H), 9.36 (s, 1H), 8.74 (s, 1H), 8.34 (d, J = 30.0 Hz, 2H), 7.71–7.59 (m, 2H), 7.42 (s, 1H), 7.28 (d, J = 29.2 Hz, 2H), 7.21–7.11 (m, 2H), 2.28 (s, 3H) 13C NMR (100 MHz, DMSO-d6) δ 162.47, 160.73, 157.43, 138.52, 136.28, 130.78, 130.63, 129.46, 127.24, 125.22, 124.84, 120.24, 114.38, 113.96, 113.71, 107.41, 20.97. HRMS calcd for C₁₉H₁₆BrN₄+ [M + H] +379.0553, found 379.0552. HPLC purity 96.65% (tR = 14.8 min).
3.1.53. 4-(5-Bromo-1H-indol-3-yl)-N-(2-methoxyphenyl) pyrimidin-2-amine B16
Yield: 36%, pale yellow solid, mp 207.9–208.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.97 (s, 1H), 8.58 (s, 1H), 8.36 (s, 1H), 8.29 (d, J = 5.4 Hz, 1H), 8.10–8.05 (m, 2H), 7.40 (d, J = 8.5 Hz, 1H), 7.31–7.24 (m, 2H), 7.12–6.98 (m, 3H), 3.84 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 162.77, 160.89, 157.80, 150.46, 136.45, 131.11, 129.35, 127.42, 125.41, 124.83, 123.74, 122.18, 121.13, 114.61, 114.15, 113.79, 111.72, 107.96, 56.31. HRMS calcd for C₁₉H₁₆BrN₄O+ [M + H] +395.0502, found 395.0502. HPLC purity 98.24% (tR = 15.0 min).
3.1.54. 4-(5-Bromo-1H-indol-3-yl)-N-(3-methoxyphenyl) pyrimidin-2-amine B17
Yield: 49%, pale yellow solid, mp 208.7–209.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.96 (s, 1H), 9.44 (s, 1H), 8.73 (s, 1H), 8.34 (s, 1H), 8.30 (d, J = 5.3 Hz, 1H), 7.39 (d, J = 8.7 Hz, 3H), 7.27 (d, J = 8.6 Hz, 1H), 7.23 (d, J = 5.4 Hz, 1H), 7.19 (t, J = 8.1 Hz, 1H), 6.51 (d, J = 8.0 Hz, 1H), 3.68 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 161.69, 159.67, 159.22, 156.56, 141.57, 135.45, 129.97, 128.87, 126.40, 124.47, 124.01, 113.54, 113.26, 112.88, 111.32, 107.07, 106.16, 104.64, 54.45. HRMS calcd for C₁₉H₁₆BrN₄O+ [M + H] +395.0502, found 395.0498. HPLC purity 97.43% (tR = 13.6 min).
3.1.55. 4-(5-Bromo-1H-indol-3-yl)-N-(4-methoxyphenyl) pyrimidin-2-amine B18
Yield: 47%, pale yellow solid, mp 245.7–246.3 °C.1H NMR (400 MHz, DMSO-d6) δ 11.96 (s, 1H), 9.24 (s, 1H), 8.72 (s, 1H), 8.38 (d, J = 2.8 Hz, 1H), 8.30 (d, J = 5.3 Hz, 1H), 7.66 (d, J = 8.6 Hz, 2H), 7.44 (d, J = 8.5 Hz, 1H), 7.32 (d, J = 8.7 Hz, 1H), 7.23 (d, J = 5.3 Hz, 1H), 6.96 (d, J = 8.6 Hz, 2H), 3.77 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 162.41, 160.86, 157.46, 154.73, 136.24, 134.11, 130.68, 127.25, 125.18, 124.84, 122.00, 114.32, 114.26, 113.91, 113.77, 107.09, 55.60. HRMS calcd for C₁₉H₁₆BrN₄O+ [M + H] +395.0502, found 395.0497. HPLC purity 95.61% (tR = 13.4 min).
