Chemodiversity of Brevetoxins and Other Potentially Toxic Metabolites Produced by Karenia spp. and Their Metabolic Products in Marine Organisms
Abstract
:1. Introduction
2. Metabolites Produced by Karenia spp.
2.1. Brevetoxins
2.2. Hemibrevetoxins
2.3. Brevenals
2.4. Brevisamide
2.5. Brevisin
2.6. Tamulamides
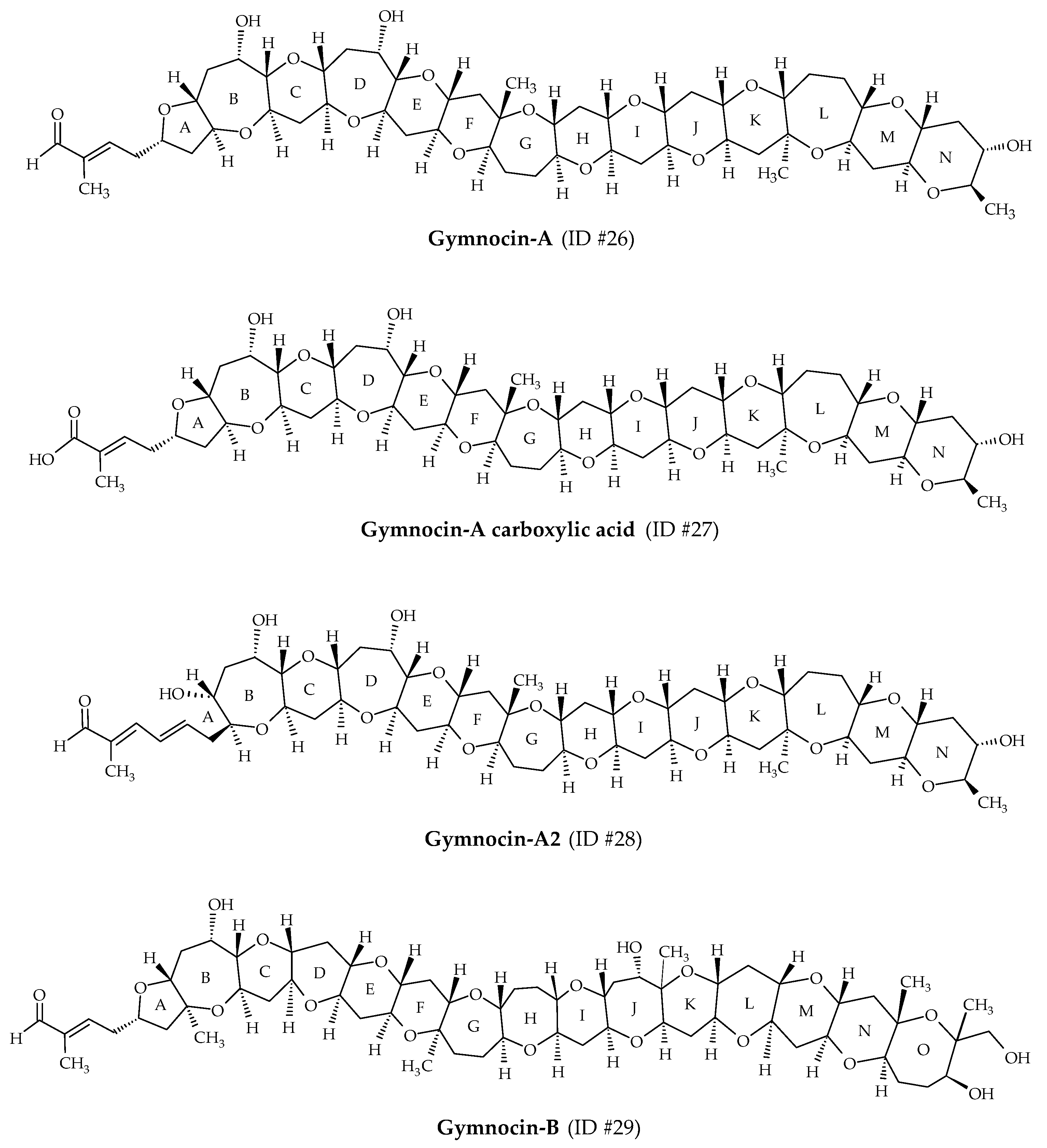
2.7. Gymnocins
2.8. Gymnodimines (GYMs)
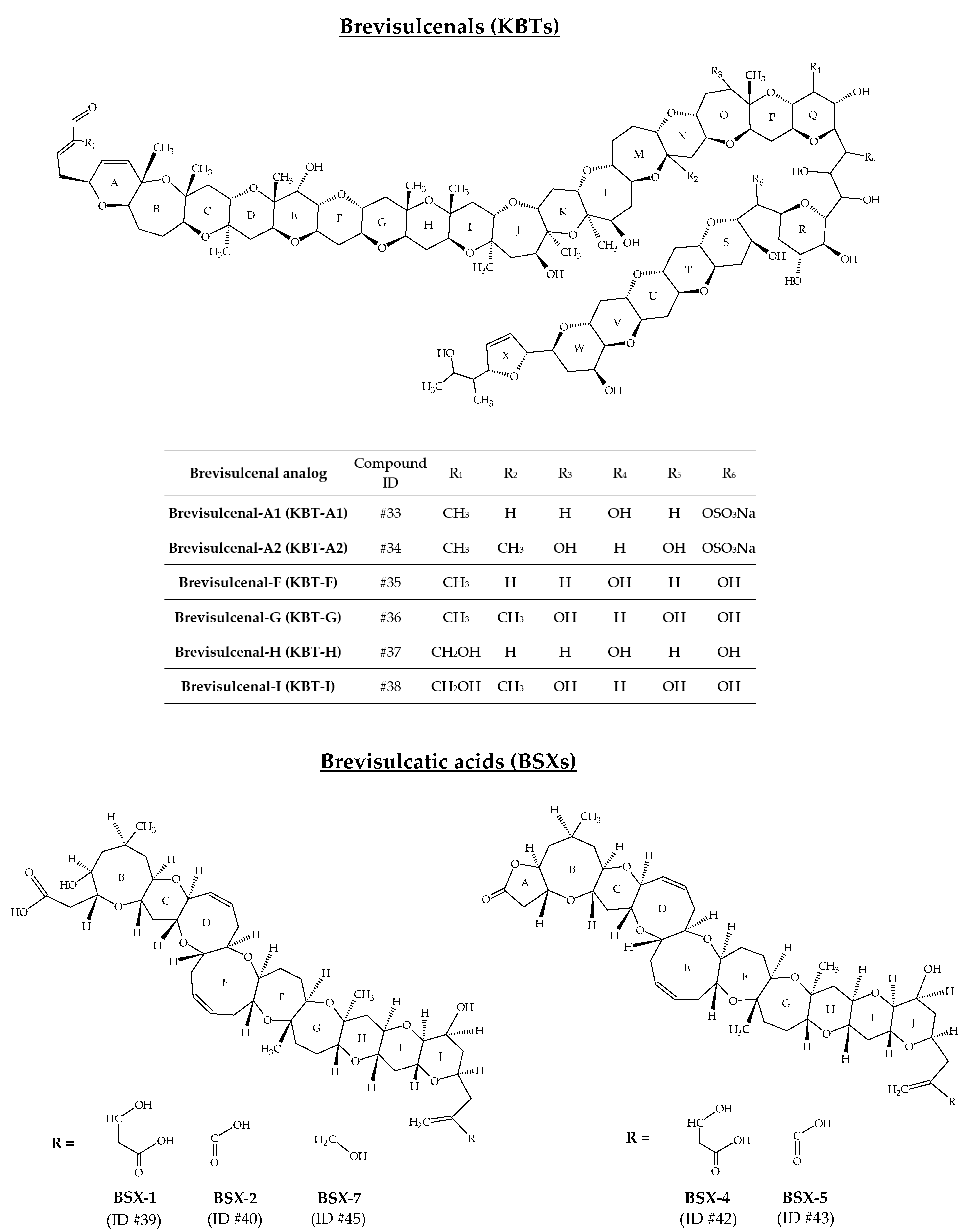
2.9. Brevisulcenals and Brevisulcatic Acids
3. Biotransformation of Metabolites Produced by Karenia spp. in Shellfish
3.1. Brevetoxins
3.1.1. Brevetoxins Already Reported from Karenia spp.
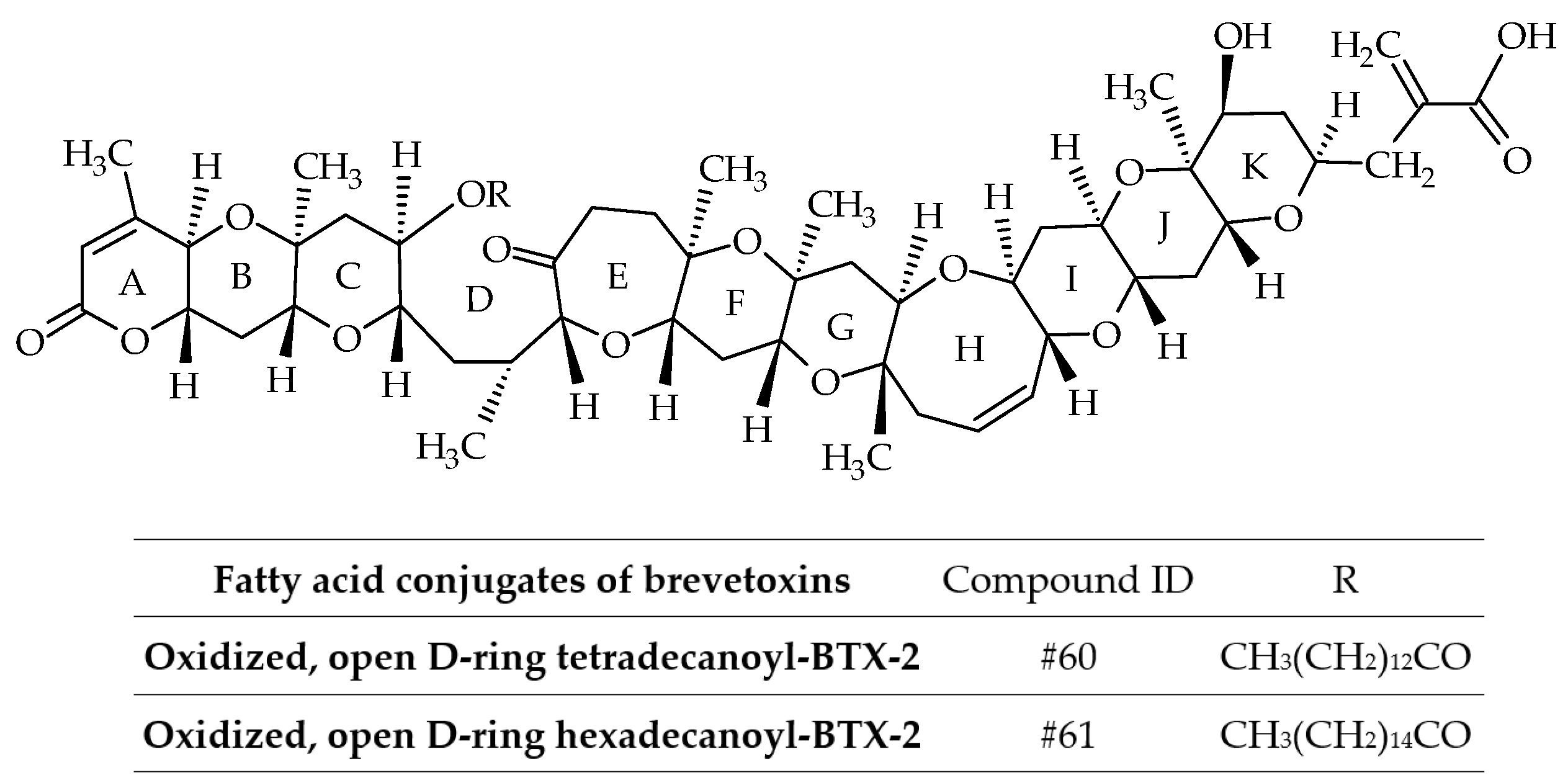
3.1.2. Fatty Acid Conjugates of Brevetoxins
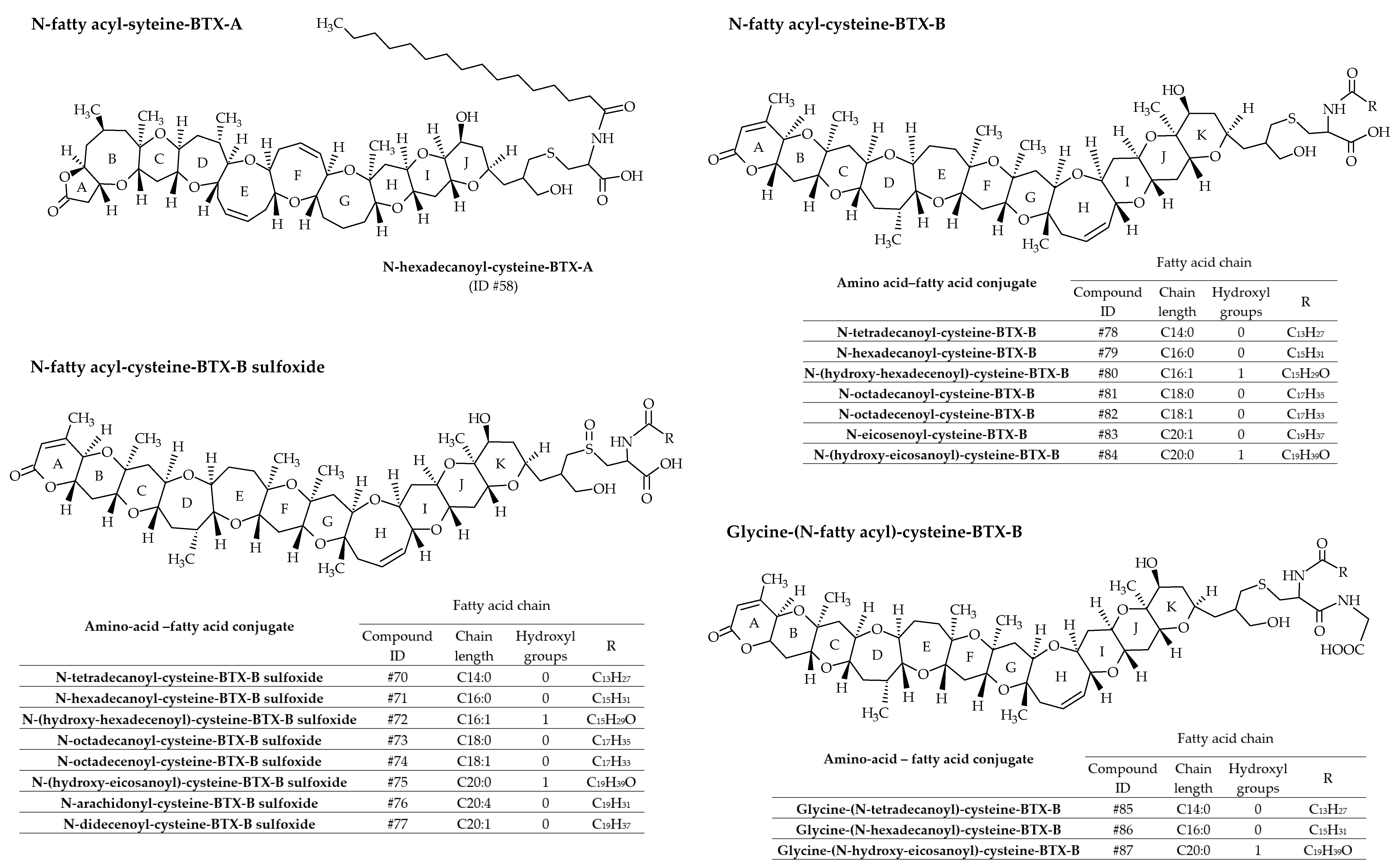
3.1.3. Amino Acid/Peptide Conjugates of Brevetoxins
3.1.4. Fatty Acid Derivatives of Brevetoxin Amino acid Conjugates
3.1.5. Other Brevetoxin Metabolites
3.1.6. Biomarkers of Brevetoxin Exposure in Shellfish
3.1.7. Monitoring Programs for Brevetoxins in Shellfish
3.2. Gymnodimines (GYMs)
4. Accumulation and Biotransformation of Metabolites Produced by Karenia spp. in Other Marine Organisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef] [Green Version]
- Bricelj, V.M.; Haubois, A.-G.; Sengco, M.R.; Pierce, R.H.; Culter, J.K.; Anderson, D.M. Trophic Transfer of Brevetoxins to the Benthic Macrofaunal Community during a Bloom of the Harmful Dinoflagellate Karenia Brevis in Sarasota Bay, Florida. Harmful Algae 2012, 16, 27–34. [Google Scholar] [CrossRef]
- Brand, L.E.; Campbell, L.; Bresnan, E. Karenia: The Biology and Ecology of a Toxic Genus. Harmful Algae 2012, 14, 156–178. [Google Scholar] [CrossRef]
- Plakas, S.M.; Dickey, R.W. Advances in Monitoring and Toxicity Assessment of Brevetoxins in Molluscan Shellfish. Toxicon 2010, 56, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Flewelling, L.J.; Naar, J.P.; Abbott, J.P.; Baden, D.G.; Barros, N.B.; Bossart, G.D.; Dechraoui Bottein, M.-Y.; Hammond, D.G.; Haubold, E.M.; Heil, C.A.; et al. Red Tides and Marine Mammal Mortalities. Nature 2005, 435, 755–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.; Xu, J.; Tsang, T.Y.; Au, D.W.T. Toxicity Comparison between Chattonella Marina and Karenia Brevis Using Marine Medaka (Oryzias Melastigma): Evidence against the Suspected Ichthyotoxins of Chattonella Marina. Chemosphere 2010, 80, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine Harmful Algal Blooms, Human Health and Wellbeing: Challenges and Opportunities in the 21st Century. J. Mar. Biol. Assoc. UK 2016, 96, 61–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, N.; Sharpe, R.A.; Barciela, R.; Nichols, G.; Davidson, K.; Berdalet, E.; Fleming, L.E. Marine Harmful Algal Blooms and Human Health: A Systematic Scoping Review. Harmful Algae 2020, 98, 101901. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.M.; Reich, A.; Fleming, L.E.; Hammond, R. Neurotoxic Shellfish Poisoning. Mar. Drugs 2008, 6, 431–455. [Google Scholar] [CrossRef] [Green Version]
- Amzil, Z.; Derrien, A.; Terre Terrillon, A.; Duval, A.; Connes, C.; Marco-Miralles, F.; Nézan, E.; Mertens, K.N. Monitoring the Emergence of Algal Toxins in Shellfish: First Report on Detection of Brevetoxins in French Mediterranean Mussels. Mar. Drugs 2021, 19, 393. [Google Scholar] [CrossRef]
- Arnich, N.; Abadie, E.; Amzil, Z.; Dechraoui Bottein, M.-Y.; Comte, K.; Chaix, E.; Delcourt, N.; Hort, V.; Mattei, C.; Molgó, J.; et al. Guidance Level for Brevetoxins in French Shellfish. Mar. Drugs 2021, 19, 520. [Google Scholar] [CrossRef]
- Caruana, A.M.N.; Amzil, Z. Chapter 13—Microalgae and Toxins. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 263–305. ISBN 978-0-12-811405-6. [Google Scholar]
- Baden, D.G. Brevetoxins: Unique Polyether Dinoflagellate Toxins. FASEB J. 1989, 3, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.A.; Mende, T.J.; Baden, D.G. Brevetoxins, Unique Activators of Voltage-Sensitive Sodium Channels, Bind to Specific Sites in Rat Brain Synaptosomes. Mol. Pharm. 1986, 30, 129–135. [Google Scholar]
- Daugbjerg, N.; Hansen, G.; Larsen, J.; Moestrup, Ø. Phylogeny of Some of the Major Genera of Dinoflagellates Based on Ultrastructure and Partial LSU RDNA Sequence Data, Including the Erection of Three New Genera of Unarmoured Dinoflagellates. Phycologia 2000, 39, 302–317. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Song, X.; Zhang, Y.; Zhang, P.; Li, J.; Song, W.; Yu, Z. Profiling of Brevetoxin Metabolites Produced by Karenia Brevis 165 Based on Liquid Chromatography-Mass Spectrometry. Toxins 2021, 13, 354. [Google Scholar] [CrossRef]
- Abraham, A.; Flewelling, L.J.; El Said, K.R.; Odom, W.; Geiger, S.P.; Granholm, A.A.; Jackson, J.T.; Bodager, D. An Occurrence of Neurotoxic Shellfish Poisoning by Consumption of Gastropods Contaminated with Brevetoxins. Toxicon 2021, 191, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; El Said, K.R.; Flewelling, L.J. Role of Biomarkers in Monitoring Brevetoxins in Karenia Brevis Exposed Shellfish. Food Saf. 2018, 6, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Marine Biotoxins in Shellfish—Emerging Toxins: Brevetoxin Group. EFSA J. 2010, 8, 1677. [Google Scholar] [CrossRef]
- Nozawa, A.; Tsuji, K.; Ishida, H. Implication of Brevetoxin B1 and PbTx-3 in Neurotoxic Shellfish Poisoning in New Zealand by Isolation and Quantitative Determination with Liquid Chromatography-Tandem Mass Spectrometry. Toxicon 2003, 42, 91–103. [Google Scholar] [CrossRef]
- Ishida, H.; Muramatsu, N.; Nukaya, H.; Kosuge, T.; Tsuji, K. Study on Neurotoxic Shellfish Poisoning Involving the Oyster, Crassostrea Gigas, in New Zealand. Toxicon 1996, 34, 1050–1053. [Google Scholar] [CrossRef]
- Abraham, A.; Wang, Y.; El Said, K.R.; Plakas, S.M. Characterization of Brevetoxin Metabolism in Karenia Brevis Bloom-Exposed Clams (Mercenaria sp.) by LC-MS/MS. Toxicon 2012, 60, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; El Said, K.R.; Wang, Y.; Jester, E.L.E.; Plakas, S.M.; Flewelling, L.J.; Henry, M.S.; Pierce, R.H. Biomarkers of Brevetoxin Exposure and Composite Toxin Levels in Hard Clam (Mercenaria sp.) Exposed to Karenia Brevis Blooms. Toxicon 2015, 96, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Satake, M.; Mackenzie, L.; Kaspar, H.F.; Yasumoto, T. Gymnodimine, a New Marine Toxin of Unprecedented Structure Isolated from New Zealand Oysters and the Dinoflagellate, Gymnodinium sp. Tetrahedron Lett. 1995, 36, 7093–7096. [Google Scholar] [CrossRef]
- Ben Naila, I.; Hamza, A.; Gdoura, R.; Diogène, J.; de la Iglesia, P. Prevalence and Persistence of Gymnodimines in Clams from the Gulf of Gabes (Tunisia) Studied by Mouse Bioassay and LC–MS/MS. Harmful Algae 2012, 18, 56–64. [Google Scholar] [CrossRef]
- Marrouchi, R.; Dziri, F.; Belayouni, N.; Hamza, A.; Benoit, E.; Molgó, J.; Kharrat, R. Quantitative Determination of Gymnodimine-A by High Performance Liquid Chromatography in Contaminated Clams from Tunisia Coastline. Mar. Biotechnol. 2010, 12, 579–585. [Google Scholar] [CrossRef]
