Molecular and Structural Characterizations of Lipases from Chlorella by Functional Genomics
Abstract
1. Introduction
2. Results
2.1. Sequence Retrieval
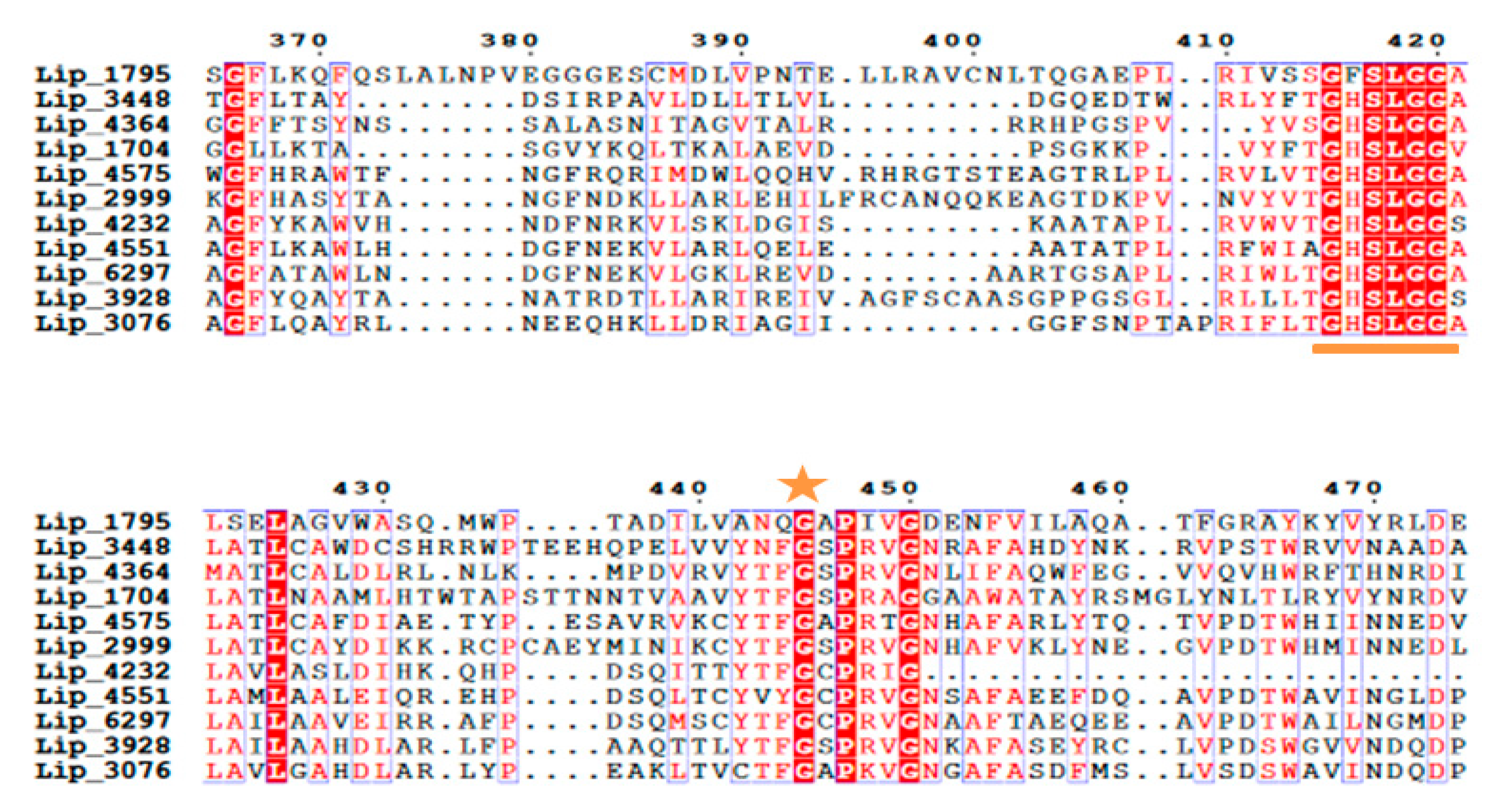
2.2. Physicochemical Characterization of Protein Sequences
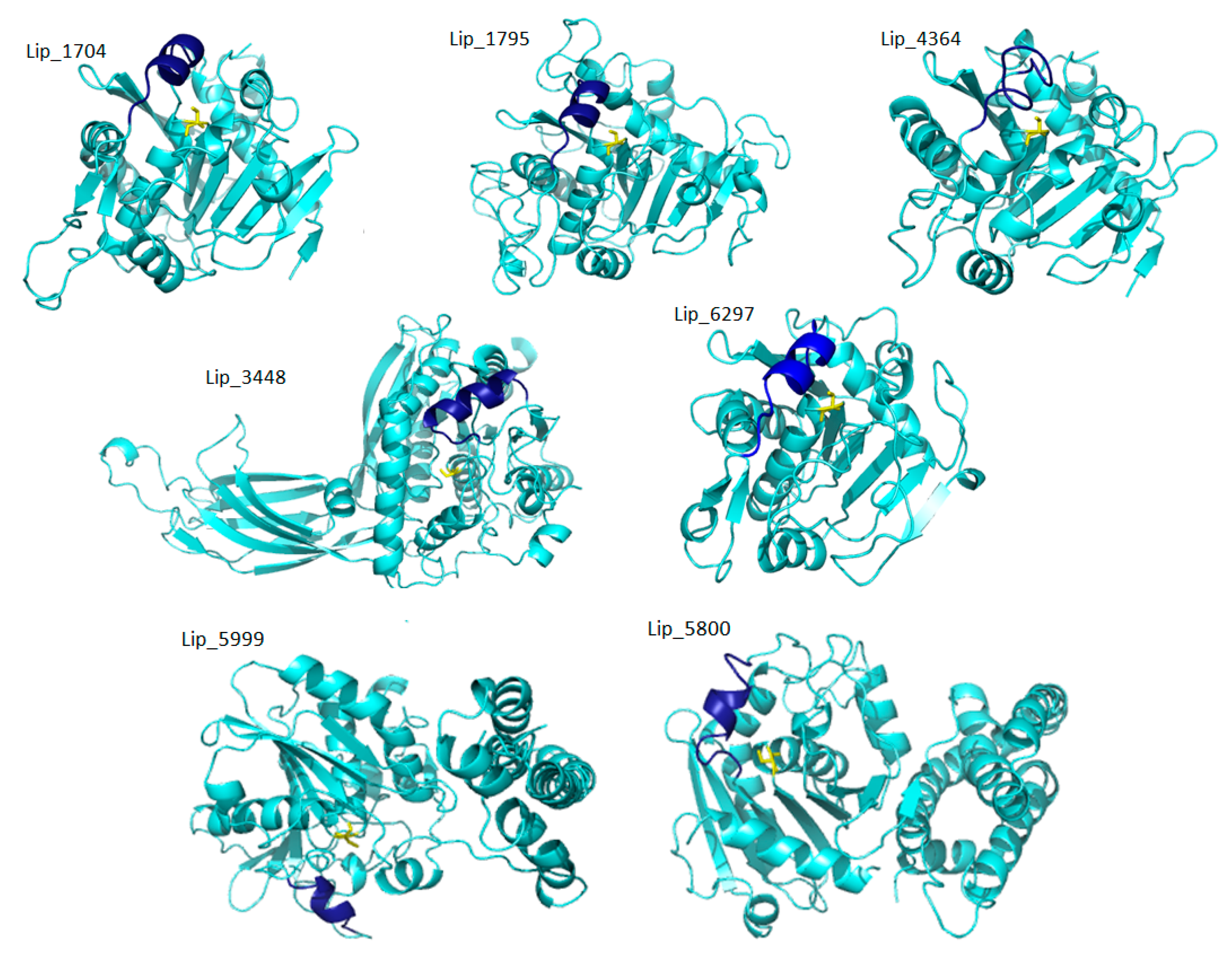
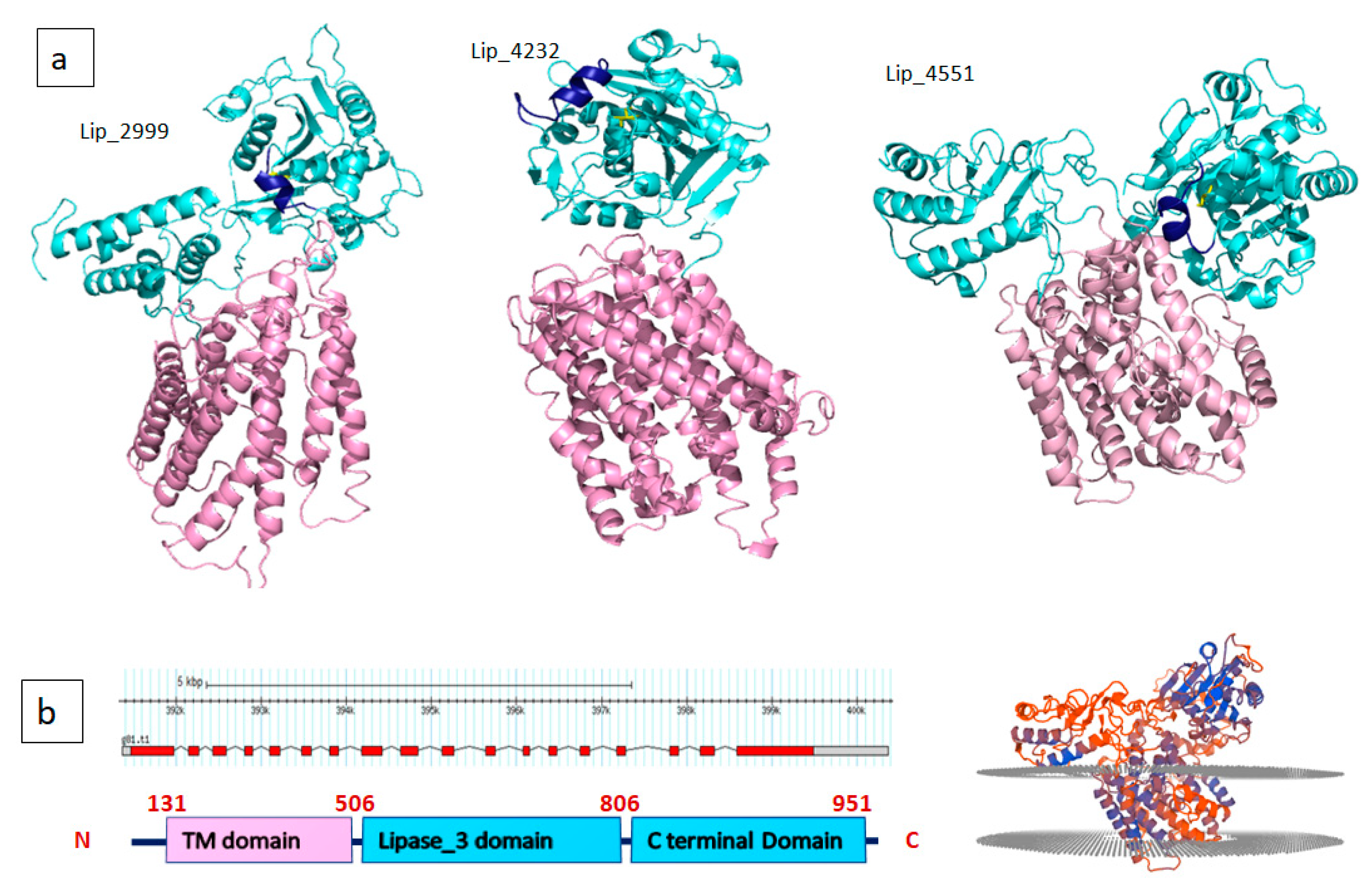
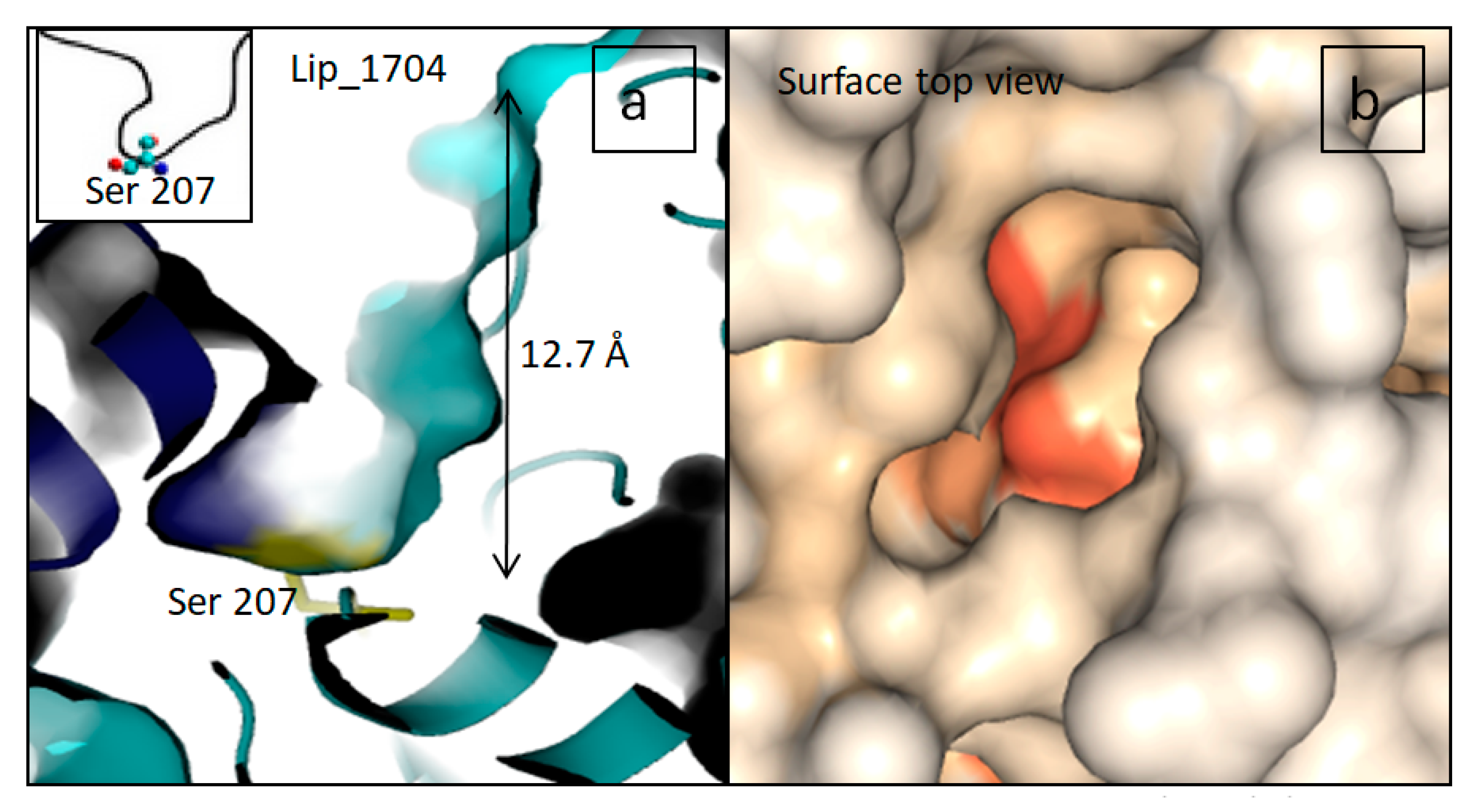
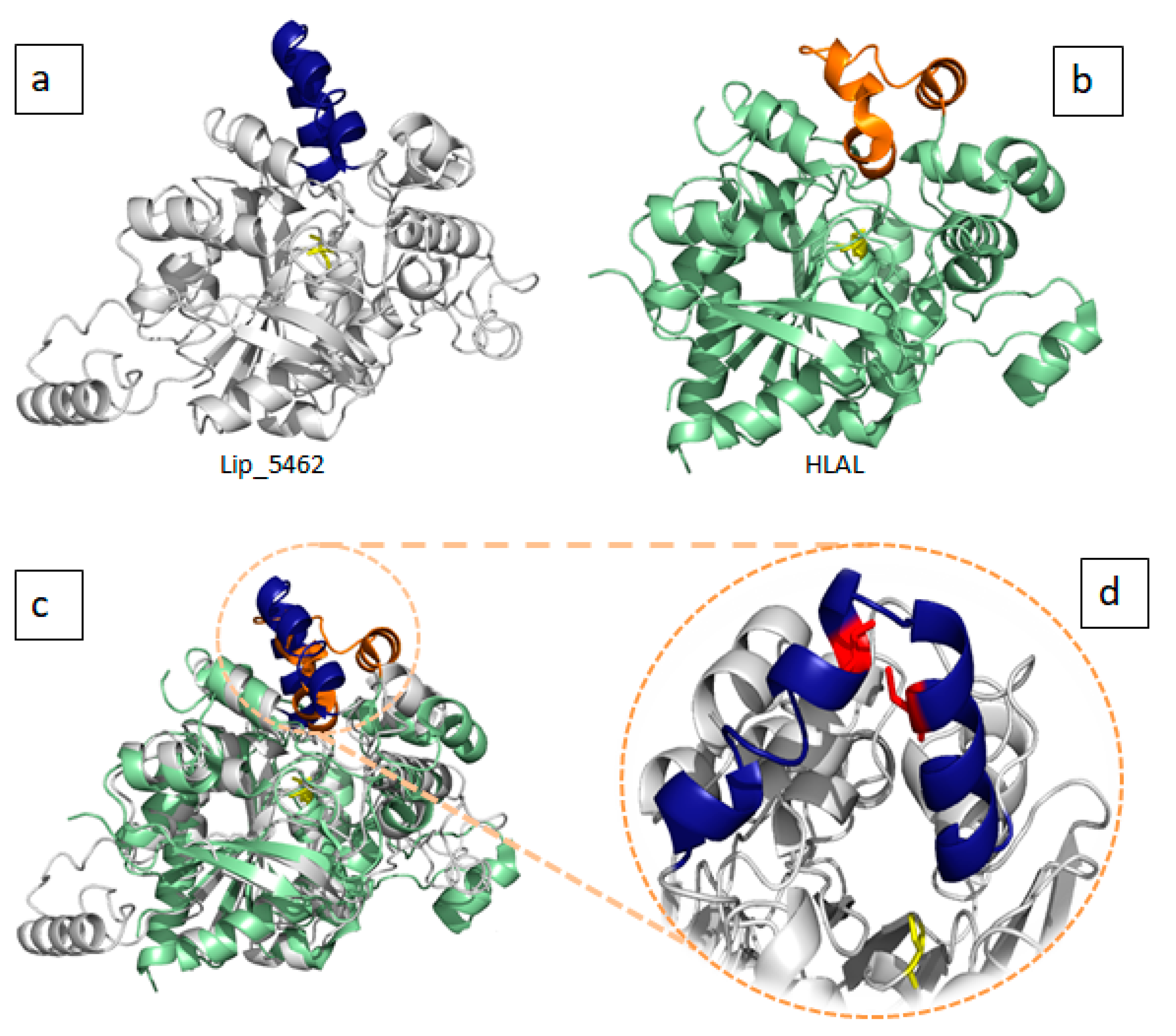
2.3. D-Structural Modeling
3. Discussion
4. Materials and Methods
4.1. Sequence Retrieval
4.2. Multiple Sequence Alignment
4.3. Physicochemical Characterization of Protein Sequences
4.4. Tertiary Structure Prediction, Structure Validation and Quality Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Market, M.N. Enzymes Market. 2020. Available online: https://www.marketsandmarkets.com.Market-Reports/enzymes-market-46202020.html (accessed on 22 June 2020).
- Prakash, D.; Nawani, N.; Prakash, M.; Bodas, M.; Mandal, A.; Khetmalas, M.; Kapadnis, B. Actinomycetes: A Repertory of Green Catalysts with a Potential Revenue Resource. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hasan, F.; Shah, A.A.; Hameed, A. Industrial applications of microbial lipases. Enzym. Microb. Technol. 2006, 39, 235–251. [Google Scholar] [CrossRef]
- Andualema, B.; Gessesse, A. Microbial Lipases and Their Industrial Applications: Review. Biotechnology 2012, 11, 100–118. [Google Scholar] [CrossRef]
- Fendri, I.; Chaari, A.; Dhouib, A.; Jlassi, B.; Abousalham, A.; Carriere, F.; Sayadi, S.; Abdelkafi, S. Isolation, identification and characterization of a new lipolytic Pseudomonas sp., strain AHD-1, from Tunisian soil. Environ. Technol. 2010, 31, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Barouh, N.; Abdelkafi, S.; Fouquet, B.; Pina, M.; Scheirlinckx, F.; Carrière, F.; Villeneuve, P. Neutral Lipid Characterization of Non-Water-Soluble Fractions of Carica Papaya Latex. J. Am. Oil Chem. Soc. 2010, 87, 987–995. [Google Scholar] [CrossRef]
- Khannous, L.; Mouna, J.; Dammak, M.; Miladi, R.; Chaaben, N.; Khemakhem, B.; Gharsallah, N.; Fendri, I. Isolation of a novel amylase and lipase-producing Pseudomonas luteola strain: Study of amylase production conditions. Lipids Health Dis. 2014, 13, 9. [Google Scholar] [CrossRef]
- Navvabi, A.; Razzaghi, M.; Fernandes, P.; Karami, L.; Homaei, A. Novel lipases discovery specifically from marine organisms for industrial production and practical applications. Process. Biochem. 2018, 70, 61–70. [Google Scholar] [CrossRef]
- Demir, B.S.; Tükel, S.S. Purification and characterization of lipase from Spirulina platensis. J. Mol. Catal. B Enzym. 2010, 64, 123–128. [Google Scholar] [CrossRef]
