Caged Dexamethasone/Quercetin Nanoparticles, Formed of the Morphogenetic Active Inorganic Polyphosphate, are Strong Inducers of MUC5AC
Abstract
1. Introduction
2. Results
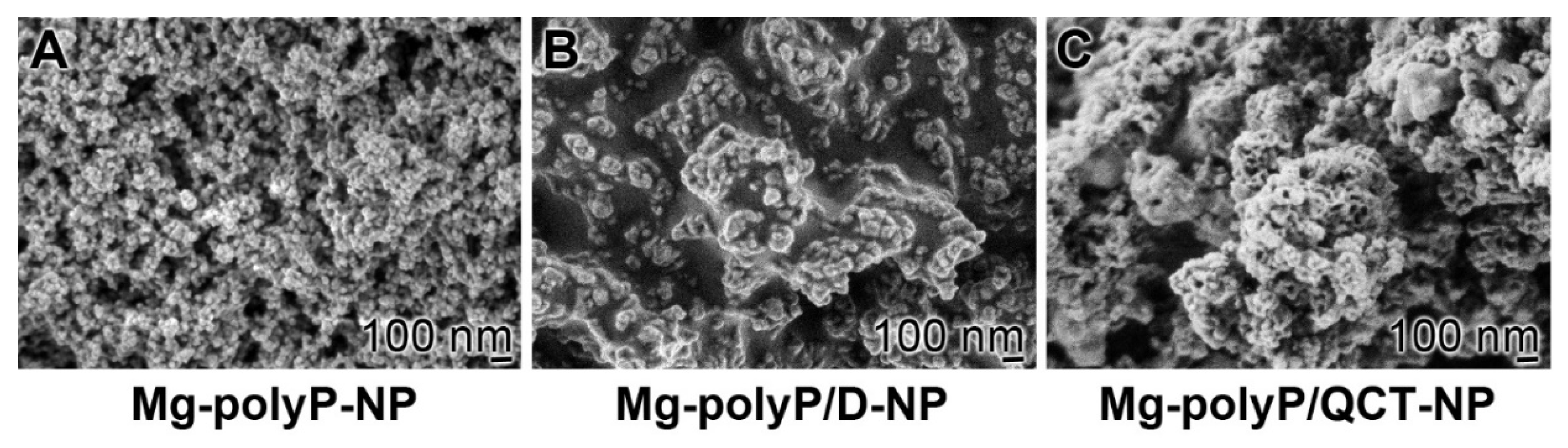
2.1. The PolyP-Based Dexamethasone and QCT NP
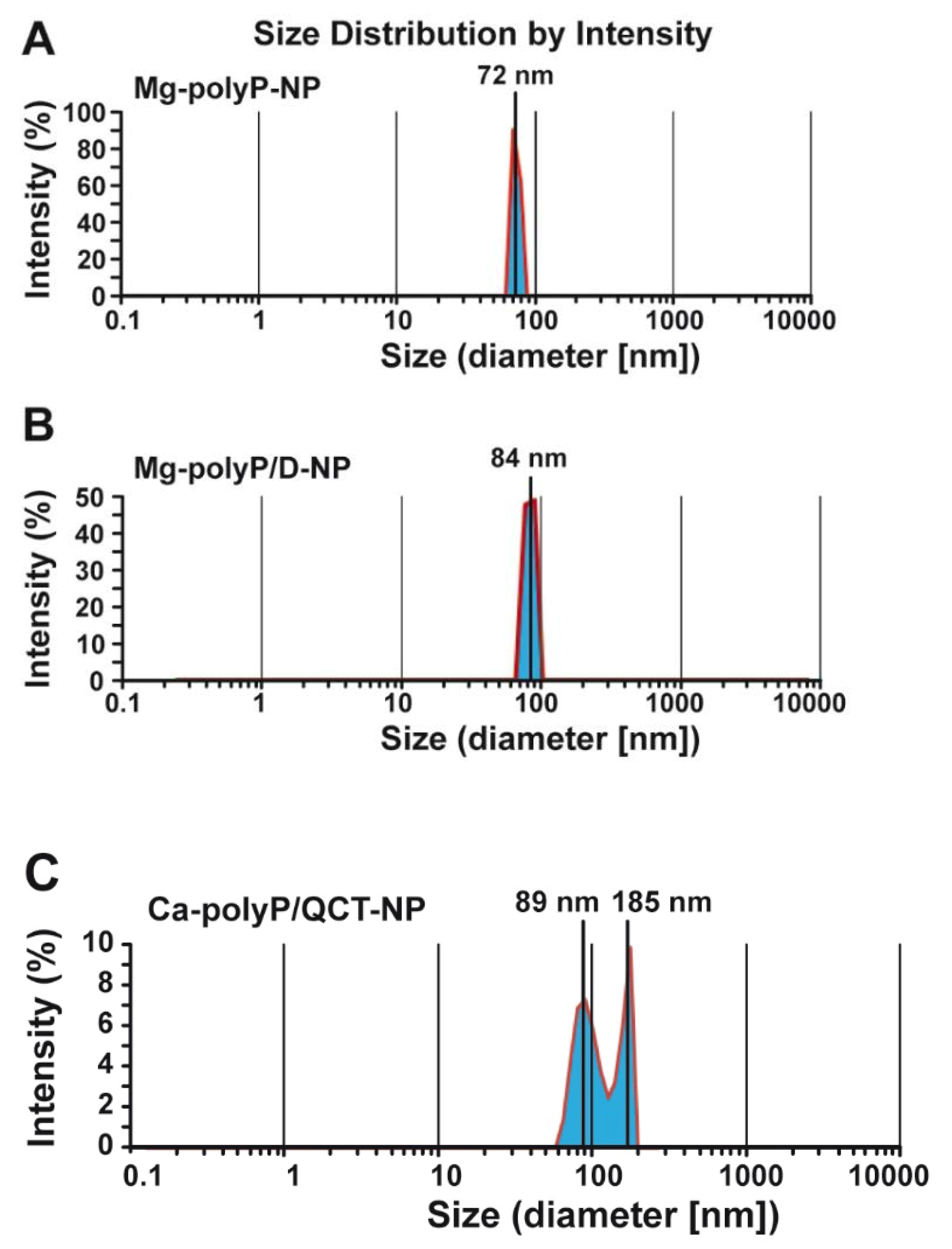
2.2. Size Determination of the Particles by Light Scattering
2.3. Interaction of Mucin with “Mg-polyP-NP”
2.4. FTIR Analysis of the Particles
2.5. Release Kinetics of Dexamethasone and QCT from the NP
2.6. Effect of Non-Capsulated and NP-Trapped Active Components: MTT
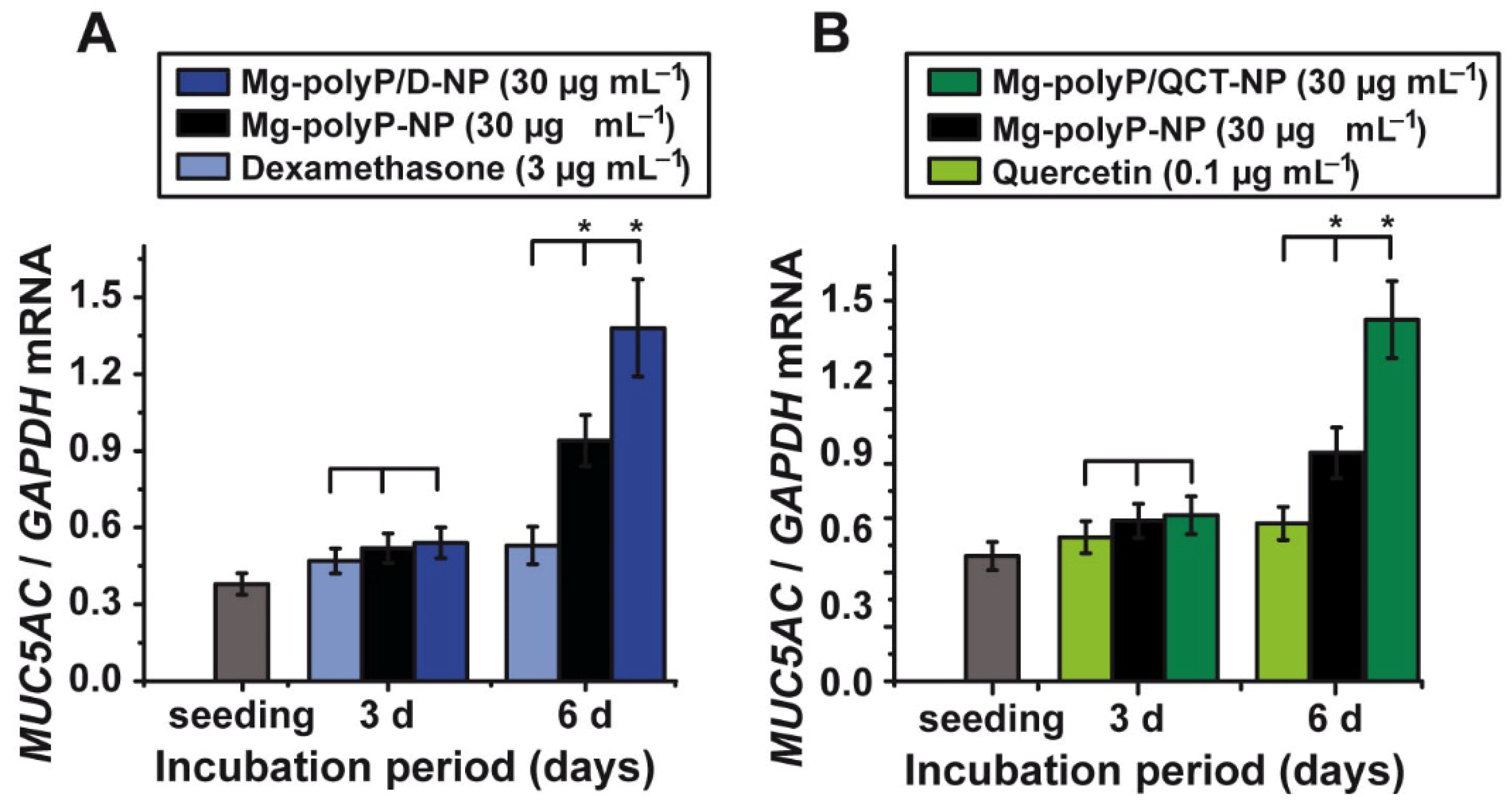
2.7. Effect of the PolyP-Based and Caged Drugs on MUC5AC Gene Expression
2.8. Immunofluorescence Analysis of MUC5AC
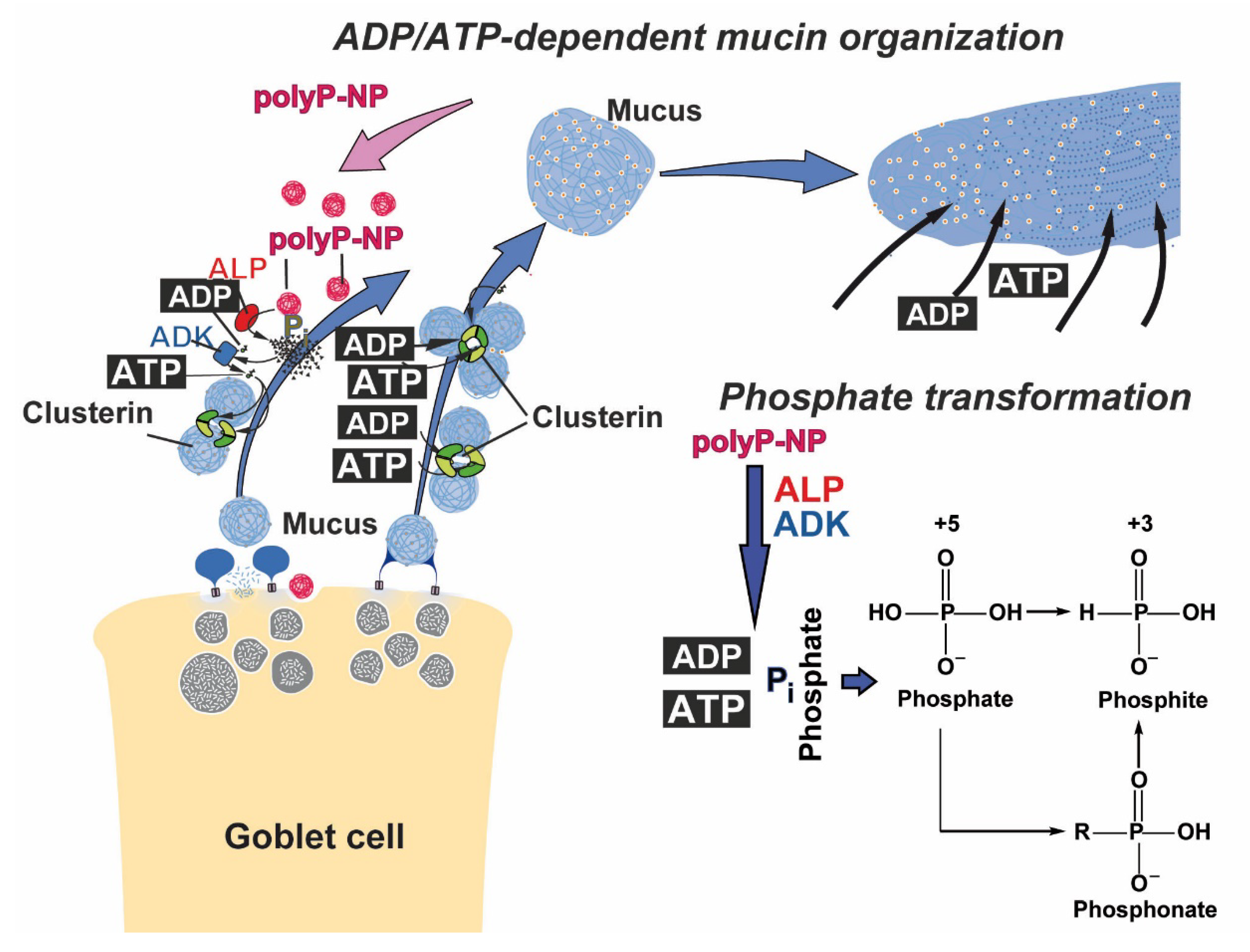
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Mg-polyP Nanoparticles and Dexamethasone-Loaded Particles
4.3. Encapsulation Efficiency of Dexamethasone
4.4. Preparation of the Quercetin-PolyP Nanoparticles
4.5. Encapsulation Efficiency of QCT
4.6. Interaction of “Mg-polyP-NP” with Mucin
4.7. QCT Release
4.8. Microscopic Analyses
4.9. Particle Size Determination
4.10. Fourier Transformed Infrared Spectroscopy
4.11. A549 Cells
4.12. MTT Metabolic Activity Assay
4.13. Quantitative Real-Time Polymerase Chain Reaction
4.14. Immunofluorescence Analysis
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mercer, J.; Greber, U.F. Virus interactions with endocytic pathways in macrophages and dendritic cells. Trends Microbiol. 2013, 21, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Schröder, H.C.; Neufurth, M.; Wang, X.H. Physiological polyanions interacting with the SARS-CoV-2 virus cell-docking machinery: A revitalization of the not fully exploited potential of innate immunity. Chem. Soc. Rev. 2020. under review. [Google Scholar]
- Fichtner, D.; Philipps, A.; Groth, M.; Schmidt-Posthaus, H.; Granzow, H.; Dauber, M.; Platzer, M.; Bergmann, S.M.; Schrudde, D.; Sauerbrei, A.; et al. Characterization of a novel picornavirus isolate from a diseased European eel (Anguilla anguilla). J. Virol. 2013, 87, 10895–10899. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.C. The History of the Smallpox; Longman: London, UK, 1815. [Google Scholar]
- Proença-Módena, J.L.; Acrani, G.O.; Snider, C.; Arruda, E. Chapter 58-Respiratory viral infections. In Tropical Infectious Diseases: Principles, Pathogens and Practice; Guerrant, R.L., Walker, D.H., Weller, P.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 378–391. [Google Scholar]
- Berman, S. Epidemiology of acute respiratory infections in children of developing countries. Rev. Infect. Dis. 1991, 13 (Suppl. 6), S454–S462. [Google Scholar] [CrossRef] [PubMed]
- Heraud, J.M.; Razanajatovo, N.H.; Viboud, C. Global circulation of respiratory viruses: From local observations to global predictions. Lancet Glob. Health 2019, 7, e982–e983. [Google Scholar] [CrossRef]