- Biré, R.; Krys, S.; Frémy, J.-M.; Dragacci, S.; Stirling, D.; Kharrat, R. First Evidence on Occurrence of Gymnodimine in Clams from Tunisia. J. Nat. Toxins 2002, 11, 269–275. [Google Scholar]
- Plakas, S.M.; El Said, K.R.; Jester, E.L.E.; Ray Granade, H.; Musser, S.M.; Dickey, R.W. Confirmation of Brevetoxin Metabolism in the Eastern Oyster (Crassostrea Virginica) by Controlled Exposures to Pure Toxins and to Karenia Brevis Cultures. Toxicon 2002, 40, 721–729. [Google Scholar] [CrossRef]
- Poli, M.A.; Musser, S.M.; Dickey, R.W.; Eilers, P.P.; Hall, S. Neurotoxic Shellfish Poisoning and Brevetoxin Metabolites: A Case Study from Florida. Toxicon 2000, 38, 981–993. [Google Scholar] [CrossRef] [Green Version]
- Dickey, R.; Jester, E.; Granade, R.; Mowdy, D.; Moncreiff, C.; Rebarchik, D.; Robl, M.; Musser, S.; Poli, M. Monitoring Brevetoxins during a Gymnodinium Breve Red Tide: Comparison of Sodium Channel Specific Cytotoxicity Assay and Mouse Bioassay for Determination of Neurotoxic Shellfish Toxins in Shellfish Extracts. Nat. Toxins 1999, 7, 157–165. [Google Scholar] [CrossRef]
- Ishida, H.; Nozawa, A.; Nukaya, H.; Tsuji, K. Comparative Concentrations of Brevetoxins PbTx-2, PbTx-3, BTX-B1 and BTX-B5 in Cockle, Austrovenus Stutchburyi, Greenshell Mussel, Perna Canaliculus, and Pacific Oyster, Crassostrea Gigas, Involved Neurotoxic Shellfish Poisoning in New Zealand. Toxicon 2004, 43, 779–789. [Google Scholar] [CrossRef]
- Ishida, H.; Nozawa, A.; Nukaya, H.; Rhodes, L.; McNabb, P.; Holland, P.T.; Tsuji, K. Confirmation of Brevetoxin Metabolism in Cockle, Austrovenus Stutchburyi, and Greenshell Mussel, Perna Canaliculus, Associated with New Zealand Neurotoxic Shellfish Poisoning, by Controlled Exposure to Karenia Brevis Culture. Toxicon 2004, 43, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Nozawa, A.; Hamano, H.; Naoki, H.; Fujita, T.; Kaspar, H.F.; Tsuji, K. Brevetoxin B5, a New Brevetoxin Analog Isolated from Cockle Austrovenus Stutchburyi in New Zealand, the Marker for Monitoring Shellfish Neurotoxicity. Tetrahedron Lett. 2004, 45, 29–33. [Google Scholar] [CrossRef]
- Plakas, S.M.; Wang, Z.; El Said, K.R.; Jester, E.L.E.; Granade, H.R.; Flewelling, L.; Scott, P.; Dickey, R.W. Brevetoxin Metabolism and Elimination in the Eastern Oyster (Crassostrea Virginica) after Controlled Exposures to Karenia Brevis. Toxicon 2004, 44, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Plakas, S.M.; Wang, Z.; Jester, E.L.E.; El Said, K.R.; Granade, H.R.; Henry, M.S.; Blum, P.C.; Pierce, R.H.; Dickey, R.W. Characterization of Polar Brevetoxin Derivatives Isolated from Karenia Brevis Cultures and Natural Blooms. Toxicon 2006, 48, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Morohashi, A.; Satake, M.; Murata, K.; Naoki, H.; Kaspar, H.F.; Yasumoto, T. Brevetoxin B3, a New Brevetoxin Analog Isolated from the Greenshell Mussel Perna Canaliculus Involved in Neurotoxic Shellfish Poisoning in New Zealand. Tetrahedron Lett. 1995, 36, 8995–8998. [Google Scholar] [CrossRef]
- Ishida, H.; Nozawa, A.; Totoribe, K.; Muramatsu, N.; Nukaya, H.; Tsuji, K.; Yamaguchi, K.; Yasumoto, T.; Kaspar, H.; Berkett, N.; et al. Brevetoxin B1, a New Polyether Marine Toxin from the New Zealand Shellfish, Austrovenus Stutchburyi. Tetrahedron Lett. 1995, 36, 725–728. [Google Scholar] [CrossRef]
- Murata, K.; Satake, M.; Naoki, H.; Kaspar, H.F.; Yasumoto, T. Isolation and Structure of a New Brevetoxin Analog, Brevetoxin B2, from Greenshell Mussels from New Zealand. Tetrahedron 1998, 54, 735–742. [Google Scholar] [CrossRef]
- Wang, Z.; Plakas, S.M.; El Said, K.R.; Jester, E.L.E.; Granade, H.R.; Dickey, R.W. LC/MS Analysis of Brevetoxin Metabolites in the Eastern Oyster (Crassostrea Virginica). Toxicon 2004, 43, 455–465. [Google Scholar] [CrossRef]
- Morohashi, A.; Satake, M.; Naoki, H.; Kaspar, H.F.; Oshima, Y.; Yasumoto, T. Brevetoxin B4 Isolated from Greenshell Mussels Perna Canaliculus, the Major Toxin Involved in Neurotoxic Shellfish Poisoning in New Zealand. Nat. Toxins 1999, 7, 45–48. [Google Scholar] [CrossRef]
- Ji, Y.; Che, Y.; Wright, E.J.; McCarron, P.; Hess, P.; Li, A. Fatty Acid Ester Metabolites of Gymnodimine in Shellfish Collected from China and in Mussels (Mytilus Galloprovincialis) Exposed to Karenia Selliformis. Harmful Algae 2020, 92, 101774. [Google Scholar] [CrossRef]
- De la Iglesia, P.; McCarron, P.; Diogène, J.; Quilliam, M.A. Discovery of Gymnodimine Fatty Acid Ester Metabolites in Shellfish Using Liquid Chromatography/Mass Spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Sun, G.; Qiu, J.; Fan, L. Lipophilic Shellfish Toxins in Dinophysis Caudata Picked Cells and in Shellfish from the East China Sea. Environ. Sci. Pollut. Res. 2015, 22, 3116–3126. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liang, Y.; Wu, X.; Xu, D.; Liu, Y.; Liu, L. First Report on the Detection of Pectenotoxin Groups in Chinese Shellfish by LC–MS/MS. Toxicon 2011, 57, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, L.; Li, Y.; Zhang, J.; Tan, Z.; Wu, H.; Jiang, T.; Lu, S. Occurrence of Marine Algal Toxins in Oyster and Phytoplankton Samples in Daya Bay, South China Sea. Chemosphere 2017, 183, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Yu, Q.; Eaglesham, G.; Connell, D.W.; McBroom, J.; Costanzo, S.; Shaw, G.R. Occurrence and Seasonal Variations of Algal Toxins in Water, Phytoplankton and Shellfish from North Stradbroke Island, Queensland, Australia. Mar. Environ. Res. 2007, 64, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Krock, B.; Pitcher, G.C.; Ntuli, J.; Cembella, A.D. Confirmed Identification of Gymnodimine in Oysters from the West Coast of South Africa by Liquid Chromatography–Tandem Mass Spectrometry. Afr. J. Mar. Sci. 2009, 31, 113–118. [Google Scholar] [CrossRef]
- Fire, S.E.; Flewelling, L.J.; Naar, J.; Twiner, M.J.; Henry, M.S.; Pierce, R.H.; Gannon, D.P.; Wang, Z.; Davidson, L.; Wells, R.S. Prevalence of Brevetoxins in Prey Fish of Bottlenose Dolphins in Sarasota Bay, Florida. Mar. Ecol. Prog. Ser. 2008, 368, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Flewelling, L.; Adams, D.; Naar, J.; Atwood, K.; Granholm, A.; O’Dea, S.; Landsberg, J. Brevetoxins in Sharks and Rays (Chondrichthyes, Elasmobranchii) from Florida Coastal Waters. Mar. Biol. 2010, 157, 1937–1953. [Google Scholar] [CrossRef]
- Twiner, M.J.; Flewelling, L.J.; Fire, S.E.; Bowen-Stevens, S.R.; Gaydos, J.K.; Johnson, C.K.; Landsberg, J.H.; Leighfield, T.A.; Mase-Guthrie, B.; Schwacke, L.; et al. Comparative Analysis of Three Brevetoxin-Associated Bottlenose Dolphin (Tursiops Truncatus) Mortality Events in the Florida Panhandle Region (USA). PLoS ONE 2012, 7, e42974. [Google Scholar] [CrossRef]