- Godet, S.; Hérault, J.; Pencreac’H, G.; Ergan, F.; Loiseau, C. Isolation and analysis of a gene from the marine microalga Isochrysis galbana that encodes a lipase-like protein. Environ. Boil. Fishes 2012, 24, 1547–1553. [Google Scholar] [CrossRef]
- Ben Hlima, H.; Dammak, M.; Karkouch, N.; Hentati, F.; Laroche, C.; Michaud, P.; Fendri, I.; Abdelkafi, S. Optimal cultivation towards enhanced biomass and floridean starch production by Porphyridium marinum. Int. J. Biol. Macromol. 2019, 129, 152–161. [Google Scholar] [CrossRef]
- Talebi, A.F.; Tohidfar, M.; Tabatabaei, M.; Bagheri, A.; Mohsenpor, M.; Mohtashami, S.K. Genetic manipulation, a feasible tool to enhance unique characteristic of Chlorella vulgaris as a feedstock for biodiesel production. Mol. Biol. Rep. 2013, 40, 4421–4428. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.T.; Levering, J.; Henard, C.A.; Boore, J.L.; Betenbaugh, M.J.; Zengler, K.; Knoshaug, E.P. Genome Sequence of the Oleaginous Green Alga, Chlorella vulgaris UTEX 395. Front. Bioeng. Biotechnol. 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, M.; Marcolungo, L.; Rossato, M.; Girolomoni, L.; Cosentino, E.; Cuine, S.; Li-Beisson, Y.; Delledonne, M.; Ballottari, M. Chlorella vulgaris genome assembly and annotation reveals the molecular basis for metabolic acclimation to high light conditions. Plant J. 2019, 100, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Lodish, H.; Berk, A.; Kaiser, C.; Krieger, M.; Bretscher, A.; Ploegh, H.; Amon, A.; Scott, M. Genes, genomics, and chromosomes. In Molecular Cell Biology, 7th ed.; W.H. Freeman Company: New York, NY, USA, 2013; pp. 227–230. [Google Scholar]
- Schatz, G.; Dobberstein, B. Common Principles of Protein Translocation across Membranes. Science 1996, 271, 1519–1526. [Google Scholar] [CrossRef]
- Bruce, B.D. Chloroplast transit peptides: Structure, function and evolution. Trends Cell Biol. 2000, 10, 440–447. [Google Scholar] [CrossRef]
- Ursu, A.-V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef]
- Lenfant, N.; Hotelier, T.; Velluet, E.; Bourne, Y.; Marchot, P.; Chatonnet, A. ESTHER, the database of the α/β-hydrolase fold superfamily of proteins: Tools to explore diversity of functions. Nucleic Acids Res. 2012, 41, D423–D429. [Google Scholar] [CrossRef]
- Janssen, B.J.; Snowden, K.C. Strigolactone and karrikin signal perception: Receptors, enzymes, or both? Front. Plant Sci. 2012, 3, 296. [Google Scholar] [CrossRef]