- Schröder, I. COVID-19: A risk assessment perspective. ACS Chem. Health Saf. 2020, 27, 160–169. [Google Scholar] [CrossRef]
- Subbarao, K.; Mahanty, S. Respiratory virus infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef]
- Uraih, L.C.; Maronpot, R.R. Normal histology of the nasal cavity and application of special techniques. Environ. Health Perspect. 1990, 85, 187–208. [Google Scholar]
- Hansson, G.C. Mucus and mucins in diseases of the intestinal and respiratory tracts. J. Intern. Med. 2019, 285, 479–490. [Google Scholar] [CrossRef]
- Thornton, D.J.; Rousseau, K.; McGuckin, M.A. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 2008, 70, 459–486. [Google Scholar] [CrossRef] [PubMed]
- Neufurth, M.; Wang, X.H.; Tolba, E.; Lieberwirth, I.; Wang, S.; Schröder, H.C.; Müller, W.E.G. The inorganic polymer, polyphosphate, blocks binding of SARS-CoV-2 spike protein to ACE2 receptor at physiological concentrations. Biochem. Pharmacol. 2020, 182, 114215. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Neufurth, M.; Schepler, H.; Wang, S.; Tolba, E.; Schröder, H.C.; Wang, X.H. The biomaterial polyphosphate blocks stoichiometrically binding of the SARS-CoV-2 S-protein to the cellular ACE2 receptor. Biomater. Sci. 2020, 8, 6603–6610. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.H.; Choi, S.H.; Smith, S.A. Polyphosphate: An ancient molecule that links platelets, coagulation, and inflammation. Blood 2012, 119, 5972–5979. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Schröder, H.C.; Müller, W.E.G. Amorphous polyphosphate, a smart bioinspired nano-/bio-material for bone and cartilage regeneration: Towards a new paradigm in tissue engineering. J. Mater. Chem. B 2018, 6, 2385–2412. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Schröder, H.C.; Wang, X.H. Inorganic polyphosphates as storage for and generator of metabolic energy in the extracellular matrix. Chem. Rev. 2019, 119, 12337–12374. [Google Scholar] [CrossRef]
- Lorenz, B.; Batel, R.; Bachinski, N.; Müller, W.E.G.; Schröder, H.C. Purification of two exopolyphosphatases from the marine sponge Tethya lyncurium. Biochim. Biophys. Acta 1995, 1245, 17–28. [Google Scholar] [CrossRef]
- Leitão, J.M.; Lorenz, B.; Bachinski, N.; Wilhelm, C.; Müller, W.E.G.; Schröder, H.C. Osmotic stress-induced synthesis and degradation of inorganic polyphosphates in the alga Phaeodactylum tricornutum. Mar. Ecol.-Prog. Ser. 1995, 121, 279–288. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Wang, S.F.; Ackermann, M.; Neufurth, M.; Steffen, R.; Mecja, E.; Muñoz-Espí, R.; Feng, Q.L.; Schröder, H.C.; Wang, X.H. Rebalancing β-amyloid-induced decrease of ATP level by amorphous nano/micro polyphosphate: Suppression of the neurotoxic effect of amyloid β-protein fragment 25–35. Int. J. Mol. Sci. 2017, 18, 2154. [Google Scholar] [CrossRef]