- Radwan, F.F.Y.; Ramsdell, J.S. Characterization of In Vitro Oxidative and Conjugative Metabolic Pathways for Brevetoxin (PbTx-2). Toxicol. Sci. 2006, 89, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; An, T.; Rein, K.S. Human Metabolites of Brevetoxin PbTx-2: Identification and Confirmation of Structure. Toxicon 2010, 56, 648–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawley, R.E.; Rein, K.S.; Jeglitsch, G.; Adams, D.J.; Theodorakis, E.A.; Tiebes, J.; Nicolaou, K.C.; Baden, D.G. The Relationship of Brevetoxin ‘Length’ and A-Ring Functionality to Binding and Activity in Neuronal Sodium Channels. Chem. Biol. 1995, 2, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Jeglitsch, G.; Rein, K.; Baden, D.G.; Adams, D.J. Brevetoxin-3 (PbTx-3) and Its Derivatives Modulate Single Tetrodotoxin-Sensitive Sodium Channels in Rat Sensory Neurons. J. Pharm. Exp. 1998, 284, 516–525. [Google Scholar]
- Purkerson-Parker, S.L.; Fieber, L.A.; Rein, K.S.; Podona, T.; Baden, D.G. Brevetoxin Derivatives That Inhibit Toxin Activity. Chem. Biol. 2000, 7, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Satake, M.; Shoji, M.; Oshima, Y.; Naoki, H.; Fujita, T.; Yasumoto, T. Gymnocin-A, a Cytotoxic Polyether from the Notorious Red Tide Dinoflagellate, Gymnodinium Mikimotoi. Tetrahedron Lett. 2002, 43, 5829–5832. [Google Scholar] [CrossRef]
- Satake, M.; Tanaka, Y.; Ishikura, Y.; Oshima, Y.; Naoki, H.; Yasumoto, T. Gymnocin-B with the Largest Contiguous Polyether Rings from the Red Tide Dinoflagellate, Karenia (Formerly Gymnodinium) Mikimotoi. Tetrahedron Lett. 2005, 46, 3537–3540. [Google Scholar] [CrossRef]
- Tanaka, Y.; Satake, M.; Yotsu-Yamashita, M.; Oshima, Y. Gymnocin-A Carboxylic Acid and Gymnocin-A2, Cytotoxic Polyethers from the Red Tide Dinoflagellate Karenia Mikimotoi. Heterocycles 2013, 87, 2037–2046. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. Gymnodimine C, an Isomer of Gymnodimine B, from Karenia Selliformis. J. Agric. Food Chem. 2003, 51, 4838–4840. [Google Scholar] [CrossRef]
- Shimizu, Y.; Chou, H.N.; Bando, H.; Duyne, G.V.; Clardy, J.C. Structure of Brevetoxin A (GB-1 Toxin), the Most Potent Toxin in the Florida Red Tide Organism Gymnodinium Breve (Ptychodiscus Brevis). J. Am. Chem. Soc. 1986, 108, 514–515. [Google Scholar] [CrossRef]
- Baden, D.; Mende, T.J.; Trainer, V.L. Derivatized Brevetoxins and Their Use as Quantitative Tools in Detection. Mycotoxins 1988, 1988, 1–2. [Google Scholar] [CrossRef]
- Baden, D.G. Metabolism and Toxinology of the Marine Dinoflagellate, Gymnodinium Breve; University of Miami: Coral Gables, FL, USA, 1977. [Google Scholar]
- Catterall, W.A.; Risk, M. Toxin T46 from Ptychodiscus Brevis (Formerly Gymnodinium Breve) Enhances Activation of Voltage-Sensitive Sodium Channels by Veratridine. Mol. Pharm. 1981, 19, 345–348. [Google Scholar]
- Lin, Y.Y.; Risk, M.; Ray, S.M.; Van Engen, D.; Clardy, J.; Golik, J.; James, J.C.; Nakanishi, K. Isolation and Structure of Brevetoxin B from the “Red Tide” Dinoflagellate Ptychodiscus Brevis (Gymnodinium Breve). J. Am. Chem. Soc. 1981, 103, 6773–6775. [Google Scholar] [CrossRef]
- Baden, D.G.; Mende, T.J. Toxicity of Two Toxins from the Florida Red Tide Marine Dinoflagellate, Ptychodiscus Brevis. Toxicon 1982, 20, 457–461. [Google Scholar] [CrossRef]
- Chou, H.-N.; Shimizu, Y. A New Polyether Toxin from Gymnodinium Breve Davis. Tetrahedron Lett. 1982, 23, 5521–5524. [Google Scholar] [CrossRef]
- Chou, H.-N.; Shimizu, Y.; Van Duyne, G.; Clardy, J. Isolation and Structures of Two New Polycyclic Ethers from Gymnodinium Breve Davis (=Ptychodiscus Brevis). Tetrahedron Lett. 1985, 26, 2865–2868. [Google Scholar] [CrossRef]
- Shimizu, Y. Recent progress in marine toxin research. Pure Appl. Chem. 1982, 54, 1973–1980. [Google Scholar] [CrossRef]
- Prasad, A.V.K.; Shimizu, Y. The Structure of Hemibrevetoxin-B: A New Type of Toxin in the Gulf of Mexico Red Tide Organism. J. Am. Chem. Soc. 1989, 111, 6476–6477. [Google Scholar] [CrossRef]
- Bourdelais, A.J.; Jacocks, H.M.; Wright, J.L.C.; Bigwarfe, P.M., Jr.; Baden, D.G. A New Polyether Ladder Compound Produced by the Dinoflagellate Karenia Brevis. J. Nat. Prod. 2005, 68, 2–6. [Google Scholar] [CrossRef] [Green Version]
- Satake, M.; Bourdelais, A.J.; Van Wagoner, R.M.; Baden, D.G.; Wright, J.L.C. Brevisamide: An Unprecedented Monocyclic Ether Alkaloid from the Dinoflagellate Karenia Brevis That Provides a Potential Model for Ladder-Frame Initiation. Org. Lett. 2008, 10, 3465–3468. [Google Scholar] [CrossRef] [Green Version]
- Satake, M.; Campbell, A.; Van Wagoner, R.M.; Bourdelais, A.J.; Jacocks, H.; Baden, D.G.; Wright, J.L.C. Brevisin: An Aberrant Polycyclic Ether Structure from the Dinoflagellate Karenia Brevis and Its Implications for Polyether Assembly. J. Org. Chem. 2009, 74, 989–994. [Google Scholar] [CrossRef]
- Truxal, L.T.; Bourdelais, A.J.; Jacocks, H.; Abraham, W.M.; Baden, D.G. Characterization of Tamulamides A and B, Polyethers Isolated from the Marine Dinoflagellate Karenia Brevis. J. Nat. Prod. 2010, 73, 536–540. [Google Scholar] [CrossRef] [Green Version]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. New Analogue of Gymnodimine from a Gymnodinium Species. J. Agric. Food Chem. 2000, 48, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Irie, R.; Holland, P.T.; Harwood, D.T.; Shi, F.; Itoh, Y.; Hayashi, F.; Zhang, H. Brevisulcenals-A1 and A2, Sulfate Esters of Brevisulcenals, Isolated from the Red Tide Dinoflagellate Karenia Brevisulcata. Toxins 2021, 13, 82. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Tachibana, K.; Holland, P.T.; Shi, F.; Beuzenberg, V.; Itoh, Y.; Satake, M. Brevisulcenal-F: A Polycyclic Ether Toxin Associated with Massive Fish-Kills in New Zealand. J. Am. Chem. Soc. 2012, 134, 4963–4968. [Google Scholar] [CrossRef] [PubMed]
- Shi, F. Chemical and Toxicological Investigation of the Toxic Dinoflagellate, Karenia Brevisulcata; Lincoln University: Christchurch, New Zealand, 2012. [Google Scholar]
- Satake, M.; Irie, R.; Hamamoto, Y.; Tachibana, K.; Holland, P.T.; Harwood, D.T.; Shi, F.; Beuzenberg, V.; Itoh, Y.; Hayashi, F.; et al. Brevisulcenal-G, -H, and -I, Polycyclic Ether Marine Toxins from the Dinoflagellate Karenia Brevisulcata. Heterocycles 2018, 96, 2096–2105. [Google Scholar] [CrossRef]
- Suzuki, R.; Irie, R.; Harntaweesup, Y.; Tachibana, K.; Holland, P.T.; Harwood, D.T.; Shi, F.; Beuzenberg, V.; Itoh, Y.; Pascal, S.; et al. Brevisulcatic Acids, Marine Ladder-Frame Polyethers from the Red Tide Dinoflagellate Karenia Brevisulcata in New Zealand. Org. Lett. 2014, 16, 5850–5853. [Google Scholar] [CrossRef]
- Irie, R.; Suzuki, R.; Kazuo, T.; Holland, P.; Harwood, T.D.; Shi, F.; McNabb, P.; Beuzenberg, V.; Hayashi, F.; Zhang, H.; et al. Brevisulcatic Acids from a Marine Microalgal Species Implicated in a Toxic Event in New Zealand. Heterocycles 2016, 92, 45. [Google Scholar] [CrossRef]
- Bourdelais, A.J.; Campbell, S.; Jacocks, H.; Naar, J.; Wright, J.L.C.; Carsi, J.; Baden, D.G. Brevenal Is a Natural Inhibitor of Brevetoxin Action in Sodium Channel Receptor Binding Assays. Cell. Mol. Neurobiol. 2004, 24, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Holland, P.T.; Shi, F.; Satake, M.; Hamamoto, Y.; Ito, E.; Beuzenberg, V.; McNabb, P.; Munday, R.; Briggs, L.; Truman, P.; et al. Novel Toxins Produced by the Dinoflagellate Karenia Brevisulcata. Harmful Algae 2012, 13, 47–57. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Hadley, W. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Fowler, N.; Tomas, C.; Baden, D.; Campbell, L.; Bourdelais, A. Chemical Analysis of Karenia Papilionacea. Toxicon 2015, 101, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, K. The Chemistry of Brevetoxins: A Review. Toxicon 1985, 23, 473–479. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Misner, I.; Tomas, C.R.; Wright, J.L.C. Occurrence of 12-Methylgymnodimine in a Spirolide-Producing Dinoflagellate Alexandrium Peruvianum and the Biogenetic Implications. Tetrahedron Lett. 2011, 52, 4243–4246. [Google Scholar] [CrossRef]