3.1.56. 4-(5-Bromo-1H-indol-3-yl)-N-(2-isopropylphenyl) pyrimidin-2-amine B19
Yield: 41%, pale yellow solid, mp 110.5–111.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.88 (s, 1H), 8.70 (d, J = 3.8 Hz, 1H), 8.29 (s, 1H), 8.21 (d, J = 17.1 Hz, 2H), 7.41–7.30 (m, 3H), 7.29–7.20 (m, 3H), 7.13 (t, J = 4.6 Hz, 1H), 3.26 (q, J = 6.4, 5.7 Hz, 1H), 1.12 (d, J = 3.1 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 162.08, 162.02, 157.01, 144.29, 136.77, 135.53, 129.74, 127.55, 126.76, 125.92, 125.88, 125.61, 124.62, 124.55, 113.53, 113.27, 113.15, 105.81, 27.28, 23.10. HRMS calcd for C₂₁H₂₀BrN₄+ [M + H] +407.0866, found 407.0863. HPLC purity 98.51% (tR = 15.1 min).
3.1.57. 4-(5-Bromo-1H-indol-3-yl)-N-(3-isopropylphenyl) pyrimidin-2-amine B20
Yield: 60%, pale yellow solid, mp 152.6–153.4 °C.1H NMR (400 MHz, DMSO-d6) δ 11.92 (s, 1H), 9.33 (s, 1H), 8.68 (d, J = 4.6 Hz, 1H), 8.32–8.23 (m, 2H), 7.70 (t, J = 6.1 Hz, 1H), 7.45 (d, J = 4.6 Hz, 1H), 7.39–7.34 (m, 1H), 7.25–7.15 (m, 3H), 6.77 (t, J = 6.2 Hz, 1H), 2.77 (q, J = 6.8 Hz, 1H), 1.13 (d, J = 6.1 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 162.33, 160.45, 157.22, 148.95, 140.94, 136.11, 130.56, 128.71, 127.07, 125.11, 124.58, 119.58, 117.56, 117.19, 114.21, 113.86, 113.58, 107.54, 33.85, 24.25. HRMS calcd for C₂₁H₂₀BrN₄+ [M + H] +407.0866, found 407.0866. HPLC purity 98.50% (tR = 15.8 min).
3.1.58. 4-(5-Bromo-1H-indol-3-yl)-N-(4-isopropylphenyl) pyrimidin-2-amine B21
Yield: 41%, pale yellow solid, mp 218.7–219.4 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.88 (s, 1H), 9.26 (s, 1H), 8.67 (s, 1H), 8.27 (s, 1H), 8.19 (d, J = 5.3 Hz, 1H), 7.56 (d, J = 8.2 Hz, 2H), 7.33 (d, J = 8.6 Hz, 1H), 7.20 (d, J = 8.6 Hz, 1H), 7.15–7.10 (m, 3H), 2.78–2.72 (m, 1H), 1.10 (d, J = 6.9 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δ 161.88, 160.12, 156.93, 141.25, 138.17, 135.72, 130.22, 126.67, 126.22, 124.66, 124.28, 119.63, 113.83, 113.38, 113.22, 106.85, 32.78, 23.99. HRMS calcd for C₂₁H₂₀BrN₄+ [M + H] +407.0866, found 407.0870. HPLC purity 98.97% (tR = 16.3 min).
3.1.59. 4-(5-Bromo-1H-indol-3-yl)-N-phenylpyrimidin-2-amine B22
Yield: 46%, pale yellow solid, mp 197.6–198.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 1H), 9.46 (s, 1H), 8.77 (s, 1H), 8.37 (s, 1H), 8.33 (d, J = 5.3 Hz, 1H), 7.79 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 8.6 Hz, 1H), 7.33 (q, J = 9.4, 8.5 Hz, 3H), 7.27 (d, J = 5.3 Hz, 1H), 6.98 (t, J = 7.3 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.36, 160.38, 157.23, 140.98, 136.21, 130.81, 128.82, 127.06, 124.97, 124.55, 121.53, 119.60, 114.31, 113.78, 113.42, 107.56. HRMS calcd for C₁₈H₁₄BrN₄+ [M + H] +365.0396, found 365.0397. HPLC purity 98.81% (tR = 10.5 min).
