The Protect Effects of Chitosan Oligosaccharides on Intestinal Integrity by Regulating Oxidative Status and Inflammation under Oxidative Stress
Abstract
1. Introduction
2. Results
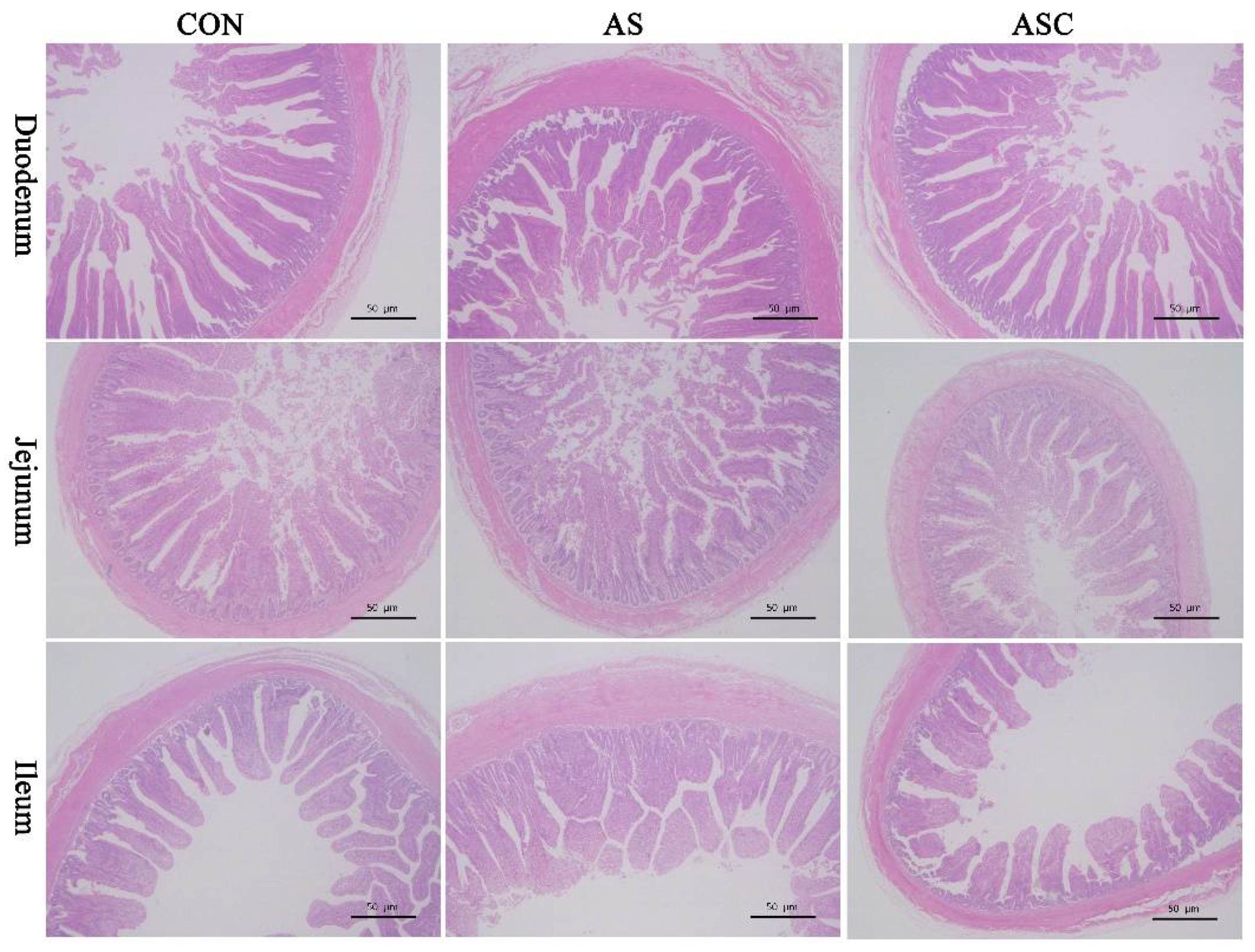
2.1. Intestinal Mucosal Morphology
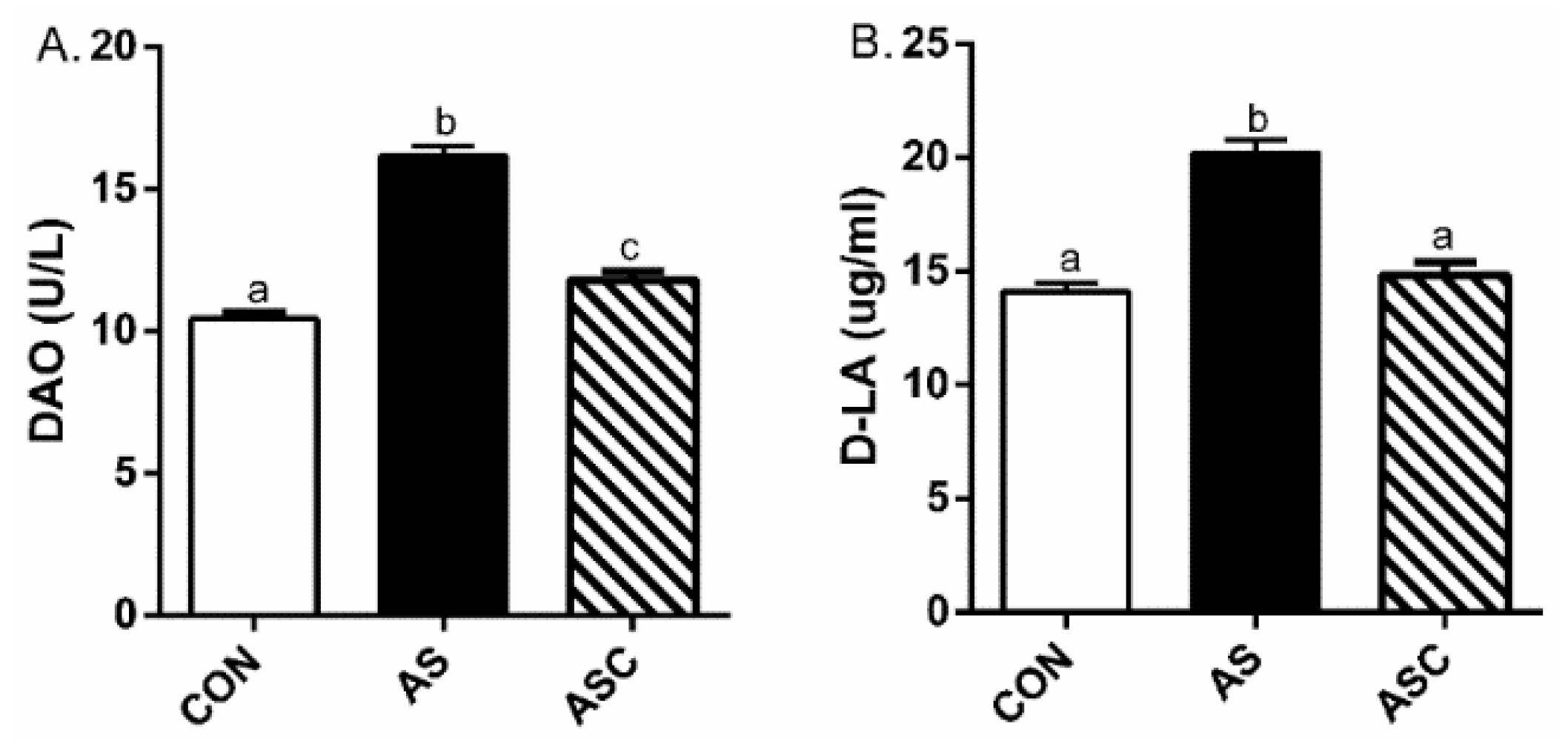
2.2. Intestinal Permeability
2.3. Antioxidant Capacity
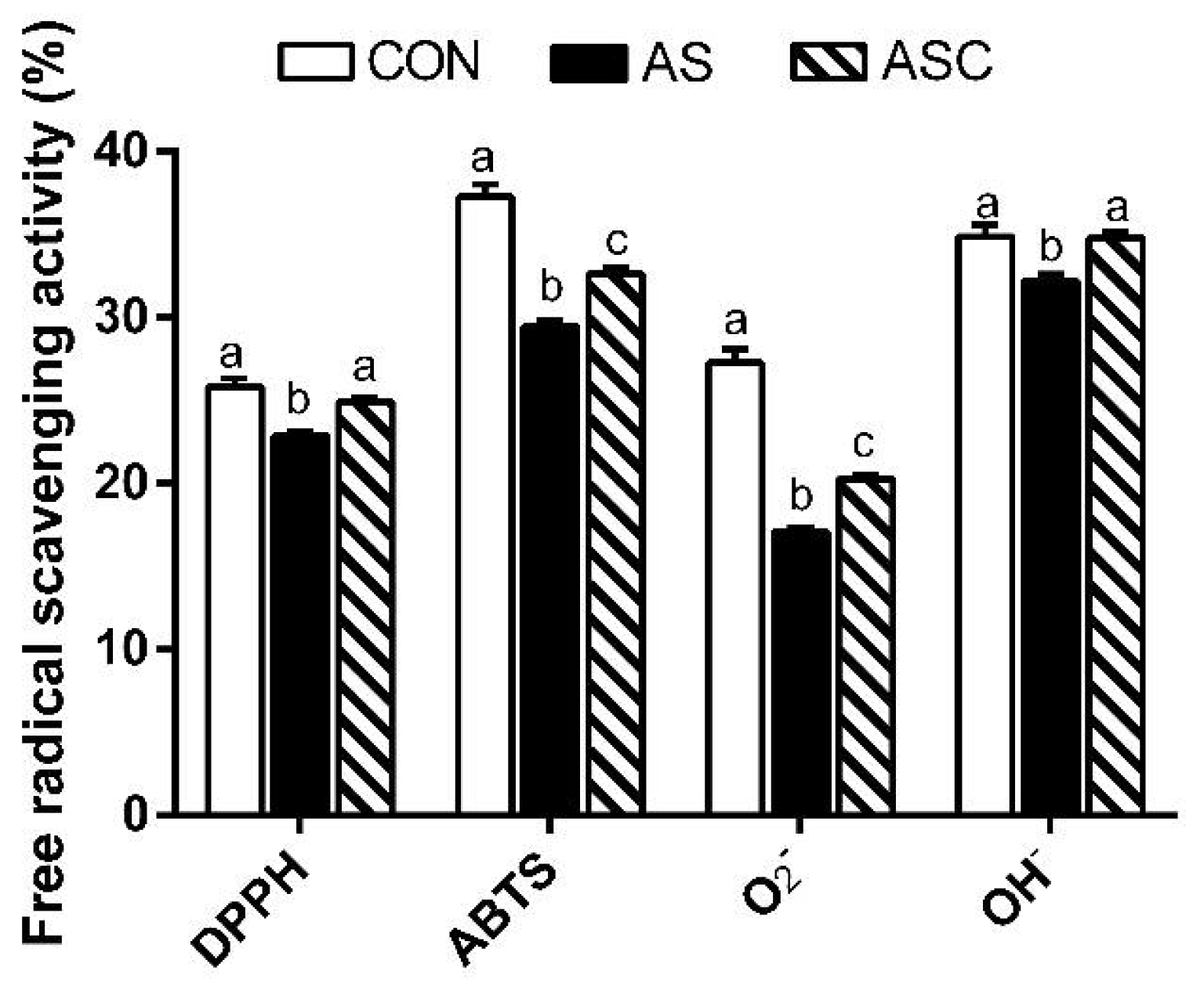
2.4. Free Radical Scavenging Activity
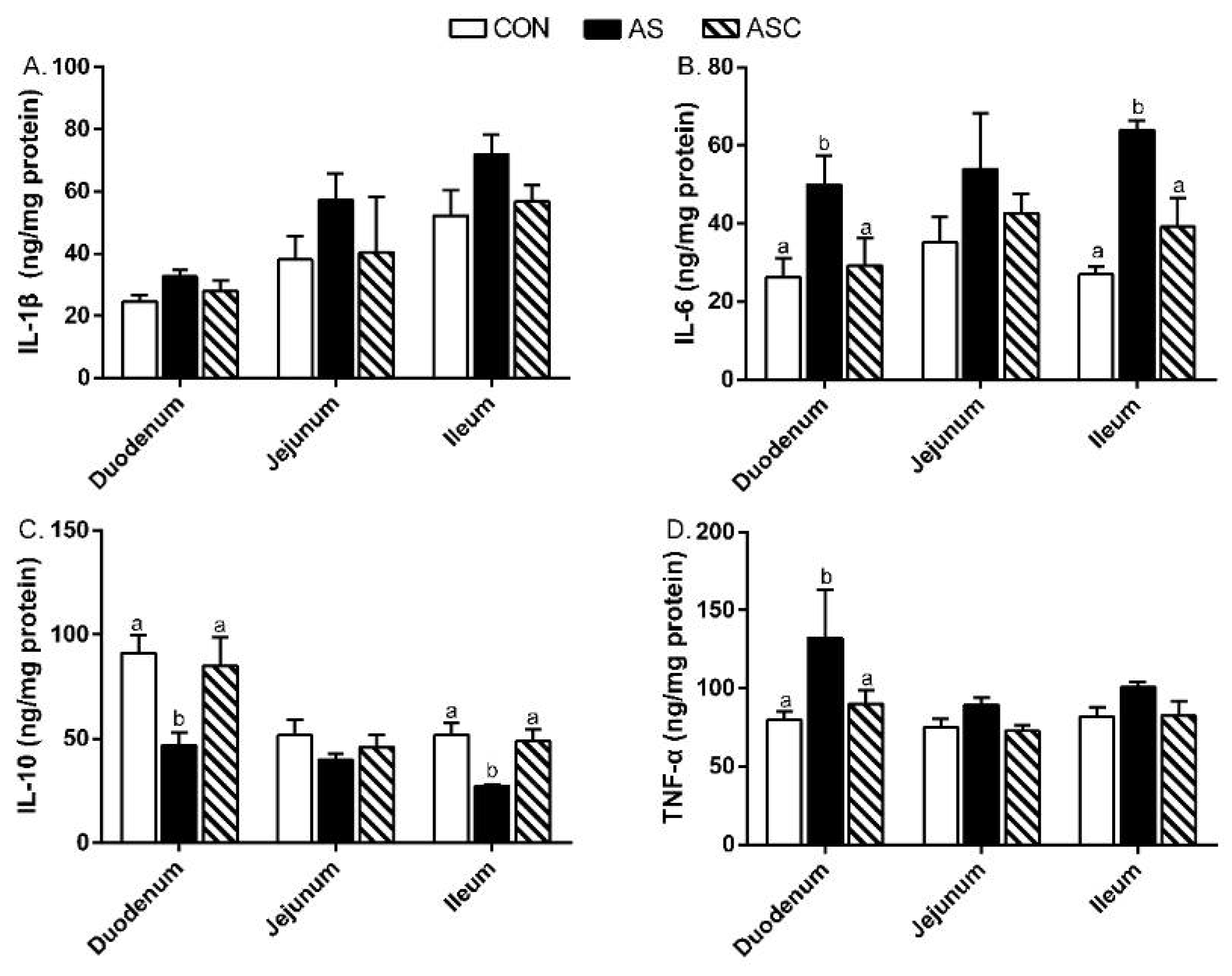
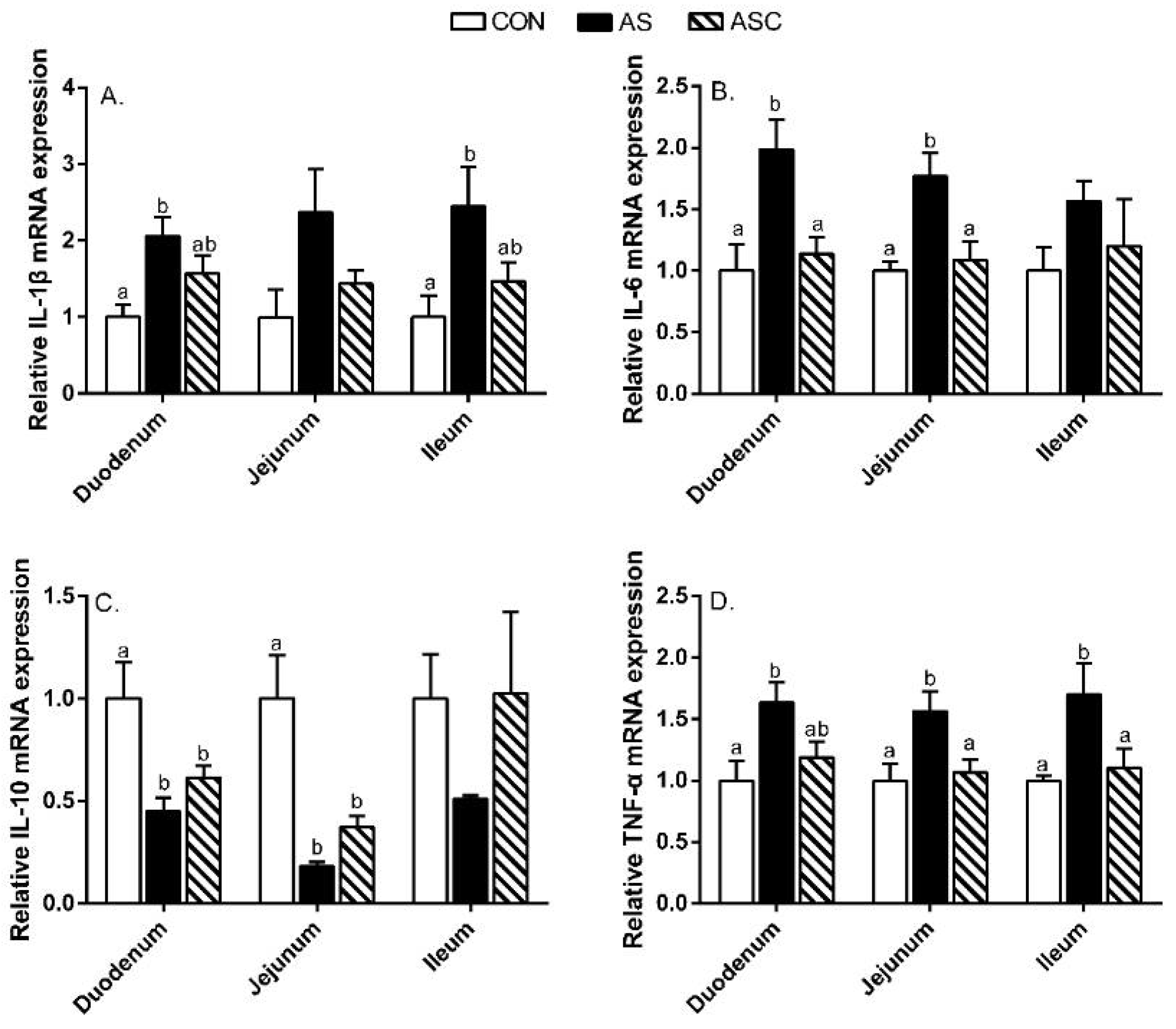
2.5. Intestinal Mucosal Immunity
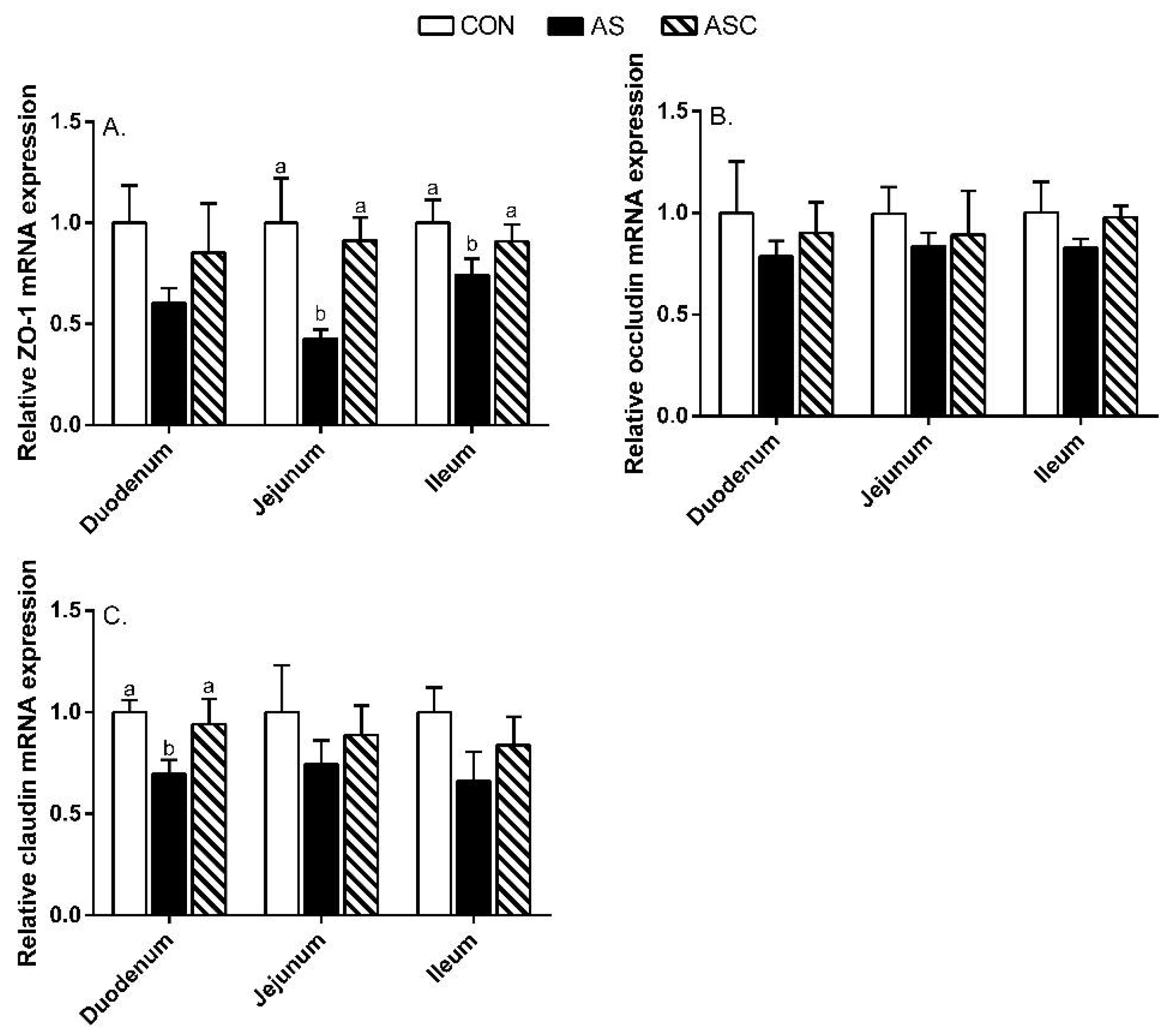
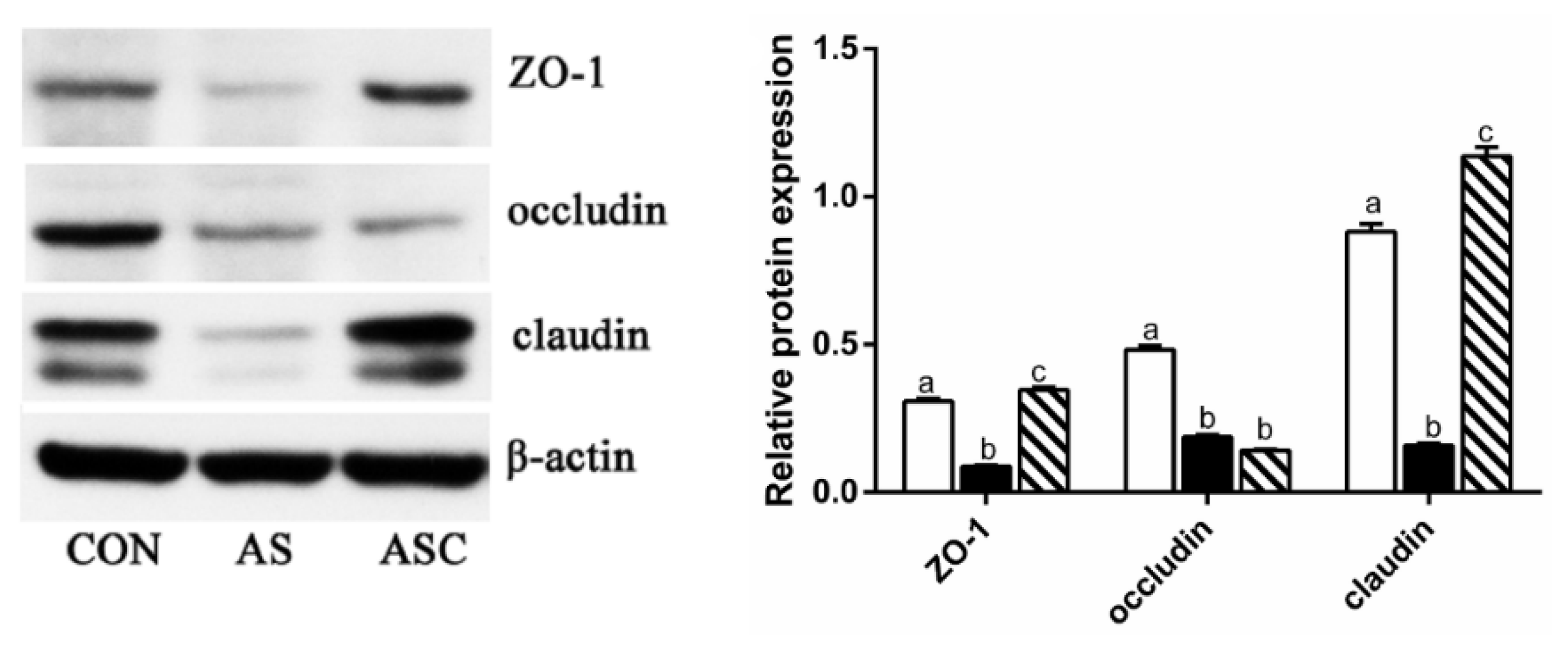
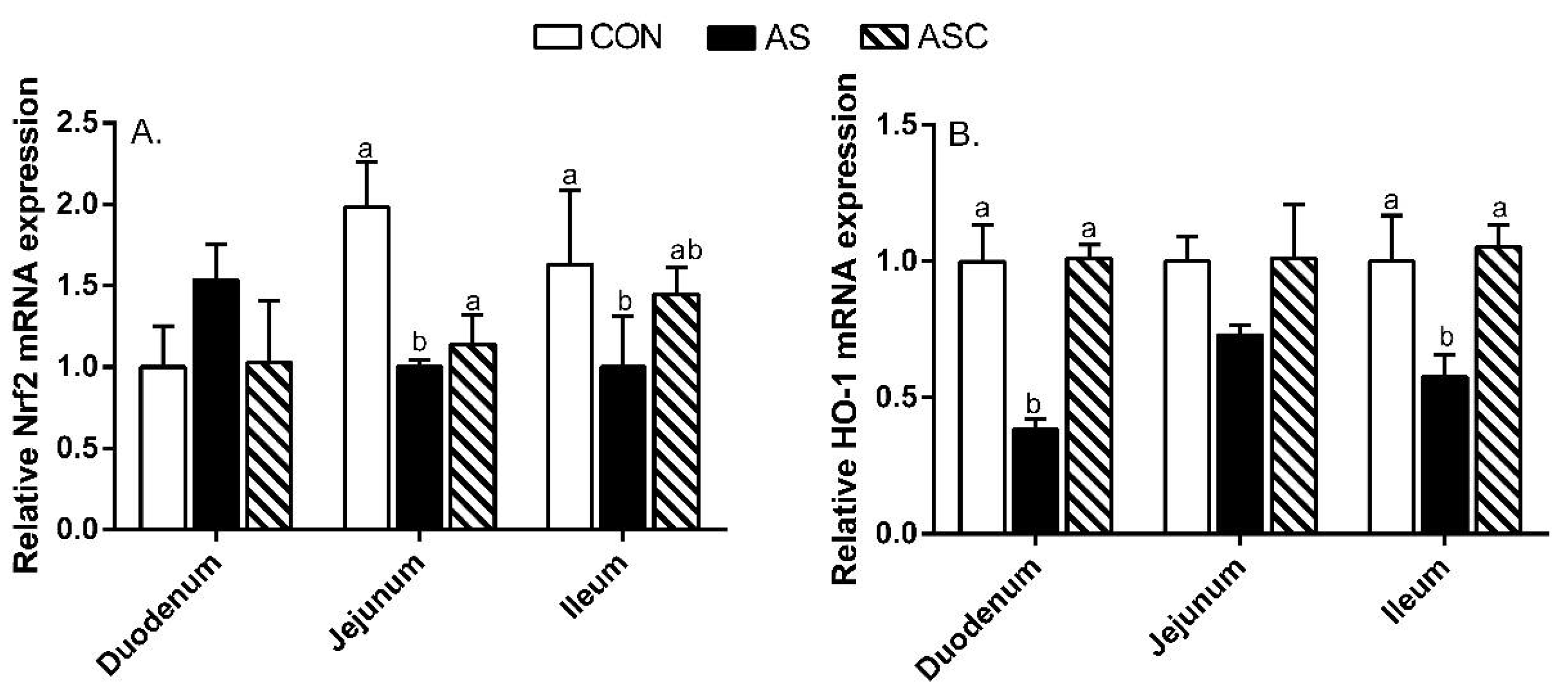
2.6. Intestinal Barrier Function-Related Gene Expression
3. Discussion
4. Materials and Methods
4.1. Animals, Diets, and Experimental Design
4.2. Sample Collection
4.3. Serum Diamine Oxidase (DAO) and d-Lactate Acid (D-LA)
4.4. Intestinal Morphology
4.5. Intestinal Antioxidant Parameters and Inflammatory Cytokines
4.6. Free Radical Scavenging Activities
4.7. Gene Expression Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Bio. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Li, Y.; Chang, Q.; Zhao, Z. Dietary chitosan oligosaccharides alleviate heat stress–induced intestinal oxidative stress and inflammatory response in yellow-feather broilers. Poult. Sci. 2020, 99, 6745–6752. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Song, Z.; Li, S.; Yan, E.; Zhang, H.; Zhang, L.; Wang, C.; Wang, T. Effects of resveratrol on intestinal oxidative status and inflammation in heat-stressed rats. J. Therm. Biol. 2019, 85, 102415. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, Y.; Chen, R.; Su, Y.; Zhang, R.; He, Q.; Wang, K.; Wen, C.; Zhou, Y. Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult. Sci. 2019, 98, 4767–4776. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Li, S.; Chang, Q.; Zhao, Z. Chitosan Oligosaccharides Protect Sprague Dawley Rats from Cyclic Heat Stress by Attenuation of Oxidative and Inflammation Stress. Animals 2019, 9, 1074. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wang, W.; Zhang, H.; Xu, S. Application of transcriptome analysis: Oxidative stress, inflammation and microtubule activity disorder caused by ammonia exposure may be the primary factors of intestinal microvilli deficiency in chicken. Sci. Total. Environ. 2019, 696, 134035. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Wu, Z.; Hou, Y. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef]
- Wu, S.; Pan, L.; Liao, H.; Yao, W.; Shen, N.; Chen, C.; Liu, D.; Ge, M. High-fat diet increased NADPH-oxidase-related oxidative stress and aggravated LPS-induced intestine injury. Life Sci. 2020, 253, 117539. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, H.; Ma, T.; Yan, Z.; Zhang, Y.; Geng, Y.; Zhu, Y.; Shi, Y. Effect of chronic cyclic heat stress on the intestinal morphology, oxidative status and cecal bacterial communities in broilers. J. Therm. Biol. 2020, 91, 102619. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, N.; Wu, X.; Wang, G.; Lin, L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018, 97, 2675–2683. [Google Scholar] [CrossRef]
- Song, Z.; Cheng, K.; Zhang, L.; Wang, T. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J. Therm. Biol. 2017, 69, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.H.; Moon, Y.S.; SoHn, S.H.; Jang, I.S. Effects of cyclic heat stress or vitamin C supplementation during cyclic heat stress on HSP70, inflammatory cytokines, and the antioxidant defense system in Sprague Dawley rats. Exp. Anim. 2012, 61, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Husain, T.; Akabar, M. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Qiao, Y.; Bai, X.; Du, Y. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011, 11, 121–127. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Xu, G.; Li, X.; Bai, X.; Wei, P.; Yu, C.; Du, Y. Chitosan oligosaccharides attenuate hydrogen peroxide-induced stress injury in human umbilical vein endothelial cells. Pharmacol. Res. 2009, 59, 167–175. [Google Scholar] [CrossRef]
- Lan, R.; Chang, Q.; An, L.; Zhao, Z. Dietary supplementation with chitosan oligosaccharides alleviates oxidative stress in rats challenged with hydrogen peroxide. Animals 2020, 10, 55. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Chen, Y.; Qu, H.; Zhao, Y.; Wen, C.; Zhou, Y. Dietary chitooligosaccharide inclusion as an alternative to antibiotics improves intestinal morphology, barrier function, antioxidant capacity, and immunity of broilers at early age. Animals 2019, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, X.; Peng, X.; Chi, X.; Cui, H.; Zuo, Z.; Fang, J. Effect of chitosan oligosaccharides on antioxidant function, lymphocyte cycle and apoptosis in ileum mucosa of broiler. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 571–577. [Google Scholar]
- Zou, Y.; Wei, H.; Xiang, Q.; Wang, J.; Zhou, Y.; Peng, J. Protective effect of quercetin on pig intestinal integrity after transport stress is associated with regulation oxidative status and inflammation. J. Vet. Med. Sci. 2016, 78, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Y.; Chang, Q.; Guo, G.; Lan, R. Effects of chitosan oligosaccharides on intestinal oxidative stress and inflammation response in heat stressed rats. Exp. Anim. 2021, 70. [Google Scholar] [CrossRef]