- Itakura, E.; Chiba, M.; Murata, T.; Matsuura, A. Heparan sulfate is a clearance receptor for aberrant extracellular proteins. J. Cell Biol. 2020, 219, e201911126. [Google Scholar] [CrossRef]
- Rohne, P.; Prochnow, H.; Wolf, S.; Renner, B.; Koch-Brandt, C. The chaperone activity of clusterin is dependent on glycosylation and redox environment. Cell. Physiol. Biochem. 2014, 34, 1626–1639. [Google Scholar] [CrossRef]
- Tsuruta, J.K.; Wong, K.; Fritz, I.B.; Griswold, M.D. Structural analysis of sulphated glycoprotein 2 from amino acid sequence. Relationship to clusterin and serum protein 40, 40. Biochem. J. 1990, 268, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Poon, S.; Treweek, T.M.; Wilson, M.R.; Easterbrook-Smith, S.B.; Carver, J.A. Clusterin is an extracellular chaperone that specifically interacts with slowly aggregating proteins on their off-folding pathway. FEBS Lett. 2002, 513, 259–266. [Google Scholar] [CrossRef]
- Hattrup, C.L.; Gendler, S.J. Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 2008, 70, 431–457. [Google Scholar] [CrossRef]
- Lu, W.; Liu, X.; Wang, T.; Liu, F.; Zhu, A.; Lin, Y.; Luo, J.; Ye, F.; He, J.; Zhao, J.; et al. Elevated MUC1 and MUC5AC mucin protein levels in airway mucus of critical ill COVID-19 patients. J. Med. Virol. 2020, 93, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, E.P.; Kato, K.; Lu, W.; Kim, K.C. Cellular and molecular biology of airway mucins. Int. Rev. Cell. Mol. Biol. 2013, 303, 139–202. [Google Scholar]
- Lieber, M.; Smith, B.; Szakal, A.; Nelson-Rees, W.; Todaro, G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer 1976, 17, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.H.; Di, Y.P.; Wu, R. Characterization of human mucin 5B gene expression in airway epithelium and the genomic clone of the amino-terminal and 5′-flanking region. Am. J. Respir. Cell Mol. Biol. 2001, 25, 542–553. [Google Scholar] [CrossRef]
- Li, N.; Li, Q.; Zhou, X.D.; Kolosov, V.P.; Perelman, J.M. The effect of quercetin on human neutrophil elastase-induced mucin5AC expression in human airway epithelial cells. Int. Immunopharmacol. 2012, 14, 195–201. [Google Scholar] [CrossRef]
- Ben Saad, H.; Gargouri, M.; Kallel, F.; Chaabene, R.; Boudawara, T.; Jamoussi, K.; Magné, C.; Mounir Zeghal, K.; Hakim, A.; Ben Amara, I. Flavonoid compounds from the red marine alga Alsidium corallinum protect against potassium bromate-induced nephrotoxicity in adult mice. Environ. Toxicol. 2017, 32, 1475–1486. [Google Scholar] [CrossRef]
- Voynow, J.A.; Young, L.R.; Wang, Y.; Horger, T.; Rose, M.C.; Fischer, B.M. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am. J. Physiol. 1999, 276, L835–L843. [Google Scholar] [PubMed]
