Cysteinolic Acid Is a Widely Distributed Compatible Solute of Marine Microalgae
Abstract
1. Introduction
2. Results and Discussion
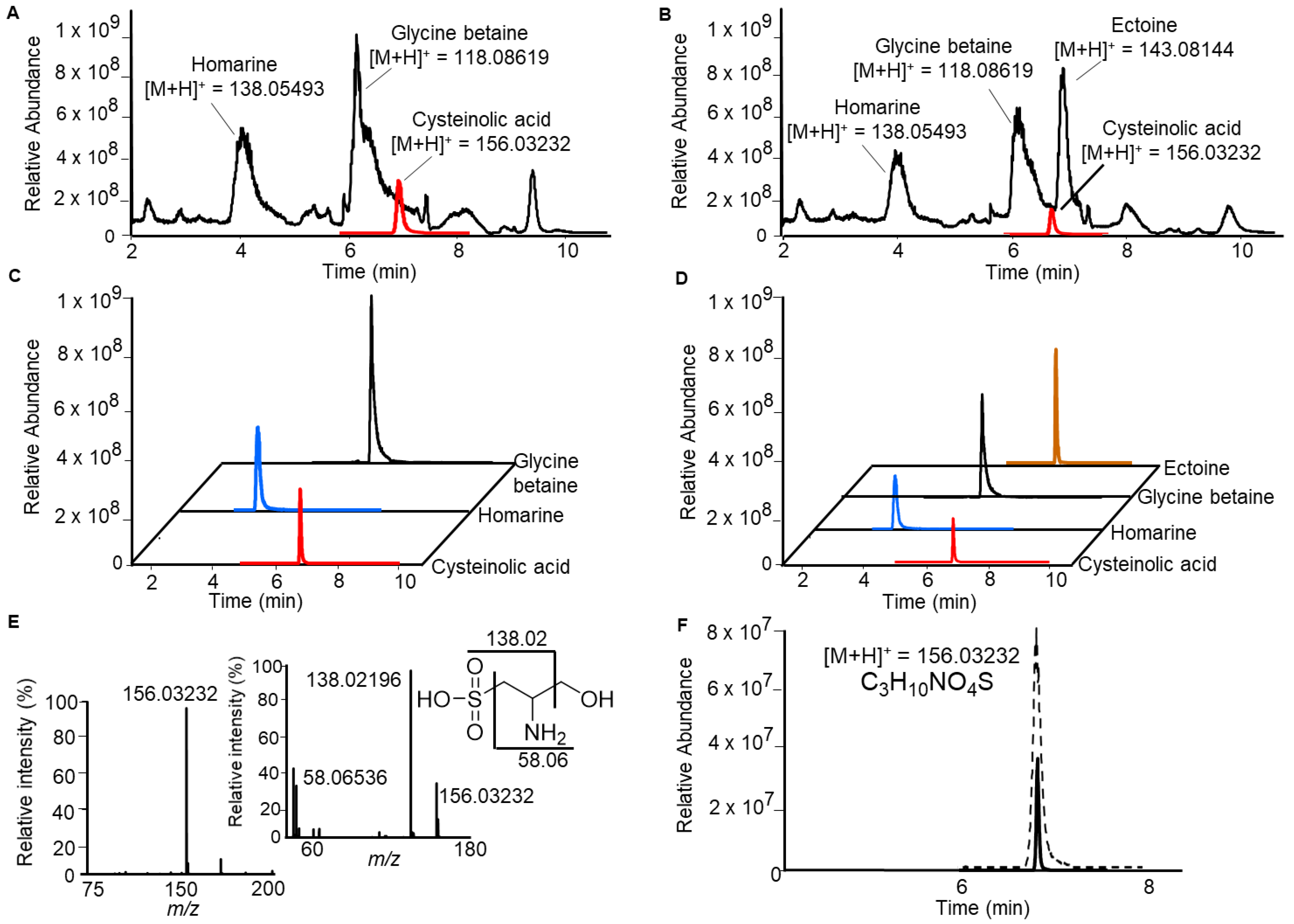
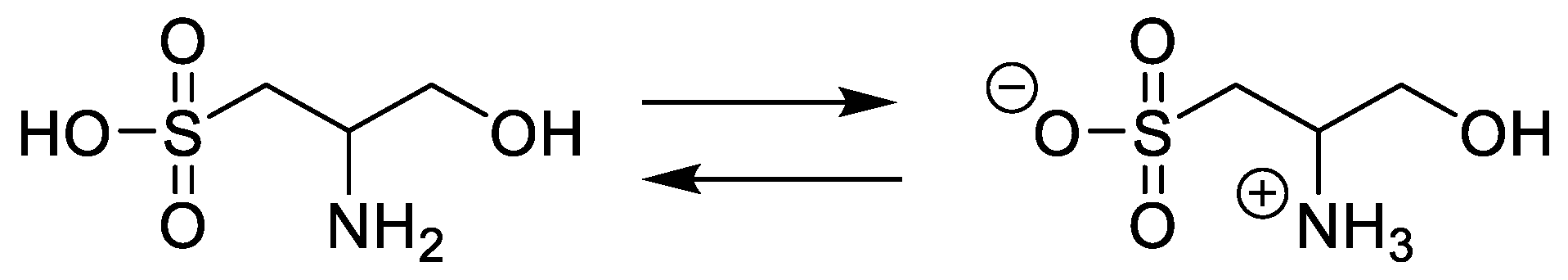
2.1. Identification of Cysteinolic Acid in the Diatom Thalassiosira weissflogii
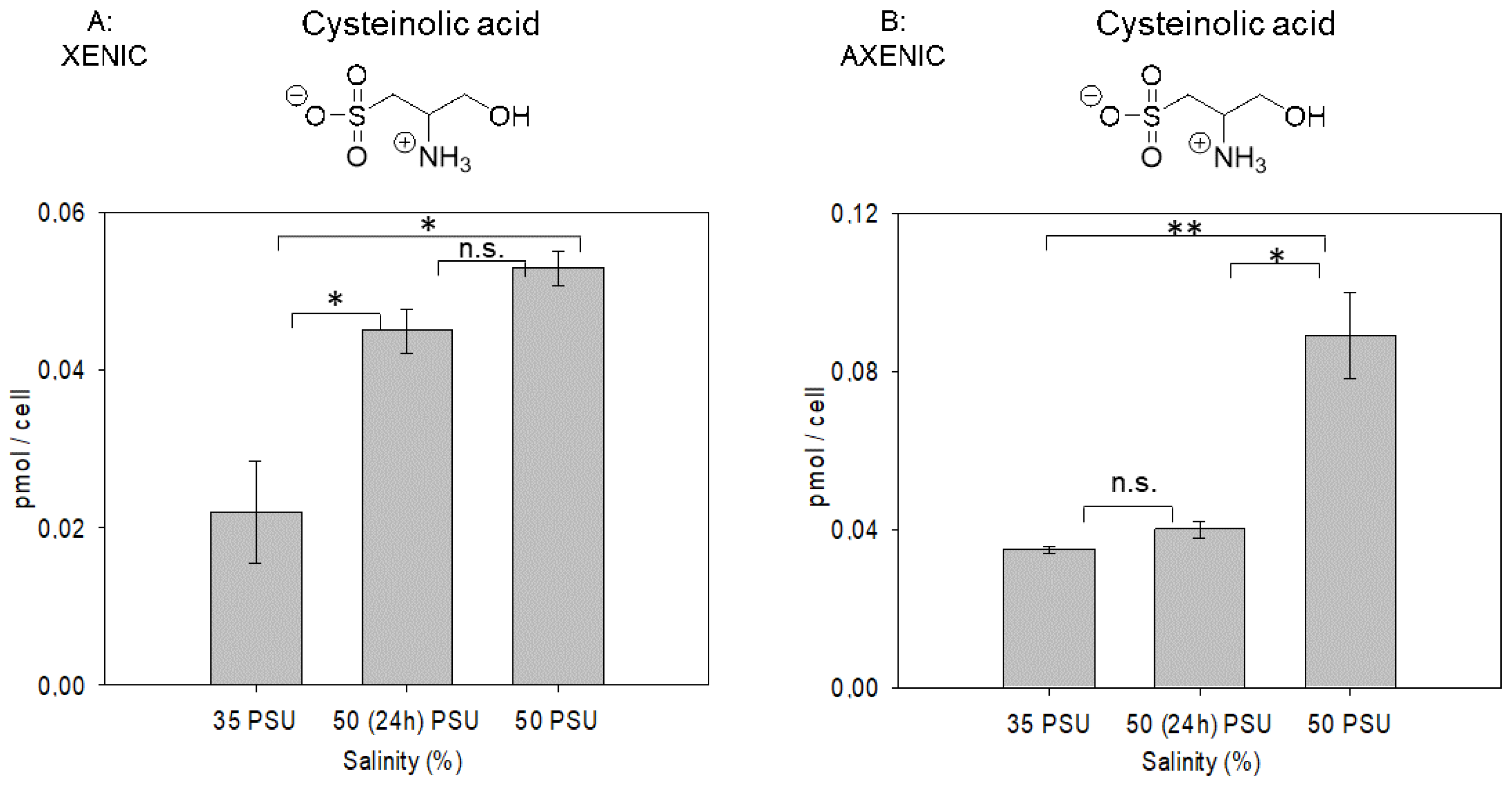
2.2. Salinity-Dependent Changes in Cysteinolic Acid Concentration in T. weissflogii
2.3. Cysteinolic Acid in Other Microalgae
3. Materials and Methods
3.1. Cultivation of Microalgae
3.2. Salinity Treatment
3.3. Cell Counting and Size Measurement
3.4. Sample Preparation
3.5. UHPLC/HRMS-Equipment and Settings
3.6. Osmolyte Analysis
3.7. Synthesis of Cysteinolic Acid
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erga, S.R.; Lie, G.C.; Aarø, L.H.; Frette, Ø.; Hamre, B. Migratory behaviour of Skeletonema grethae (Bacillariophyceae) in stratified waters. Diatom Res. 2014, 30, 13–25. [Google Scholar] [CrossRef]
- Mousing, E.A.; Richardson, K.; Bendtsen, J.; Cetinić, I.; Perry, M.J.; Cornell, W. Evidence of small-scale spatial structuring of phytoplankton alpha- and beta-diversity in the open ocean. J. Ecol. 2016, 104, 1682–1695. [Google Scholar] [CrossRef]
- Smyth, K.; Elliott, M. Effects of changing salinity on the ecology of the marine environment. In Stressors in the Marine Environment; Solan, M., Whiteley, N., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 161–174. [Google Scholar] [CrossRef]
- Gebser, B.; Pohnert, G. Synchronized regulation of different zwitterionic metabolites in the osmoadaption of phytoplankton. Mar. Drugs 2013, 11, 2168–2182. [Google Scholar] [CrossRef]
- Welsh, D.T. Ecological significance of compatible solute accumulation by micro-organisms: From single cells to global climate. FEMS Microbio. Rev. 2000, 24, 263–290. [Google Scholar] [CrossRef]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef]