- Zhao, P.; Piao, X.; Zeng, Z.; Li, P.; Xu, X.; Wang, H. Effect of Forsythia suspensa extract and chito-oligosaccharide alone or in combination on performance, intestinal barrier function, antioxidant capacity and immune characteristics of weaned piglets. Anim. Sci. J. 2017, 88, 854–862. [Google Scholar] [CrossRef]
- Liu, P.; Piao, X.; Kim, S.; Wang, L.; Shen, Y.; Lee, H.; Li, S. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs. J. Anim. Sci. 2008, 86, 2609–2618. [Google Scholar] [CrossRef]
- Liu, P.; Piao, X.; Thacker, P.; Zeng, Z.; Li, P.; Wang, D.; Kim, S. Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J. Anim. Sci. 2010, 88, 3871–3879. [Google Scholar] [CrossRef]
- Song, D.; Cheng, Y.; Li, X.; Wang, F.; Lu, Z.; Xiao, X.; Wang, Y. Biogenic nanoselenium particles effectively attenuate oxidative stress-induced intestinal epithelial barrier injury by activating the Nrf2 antioxidant pathway. ACS Appl. Mater. Interfaces 2017, 9, 14724–14740. [Google Scholar] [CrossRef]
- He, N.; Wang, S.; Lv, Z.; Zhao, W.; Li, S. Low molecular weight chitosan oligosaccharides (LMW-COSs) prevent obesity-related metabolic abnormalities in association with the modification of gut microbiota in high-fat diet (HFD)-fed mice. Food Funct. 2020, 11, 9947–9959. [Google Scholar] [CrossRef]
- Osho, S.; Adeola, O. Chitosan oligosaccharide supplementation alleviates stress stimulated by in-feed dexamethasone in broiler chickens. Poult. Sci. 2020, 99, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jiang, F.; Xu, Q.