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and Derivative Brevetoxins: Historical Background, Multiplicity, and Effects. Environ. Health Perspect. 2005, 113, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Baden, D.G.; Mende, T.J.; Szmant, A.M.; Trainer, V.L.; Edwards, R.A.; Roszell, L.E. Brevetoxin Binding: Molecular Pharmacology versus Immunoassay. Toxicon 1988, 26, 97–103. [Google Scholar] [CrossRef]
- Abraham, W.M.; Bourdelais, A.J.; Sabater, J.R.; Ahmed, A.; Lee, T.A.; Serebriakov, I.; Baden, D.G. Airway Responses to Aerosolized Brevetoxins in an Animal Model of Asthma. Am. J. Respir. Crit. Care Med. 2005, 171, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Golik, J.; James, J.C.; Nakanishi, K.; Lin, Y.-Y. The Structure of Brevetoxin C. Tetrahedron Lett. 1982, 23, 2535–2538. [Google Scholar] [CrossRef]
- Fuwa, H.; Ebine, M.; Bourdelais, A.J.; Baden, D.G.; Sasaki, M. Total Synthesis, Structure Revision, and Absolute Configuration of (−)-Brevenal. J. Am. Chem. Soc. 2006, 128, 16989–16999. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, A.T.; Martinez, S.R.; Zakarian, A. A Concise Asymmetric Total Synthesis of (+)-Brevisamide. Org. Lett. 2011, 13, 3636–3639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, T.; Fukuta, A.; Nakamura, K.; Nakano, M.; Mori, Y. Total Synthesis of Brevisamide Using an Oxiranyl Anion Strategy. J. Org. Chem. 2016, 81, 3799–3808. [Google Scholar] [CrossRef]
- Lee, J.; Oh, H.-S.; Kang, H.-Y. A Formal Total Synthesis of (−)-Brevisamide, a Marine Monocyclic Ether Amide. Tetrahedron Lett. 2015, 56, 1099–1102. [Google Scholar] [CrossRef]
- Van Wagoner, R.M.; Satake, M.; Bourdelais, A.J.; Baden, D.G.; Wright, J.L.C. Absolute Configuration of Brevisamide and Brevisin: Confirmation of a Universal Biosynthetic Process for Karenia Brevis Polyethers. J. Nat. Prod. 2010, 73, 1177–1179. [Google Scholar] [CrossRef] [Green Version]
- Kuranaga, T.; Ohtani, N.; Tsutsumi, R.; Baden, D.G.; Wright, J.L.C.; Satake, M.; Tachibana, K. Total Synthesis of (−)-Brevisin: A Concise Synthesis of a New Marine Polycyclic Ether. Org. Lett. 2011, 13, 696–699. [Google Scholar] [CrossRef] [Green Version]
- Tsukano, C.; Sasaki, M. Total Synthesis of Gymnocin-A. J. Am. Chem. Soc. 2003, 125, 14294–14295. [Google Scholar] [CrossRef]
- Stewart, M.; Blunt, J.W.; Munro, M.H.; Robinson, W.T.; Hannah, D.J. The Absolute Stereochemistry of the New Zealand Shellfish Toxin Gymnodimine. Tetrahedron Lett. 1997, 38, 4889–4890. [Google Scholar] [CrossRef]
- Medhioub, W.; Guéguen, M.; Lassus, P.; Bardouil, M.; Truquet, P.; Sibat, M.; Medhioub, N.; Soudant, P.; Kraiem, M.; Amzil, Z. Detoxification Enhancement in the Gymnodimine-Contaminated Grooved Carpet Shell, Ruditapes Decussatus (Linné). Harmful Algae 2010, 9, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Otero, A.; Chapela, M.-J.; Atanassova, M.; Vieites, J.M.; Cabado, A.G. Cyclic Imines: Chemistry and Mechanism of Action: A Review. Chem. Res. Toxicol. 2011, 24, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Harju, K.; Koskela, H.; Kremp, A.; Suikkanen, S.; de la Iglesia, P.; Miles, C.O.; Krock, B.; Vanninen, P. Identification of Gymnodimine D and Presence of Gymnodimine Variants in the Dinoflagellate Alexandrium Ostenfeldii from the Baltic Sea. Toxicon 2016, 112, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strangman, W.; Anttila, M.; Tomas, C.; Wright, J.L.C. (5S)-5-[(4aR,8aS,9E,11S,13R,14S,16R,17R,19S)-11,19-Dihydroxy-8,10,13,16-Tetramethyl-18-Methylidene-3,4,5,6,8a,11,12,13,14,15,16,17,18,19,20,21-Hexadecahydro-2H-14,17-Epoxybenzo[2,3]Cyclohexadeca[1,2-b]Pyridine-7-Yl]-3-Methylfuran-2(5H)-One (12-Methylgymnodimine B). Molbank 2016, 2016, M896. [Google Scholar] [CrossRef] [Green Version]
- Zurhelle, C.; Nieva, J.; Tillmann, U.; Harder, T.; Krock, B.; Tebben, J. Identification of Novel Gymnodimines and Spirolides from the Marine Dinoflagellate Alexandrium Ostenfeldii. Mar. Drugs 2018, 16, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, P.; Riobó, P.; Rodríguez, F.; Franco, J.M.; Bravo, I. Differences in the Toxin Profiles of Alexandrium Ostenfeldii (Dinophyceae) Strains Isolated from Different Geographic Origins: Evidence of Paralytic Toxin, Spirolide, and Gymnodimine. Toxicon 2015, 103, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Munday, R.; Towers, N.R.; Mackenzie, L.; Beuzenberg, V.; Holland, P.T.; Miles, C.O. Acute Toxicity of Gymnodimine to Mice. Toxicon 2004, 44, 173–178. [Google Scholar] [CrossRef]
- Kharrat, R.; Servent, D.; Girard, E.; Ouanounou, G.; Amar, M.; Marrouchi, R.; Benoit, E.; Molgó, J. The Marine Phycotoxin Gymnodimine Targets Muscular and Neuronal Nicotinic Acetylcholine Receptor Subtypes with High Affinity. J. Neurochem. 2008, 107, 952–963. [Google Scholar] [CrossRef]
- Chang, F.H. Gymnodinium Brevisulcatum sp. Nov. (Gymnodiniales, Dinophyceae), a New Species Isolated from the 1998 Summer Toxic Bloom in Wellington Harbour, New Zealand. Phycologia 1999, 38, 377–384. [Google Scholar] [CrossRef]
- Keyzers, R.A. The Isolation of Biologically Active Secondary Metabolites from New Zealand Marine Organisms; Victoria University: Wellington, New Zealand, 2003. [Google Scholar]
- Harwood, D.T.; Shi, F.; Satake, M.; Holland, P.T. A Sensitive LC-MS/MS Assay for Brevisulcenal and Brevisulcatic Acid Toxins Produced by the Dinoflagellate Karenia Brevisulcata. Toxicon 2014, 84, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Naar, J.; Kubanek, J.; Weidner, A.; Flewelling, L.; Bourdelais, A.; Steidinger, K.; Baden, D.G. Brevetoxin Depuration in Shellfish via Production of Non-Toxic Metabolites: Consequences for Seafood Safety and the Environmental Fate of Biotoxins. Harmful Algae 2004, 10, 488–490. [Google Scholar]
- Pérez Linares, J.; Ochoa, J.L.; Gago Martínez, A. Retention and Tissue Damage of PSP and NSP Toxins in Shrimp: Is Cultured Shrimp a Potential Vector of Toxins to Human Population? Toxicon 2009, 53, 185–195. [Google Scholar] [CrossRef]
- Plakas, S.M.; Jester, E.L.E.; El Said, K.R.; Granade, H.R.; Abraham, A.; Dickey, R.W.; Scott, P.S.; Flewelling, L.J.; Henry, M.; Blum, P.; et al. Monitoring of Brevetoxins in the Karenia Brevis Bloom-Exposed Eastern Oyster (Crassostrea Virginica). Toxicon 2008, 52, 32–38. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). National Shellfish Sanitation Program (NSSP). Guide for the Control of Molluscan Shellfish. 2019; pp. 1–491. Available online: https://www.fda.gov/media/143238/download (accessed on 22 November 2021).