3.1.60. 4-(5-Bromo-1H-indol-3-yl)-N-(3,5-difluorophenyl) pyrimidin-2-amine B23
Yield: 52%, pale yellow solid, mp 251.3–252.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 1H), 9.92 (s, 1H), 8.73 (d, J = 2.0 Hz, 1H), 8.52–8.24 (m, 2H), 7.63–7.58 (m, 2H), 7.46 (d, J = 8.6 Hz, 1H), 7.38 (d, J = 5.4 Hz, 1H), 7.35–7.32 (m, 1H), 6.74 (t, J = 9.3 Hz, 1H).13C NMR (100 MHz, DMSO-d6) δ 163.86 (d, J = 16.1 Hz), 162.34, 161.47 (d, J = 16.0 Hz), 159.54, 156.97, 143.53 (d, J = 14.6 Hz), 135.92, 130.73, 126.78, 125.01, 124.17, 114.10, 113.79, 113.02, 108.62, 101.42 (d, J = 8.1 Hz). HRMS calcd for C₁₈H₁₂BrF₂N₄+ [M + H] + 401.0208, found 401.0201 HPLC purity 96.39% (tR = 14.7 min).
3.1.61. 4-(5-Bromo-1H-indol-3-yl)-N-(3,5-dichlorophenyl) pyrimidin-2-amine B24
Yield: 63%, pale yellow solid, mp 223.6–224.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 12.02 (s, 1H), 9.84 (s, 1H), 8.66 (d, J = 2.0 Hz, 1H), 8.39 (s, 1H), 8.37 (d, J = 3.0 Hz, 1H), 7.91 (d, J = 1.9 Hz, 2H), 7.41 (d, J = 8.6 Hz, 1H), 7.34 (d, J = 5.4 Hz, 1H), 7.31–7.27 (m, 1H), 7.06 (t, J = 1.8 Hz, 1H).13C NMR (100 MHz, DMSO-d6) δ 162.82, 159.84, 157.34, 143.86, 136.33, 134.40, 131.13, 127.23, 125.49, 124.60, 120.30, 117.01, 114.50, 114.27, 113.39, 109.14. HRMS calcd for C₁₈H₁₂BrCl₂N₄+ [M + H] +432.9617, found 432. 9611. HPLC purity 97.08% (tR = 16.7 min).
3.1.62. 4-(5-Bromo-1H-indol-3-yl)-N-(3,5-dibromophenyl) pyrimidin-2-amine B25
Yield: 48%, pale yellow solid, mp 193.4–193.7 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.91 (s, 1H), 9.68 (s, 1H), 8.54 (s, 1H), 8.29–8.23 (m, 2H), 7.98–7.94 (m, 2H), 7.30 (d, J = 8.5 Hz, 1H), 7.21 (d, J = 5.4 Hz, 1H), 7.18 (d, J = 4.9 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 162.28, 159.25, 156.82, 143.68, 135.80, 130.53, 126.69, 125.08, 125.00, 124.09, 122.23, 119.67, 113.99, 113.79, 112.85, 108.61. HRMS calcd for C₁₈H₁₄Br₃N₄+ [M + H] +522.8763, found 522.8587. HPLC purity 97.05% (tR = 17.3 min).
3.1.63. N-(3,5-bis(trifluoromethyl)phenyl)-4-(5-bromo-1H-indol-3-yl) pyrimidin-2-amine B26
Yield: 59%, pale yellow solid, mp 220.9–221.3 °C.1H NMR (400 MHz, DMSO-d6) δ 12.06 (s, 1H), 10.17 (s, 1H), 8.68 (s, 1H), 8.55 (s, 2H), 8.45 (d, J = 5.4 Hz, 1H), 8.40 (s, 1H), 7.56 (s, 1H), 7.42 (t, J = 7.8 Hz, 2H), 7.31 (d, J = 7.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 162.54, 159.37, 156.90, 142.96, 135.93, 130.93 (d, J = 34.2 Hz), 130.30 (d, J = 32.3 Hz), 126.79, 125.08, 124.94 (q, J = 273.7 Hz),124.12, 118.04, 114.09, 113.84, 113.17, 112.88, 109.08. HRMS calcd for C₂₀H₁₂BrF₆N₄+, [M + H] +501.0144, found 501.0138. HPLC purity 97.89% (tR = 16.1 min).
3.1.64. 4-(5-Bromo-1H-indol-3-yl)-N-(3, 5-dimethylphenyl) pyrimidin-2-amine B27
Yield: 42%, pale yellow solid, mp 184.6–185.3 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.94 (s, 1H), 9.29 (s, 1H), 8.66 (s, 1H), 8.30 (d, J = 16.5 Hz, 2H), 7.38 (s, 3H), 7.23 (d, J = 30.4 Hz, 2H), 6.55 (s, 1H), 2.21 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 161.84, 159.96, 156.81, 140.44, 137.19, 135.67, 130.06, 126.70, 124.71, 124.15, 122.78, 116.73, 113.77, 113.37, 113.20, 107.03, 21.21. HRMS calcd for C₂₀H₁₈BrN₄+ [M + H] +393.0709, found 393.0712 HPLC purity 97.05% (tR = 15.3 min).
