Zhaoshumycins A and B, Two Unprecedented Antimycin-Type Depsipeptides Produced by the Marine-Derived Streptomyces sp. ITBB-ZKa6
Abstract
:1. Introduction
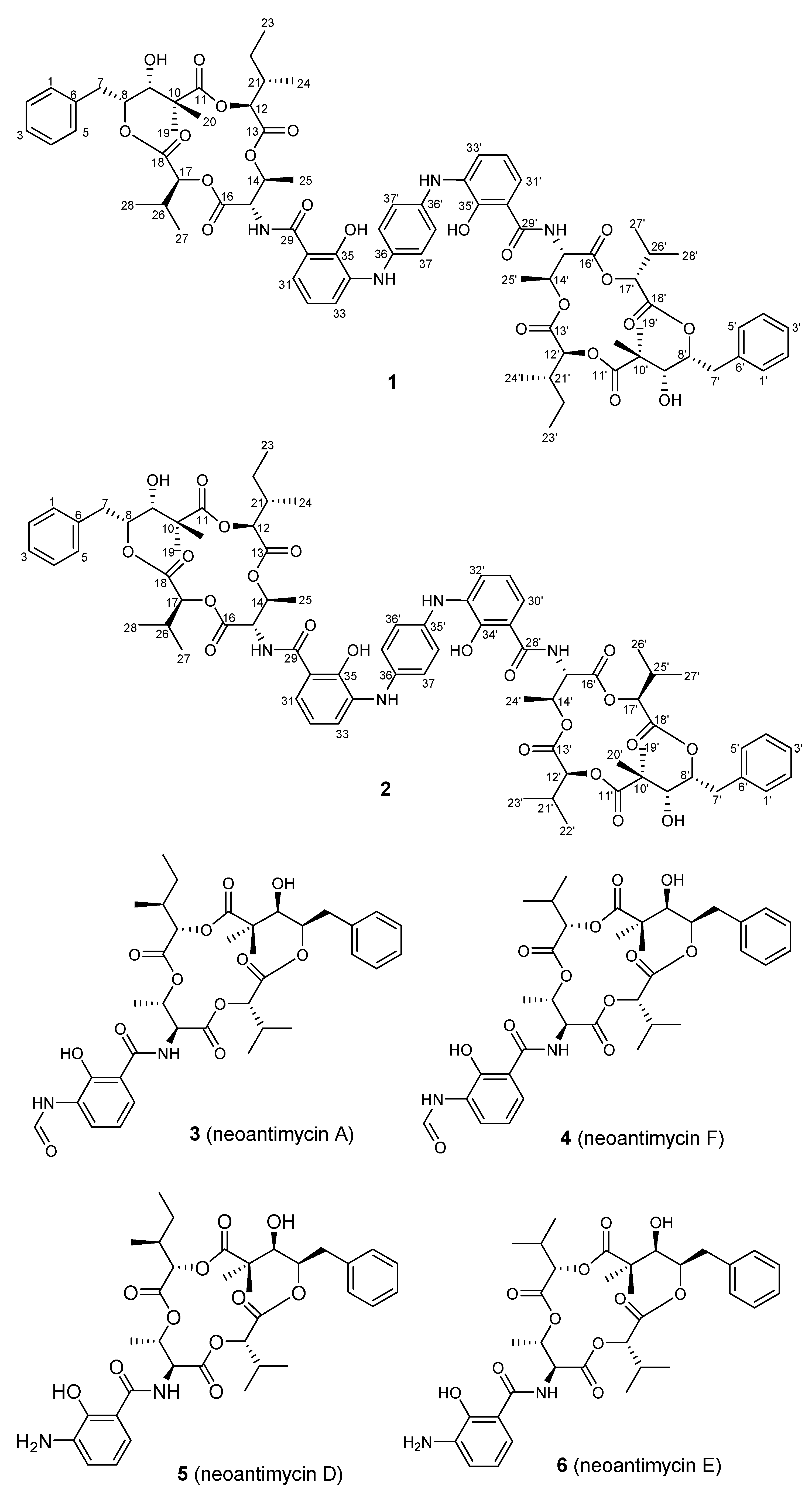
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Strain Isolation and Identification
3.3. Extraction and Isolation
3.4. Cytotoxic Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schinke, C.; Martins, T.; Queiroz, S.C.N.; Melo, I.S.; Reyes, F.G.R. Antibacterial compounds from marine bacteria, 2010–2015. J. Nat. Prod. 2017, 80, 1215–1228. [Google Scholar] [CrossRef]
- Yang, C.L.; Wang, Y.S.; Liu, C.L.; Zeng, Y.J.; Cheng, P.; Jiao, R.H.; Bao, S.X.; Huang, H.Q.; Tan, R.X.; Ge, H.M. Strepchazolins A and B: Two new alkaloids from a marine Streptomyces chartreusis NA02069. Mar. Drugs 2017, 15, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.S.; Zhang, B.; Zhu, J.; Yang, C.L.; Guo, Y.; Liu, C.L.; Liu, F.; Huang, H.; Zhao, S.; Liang, Y.; et al. Molecular basis for the final oxidative rearrangement steps in chartreusin biosynthesis. J. Am. Chem. Soc. 2018, 140, 10909–10914. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Q.; Sun, C.; Tian, X.P.; Gui, C.; Qin, X.; Zhang, H.; Ju, J. Cytotoxic kendomycins containing the carbacylic ansa scaffold from the marine-derived Verrucosispora sp. SCSIO 07399. J. Nat. Prod. 2019, 82, 3366–3371. [Google Scholar] [CrossRef]
- Jiao, F.W.; Wang, Y.S.; You, X.T.; Wei, W.; Chen, Y.; Yang, C.L.; Guo, Z.K.; Zhang, B.; Liang, Y.; Tan, R.X.; et al. An NADPH-dependent ketoreductase catalyses the tetracyclic to the pentacyclic ring rearrangement in chartreusin biosynthesis. Angew. Chem. Int. Ed. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Cui, C.; Liu, Y.; Zhou, Z.; Zhang, S.; Hu, Y.; Gu, Y.C.; Huang, H.; Ju, J. Angucycline glycosides from mangrove-derived Streptomyces diastaticus subsp. SCSIO GJ056. Mar. Drugs 2018, 16, 185. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Yang, Z.; Zhang, C.; Liu, Z.; He, J.; Liu, Q.; Zhang, T.; Ju, J.; Ma, J. Genome mining of Streptomyces atratus SCSIO ZH16: Discovery of atratumycin and identification of its biosynthetic gene cluster. Org. Lett. 2019, 21, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ding, W.; Qin, X.; Ju, J. Genome