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Yu, J.; He, J. New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. Rsc. Adv. 2017, 7, 9669–9679. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Wen, X.; Wang, L.; Jiang, Z.; Zheng, C. Effects of low-molecular-weight chitosan on the growth performance, intestinal morphology, barrier function, cytokine expression and antioxidant system of weaned piglets. BMC Vet. Res. 2018, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, W.; Jia, G.; Zhao, H.; Chen, X.; Wu, C.; Tang, J.; Wang, J.; Liu, G. New insights into the role of spermine in enhancing the antioxidant capacity of rat spleen and liver under oxidative stress. Anim. Nutr. 2017, 3, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.; Haq, E.; Hamid, A.; Masood, A.; Zargar, M. Long dose exposure of hydrogen peroxide (H2O2) in albino rats and effect of Podophyllum hexandrum on oxidative stress. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 906–915. [Google Scholar] [CrossRef]
- Duan, J.; Yin, J.; Ren, W.; Liu, T.; Cui, Z.; Huang, X.; Wu, L.; Kim, S.W.; Liu, G.; Wu, X. Dietary supplementation with L-glutamate and L-aspartate alleviates oxidative stress in weaned piglets challenged with hydrogen peroxide. Amino Acids 2016, 48, 53–64. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Alotaibi, M.F.; Bin-Jumah, M.; Elgebaly, H.; Mahmoud, A.M. Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed. Pharmacother. 2019, 111, 676–685. [Google Scholar] [CrossRef]
- Zhang, Y.; Ahmad, K.A.; Khan, F.U.; Yan, S.; Ihsan, A.U.; Ding, Q. Chitosan oligosaccharides prevent doxorubicin-induced oxidative stress and cardiac apoptosis through activating p38 and JNK MAPK mediated Nrf2/ARE pathway. Chem. Biol. Interact. 2019, 305, 54–65. [Google Scholar] [CrossRef]