- Fenizia, S.; Thume, K.; Wirgenings, M.; Pohnert, G. Ectoine from bacterial and algal origin is a compatible solute in microalgae. Mar. Drugs 2020, 18, 42. [Google Scholar] [CrossRef]
- Ksionzek, K.B.; Lechtenfeld, O.J.; McCallister, S.L.; Schmitt-Kopplin, P.; Geuer, J.K.; Geibert, W.; Koch, B.P. Dissolved organic sulfur in the ocean: Biogeochemistry of a petagram inventory. Science 2016, 354, 456–459. [Google Scholar] [CrossRef]
- Curson, A.R.; Liu, J.; Bermejo-Martinez, A.; Green, R.T.; Chan, Y.; Carrion, O.; Williams, B.T.; Zhang, S.H.; Yang, G.P.; Bulman-Page, P.C.; et al. Dimethylsulfoniopropionate biosynthesis in marine bacteria and identification of the key gene in this process. Nat. Microbiol. 2017, 2, 17009. [Google Scholar] [CrossRef]
- Lana, A.; Bell, T.G.; Simó, R.; Vallina, S.M.; Ballabrera-Poy, J.; Kettle, A.J.; Dachs, J.; Bopp, L.; Saltzman, E.S.; Stefels, J.; et al. An updated climatology of surface dimethlysulfide concentrations and emission fluxes in the global ocean. Global Biogeochem. Cycles 2011, 25. [Google Scholar] [CrossRef]
- Thume, K.; Gebser, B.; Chen, L.; Meyer, N.; Kieber, D.J.; Pohnert, G. The metabolite dimethylsulfoxonium propionate extends the marine organosulfur cycle. Nature 2018, 563, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Charlson, R.J.; Lovelock, J.E.; Andreae, M.O.; Warren, S.G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 1987, 326, 655–661. [Google Scholar] [CrossRef]
- Andreae, M.O.; Crutzen, P.J. Atmospheric aerosols: Biogeochemical sources and role in atmospheric chemistry. Science 1997, 276, 1052–1058. [Google Scholar] [CrossRef]
- Spielmeyer, A.; Pohnert, G. Direct quantification of dimethylsulfoniopropionate (DMSP) with hydrophilic interaction liquid chromatography/mass spectrometry. J. Chromatogr. B 2010, 878, 3238–3242. [Google Scholar] [CrossRef]