- Savvidou, M.G.; Katsabea, A.; Kotidis, P.; Mamma, D.; Lymperopoulou, T.V.; Kekos, D.; Kolisis, F.N. Studies on the catalytic behavior of a membrane-bound lipolytic enzyme from the microalgae Nannochloropsis oceanica CCMP1779. Enzym. Microb. Technol. 2018, 116, 64–71. [Google Scholar] [CrossRef]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The Lid Domain in Lipases: Structural and Functional Determinant of Enzymatic Properties. Front. Bioeng. Biotechnol. 2017, 5, 16. [Google Scholar] [CrossRef]
- Ramnath, L.; Sithole, B.; Govinden, R. Classification of lipolytic enzymes and their biotechnological applications in the pulping industry. Can. J. Microbiol. 2017, 63, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.L.; Buchholz, P.C.; Pleiss, J. The modular structure of α/β-hydrolases. FEBS J. 2020, 287, 1035–1053. [Google Scholar] [CrossRef] [PubMed]
- Nomaguchi, T.; Maeda, Y.; Liang, Y.; Yoshino, T.; Asahi, T.; Tanaka, T. Comprehensive analysis of triacylglycerol lipases in the oleaginous diatom Fistulifera solaris JPCC DA0580 with transcriptomics under lipid degradation. J. Biosci. Bioeng. 2018, 126, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat Mobilization in Adipose Tissue Is Promoted by Adipose Triglyceride Lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef]
- Leyland, B.; Boussiba, S.; Khozin-Goldberg, I. A Review of Diatom Lipid Droplets. Biology 2020, 9, 38. [Google Scholar] [CrossRef]
- Gupta, R.; Kumari, A.; Syal, P.; Singh, Y. Molecular and functional diversity of yeast and fungal lipases: Their role in biotechnology and cellular physiology. Prog. Lipid Res. 2015, 57, 40–54. [Google Scholar] [CrossRef]
- Pleiss, J.; Fischer, M.; Peiker, M.; Thiele, C.; Schmid, R.D. Lipase engineering database: Understanding and exploiting sequence-structure-function relationships. J. Mol. Cat. B Enzym. 2000, 10, 491–508. [Google Scholar] [CrossRef]
- Abdelkafi, S.; Ogata, H.; Barouh, N.; Fouquet, B.; Lebrun, R.; Pina, M.; Scheirlinckx, F.; Villeneuve, P.; Carrière, F. Identification and biochemical characterization of a GDSL-motif carboxylester hydrolase from Carica papaya latex. Biochim. Biophys. Acta-(BBA) Mol. Cell Biol. Lipids 2009, 1791, 1048–1056. [Google Scholar] [CrossRef]
- Martínez-Corona, R.; Marrufo, G.V.; Penagos, C.C.; Madrigal-Perez, L.A.; González-Hernández, J.C. Bioinformatic characterization of the extracellular lipases from Kluyveromyces marxianus. Yeast 2020, 37, 149–162. [Google Scholar] [CrossRef]
- Hitch, T.C.A.; Clavel, T. A proposed update for the classification and description of bacterial lipolytic enzymes. PeerJ 2019, 7, e7249. [Google Scholar] [CrossRef]