- NSW Government. Marine Biotoxin Managment Plan—NSW Shellfish Program; NSW Government: Newington, NSW, Australia, 2015; pp. 1–44. Available online: https://www.foodauthority.nsw.gov.au/sites/default/files/_Documents/industry/marine_biotoxin_management_plan.pdf (accessed on 11 November 2021).
- Victorian Fisheries Authority. Marine Biotoxin Management Plan. Available online: https://vfa.vic.gov.au/aquaculture/publications/shellfish-quality-asurance/marine-biotoxin-management-plan (accessed on 2 September 2021).
- McNabb, P.S.; Selwood, A.I.; Van Ginkel, R.; Boundy, M.; Holland, P.T. Determination of Brevetoxins in Shellfish by LC/MS/MS: Single-Laboratory Validation. J. AOAC Int. 2012, 95, 1097–1105. [Google Scholar] [CrossRef]
- New Zealand Government. Regulated Control Scheme—Bivalve Molluscan Shellfish for Human Consumption; New Zealand Government: Wellington, New Zealand, 2021; pp. 1–68. Available online: https://www.mpi.govt.nz/dmsdocument/30282-Animal-Products-Notice-Regulated-Control-Scheme-Bivalve-Molluscan-Shellfish-for-Human-Consumption-2018 (accessed on 22 November 2021).
- ANSES. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the State of Knowledge on Brevetoxins in Shellfish, Data on Toxicity, Occurrence and Brevetoxin-Producing Microalgae; ANSES: Maisons-Alfort, France, 2021; pp. 1–18. Available online: https://www.anses.fr/en/system/files/ERCA2020SA0020EN.pdf (accessed on 22 November 2021).
- Van Deventer, M.; Atwood, K.; Vargo, G.A.; Flewelling, L.J.; Landsberg, J.H.; Naar, J.P.; Stanek, D. Karenia Brevis Red Tides and Brevetoxin-Contaminated Fish: A High Risk Factor for Florida’s Scavenging Shorebirds? Botanica Marina 2012, 55, 31–37. [Google Scholar] [CrossRef]
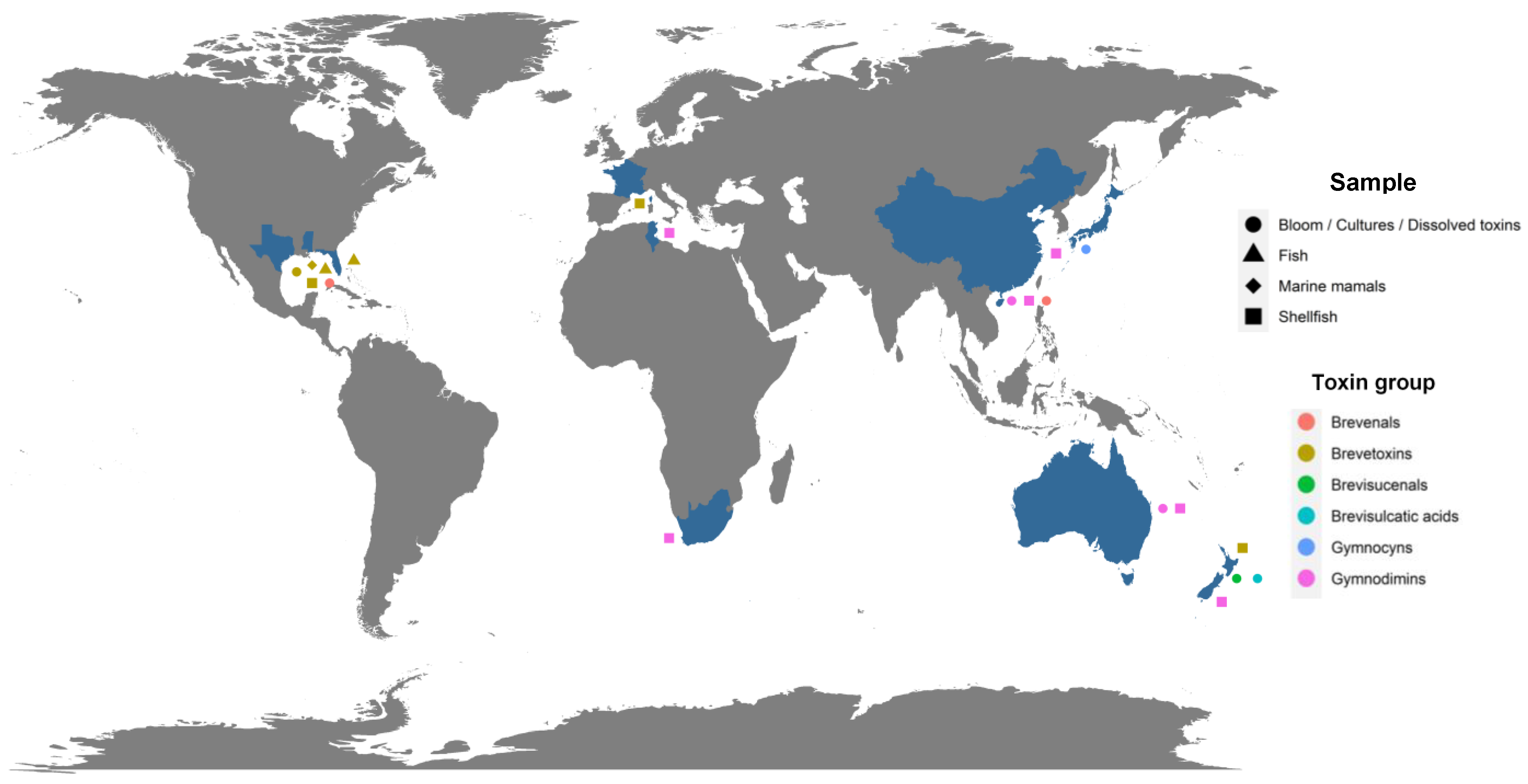

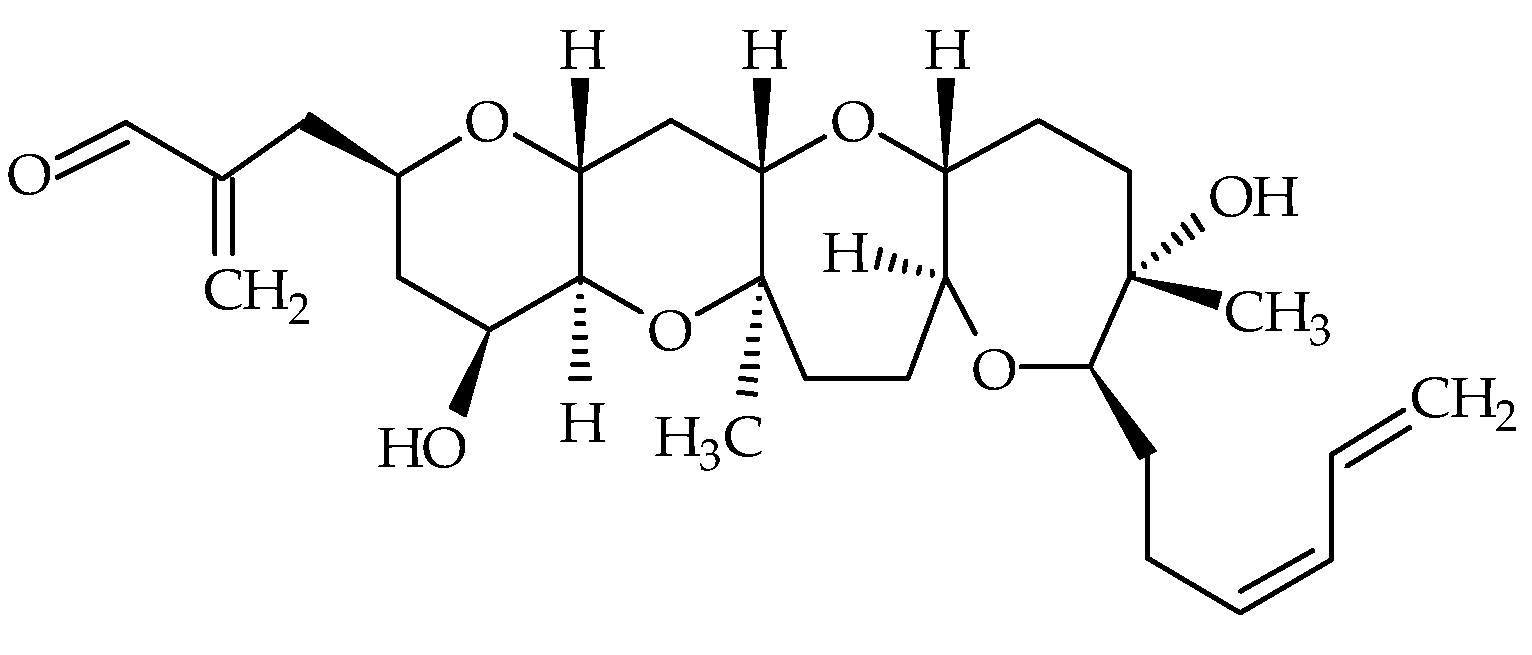

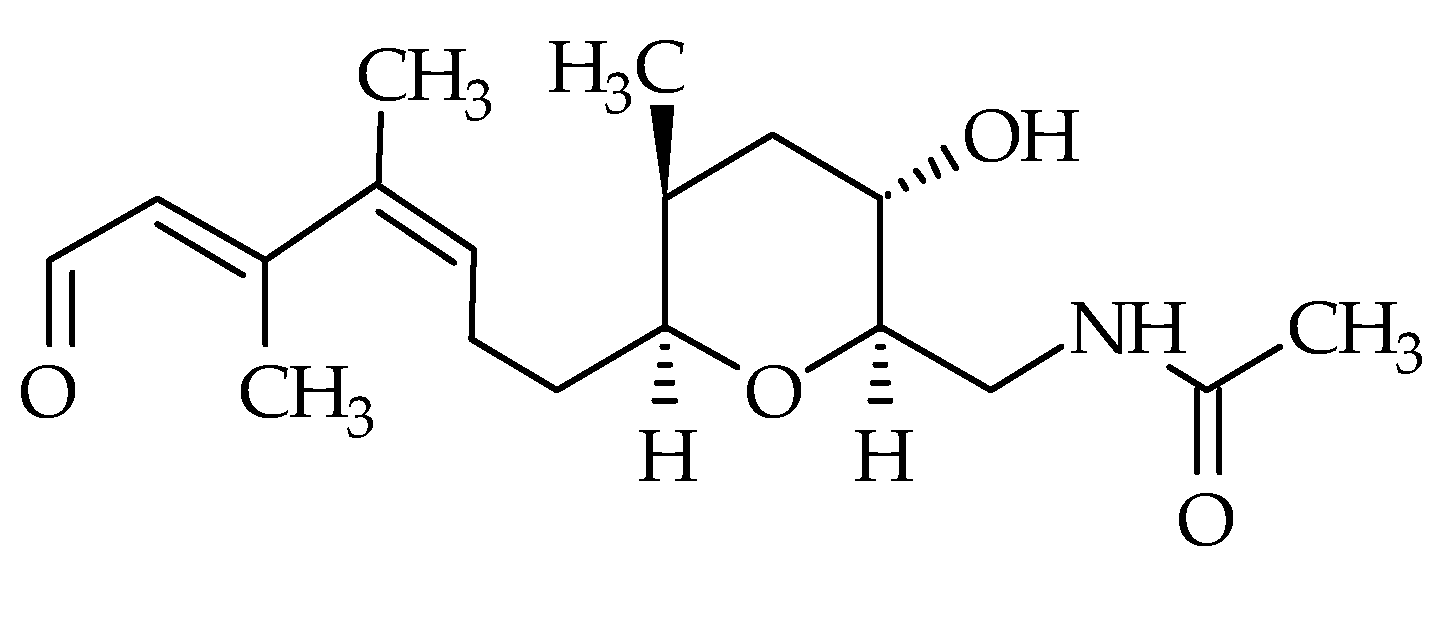
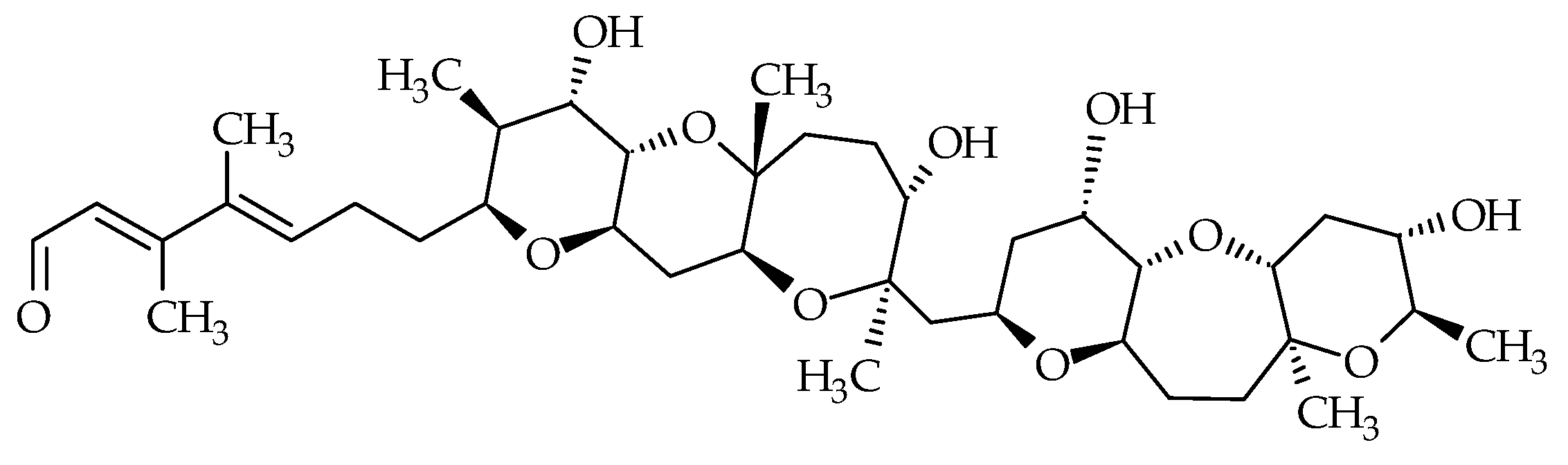




| Group | Compound Identification Number (ID) | Metabolite | Other Existing Names | Molecular Formula | Monoisotopic Mass (Da) | LogP 1 | Species | First Identification/Structural Elucidation References |
|---|---|---|---|---|---|---|---|---|
| A-type brevetoxins (BTX-A) | #1 | BTX-1 | PbTx-1; GB-1; T46, BTX-A | C49H70O13 | 866.5 | 5.8 | K. brevis | [60] |
| #2 | BTX-7 | PbTx-7; GB-7; Aldehyde-reduced PbTx-1 | C49H72O13 | 868.5 | 5.7 | K. brevis | [60] | |
| #3 | BTX-10 | / | C49H74O13 | 870.5 | 5.5 | K. brevis | [13,61] 2 | |
| #4 | Oxidized BTX-1 | Oxidized PbTx-1 PbTx-1-carboxylic acid | C49H70O14 | 882.5 | / | K. brevis | [35] 3 | |
| #5 | Open A-ring BTX-1 | Open-ring PbTx-1 | C49H72O14 | 884.5 | / | K. brevis | [35] 3 | |
| #6 | Open A-ring, oxidized BTX-1 | Open-ring, oxidized PbTx-1; PbTx-1-open ring-carboxylic acid | C49H72O15 | 900.5 | / | K. brevis | [35] 3 | |
| #7 | Open A-ring BTX-7 | Open-ring PbTx-7 | C49H74O14 | 886.5 | / | K. brevis | [35] 3 | |
| B-type brevetoxins (BTX-B) | #8 | BTX-2 | PbTx-2; T47; GB-2, T34, BTX-B | C50H70O14 | 894.5 | 6.4 | K. brevis | [62,63,64] |
| #9 | BTX-3 | PbTx-3; GB-3; T17; Dihydro-BTX-B; Aldehyde-reduced PbTx-2 | C50H72O14 | 896.5 | 6.4 | K. brevis | [65,66] | |
| #10 | BTX-5 | PbTx-5; GB-5; acetylated PbTx-2 | C52H72O15 | 936.5 | 7.1 | K. brevis | [67] | |
| #11 | BTX-6 | PbTx-6; GB-6; 27,28 epoxyde of PbTx-2 | C50H70O15 | 910.5 | 7.3 | K. brevis | [67] | |
| #12 | BTX-9 | PbTx-9; α-methylene reduced PbTx-3 | C50H74O14 | 898.5 | 6.3 | K. brevis | [13,14,61] | |
| #13 | BTX-B5 | Oxidized BTX-2; Oxidized PbTx-2 | C50H70O15 | 910.5 | / | K. brevis | [32,33,35] | |
| #14 | Open A-ring BTX-2 | Open A-ring PbTx-2 | C50H72O15 | 912.5 | / | K. brevis | [35] 3 | |
| #15 | Open A-ring, oxidized BTX-2 | Open A-ring, Oxidized PbTx-2; Open A-ring BTX-B5; PbTx-2-open ring-carboxylic acid | C50H72O16 | 928.5 | / | K. brevis | [35] 3 | |
| #16 | Open A-ring BTX-3 | Open A-ring PbTx-3 | C50H74O15 | 914.5 | / | K. brevis | [35] 3 | |
| Hemi- Brevetoxins (Hemi-BTXs) | #17 | Hemi-BTX-A | GB-M | / | / | / | K. brevis | [68,69] |
| #18 | Hemi-BTX-B | GB-N | C28H42O7 | 490.3 | 3.5 | K. brevis | [69] | |
| #19 | Hemi-BTX-C | GB-4 | / | / | / | K. brevis | [69] | |
| Brevenals | #20 | Brevenal | / | C39H60O8 | 656.4 | 6.9 | K. brevis | [70] |
| #21 | Dimethyl acetal brevenal | / | C41H66O9 | 702.5 | / | K. brevis | [70] | |
| Brevisamide | #22 | Brevisamide | / | C18H29NO4 | 323.2 | / | K. brevis | [71] |
| Brevisin | #23 | Brevisin | / | C39H62O11 | 706.4 | 3.6 | K. brevis | [72] |
| Tamulamides (Tams) | #24 | Tam-A | / | C35H45NO10 | 639.3 | 1.0 | K. brevis | [73] |
| #25 | Tam-B | / | C34H43NO10 | 625.3 | 0.7 | K. brevis | [73] | |
| Gymnocins | #26 | Gymnocin-A | / | C55H80O18 | 1028.5 | 3.3 | K. mikimotoi | [56] |
| #27 | Gymnocin-A carboxylic acid | / | C55H80O19 | 1044.5 | 3.6 | K. mikimotoi | [58] | |
| #28 | Gymnocin-A2 | / | C55H80O18 | 1028.5 | / | K. mikimotoi | [58] | |
| #29 | Gymnocin-B | / | C62H92O20 | 1156.6 | 5.0 | K. mikimotoi | [57] | |
| Gymnodimines (GYMs) | #30 | GYM-A | / | C32H45NO4 | 509.4 | 6.4 | K. mikimotoi K. selliformis | [24] |
| #31 | GYM-B | / | C32H45NO5 | 523.3 | 5.1 | K. selliformis | [74] | |
| #32 | GYM-C | GYM-B isomer | C32H45NO5 | 523.3 | / | K. selliformis | [59] | |
| Brevisucenals (KBTs) | #33 | KBT-A1 (sodium salt) | KBT-F sulfate ester | C107H159O41SNa | 2155.0 | / | K. brevisulcata | [75] |
| #34 | KBT-A2 (sodium salt) | KBT-G sulfate ester | C108H161O42SNa | 2185.0 | / | K. brevisulcata | [75] | |
| #35 | KBT-F | / | C107H160O38 | 2053.1 | 7.9 | K. brevisulcata | [76,77] | |
| #36 | KBT-G | / | C108H162O39 | 2083.1 | / | K. brevisulcata | [77,78] | |
| #37 | KBT-H | / | C107H160O39 | 2069.1 | / | K. brevisulcata | [78] | |
| #38 | KBT-I | / | C108H162O40 | 2099.1 | / | K. brevisulcata | [78] | |
| Brevisulcatic acids (BSXs) | #39 | BSX-1 | / | C49H72O16 | 916.5 | 3.6 | K. brevisulcata | [77,79] |