sequencing of Streptomyces olivaceus SCSIO T05 and activated production of lobophorin CR4 via metabolic engineering and genome mining. Mar. Drugs 2019, 17, 593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Song, Y.; Tang, M.C.; Li, M.; Deng, J.; Wong, N.K.; Ju, J. Genome-directed discovery of tetrahydroisoquinolines from deep-sea derived Streptomyces niveus SCSIO 3406. J. Org. Chem. 2021, 86, 11107–11116. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Tu, J.; Zhang, H.; Wei, X.; Ju, J.; Li, Q. Genome mining and metabolic profiling uncover polycyclic tetramate macrolactams from Streptomyces koyangensis SCSIO 5802. Mar. Drugs 2021, 19, 440. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, X.; Kim, S.J.; Zhang, W. Antimycin-type depsipeptides: Discovery, biosynthesis, chemical synthesis, and bioactivities. Nat. Prod. Rep. 2016, 33, 1146–1165. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zvanych, R.; Vanner, S.A.; Wang, W.; Magarvey, N.A. Chemical variation from the neoantimycin depsipeptide assembly line. Bioorg. Med. Chem. Lett. 2013, 23, 5123–5127. [Google Scholar] [CrossRef] [PubMed]
- Vanner, S.A.; Li, X.; Zvanych, R.; Torchia, J.; Sang, J.; Andrews, D.W.; Magarvey, N.A. Chemical and biosynthetic evolution of the antimycin-type depsipeptides. Mol. Biosyst. 2013, 9, 2712–2719. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, F.; Zhang, L.; Cheng, Y.; Zhu, H.; Wang, S.P.; Jiao, W.H.; Leadlay, P.F.; Zhou, Y.; Lin, H.W. Biosynthesis of depsipeptides with a 3-hydroxybenzoate moiety and selective anticancer activities involves a chorismatase. J. Biol. Chem. 2020, 295, 5509–5518. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.S.; Zhang, B.; Zhang, M.; Guo, Z.K.; Deng, X.Z.; Shi, J.; Li, W.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Rifamorpholines A-E, potential antibiotics from a locust-associated actinobacteria Amycolatopsis sp. Hca4. Org. Biomol. Chem. 2017, 15, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.K.; Wang, R.; Chen, F.X.; Liu, T.M.; Yang, M.Q. Bioactive aromatic metabolites from the sea urchin-derived actinomycete Streptomyces spectabilis strain HDa1. Phytochem. Lett. 2018, 25, 132–135. [Google Scholar] [CrossRef]