- Barriuso, J.; Vaquero, M.E.; Prieto, A.; Martínez, M.J. Structural traits and catalytic versatility of the lipases from the Candida rugosa-like family: A review. Biotechnol. Adv. 2016, 34, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Pleiss, J.; Fischer, M.; Schmid, R.D. Anatomy of lipase binding sites: The scissile fatty acid binding site. Chem. Phys. Lipids 1998, 93, 67–80. [Google Scholar] [CrossRef]
- Settembre, C.; Ballabio, A. Lysosome: Regulator of lipid degradation pathways. Trends Cell Biol. 2014, 24, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Lübke, T.; Lobel, P.; Sleat, D.E. Proteomics of the lysosome. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Rismani-Yazdi, H.; Haznedaroglu, B.Z.; Hsin, C.; Peccia, J. Transcriptomic analysis of the oleaginous microalga Neochloris oleoabundans reveals metabolic insights into triacylglyceride accumulation. Biotechnol. Biofuels 2012, 5, 74. [Google Scholar] [CrossRef]
- Anderson, R.A.; Bryson, G.M.; Parks, J.S. Lysosomal acid lipase mutations that determine phenotype in Wolman and choles-terol ester storage disease. Mol. Gene. Metab. 1999, 68, 345–346. [Google Scholar]
- Ries, S.; Aslanidis, C.; Fehringer, P.; Carel, J.C.; Gendrel, D.; Schmitz, G. A new mutation in the gene for lysosomal acid li-pase leads to Wolman disease in an African kindred. J. Lipid Res. 1996, 37, 1761–1765. [Google Scholar] [CrossRef]
- Frampton, J.E. Sebelipase Alfa: A Review in Lysosomal Acid Lipase Deficiency. Am. J. Cardiovasc. Drugs 2016, 16, 461–468. [Google Scholar] [CrossRef]
- Gomaraschi, M.; Bonacina, F.; Norata, G.D. Lysosomal Acid Lipase: From Cellular Lipid Handler to Immunometabolic Target. Trends Pharmacol. Sci. 2019, 40, 104–115. [Google Scholar] [CrossRef]
- Rombel, I.T.; Sykes, K.F.; Rayner, S.; Johnston, S.A. ORF-FINDER: A vector for high-throughput gene identification. Gene 2002, 282, 33–41. [Google Scholar] [CrossRef]
- Hoff, K.J.; Stanke, M. WebAUGUSTUS—A web service for training AUGUSTUS and predicting genes in eukaryotes. Nucleic Acids Res. 2013, 41, W123–W128. [Google Scholar] [CrossRef] [PubMed]
- Gouet, P.; Robert, X.; Courcelle, E. ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 2003, 31, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Attwood, T.K.; Babbitt, P.C.; Bateman, A.; Bork, P.; Bridge, A.J.; Chang, H.-Y.; Dosztányi, Z.; El-Gebali, S.; Fraser, M.; et al. InterPro in 2017—Beyond protein family and domain annotations. Nucleic Acids Res. 2017, 45, D190–D199. [Google Scholar] [CrossRef] [PubMed]