- Kim, J.E.; Lee, M.R.; Park, J.J.; Choi, J.Y.; Song, B.R.; Son, H.J.; Choi, Y.W.; Kim, K.M.; Hong, J.T.; Hwang, D.Y. Quercetin promotes gastrointestinal motility and mucin secretion in loperamide-induced constipation of SD rats through regulation of the mAChRs downstream signal. Pharm. Biol. 2018, 56, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Sasso, A.; De Felice, B.; Di Gregorio, I.; La Rosa, G.; Lupoli, G.A.; Belfiore, A.; Mondola, P.; Santillo, M. Quercetin increases MUC2 and MUC5AC gene expression and secretion in intestinal goblet cell-like LS174T via PLC/PKCα/ERK1-2 pathway. Front. Physiol. 2018, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. eLife 2020, 9, e57309. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Schröder, H.C.; Feng, Q.; Diehl-Seifert, B.; Grebenjuk, V.A.; Müller, W.E.G. Isoquercitrin and polyphosphate co-enhance mineralization of human osteoblast-like SaOS-2 cells via separate activation of two RUNX2 cofactors AFT6 and Ets1. Biochem. Pharmacol. 2014, 89, 413–421. [Google Scholar] [CrossRef]
- Lorenz, B.; Schröder, H.C. Mammalian intestinal alkaline phosphatase acts as highly active exopolyphosphatase. Biochim. Biophys. Acta 2001, 1547, 254–261. [Google Scholar] [CrossRef]
- Matthay, M.A.; Thompson, B.T. Dexamethasone in hospitalised patients with COVID-19: Addressing uncertainties. Lancet Respir. Med. 2020, 8, 1170–1172. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Wang, S.; Glaßer, G.; Muñoz-Espí, R.; Link, T.; Wang, X.H. A new polyphosphate calcium material with morphogenetic activity. Mater. Lett. 2015, 148, 163–166. [Google Scholar] [CrossRef]
- Wang, X.H.; Ackermann, M.; Tolba, E.; Neufurth, M.; Wurm, F.; Feng, Q.; Wang, S.; Schröder, H.C.; Müller, W.E.G. Artificial cartilage bio-matrix formed of hyaluronic acid and Mg2+-polyphosphate. Eur. Cell. Mater. 2016, 32, 271–283. [Google Scholar] [CrossRef]
- Petrou, G.; Crouzier, T. Mucins as multifunctional building blocks of biomaterials. Biomater. Sci. 2018, 6, 2282–2297. [Google Scholar] [CrossRef]
- Stutman, J.M.; Termine, J.D.; Posner, A.S. Vibrational spectra and structure of the phosphate ion in some calcium phosphates. Trans. N. Y. Acad. Sci. 1965, 27, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Z.C.; Yu, S.H.; Chao, A.C.; Dong, G.C. Preparation and characterization of dexamethasone-immobilized chitosan scaffold. J. Biosci. Bioeng. 2012, 113, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Papale, F.; Bollino, F.; Piccolella, S.; Marciano, S.; Nocera, P.; Pacifico, S. Silica/quercetin sol-gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015, 16, 035001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Q.; Wang, J.; Zhang, X.; Yu, X.; Wan, C. Degradation kinetics of calcium polyphosphate bioceramic: An experimental and theoretical study. Mater. Res. 2009, 12, 495–501. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, E.-S.A.; Hassan, M.A.; El-Mahdy, M.M.; Mohamed, A.S. Formulation and evaluation of quercetin in certain dermatological preparations. J. Drug Deliv. Sci. Technol. 2007, 17, 431–436. [Google Scholar] [CrossRef]