- Spielmeyer, A.; Gebser, B.; Pohnert, G. Dimethylsulfide sources from microalgae: Improvement and application of a derivatization-based method for the determination of dimethylsulfoniopropionate and other zwitterionic osmolytes in phytoplankton. Mar. Chem. 2011, 124, 48–56. [Google Scholar] [CrossRef]
- Boysen, A.K.; Heal, K.R.; Carlson, L.T.; Ingalls, A.E. Best-matched internal standard normalization in liquid chromatography-mass spectrometry metabolomics applied to environmental samples. Anal. Chem. 2018, 90, 1363–1369. [Google Scholar] [CrossRef]
- Heal, K.R.; Kellogg, N.A.; Carlson, L.T.; Lionheart, R.M.; Ingalls, A.E. Metabolic consequences of cobalamin scarcity in the diatom Thalassiosira pseudonana as revealed through metabolomics. Protist 2019, 170, 328–348. [Google Scholar] [CrossRef]
- Wang, R.; Gallant, E.; Seyedsayamdost, M.R. Investigation of the genetics and biochemistry of Roseobacticide production in the Roseobacter clade bacterium Phaeobacter inhibens. MBio 2016, 7, e02118-15. [Google Scholar] [CrossRef] [PubMed]
- Landa, M.; Burns, A.S.; Durham, B.P.; Esson, K.; Nowinski, B.; Sharma, S.; Vorobev, A.; Nielsen, T.; Kiene, R.P.; Moran, M.A. Sulfur metabolites that facilitate oceanic phytoplankton-bacteria carbon flux. ISME J. 2019, 13, 2536–2550. [Google Scholar] [CrossRef]
- Durham, B.P.; Boysen, A.K.; Carlson, L.T.; Groussman, R.D.; Heal, K.R.; Cain, K.R.; Morales, R.L.; Coesel, S.N.; Morris, R.M.; Ingalls, A.E.; et al. Sulfonate-based networks between eukaryotic phytoplankton and heterotrophic bacteria in the surface ocean. Nat. Microbiol. 2019, 4, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Kamp, A.; Stief, P.; Knappe, J.; de Beer, D. Response of the ubiquitous pelagic diatom Thalassiosira weissflogii to darkness and anoxia. PLoS ONE 2013, 8, e82605. [Google Scholar] [CrossRef]
- Bussard, A.; Corre, E.; Hubas, C.; Duvernois-Berthet, E.; Le Corguille, G.; Jourdren, L.; Coulpier, F.; Claquin, P.; Lopez, P.J. Physiological adjustments and transcriptome reprogramming are involved in the acclimation to salinity gradients in diatoms. Environ. Microbiol. 2017, 19, 909–925. [Google Scholar] [CrossRef]