- Savojardo, C.; Bruciaferri, N.; Tartari, G.; Martelli, P.L.; Casadio, R. DeepMito: Accurate prediction of protein sub-mitochondrial localization using convolutional neural networks. Bioinformatics 2020, 36, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Claros, M.G.; Vincens, P. Computational Method to Predict Mitochondrially Imported Proteins and their Targeting Sequences. JBIC J. Biol. Inorg. Chem. 1996, 241, 779–786. [Google Scholar] [CrossRef]
- Gschloessl, B.; Guermeur, Y.; Cock, J.M. HECTAR: A method to predict subcellular targeting in heterokonts. BMC Bioinform. 2008, 9, 393. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; Von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Petersen, T.N.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Wang, S.; Mao, Y.; Narimatsu, Y.; Ye, Z.; Tian, W.; Goth, C.K.; Lira-Navarrete, E.; Pedersen, N.B.; Benito-Vicente, A.; Martin, C.; et al. Site-specific O-glycosylation of members of the low-density lipoprotein receptor superfamily enhances ligand interactions. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef]
- Eisenberg, D.; Lüthy, R.; Bowie, J.U. VERIFY3D: Assessment of protein models with three-dimensional profiles. Methods Enzymol. 1997, 277, 396–404. [Google Scholar]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web servicefor the recognition of errors in three-dimensional structures ofproteins. Nucleic Acids Res. 2007, 35, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Kuriata, A.; Gierut, A.M.; Oleniecki, T.; Ciemny, M.P.; Kolinski, A.; Kurcinski, M.; Kmiecik, S. CABS-flex 2.0: A web server for fast simulations of flexibility of protein structures. Nucleic Acids Res. 2018, 46, W338–W343. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.P.; Nguyen, T.B.; Patel, S.; Varadarajan, R.; Madhusudhan, M.S. Depth: A web server to compute depth, cavity sizes, detect potential small-molecule ligand-binding cavities and predict the pKa of ionizable residues in proteins. Nucleic Acids Res. 2013, 41, W314–W321. [Google Scholar] [CrossRef] [PubMed]
| TSA ID | Family InterPro | Family Pfam | Acyl Hydrolase Motif (GXSXG) | Highest Identity in ESTHER Database | Accession Number ESTHER Database |
|---|---|---|---|---|---|
| GHLX01005462.1 | IPR029058, AB_hydrolase | PF04083, Abhydro_lipase | GHSQG | 62.2% Lipase (M. conductrix) | A0A2P6V8F3 |
| GHLX01003448.1 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 46.6% Phospholipase A(1) chloroplastic (M. conductrix) | A0A2P6VDJ3 |
| GHLX01004364.1 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 79.9% Lipase_3 domain-containing protein (C. variabilis) | E1ZB31 |