| #40 | BSX-2 | / | C47H68O15 | 872,5 | / | K. brevisulcata | [77,80] | |
| #41 | BSX-3 | / | / | 856.5 | / | K. brevisulcata | [77] 4 | |
| #42 | BSX-4 | / | C49H70O15 | 898.5 | 3.8 | K. brevisulcata | [68,79] | |
| #43 | BSX-5 | / | C47H66O14 | 854.4 | / | K. brevisulcata | [77,80] | |
| #44 | BSX-6 | Lactone derivative of BSX-3 | / | 838.5 | / | K. brevisulcata | [77] 4 | |
| #45 | BSX-7 | / | C47H70O14 | 858.5 | / | K. brevisulcata | [80] |
| Group | Compound Identification Number (ID) | Metabolite | Other Existing Names | Molecular Formula | Monoisotopic Mass (Da) | LogP 1 | Sample | References |
|---|---|---|---|---|---|---|---|---|
| A-type BTXs | #4 | Oxidized BTX-1 | Oxidized PbTx-1; PbTx-1-carboxylic acid | C49H70O14 | 882.5 | / | Clams (mercenaria sp.) | [22] |
| #6 | Open A-ring, oxidized BTX-1 | Open A-ring of oxidized PbTx-1; A-type opened A-ring derivative | C49H72O15 | 900.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) | [22,35] | |
| #7 | Open A-ring BTX-7 | Open A-ring PbTx-7 | C49H74O14 | 886.5 | / | Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Spot (Leiostomus xanthurus) | [35,39,48] | |
| #52 | Taurine-BTX-A | N-taurine conjugate of oxidized BTX-1 | C51H75NO16S | 989.5 | / | Clams (mercenaria sp.) | [22] | |
| #53 | Cysteine–BTX-A | Cysteine–PbTx-A; Cysteine-PbTx-1; Cysteine-BTX-1 | C52H79NO15S | 989.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Spot (Leiostomus xanthurus) | [23,35,39,48] | |
| #54 | Cysteine–BTX-A sulfoxide | Cysteine–PbTx-A sulfoxide; Sulfoxide cysteine conjugate of PbTx-1 | C52H79NO16S | 1005.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Spot (Leiostomus xanthurus) | [23,35,39,48] | |
| #55 | Open A-ring cysteine-BTX-A | Open A-ring cysteine–PbTx-A | C52H81NO16S | 1007.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) | [22,34,35,39] | |
| #56 | Glutathione-BTX-A | Glutathione-PbTx-A | C59H89N3O19S | 1175.6 | / | Oysters (Crassostrea virginica) | [23,34,39] | |
| #57 | Glycine-cysteine-BTX-A | Glycine-cysteine-PbTx-A | C54H82N2O16S | 1046.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) | [23,39] | |
| #58 | N-hexadecanoyl-cysteine–BTX-A | N-hexadecanoyl-cysteine–PbTx-A | C68H109NO16S | 1227.7 | / | Oysters (Crassostrea virginica) | [39] | |
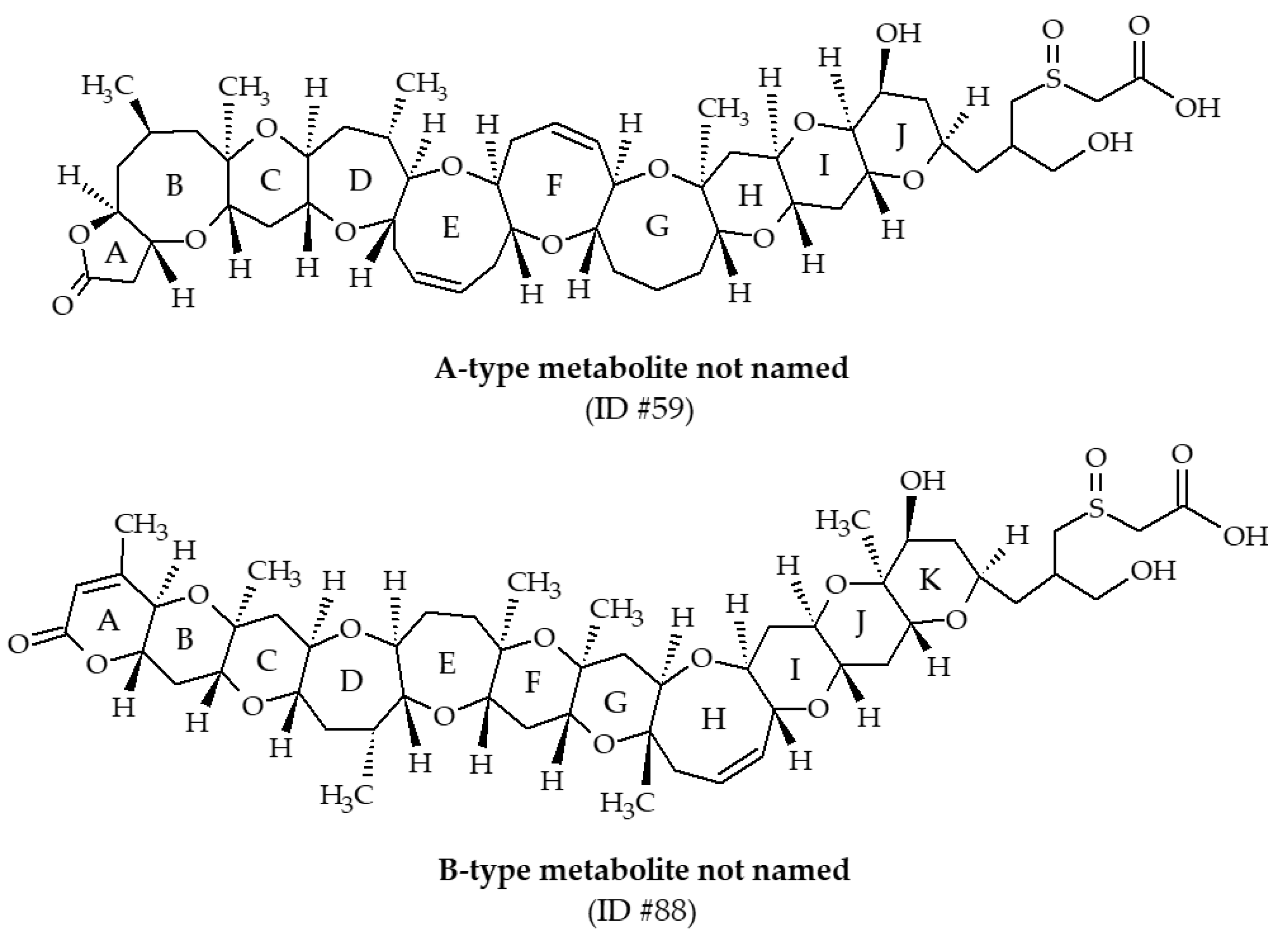
| #59 | Metabolite not named | / | C51H76O16S | 976.5 | / | Oysters (Crassostrea virginica) | [39] | |
| B-type BTXs | #8 | BTX-2 | PbTx-2; T47; GB-2; T34; BTX-B | C50H70O14 | 894.5 | 6.2 | Cockles (Austrovenus stutchburyi); Mussels (Perna canaliculus); Oysters (Crassostrea virginica, Crassostrea gigas); Sharks (Rhizoprionodon terraenovae, Sphyrna tiburo); Shrimps (Litopenaeus vannamei) | [10,21,30,31,32,49,64,112] |
| #9 | BTX-3 | PbTx-3; GB-3; T17; dihydro-BTX-B; aldehyde-reduced PbTx-2 | C50H72O14 | 896.5 | 6.4 | Bottlenose dolphins (Tursiops Truncatus); Clams (Mercenaria sp.); Cockles (Austrovenus stutchburyi); Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Mussels (Perna canaliculus); Oysters (Crassostrea gigas, Crassostrea virginica); Pinfish (Lagodon rhomboides); Rays (Dasyatis sabina); Sharks (Carcharhinus limbatus, Rhizoprionodon terraenovae, Sphyrna tiburo); Spot (Leiostomus xanthurus) | [10,17,20,21,22,31,32,33,48,49,50,66] | |
| #16 | Open A-ring BTX-3 | Open-ring PbTx-3 | C50H74O15 | 914.5 | / | Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Spot (Leiostomus xanthurus) | [17,35,48] | |
| #12 | BTX-9 | Reduced-BTX-2 | C50H74O14 | 898.5 | 6.3 | Oysters (Crassostrea virginica) | [34,39] | |
| #13 | BTX-B5 | Oxidized BTX-2; Oxidized PbTx-2; PbTx-2-carboxylic acid | C50H70O15 | 910.5 | 6.7 | Clams (Mercenaria sp., Macrocallista nimbosa); Cockles (Austrovenus stutchburyi); Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Mussels (Perna canaliculus); Oysters (Crassostrea gigas, Crassostrea virginica) | [17,22,23,31,32,33,39] | |
| #15 | Open A-ring, oxidized BTX-2 | Open ring, oxidized PbTx-2; Open A-ring BTX-B5; PbTx-2-open ring-carboxylic acid | C50H72O16 | 928.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) | [22,23,35] | |
| #60 | Oxidized, open D-ring tetradecanoyl-BTX-2 | BTX-B3; Oxidized, open D-ring myristoyl-BTX-2 | C64H96O17 | 1136.7 | / | Mussels (Perna canaliculus) | [36] | |
| #61 | Oxidized, open D-ring hexadecanoyl-BTX-2 | BTX-B3; Oxidized, open D-ring palmitoyl-BTX-2 | C66H100O17 | 1164.7 | / | Mussels (Perna canaliculus) | [36] | |
| #62 | Taurine-BTX-B | BTX-B1; N-taurine conjugate of oxidized PbTx-2; N-taurine conjugate of BTX-B5 | C52H75NO17S 2 | 1017.5 2 | / | Clams (Mercenaria sp.; Macrocallista nimbosa); Cockles (Austrovenus stutchburyi); Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Mussels (Perna canaliculus); Oysters (Crassostrea gigas) | [17,20,22,23,31,32,33,37] | |
| #63 | Cysteine-BTX-B | S-desoxy-BTX-B2; S-deoxy-BTX-B2; Cysteine-BTX-B; Cysteine-PbTx; Cysteine-PbTx-B; Cysteine conjugate of PbTx-2 | C53H79NO16S | 1017.5 | / | Clams (Mercenaria sp.; Macrocallista nimbosa); Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Sharks (Carcharhinus limbatus, Rhizoprionodon terraenovae, Sphyrna tiburo); Spot (Leiostomus xanthurus); Rays (Dasyatis sabina) | [17,22,23,28,34,35,48,49] | |
| #64 | Cysteine-BTX-B sulfoxide | BTX-B2; Cysteine-PbTx-B sulfoxide; Cysteine-PbTx sulfoxide; Oxidised-S-desoxy-BTX-B2 | C53H79NO17S | 1033.5 | 4.3 | Clams (Mercenaria sp., Macrocallista nimbosa); Gastropods (Triplofusus giganteus, Sinistrofulgur sinistrum, Cinctura hunteria, Strombus alatus, Fulguropsis spirata); Mussels (Perna canaliculus); Oysters (Crassostrea virginica); Pinfish (Lagodon rhomboides); Sharks (Carcharhinus limbatus, Rhizoprionodon terraenovae, Sphyrna tiburo); Spot (Leiostomus xanthurus); Rays (Dasyatis sabina) | [17,22,23,28,34,35,38,48,49] | |