- Tao, W.; Sun, W.; Liu, L.; Wang, G.; Xiao, Z.; Pei, X.; Wang, M. Chitosan oligosaccharide attenuates nonalcoholic fatty liver disease induced by high fat diet through reducing lipid accumulation, inflammation and oxidative stress in C57BL/6 mice. Mar. Drugs 2019, 17, 645. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Lu, C.; Bai, K.; Zhang, L.; Wang, T. Effect of various levels of dietary curcumin on meat quality and antioxidant profile of breast muscle in broilers. J. Agr. Food Chem. 2015, 63, 3880–3886. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2009, 14, 2765. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xiao, K.; Luan, Z.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Pié, S.; Lallès, J.P.; Blazy, F.; Laffitte, J.; Sève, B.; Oswald, I. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Liu, S.; Zhou, Y.; Mi, S.; Liu, G.; Wu, X.; Yao, K.; Assaad, H.; Deng, Z.; Hou, Y. Chlorogenic acid decreases intestinal permeability and increases expression of intestinal tight junction proteins in weaned rats challenged with LPS. PLoS ONE 2014, 9, e97815. [Google Scholar] [CrossRef]
- Hyung, J.H.; Ahn, C.B.; Kim, B.I.; Kim, K.; Je, J.Y. Involvement of Nrf2-mediated heme oxygenase-1 expression in anti-inflammatory action of chitosan oligosaccharides through MAPK activation in murine macrophages. Eur. J. Pharmacol. 2016, 793, 43–48. [Google Scholar] [CrossRef]
- Liu, H.T.; Huang, P.; Ma, P.; Liu, Q.S.; Yu, C.; Du, Y.G. Chitosan oligosaccharides suppress LPS-induced IL-8 expression in human umbilical vein endothelial cells through blockade of p38 and Akt protein kinases. Acta Pharmacol. Sin. 2011, 32, 478–486. [Google Scholar] [CrossRef]