| GHLX01003076.1 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 41.5% Lipase_3 domain-containing protein (C. variabilis) | E1ZMR0 |
| GHLX01002999.1 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 56.8% Lipase_3 domain-containing protein (C. variabilis) | E1Z559 |
| GHLX01001704.1 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 56.1%Lipase_3 domain-containing protein (C. variabilis) | E1ZAU0 |
| GHLX01004551.1 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 53.5% Lipase_3 domain-containing protein (C. variabilis) | E1Z6D5 |
| GHLX01003928.1 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 54.5% Lipase_3 domain-containing protein (C. variabilis) | E1ZMR0 |
| GHLX01006297.1 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 61.7% Lipase_3 domain-containing protein (C. variabilis) | E1Z6D6 |
| GHLX01001795.1 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GFSLG | 60.8% Lipase_3 domain-containing protein (C.a variabilis) | E1Z814 |
| GHLX01004575.1 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 55.3% Lipase_3 domain-containing protein (C. variabilis) | E1Z559 |
| GHLX01004232.1 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 52.4% Alpha beta-hydrolase (C. sorokiniana) | A0A2P6TJS1 |
| GHLX01005999 | IPR029058, AB_hydrolase IPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 62.2% sn1-specific diacylglycerol lipase alpha (Auxenochlorella protothecoides) | A0A087SMB1 |
| GHLX01005800 | IPR029058, AB_hydrolaseIPR002921, Fungal_lipase-like | PF01764, Lipase_3 | GHSLG | 44.5% sn1-specific diacylglycerol lipase alpha (M. conductrix) | A0A2P6V840 |
| Transcriptome Shotgun Assembly ID | Genome Survey Sequences ID | Start | End | Gene Length | Strand | 5′ UTR | 3′ UTR | StartCodon | StopCodon | Exon Number |
|---|---|---|---|---|---|---|---|---|---|---|
| GHLX01005462.1 | VATW01000002.1 | 1,249,709 | 1,253,360 | 3651 | + | 1,249,709 | 1,253,181 | 1,249,877 | 1,253,178 | 8 |
| GHLX01003448.1 | VATW01000019.1 | 480,480 | 486,471 | 5991 | + | 480,893 | 485,948 | 480,896 | 485,947 | 15 |
| GHLX01004364.1 | VATW01000012.1 | 444,856 | 447,981 | 3125 | − | 447,981 | 444,884 | 447,869 | 444,885 | 9 |
| GHLX01003076.1 | VATW01000017.1 | 300,534 | 306,599 | 6065 | − | 306,599 | 300,680 | 306,266 | 300,681 | 16 |
| GHLX01002999.1 | VATW01000004.1 | 234,154 | 243,389 | 9235 | − | 243,389 | 235,621 | 243,213 | 235,622 | 23 |
| GHLX01001704.1 | VATW01000077.1 | 53,966 | 57,537 | 3571 | + | 54,324 | 57,537 | 54,485 | 57,503 | 12 |
| GHLX01004551.1 | VATW01000014.1 | 391,368 | 400,369 | 9001 | + | 391,465 | 400,369 | 391,466 | 399,488 | 18 |