- Guo, Z.K.; Wang, R.; Chen, F.X.; Liu, T.M. Streptoxamine, an unprecedented benzoisoindole-deferoxamine hybrid from the locust-derived Streptomyces sp. HKHCa2. Fitoterapia 2018, 127, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Guo, Z.K.; Zhang, B.; Zhang, M.; Shi, J.; Li, W.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Bioactive phenazines from an earwig-associated Streptomyces sp. Chin. J. Nat. Medicines 2019, 17, 475–480. [Google Scholar] [CrossRef]
- Caglioti, L.; Misiti, D.; Mondelli, R.; Selva, A.; Arcamone, F.; Cassinelli, G. The structure of neoantimycin. Tetrahedron 1969, 25, 2193–2221. [Google Scholar] [CrossRef]
- Ma, S.Y.; Xiao, Y.S.; Zhang, B.; Shao, F.L.; Guo, Z.K.; Zhang, J.J.; Jiao, R.H.; Sun, Y.; Xu, Q.; Tan, R.X.; et al. Amycolamycins A and B, two enediyne-derived compounds from a locust-associated actinomycete. Org. Lett. 2017, 19, 6208–6211. [Google Scholar] [CrossRef] [PubMed]

| Position | δC, Type | δH, Mult. (J in Hz) | Position | δC, Type | δH, Mult. (J in Hz) |
|---|---|---|---|---|---|
| 1, 1′ | 129.2, CH | 7.21, d (6.5) | 21, 21′ | 36.0, CH | 1.96, m |
| 2, 2′ | 128.7, CH | 7.27, t (6.5) | 22, 22′ | 24.8, CH2 | 1.52, m; 1.20, m |
| 3, 3′ | 126.9, CH | 7.21, t (6.5) | 23, 23′ | 10.6, CH3 | 0.88, overlap |
| 4, 4′ | 128.7, CH | 7.27, t (6.5) | 24, 24′ | 14.3, CH3 | 0.89, overlap |
| 5, 5′ | 129.2, CH | 7.21, d (6.5) | 25, 25′ | 16.3, CH3 | 1.35, d (6.5) |
| 6, 6′ | 136.8, C | 26, 26′ | 30.7, CH | 1.80, m | |
| 7, 7′ | 40.3, CH2 | 2.94, dd (14.0, 9.6); 3.16, dd (14.0, 9.6) | 27, 27′ | 16.1, CH3 | 0.46, d (6.9) |
| 8, 8′ | 71.8, CH | 5.52, dd (9.6, 5.8) | 28, 28′ | 18.7, CH3 | 0.82, d (6.9) |
| 9, 9′ | 79.1, CH | 3.20, s | 29, 29′ | 170.8, C | |
| 10, 10′ | 45.4, C | 30, 30′ | 112.7, C | ||
| 11, 11′ | 176.9, C | 31, 31′ | 114.7, CH | 7.02, d (7.8) | |
| 12, 12′ | 75.2, CH | 4.67, d (8.3) | 32, 32′ | 118.8, CH | 6.81, t (7.8) |
| 13, 13′ | 168.2, C | 33, 33′ | 116.2, CH | 7.30, d (7.8) | |
| 14, 14′ | 72.6, CH | 5.74, dd (6.5, 2.6) | 34, 34′ | 135.0, C | |
| 15, 15′ | 55.1, CH | 5.16, dd (8.9, 2.6) | 35, 35′ | 150.2, C | |
| 16, 16′ | 168.4, C | 35-OH, 35′-OH | 12.50, brs | ||
| 17, 17′ | 76.6, CH | 5.45, d (3.5) | |||
| 18, 18′ | 168.5, C | 36, 36′ | 136.6, C | ||