| #65 | Oxidized cysteine-BTX-2 | Cysteine conjugate of oxidized PbTx-2 | C53H77NO17S | 1031.5 | / | Oysters (Crassostrea virginica) | [39] | |
| #66 | Open A-ring cysteine-BTX-B | Open A-ring S-desoxy-BTX-B2; Open A-ring cysteine–PbTx-B; Open A-ring cysteine–PbTx-2 | C53H81NO17S | 1035.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) | [22,34,35,39] | |
| #67 | Glycine-cysteine-BTX-B | Glycine-cysteine-PbTx-B | C55H82N2O17S | 1074.5 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) | [22,34,39] | |
| #68 | γ-glutamyl-cysteine-BTX-B | γ-glutamyl-cysteine-PbTx-B | C58H86N2O19S | 1146.6 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) | [22,34,39] | |
| #69 | Glutathione-BTX-B | Glutathione-PbTx-B | C60H89N3O20S | 1203.6 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) | [22,34,39] | |
| #70 | N-tetradecanoyl-cysteine-BTX-B sulfoxide | BTX-B4; N-tetradecanoyl-BTX-B2; N-myristoyl-BTX-B2; N-myristoyl-cysteine- PbTx-B sulfoxide | C67H105NO18S | 1243.7 | 11.3 | Mussels (Perna canaliculus); Oysters (Crassostrea virginica) | [39,40] | |
| #71 | N-hexadecanoyl-cysteine-BTX-B sulfoxide | BTX-B4; N-hexadecanoyl-BTX-B2; N-palmitoyl-BTX-B2; N-hexadecanoyl-cysteine–PbTx-B sulfoxide | C69H109NO18S | 1271.7 | 12.3 | Clams (mercenaria sp.); Mussels (Perna canaliculus); Oysters (Crassostrea virginica) | [22,39,40] | |
| #72 | N- (hydroxy-hexadecenoyl)-cysteine-BTX-B sulfoxide | N- (hydroxy-hexadecenoyl)-BTX-B2 | C69H107NO19S | 1285.7 | / | Clams (mercenaria sp.) | [22] | |
| #73 | N-octadecanoyl-cysteine-BTX-B sulfoxide | N-octadecanoyl-BTX-B2 | C71H113NO18S | 1299.8 | / | Clams (mercenaria sp.) | [22] | |
| #74 | N-octadecenoyl-cysteine-BTX-B sulfoxide | N-octadecenoyl-BTX-B2 | C71H111NO18S | 1297.8 | / | Clams (mercenaria sp.) | [22] | |
| #75 | N- (hydroxy-eicosanoyl)-cysteine-BTX-B sulfoxide | N- (hydroxy-eicosanoyl)-BTX-B2 | C73H117NO19S | 1343.8 | / | Clams (mercenaria sp.) | [22] | |
| #76 | N-arachidonyl-cysteine-BTX-B sulfoxide | N-arachidonyl-BTX-B2 | C73H109NO18S | 1319.7 | / | Clams (mercenaria sp.) | [22] | |
| #77 | N-didecenoyl-cysteine-BTX-B sulfoxide | N-didecenoyl-BTX-B2 | C73H115NO18S | 1325.8 | / | Clams (mercenaria sp.) | [22] | |
| #78 | N-tetradecanoyl-cysteine-BTX-B | N-tetradecanoyl-cysteine–PbTx-B | C67H105NO18S | 1243.7 | / | Oysters (Crassostrea virginica) | [39] | |
| #79 | N-hexadecanoyl-cysteine-BTX-B | N-hexadecanoyl-cysteine–PbTx-B; N-hexadecanoyl-S-deoxy-BTX-B2 | C69H109NO17S | 1255.7 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) | [22,39] | |
| #80 | N-(hydroxy-hexadecenoyl)-cysteine-BTX-B | N-(hydroxy-hexadecenoyl)-S-deoxy-BTX-B2 | C69H107NO18S | 1269.7 | / | Clams (mercenaria sp.) | [22] | |
| #81 | N-octadecanoyl-cysteine-BTX-B | N-octadecanoyl-cysteine–PbTx-B; N-octadecanoyl-S-deoxy-BTX-B2 | C71H113NO17S | 1283.8 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) | [22,39] | |
| #82 | N-octadecenoyl-cysteine-BTX-B | N-octadecenoyl-cysteine–PbTx-B | C71H111NO17S | 1281.8 | / | Oysters (Crassostrea virginica) | [39] | |
| #83 | N-eicosenoyl-cysteine-BTX-B | N-eicosenoyl-cysteine–PbTx-B | C73H115NO17S | 1309.8 | / | Oysters (Crassostrea virginica) | [39] | |
| #84 | N- (hydroxy-eicosanoyl)-cysteine-BTX-B | N-(hydroxy-eicosanoyl)-cysteine–PbTx-B; N-(hydroxy-eicosanoyl)-S-deoxy-BTX-B2 | C73H117NO18S | 1327.8 | / | Clams (mercenaria sp.); Oysters (Crassostrea virginica) | [22,39] | |
| #85 | Glycine-(N-tetradecanoyl)-cysteine-BTX-B | Glycine-(N-tetradecanoyl-cysteine)–PbTx-B | C69H108N2O18S | 1284.7 | / | Oysters (Crassostrea virginica) | [39] | |
| #86 | Glycine-(N-hexadecanoyl)-cysteine-BTX-B | Glycine-(N-hexadecanoyl-cysteine)– PbTx-B | C71H112N2O18S | 1312.8 | / | Oysters (Crassostrea virginica) | [39] | |
| #87 | Glycine–(N-hydroxy-eicosanoyl)-cysteine-BTX-B | Glycine–(N-hydroxy-eicosanoyl)-cysteine–PbTx-B | C75H120N2O19S | 1384.8 | / | Oysters (Crassostrea virginica) | [39] | |
| #88 | Metabolite not named | / | C52H76O17S | 1004.5 | / | Oysters (Crassostrea virginica) | [30,39] |
| Gymnodimine (GYM) Metabolite | Compound Identification Number (ID) | Carboxyl Group of the Ester (Carbon:Unsaturation) | Molecular Formula | Monoisotopic Mass (Da) | LogP 1 | Sample | References |
|---|---|---|---|---|---|---|---|
| GYM-A | #30 | / | C32H45NO4 | 507.3 | 6.4 | Clams (Antigona lamella, Ruditapes decussatus); Gastropods (Batillaria zonalis); Oysters (Crassostrea angulata, Crassostrea ariakensis, Crassostrea giga, Dendostrea crenulifrea, Saccostrea glomerata, Tiostrea chilensis); Mussels (Choromytilus meridionalis, Mytilus galloprovincialis, Modiolus proclivis); Pipis (Donax deltoides); Pen shells (Atrina pectinata); | [24,25,26,27,41,44,45,46,47] |
| GYM-B | #31 | / | C32H45NO5 | 523.3 | 5.1 | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [25,41,42] |
| GYM-C | #32 | / | C32H45NO5 | 523.3 | / | Clams (Ruditapes decussatus) | [25,42] |
| 10-O-dodecanoyl-GYM-A | #89 | 12:0 | C44H67NO5 | 689.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-dodecenoyl-GYM-A | #90 | 12:1 | C44H65NO5 | 687.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-tetradecanoyl GYM-A | #91 | 14:0 | C46H71NO5 | 717.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-tetradecenoyl GYM-A | #92 | 14:1 | C46H69NO5 | 715.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-tetradecatrienoyl-GYM-A | #93 | 14:3 | C46H65NO5 | 711.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-pentadecanoyl-GYM-A | #94 | 15:0 | C47H73NO5 | 731.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-pentadecenoyl-GYM-A | #95 | 15:1 | C47H71NO5 | 729.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-hexadecanoyl-GYM-A | #96 | 16:0 | C48H75NO5 | 745.6 | / | Clams (Ruditapes decussatus, Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-hexadecenoyl-GYM-A | #97 | 16:1 | C48H73NO5 | 743.