| GHLX01003928.1 | VATW01000021.1 | 364,412 | 371,783 | 7371 | + | 364,615 | 371,704 | 364,616 | 371,701 | 17 |
| GHLX01006297.1 | VATW01000014.1 | 387,248 | 391,356 | 4108 | + | 387,638 | 391,356 | 387,830 | 391,200 | 10 |
| GHLX01001795.1 | VATW01000003.1 | 1,009,232 | 1,012,963 | 3731 | + | 1,009,437 | 1,012,963 | 1,009,559 | 1,012,785 | 9 |
| GHLX01004575.1 | VATW01000004.1 | 243,414 | 249,169 | 5755 | − | 248,807 | 243,564 | 248,803 | 243,565 | 19 |
| GHLX01004232.1 | VATW01000004.1 | 387,912 | 396,833 | 8921 | + | 388,099 | 393,753 | 388,100 | 393,622 | 16 |
| GHLX01005999.1 | VATW01000002.1 | 467,247 | 474,411 | 7164 | − | 474,331 | 467,332 | 474,153 | 467,333 | 16 |
| GHLX01005800.1 | VATW01000021.1 | 56,136 | 59,601 | 3465 | + | 56,233 | 59,492 | 56,234 | 59,489 | 8 |
| Protein Name | Length (Amino Acids) | Molecular Mass (Da) | Theoretical Ip | Total Number of Negatively Charged Residues (Asp + Glu) | Total Number of Positively Charged Residues (Arg + Lys) | Molar Extinction (M−1 cm−1) | Half-Life | Grand Average of Hydropathicity Index (GRAVY) |
|---|---|---|---|---|---|---|---|---|
| Lip_5462 | 469 | 50,526.70 | 6.94 | 35 | 34 | 58,830 58,330 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | 0.056 |
| Lip_3448 | 810 | 87,834.98 | 4.50 | 125 | 55 | 113,955 113,330 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | −0.223 |
| Lip_4364 | 433 | 47,169.73 | 6.08 | 35 | 28 | 105,475 104,850 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | 0.083 |
| Lip_3076 | 966 | 104,984.87 | 8.78 | 80 | 91 | 164,875 163,750 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | 0.086 |
| Lip_2999 | 1104 | 121,199.69 | 8.67 | 98 | 110 | 193,210 191,710 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | −0.125 |
| Lip_1704 | 421 | 44,824.88 | 8.57 | 29 | 35 | 47,050 46,300 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | 0.057 |
| Lip_4551 | 1145 | 124,302.95 | 7.43 | 103 | 103 | 157,425 156,300 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | −0.057 |
| Lip_3928 | 934 | 100,739.17 | 8.92 | 80 | 93 | 132,905 131,780 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | −0.013 |
| Lip_6297 | 530 | 56,818.32 | 6.12 | 51 | 47 | 69,940 69,440 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | 0.101 |
| Lip_1795 | 557 | 59,817.51 | 4.09 | 67 | 23 | 97,330 96,830 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | 0.110 |
| Lip_4575 | 726 | 81,163.18 | 9.26 | 67 | 28 | 162,885 162,260 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | −0.117 |
| Lip_4232 | 779 | 85,389.08 | 9.34 | 62 | 83 | 107,675 106,800 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | 0.082 |