- He, J.; Zhou, J.; Yang, W.; Zhou, Q.; Liang, X.; Pang, X.; Li, J.; Pan, F.; Liang, H. Dexamethasone affects cell growth/apoptosis/chemosensitivity of colon cancer via glucocorticoid receptor α/NF-κB. Oncotarget 2017, 8, 67670–67683. [Google Scholar] [CrossRef]
- Barnes, P.J. Corticosteroid effects on cell signalling. Eur. Respir. J. 2006, 27, 413–426. [Google Scholar] [CrossRef]
- Sadeghirad, B.; Siemieniuk, R.A.C.; Brignardello-Petersen, R.; Papola, D.; Lytvyn, L.; Vandvik, P.O.; Merglen, A.; Guyatt, G.H.; Agoritsas, T. Corticosteroids for treatment of sore throat: Systematic review and meta-analysis of randomised trials. BMJ 2017, 358, j3887. [Google Scholar] [CrossRef]
- Holte, K.; Kehlet, H. Perioperative single-dose glucocorticoid administration: Pathophysiologic effects and clinical implications. J. Am. Coll. Surg. 2002, 195, 694–712. [Google Scholar] [CrossRef]
- Murani, E.; Trakooljul, N.; Hadlich, F.; Ponsuksili, S.; Wimmers, K. Transcriptome responses to dexamethasone depending on dose and glucocorticoid receptor sensitivity in the liver. Front. Genet. 2019, 10, 559. [Google Scholar] [CrossRef]
- He, Y.; Yi, W.; Suino-Powell, K.; Zhou, X.E.; Tolbert, W.D.; Tang, X.; Yang, J.; Yang, H.; Shi, J.; Hou, L.; et al. Structures and mechanism for the design of highly potent glucocorticoids. Cell Res. 2014, 24, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Bas, E.; Gupta, C.; Van De Water, T.R. A novel organ of corti explant model for the study of cochlear implantation trauma. Anat. Rec. 2012, 295, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.N.; Paasche, G.; Reich, U.; Lenarz, T.; Warnecke, A. Differential effects of low- and high-dose dexamethasone on electrically induced damage of the cultured organ of corti. Neurotox. Res. 2020, 38, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Krishn, S.R.; Ganguly, K.; Kaur, S.; Batra, S.K. Ramifications of secreted mucin MUC5AC in malignant journey: A holistic view. Carcinogenesis 2018, 39, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wang, L.; Sun, J.; Li, C. Drug delivery based pharmacological enhancement and current insights of quercetin with therapeutic potential against oral diseases. Biomed. Pharmacother. 2020, 128, 110372. [Google Scholar] [CrossRef]
- Kim, H.; Chin, J.; Choi, H.; Baek, K.; Lee, T.G.; Park, S.E.; Wang, W.; Hahn, D.; Yang, I.; Lee, J.; et al. Phosphoiodyns A and B, unique phosphorus-containing iodinated polyacetylenes from a Korean sponge Placospongia sp. Org. Lett. 2013, 15, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, C.G.; Haenen, G.R.; Oostveen, R.M.; Stalpers, E.M.; Bast, A. Protection of flavonoids against lipid peroxidation: The structure activity relationship revisited. Free Radic. Res. 2002, 36, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.M.; Boersma, M.G.; Vervoort, J.; Rietjens, I.M. Peroxidase-catalyzed formation of quercetin quinone methide-glutathione adducts. Arch. Biochem. Biophys. 