- Spielmeyer, A.; Gebser, B.; Pohnert, G. Investigations of the uptake of dimethylsulfoniopropionate by phytoplankton. ChemBioChem Comb. Chem. Biol. 2011, 12, 2276–2279. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Y.; Li, Z.; Xu, J.; Gao, K. Effects of seawater acidification on the growth rates of the diatom Thalassiosira (Conticribra) weissflogii under different nutrient, light, and UV radiation regimes. J. Appl. Phycol. 2016, 29, 133–142. [Google Scholar] [CrossRef]
- Walter, B.; Peters, J.; van Beusekom, J.E.E.; St. John, M.A. Interactive effects of temperature and light during deep convection: A case study on growth and condition of the diatom Thalassiosira weissflogii. ICES J. Mar. Sci. 2015, 72, 2061–2071. [Google Scholar] [CrossRef][Green Version]
- Garcia, N.; Lopez-Elias, J.A.; Miranda, A.; Martinez-Porchas, M.; Huerta, N.; Garcia, A. Effect of salinity on growth and chemical composition of the diatom Thalassiosira weissflogii at three culture phases. Lat. Am. J. Aquat. Res. 2012, 40, 435–440. [Google Scholar] [CrossRef]
- Takagi, M.; Yoshida, T. Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J. Biosci. Bioeng. 2006, 101, 223–226. [Google Scholar] [CrossRef]
- Duhrkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Bocker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching molecular structure databases with tandem mass spectra using CSI: Finger ID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef]
- Wickberg, B. Isolation of 2-L-amino-3-hydroxy-1-propane sulfonic acid from Polysiphonia fastigiata. Acta Chem. Scand. 1957, 11, 506–511. [Google Scholar] [CrossRef]
- Ito, K. Distribution of d-cysteinolic acid in marine algae. Bull. Japan. Soc. Sci. Fish 1963, 29, 771–775. [Google Scholar] [CrossRef][Green Version]