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-hexadecadienoyl-GYM-A | #98 | 16:2 | C48H71NO5 | 741.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-hexadecatrienoyl-GYM-A | #99 | 16:3 | C48H69NO5 | 739.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-hexadecatetraenoyl-GYM-A | #100 | 16:4 | C48H67NO5 | 737.5 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heptadecanoyl-GYM-A | #101 | 17:0 | C49H77NO5 | 759.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-heptadecenoyl-GYM-A | #102 | 17:1 | C49H75NO5 | 757.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-heptadecadienoyl-GYM-A | #103 | 17:2 | C49H73NO5 | 755.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-octadecanoyl-GYM-A | #104 | 18:0 | C50H79NO5 | 773.6 | / | Clams (Ruditapes decussatus, Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-octadecenoyl-GYM-A | #105 | 18:1 | C50H77NO5 | 771.6 | / | Clams (Ruditapes decussatus; Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-octadecadienoyl-GYM-A | #106 | 18:2 | C50H75NO5 | 769.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-octadecatrienoyl-GYM-A | #107 | 18:3 | C50H73NO5 | 767.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-octadecatetraenoyl-GYM-A | #108 | 18:4 | C50H71NO5 | 765.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-nonadecanoyl-GYM-A | #109 | 19:0 | C51H81NO5 | 787.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-nonadecenoyl-GYM-A | #110 | 19:1 | C51H79NO5 | 785.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-nonadecadienoyl-GYM-A | #111 | 19:2 | C51H77NO5 | 783.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-eicosanoyl-GYM-A | #112 | 20:0 | C52H83NO5 | 801.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-eicosenoyl-GYM-A | #113 | 20:1 | C52H81NO5 | 799.6 | / | Clams (Ruditapes decussatus; Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-eicosadienoyl-GYM-A | #114 | 20:2 | C52H79NO5 | 797.6 | / | Clams (Ruditapes decussatus, Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-eicosatrienoyl-GYM-A | #115 | 20:3 | C52H77NO5 | 795.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-eicosatetraenoyl-GYM-A | #116 | 20:4 | C52H75NO5 | 793.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-eicosapentaenoyl-GYM-A | #117 | 20:5 | C52H72NO5 | 790.5 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-heneicosanoyl-GYM-A | #118 | 21:0 | C53H85NO5 | 815.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heneicosenoyl-GYM-A | #119 | 21:1 | C53H83NO5 | 813.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heneicosadienoyl-GYM-A | #120 | 21:2 | C53H81NO5 | 811.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heneicosatrienoyl-GYM-A | #121 | 21:3 | C53H79NO5 | 809.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heneicosatetraenoyl-GYM-A | #122 | 21:4 | C53H77NO5 | 807.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-heneicosapentaenoyl-GYM-A | #123 | 21:5 | C53H75NO5 | 805.6 | / | Mussels (M. galloprovincialis) | [41] |
| 10-O-docosanoyl-GYM-A | #124 | 22:0 | C54H87NO5 | 829.7 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-docosenoyl-GYM-A | #125 | 22:1 | C54H85NO5 | 827.6 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-docosadienoyl-GYM-A | #126 | 22:2 | C54H83NO5 | 825.6 | / | Clams (Ruditapes decussatus, Antigona lamellaris); Pen shell (Atrina pectinata); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-docosatrienoyl-GYM-A | #127 | 22:3 | C54H81NO5 | 823.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-docosatetraenoyl-GYM-A | #128 | 22:4 | C54H79NO5 | 821.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-docosapentaenoyl-GYM-A | #129 | 22:5 | C54H77NO5 | 819.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-docosahexaenoyl-GYM-A | #130 | 22:6 | C54H75NO5 | 817.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-tetracosanoyl-GYM-A | #131 | 24:0 | C56H91NO5 | 857.7 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-tetracosenoyl-GYM-A | #132 | 24:1 | C56H89NO5 | 855.7 | / | Clams (Ruditapes decussatus) | [42] |
| 10-O-tetracosapentaenoyl-GYM-A | #133 | 24:5 | C56H81NO5 | 847.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| 10-O-tetracosahexaenoyl-GYM-A | #134 | 24:6 | C56H79NO5 | 845.6 | / | Clams (Ruditapes decussatus); Mussels (M. galloprovincialis) | [41,42] |
| O-octadecanoyl-GYM-B | #135 | 18:0 | C50H79NO6 | 789.6 | / | Clams (Ruditapes decussatus) | [42] |
| O-octadecanoyl-GYM-C | #136 | 18:0 | C50H79NO6 | 789.6 | / | Clams (Ruditapes decussatus) | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hort, V.; Abadie, E.; Arnich, N.; Dechraoui Bottein, M.-Y.; Amzil, Z. Chemodiversity of Brevetoxins and Other Potentially Toxic Metabolites Produced by Karenia spp. and Their Metabolic Products in Marine Organisms. Mar. Drugs 2021, 19, 656. https://doi.org/10.3390/md19120656
Hort V, Abadie E, Arnich N, Dechraoui Bottein M-Y, Amzil Z. Chemodiversity of Brevetoxins and Other Potentially Toxic Metabolites Produced by Karenia spp. and Their Metabolic Products in Marine Organisms. Marine Drugs. 2021; 19(12):656. https://doi.org/10.3390/md19120656
Chicago/Turabian StyleHort, Vincent, Eric Abadie, Nathalie Arnich, Marie-Yasmine Dechraoui Bottein, and Zouher Amzil. 2021. "Chemodiversity of Brevetoxins and Other Potentially Toxic Metabolites Produced by Karenia spp. and Their Metabolic Products in Marine Organisms" Marine Drugs 19, no. 12: 656. https://doi.org/10.3390/md19120656
APA StyleHort, V., Abadie, E., Arnich, N., Dechraoui Bottein, M.-Y., & Amzil, Z. (2021). Chemodiversity of Brevetoxins and Other Potentially Toxic Metabolites Produced by Karenia spp. and Their Metabolic Products in Marine Organisms. Marine Drugs, 19(12), 656. https://doi.org/10.3390/md19120656