- Lan, R.; Liu, F.; He, Z.; Chen, C.; Liu, S.; Shi, Y.; Liu, Y.; Yoshimura, Y.; Zhang, M. Immunolocalization of GnRHRI, gonadotropin receptors, PGR, and PGRMCI during follicular development in the rabbit ovary. Theriogenology 2014, 81, 1139–1147. [Google Scholar] [CrossRef]

| Items 1 | CON | AS | ASC | SEM 2 | p-Value |
|---|---|---|---|---|---|
| Duodenum | |||||
| VH (μm) | 277.71 a | 219.07 b | 258.72 c | 5.70 | <0.0001 |
| CD (μm) | 104.32 | 97.34 | 104.08 | 2.48 | 0.1265 |
| VH:CD | 2.67 a | 2.26 b | 2.50 a | 0.06 | 0.0021 |
| Jejunum | |||||
| VH (μm) | 209.73 a | 157.59 b | 190.96 a | 6.86 | 0.0010 |
| CD (μm) | 84.68 | 79.47 | 81.85 | 3.41 | 0.5751 |
| VH:CD | 2.48 a | 2.00 b | 2.34 a | 0.05 | 0.0002 |
| Ileum | |||||
| VH (μm) | 170.16 a | 116.56 b | 150.19 a | 7.26 | 0.0013 |
| CD (μm) | 67.69 | 61.19 | 65.81 | 2.41 | 0.1969 |
| VH:CD | 2.52 a | 1.91 b | 2.29 ab | 0.13 | 0.0221 |
| Ingredients (g/kg) | Basal Diet | Basal Diet with 200 mg/kg COS |
|---|---|---|
| Cornstarch | 464.00 | 463.80 |
| Chitosan oligosaccharides | 0 | 0.20 |
| Casein | 140.00 | 140.00 |
| Dextrinized cornstarch | 155.00 | 155.00 |
| Sucrose | 100.00 | 100.00 |
| Soybean oil | 40.00 | 40.00 |
| Cellulose acetate | 50.00 | 50.00 |
| Mineral premix 1 | 35.00 | 35.00 |
| Vitamin premix 2 | 10.00 | 10.00 |
| l-Methionine | 1.80 | 1.80 |
| l-Cystine | 1.80 | 1.80 |
| Choline bitartrate | 2.30 | 2.30 |
| Tert-butylhydroquinone | 0.10 | 0.10 |
| Gross energy (MJ/kg) | 16.22 | 16.20 |
| Gene | Accession NO. | Primer Sequence (5′ to 3′) | Product Size (bp) |
|---|---|---|---|
| GAPDH | NM_017008.4 | F: GGCAAGTTCAACGGCACAG R: GACGCCAGTAGACTCCACGAC | 144 |
| IL-1β | NC_005102.4 | F: CCACCTCCAGGGACAGGATA R: TGGGATCTACACTCTCCAGC | 132 |