- Busby, W.F. Sulfopropanedial and cysteinolic acid in the diatom. Biochim. Biophys. Acta 1966, 121, 160–161. [Google Scholar] [CrossRef]
- Busby, W.F.; Benson, A.A. Sulfonic acid metabolism in the diatom Navicula pelliculosa. Plant Cell Physiol. 1973, 14, 1123–1132. [Google Scholar] [CrossRef][Green Version]
- Gotz, F.; Longnecker, K.; Kido Soule, M.C.; Becker, K.W.; McNichol, J.; Kujawinski, E.B.; Sievert, S.M. Targeted metabolomics reveals proline as a major osmolyte in the chemolithoautotroph Sulfurimonas denitrificans. MicrobiologyOpen 2018, 7, e00580. [Google Scholar] [CrossRef] [PubMed]
- Durham, B.P.; Sharma, S.; Luo, H.; Smith, C.B.; Amin, S.A.; Bender, S.J.; Dearth, S.P.; Van Mooy, B.A.; Campagna, S.R.; Kujawinski, E.B.; et al. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc. Natl. Acad. Sci. USA 2015, 112, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.T.; Todd, J.D. A day in the life of marine sulfonates. Nat. Microbiol. 2019, 4, 1610–1611. [Google Scholar] [CrossRef]
- Azam, F.; Malfatti, F. Microbial structuring of marine ecosystems. Nat. Rev. Microbiol. 2007, 5, 782–791. [Google Scholar] [CrossRef]
- McParland, E.L.; Levine, N.M. The role of differential DMSP production and community composition in predicting variability of global surface DMSP concentrations. Limnol. Oceanogr. 2018, 64, 757–773. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Utsa Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Maier, I.; Calenberg, M. Effect of extracellular Ca2+ and Ca2+-antagonists on the movement and chemoorientation of male gametes of Ectocarpus siliculosus (Phaeophyceae). Bot. Acta 1994, 107, 451–458. [Google Scholar] [CrossRef]
- Xu, J. A new and expeditious asymmetric synthesis of (R)- and (S)-2-aminoalkanesulfonic acids from chiral amino alcohols. Tetrahedron Asymmetry 2002, 13, 1129–1134. [Google Scholar] [CrossRef]
| Species | DMSA | Gonyol | GBT (Fmol Cell−1) | Homarine (Fmol Cell−1) | DMSP (Fmol Cell−1) | DMSOP (Fmol Cell−1) | Ectoine (Fmol Cell−1) | Cysteinolic Acid (Fmol Cell−1) | Cysteinolic Acid (mM) |