| Lip_5999 | 1003 | 102,522.28 | 5.31 | 112 | 86 | 85,425 84,800 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | −0.143 |
| Lip_5800 | 629 | 67,075.99 | 4.98 | 98 | 66 | 58,160 57,410 | 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), >10 h (Escherichia coli, in vivo). | −0.307 |
| Protein Name | Predicted Localization | Signal Peptide Sequence | Membrane Helix | N Glycosylation Sites |
|---|---|---|---|---|
| Lip_5462 | Ps (extracellular space) | MNVGRVAALFACLLQGACLALAVQ | - | 325 |
| Lip_3448 | Mito | - | - | 170 |
| Lip_4364 | Ps (extracellular space) | MRPAITEALLAVLVCLVVGANGA | - | 134/180/273/280 |
| Lip_3076 | Chloro (membrane) | - | 42–64/84–106/129–151/161–183/266–288/314–336/348–370 | 8/676 |
| Lip_2999 | Chloro (membrane) | - | 120–142/162–184/203–225/245–267/293–315/341–363/400–422 | 187/349/383/1030 |
| Lip_1704 | Chloro | MKLGLPLLLAALLLAAAAPATAR | - | 230/260/305/369 |
| Lip_4551 | Chloro (membrane) | - | 139–161/176–198/222–244/254–276/313–330/362–384/411–433/453–475/482–504 | 279/946 |
| Lip_3928 | plasma membrane | - | 62–84/174–196/220–242/254–276 | - |
| Lip_6297 | Ps (extracellular space) | MFIRVQSRVVSAVFTAIIFSLLFMSLVPTLQGN | 392 | |
| Lip_1795 | cyto | - | - | 19/53/307 |
| Lip_4575 | plasma membrane | MYIANTSVGGVLTLASFAMLAHGL | 6–28/48–70/80–102/115–137/170–189/196–218 | 5 |
| Lip_4232 | plasma membrane | - | 31–53/66–88/108–130/145–167/202–224/251–273/300–322 | 475 |
| Lip_5999 | chloro | - | - | - |
| Lip_5800 | chloro | - | - | 487 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Hlima, H.; Dammak, M.; Karray, A.; Drira, M.; Michaud, P.; Fendri, I.; Abdelkafi, S. Molecular and Structural Characterizations of Lipases from Chlorella by Functional Genomics. Mar. Drugs 2021, 19, 70. https://doi.org/10.3390/md19020070
Ben Hlima H, Dammak M, Karray A, Drira M, Michaud P, Fendri I, Abdelkafi S. Molecular and Structural Characterizations of Lipases from Chlorella by Functional Genomics. Marine Drugs. 2021; 19(2):70. https://doi.org/10.3390/md19020070
Chicago/Turabian StyleBen Hlima, Hajer, Mouna Dammak, Aida Karray, Maroua Drira, Philippe Michaud, Imen Fendri, and Slim Abdelkafi. 2021. "Molecular and Structural Characterizations of Lipases from Chlorella by Functional Genomics" Marine Drugs 19, no. 2: 70. https://doi.org/10.3390/md19020070
APA StyleBen Hlima, H., Dammak, M., Karray, A., Drira, M., Michaud, P., Fendri, I., & Abdelkafi, S. (2021). Molecular and Structural Characterizations of Lipases from Chlorella by Functional Genomics. Marine Drugs, 19(2), 70. https://doi.org/10.3390/md19020070