3.1.65. 4-(5-Bromo-1H-indol-3-yl)-N-(3, 5-dimethoxyphenyl) pyrimidin-2-amine B28
Yield: 48%, pale yellow solid, mp 255.6–256.8 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.96 (s, 1H), 9.40 (s, 1H), 8.71 (s, 1H), 8.33 (d, J = 2.5 Hz, 1H), 8.30 (d, J = 5.3 Hz, 1H), 7.39 (d, J = 8.6 Hz, 1H), 7.29–7.25 (m, 1H), 7.23 (d, J = 5.4 Hz, 1H), 7.04 (d, J = 2.2 Hz, 2H), 6.09 (s, 1H), 3.67 (s, 6H).13C NMR (100 MHz, DMSO-d6) δ 162.53, 160.96, 160.46, 157.37, 142.89, 136.29, 130.76, 127.25, 125.37, 124.87, 114.39, 114.16, 113.70, 108.04, 97.92, 93.74, 55.40. HRMS calcd for C₂₀H₁₈BrN₄O₂+ [M + H] +425.0608, found 425.0601. HPLC purity 96.76% (tR = 13.3 min).
3.1.66. 4-(5-Bromo-1H-indol-3-yl)-N-(2,6-difluorophenyl) pyrimidin-2-amine B29
Yield: 40%, pale yellow solid, mp 263.3–264.2 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.87 (s, 1H), 8.91 (s, 1H), 8.23 (d, J = 40.8 Hz, 3H), 7.53–7.01 (m, 7H). 13C NMR (100 MHz, DMSO-d6) δ 162.85, 161.48, 160.29 (d, J = 5.2 Hz), 157.83 (d, J = 5.1 Hz), 157.57, 136.15, 130.69, 127.36, 125.23, 124.72, 114.23, 113.91, 113.41, 112.50 (d, J = 5.9 Hz), 112.32 (d, J = 5.2 Hz), 107.43. HRMS calcd for C₁₈H₁₂BrF₂N₄+, [M + H] +401.0208, found 401.0201. HPLC purity 97.49% (tR = 12.4 min).
3.1.67. 4-(5-Bromo-1H-indol-3-yl)-N-(2,6-dimethylphenyl) pyrimidin-2-amine B30
Yield: 34%, pale yellow solid, mp 228.5–229.4 °C.1H NMR (400 MHz, DMSO-d6) δ 11.85 (s, 1H), 8.54 (s, 1H), 8.24 (d, J = 38.1 Hz, 2H), 7.82 (s, 1H), 7.33 (s, 1H), 7.25–6.98 (m, 5H), 2.20 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 162.27, 161.60, 157.27, 148.03, 137.14, 135.62, 129.80, 128.05, 126.99, 126.08, 124.73, 124.44, 113.64, 113.40, 113.32, 105.52, 18.58. HRMS calcd for C₂₀H₁₈BrN₄+ [M + H] +393.0709, found 393.0715. HPLC purity 96.56% (tR = 14.2 min).
3.1.68. 4-(5-Bromo-1H-indol-3-yl)-N-(2,6-dimethoxyphenyl) pyrimidin-2-amine B31
Yield: 62%, pale yellow solid, mp 244.3–245.6 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.74 (s, 1H), 8.15 (s, 1H), 8.01 (d, J = 5.3 Hz, 1H), 7.92 (s, 1H), 7.25 (d, J = 8.5 Hz, 1H), 7.15–7.09 (m, 2H), 6.95 (d, J = 5.3 Hz, 1H), 6.65 (d, J = 8.4 Hz, 2H), 3.60 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 162.50, 162.37, 157.25, 156.70, 136.12, 130.09, 127.49, 127.38, 125.20, 125.04, 117.15, 114.14, 113.93, 113.82, 106.08, 105.16, 56.00. HRMS calcd for C₂₀H₁₈BrN₄O₂+ [M + H] +425.0608, found 425.0600. HPLC purity 97.85% (tR = 12.2 min).