2000, 378, 224–233. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Ackermann, M.; Tolba, E.; Neufurth, M.; Ivetac, I.; Kokkinopoulou, M.; Schröder, H.C.; Wang, X.H. Role of ATP during the initiation of microvascularization. Acceleration of an autocrine sensing mechanism facilitating chemotaxis by inorganic polyphosphate. Biochem. J. 2018, 475, 3255–3273. [Google Scholar] [CrossRef]
- Weilheimer, D.M.; Regenstein, J.M. Antioxidant properties of phosphates and other additives during the storage of raw Mackerel and Lake Trout. JFS Food Chem. Toxicol. 2004, 69, fct102–fct108. [Google Scholar] [CrossRef]
- Button, B.; Okada, S.F.; Frederick, C.B.; Thelin, W.R.; Boucher, R.C. Mechanosensitive ATP release maintains proper mucus hydration of airways. Sci. Signal 2013, 6, ra46. [Google Scholar] [CrossRef] [PubMed]
- Salathe, M. Effects of beta-agonists on airway epithelial cells. J. Allergy Clin. Immunol. 2002, 110, S275–S281. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz-Scroggins, M.E.; Finkbeiner, W.E.; Gordon, E.D.; Yuan, S.; Zlock, L.; Bhakta, N.R.; Woodruff, P.G.; Fahy, J.V.; Boushey, H.A. Corticosteroid and long-acting ß-agonist therapy reduces epithelial goblet cell metaplasia. Clin. Exp. Allergy 2017, 47, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Hang, H.C.; Yu, C.; Kato, D.L.; Bertozzi, C.R. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc. Natl. Acad. Sci. USA 2003, 100, 14846–14851. [Google Scholar] [CrossRef]
- Olgun, A. Biological effects of deuteronation: ATP synthase as an example. Theor. Biol. Med. Model. 2007, 4, 9. [Google Scholar] [CrossRef]
- Damiano, S.; Morano, A.; Ucci, V.; Accetta, R.; Mondola, P.; Paternò, R.; Avvedimento, V.E.; Santillo, M. Dual oxidase 2 generated reactive oxygen species selectively mediate the induction of mucins by epidermal growth factor in enterocytes. Int. J. Biochem. Cell Biol. 2015, 60, 8–18. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Ghosh, N.; Chakraborty, T.; Mallick, S.; Mana, S.; Singha, D.; Ghosh, B.; Roy, S. Synthesis, characterization and study of antioxidant activity of quercetin-magnesium complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 807–813. [Google Scholar] [CrossRef]
- Gray, M.J.; Jakob, U. Oxidative stress protection by polyphosphate—New roles for an old player. Curr. Opin. Microbiol. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Hsu, S.C.; Qi, M.; DeFranco, D.B. Cell cycle regulation of glucocorticoid receptor function. EMBO J. 1992, 11, 3457–3468. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.M.; Li, L.; Chen, M.; Lagunero, F.T.; Go, V.L.; Boros, L.G. Diverse mechanisms of growth inhibition by luteolin, resveratrol, and quercetin in MIA PaCa-2 cells: A comparative glucose tracer study with the fatty acid synthase inhibitor C75. Metabolomics 2012, 8, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, S.; Tolba, E.; Neufurth, M.; Ackermann, M.; Muñoz-Espí, R.; Lieberwirth, I.; Glasser, G.; Schröder, H.C.; Wang, X.H. Transformation of amorphous polyphosphate nanoparticles into coacervate complexes: An approach for the encapsulation of mesenchymal stem cells. Small 2018, 14, e1801170. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Ackermann, M.; Tolba, E.; Neufurth, M.; Wang, S.; Schröder, H.C.; Wang, X.H. A bio-imitating approach to fabricate an artificial matrix for cartilage tissue engineering using magnesium-polyphosphate and hyaluronic acid. RSC Adv. 2016, 6, 88559–88570. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Tolba, E.; Wang, S.; Neufurth, M.; Lieberwirth, I.; Ackermann, M.; Schröder, H.C.; Wang, X.H. Nanoparticle-directed and ionically forced polyphosphate coacervation: A versatile and reversible core-shell system for drug delivery. Sci. Rep. 2020, 10, 17147. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, R.A.; Cichota, L.C.; Rolim, C.M.B.; Beck, C.R. Validation of a simple and rapid UV spectrophotometric method for dexamethasone assay in tablets. Quim. Nova 2009, 32, 1052–1054. [Google Scholar]
- Pakade, V.E.; Lesaoana, M.; Tavengwa, N.T. Effect of pH, time and temperature on forced degradation studies of quercetin in presence of polymers. Asian J. Chem. 2016, 28, 2181–2187. [Google Scholar] [CrossRef]
- Jurasekova, Z.; Domingo, C.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Effect of pH on the chemical modification of quercetin and structurally related flavonoids characterized by optical (UV-visible and Raman) spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 12802–12811. [Google Scholar] [CrossRef]
- Nordgård, C.T.; Nonstad, U.; Olderøy, M.Ø.; Espevik, T.; Draget, K.I. Alterations in mucus barrier function and matrix structure induced by guluronate oligomers. Biomacromolecules 2014, 15, 2294–2300. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, P.; Liu, J.; Ke, Q.; Zhang, C.; Guo, Y.; Ding, H. Lanthanum phosphate/chitosan scaffolds enhance cytocompatibility and osteogenic efficiency via the Wnt/β-catenin pathway. J. Nanobiotechnol. 2018, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.R.; Abdullatif, M.B.; Burnett, E.C.; Kempsell, K.E.; Conforti, F.; Tolley, H.; Collins, J.E.; Davies, D.E. Long term culture of the A549 cancer cell line promotes multilamellar body formation and differentiation towards an alveolar type II pneumocyte phenotype. PLoS ONE 2016, 11, e0164438. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Wang, X.H.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Lieberwirth, I.; Glasser, G.; Wiens, M.; Schröder, H.C. Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro. Acta Biomater. 2011, 7, 2661–2671. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bodhak, S.; Bose, S.; Kinsel, W.C.; Bandyopadhyay, A. Investigation of in vitro bone cell adhesion and proliferation on Ti using direct current stimulation. Mater. Sci. Eng. C Mater. Biol. Appl. 2012, 32, 2163–2168. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Belikov, S.I.; Tremel, W.; Perry, C.C.; Gieskes, W.W.C.; Boreiko, A.; Schröder, H.C. Siliceous spicules in marine demosponges (example Suberites domuncula). Micron 2006, 37, 107–120. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An analysis of transformations. J. R. Statist. Soc. B 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Pasek, M.A.; Sampson, J.M.; Atlas, Z. Redox chemistry in the phosphorus biogeochemical cycle. Proc. Natl. Acad. Sci. USA 2014, 111, 15468–15473. [Google Scholar] [CrossRef]
- Glonek, T.; Henderson, T.O.; Hilderbrand, R.L.; Myers, T.C. Biological phosphonates: Determination by phosphorus-31 nuclear magnetic resonance. Science 1970, 169, 172–174. [Google Scholar] [CrossRef]
- Vrtis, J.M.; White, A.K.; Metcalf, W.W.; van der Donk, W.A. Phosphite dehydrogenase: An unusual phosphoryl transfer reaction. J. Am. Chem. Soc. 2001, 123, 2672–2673. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Neufurth, M.; Wang, S.; Tan, R.; Schröder, H.C.; Wang, X.H. Morphogenetic (mucin expression) as well as potential anti-corona viral activity of the marine secondary metabolite polyphosphate on A549 cells. Mar. Drugs 2020, 18, 639. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.W.; Dowell, M.L.; Lethem, M.; Van Scott, M. Goblet cell degranulation in isolated canine tracheal epithelium: Response to exogenous ATP, ADP, and adenosine. Am. J. Physiol. 1992, 262, C1313–C1323. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neufurth, M.; Wang, X.; Wang, S.; Schröder, H.C.; Müller, W.E.G. Caged Dexamethasone/Quercetin Nanoparticles, Formed of the Morphogenetic Active Inorganic Polyphosphate, are Strong Inducers of MUC5AC. Mar. Drugs 2021, 19, 64. https://doi.org/10.3390/md19020064
Neufurth M, Wang X, Wang S, Schröder HC, Müller WEG. Caged Dexamethasone/Quercetin Nanoparticles, Formed of the Morphogenetic Active Inorganic Polyphosphate, are Strong Inducers of MUC5AC. Marine Drugs. 2021; 19(2):64. https://doi.org/10.3390/md19020064
Chicago/Turabian StyleNeufurth, Meik, Xiaohong Wang, Shunfeng Wang, Heinz C. Schröder, and Werner E. G. Müller. 2021. "Caged Dexamethasone/Quercetin Nanoparticles, Formed of the Morphogenetic Active Inorganic Polyphosphate, are Strong Inducers of MUC5AC" Marine Drugs 19, no. 2: 64. https://doi.org/10.3390/md19020064
APA StyleNeufurth, M., Wang, X., Wang, S., Schröder, H. C., & Müller, W. E. G. (2021). Caged Dexamethasone/Quercetin Nanoparticles, Formed of the Morphogenetic Active Inorganic Polyphosphate, are Strong Inducers of MUC5AC. Marine Drugs, 19(2), 64. https://doi.org/10.3390/md19020064