|---|---|---|---|---|---|---|---|---|---|
| Prorocentrum minimum | + | + | 37.9 ± 4.8 | 0.27 ± 0.06 | 463.4 ± 52.6 | 3.66 ± 1.23 | 42.0 ± 6.1 | 50.6 ± 8.1 | 71.1 ± 11.4 |
| Prymnesium parvum | − | + | 1.5 ± 0.7 | 0.04 ± 0.02 | 47.4 ± 5.3 | 0.029 ± 0.005 | 4.8 ± 1.2 | 1.9 ± 0.8 | 17.9 ± 7.3 |
| Amphidinium carterae | + | + | + | 0.09 ± 0.04 | + | − | 9.1 ± 1.5 | 17.2 ± 2.8 | 18.5 ± 3.0 |
| Thalassiosira pseudonana | + | − | 7.7 ± 0.9 | 2.0 ± 0.2 | 4.8 ± 0.6 | − | 1.9 ± 0.2 | 2.8 ± 0.3 | 17.5 ± 2.0 |
| Skeletonema costatum | − | − | + | 0.06 ± 0.01 | 28.5 ± 4.1 | 0.029 ± 0.005 | 10.0 ± 1.5 | 10.9 ± 0.5 | 42.2 ± 2.1 |
| Emiliania huxleyi | − | + | 0.73 ± 0.04 | 0.41 ± 0.03 | 7.3 ± 0.7 | 0.029 ± 0.013 | 0.54 ± 0.06 | 1.0 ± 0.1 | 11.9 ± 0.7 |
| Isochrysis galbana | − | + | 4.8 ± 0.2 | 1.3 ± 0.1 | 13.8 ± 0.6 | 0.017 ± 0.003 | 1.6 ± 0.1 | 1.0 ± 0.03 | 10.8 ± 0.4 |
| Thalassiosira weissflogii | − | − | 234.7 ± 35.7 | 35.4 ± 2.5 | − | − | 85.2 ± 13.1 | 22.3 ± 11.3 | 8.0 ± 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenizia, S.; Weissflog, J.; Pohnert, G. Cysteinolic Acid Is a Widely Distributed Compatible Solute of Marine Microalgae. Mar. Drugs 2021, 19, 683. https://doi.org/10.3390/md19120683
Fenizia S, Weissflog J, Pohnert G. Cysteinolic Acid Is a Widely Distributed Compatible Solute of Marine Microalgae. Marine Drugs. 2021; 19(12):683. https://doi.org/10.3390/md19120683
Chicago/Turabian StyleFenizia, Simona, Jerrit Weissflog, and Georg Pohnert. 2021. "Cysteinolic Acid Is a Widely Distributed Compatible Solute of Marine Microalgae" Marine Drugs 19, no. 12: 683. https://doi.org/10.3390/md19120683
APA StyleFenizia, S., Weissflog, J., & Pohnert, G. (2021). Cysteinolic Acid Is a Widely Distributed Compatible Solute of Marine Microalgae. Marine Drugs, 19(12), 683. https://doi.org/10.3390/md19120683