| IL-6 | NM_012589.2 | F: CAAGTCCGGAGAGGAGACT R: TTCTGACAGTGCATCATCGC | 172 |
| IL-10 | NM_012854.2 | F: TGCGACGCTGTCATCGATTT R: GTAGATGCCGGGTGGTTCAA | 186 |
| TNF-α | NM_012675.3 | F: ACACACGAGACGCTGAAGT R: TCCAGTGAGTTCCGAAAGCC | 93 |
| ZO-1 | NM_001106266.1 | F: GCCAGCTTTAAGCCTCCAGA R: TGGCTTCGCTTGAGGTTTCT | 144 |
| Occludin | NM_031329.2 | F: GATCTAGAGCCTGGAGCAACG R: ATTGGGTTTGAATTCATCCGGC | 166 |
| Claudin-1 | NM_031699.2 | F: GCTGTCATCGGGGGCATAAT R: CCTGGCCAAATTCATACCTGG | 136 |
| Nrf2 | NM_031789.2 | F: TTTGTAGATGACCATGAGTCG R: TGTCCTGCTGTATGCTGCTT | 142 |
| HO-1 | NM_012580.2 | F: TTAAGCTGGTGATGGCCTCC R: GTGGGGCATAGACTGGGTTC | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, R.; Chang, Q.; Wei, L.; Zhao, Z. The Protect Effects of Chitosan Oligosaccharides on Intestinal Integrity by Regulating Oxidative Status and Inflammation under Oxidative Stress. Mar. Drugs 2021, 19, 57. https://doi.org/10.3390/md19020057
Lan R, Chang Q, Wei L, Zhao Z. The Protect Effects of Chitosan Oligosaccharides on Intestinal Integrity by Regulating Oxidative Status and Inflammation under Oxidative Stress. Marine Drugs. 2021; 19(2):57. https://doi.org/10.3390/md19020057
Chicago/Turabian StyleLan, Ruixia, Qingqing Chang, Linlin Wei, and Zhihui Zhao. 2021. "The Protect Effects of Chitosan Oligosaccharides on Intestinal Integrity by Regulating Oxidative Status and Inflammation under Oxidative Stress" Marine Drugs 19, no. 2: 57. https://doi.org/10.3390/md19020057
APA StyleLan, R., Chang, Q., Wei, L., & Zhao, Z. (2021). The Protect Effects of Chitosan Oligosaccharides on Intestinal Integrity by Regulating Oxidative Status and Inflammation under Oxidative Stress. Marine Drugs, 19(2), 57. https://doi.org/10.3390/md19020057