| 19, 19′ | 21.9, CH3 | 1.30, s | 37, 37′ | 121.8, CH | 7.15, overlap |
| 20, 20′ | 26.9, CH3 | 1.40, s | 38, 38′ | 121.8, CH | 7.15, overlap |
| Position | δC, Type | δH, Mult. (J in Hz) | Position | δC, Type | δH, Mult. (J in Hz) |
|---|---|---|---|---|---|
| 1 | 129.2, CH | 7.21, d (6.5) | 1′ | 129.2, CH | 7.21, d (6.5) |
| 2 | 128.7, CH | 7.27, m | 2′ | 128.7, CH | 7.27, m |
| 3 | 126.9, CH | 7.21, t (6.5) | 3′ | 126.9, CH | 7.21, t (6.5) |
| 4 | 128.7, CH | 7.27, m | 4′ | 128.7, CH | 7.27, m |
| 5 | 129.2, CH | 7.21, d (6.5) | 5′ | 129.2, CH | 7.21, d (6.5) |
| 6 | 136.8, C | 6′ | 136.8, C | ||
| 7 | 40.3, CH2 | 2.94, dd (14.0,5.7); 3.16, dd (14.0,9.6) | 7′ | 40.3, CH2 | 2.94, dd (14.0, 5.7); 3.16, dd (14.0, 9.6) |
| 8 | 71.8, CH | 5.52, dd (9.6,5.8) | 8′ | 71.8, CH | 5.52, dd (9.6, 5.8) |
| 9 | 79.1, CH | 3.20, s | 9′ | 79.1, CH | 3.20, s |
| 10 | 45.4, C | 10′ | 45.4, C | ||
| 11 | 176.9, C | 11′ | 176.9, C | ||
| 12 | 75.2, CH | 4.67, d (8.3) | 12′ | 75.2, CH | 4.58, d (7.9) |
| 13 | 168.2, C | 13′ | 168.2, C | ||
| 14 | 72.6, CH | 5.74, dd (6.5,2.6) | 14′ | 72.6, CH | 5.74, dd (6.5, 2.6) |
| 15 | 55.1, CH | 5.15, dd (8.9,2.6) | 15′ | 55.1, CH | 5.15, dd (8.9, 2.6) |
| 16 | 168.4, C | 16′ | 168.4, C | ||
| 17 | 76.6, CH | 5.45, d (3.5) | 17′ | 76.6, CH | 5.45, d (3.5) |
| 18 | 168.5, C | 18′ | 168.5, C | ||
| 19 | 21.9, CH3 | 1.30, s | 19′ | 21.9, CH3 | 1.30, s |
| 20 | 26.9, CH3 | 1.41, d (2.7) | 20′ | 26.9, CH3 | 1.41, d (2.7) |
| 21 | 36.0, CH | 1.97, m | 21′ | 36.0, CH | 1.97, m |
| 22 | 24.8, CH2 | 1.51, m; 1.20, m | 22′ | 10.6, CH3 | 0.92, d (6.8) |
| 23 | 10.6, CH3 | 0.88, m | 23′ | 14.3, CH3 | 0.97, d (6.8) |
| 24 | 14.3, CH3 | 0.89, m | 24′ | 16.3, CH3 | 1.35, d (6.5) |
| 25 | 16.3, CH3 | 1.35, d (6.5) | 25′ | 30.7, CH | 1.81, m |
| 26 | 30.7, CH | 1.81, m | 26′ | 16.1, CH3 | 0.46, d (6.8) |
| 27 | 16.1, CH3 | 0.46, d (6.8) | 27′ | 18.7, CH3 | 0.82, d (6.8) |
| 28 | 18.7, CH3 | 0.82, d (6.8) | 28′ | 170.8, C | |
| 29 | 170.8, C | 29′ | 112.7, C | ||
| 30 | 112.7, C | 30′ | 114.7, CH | 7.01, m | |
| 31 | 114.7, CH | 7.01, m | 31′ | 118.8, CH | 6.81, t (7.8) |
| 32 | 118.8, CH | 6.81, t (7.8) | 32′ | 116.2, CH | 7.30, d (7.8) |
| 33 | 116.2, CH | 7.30, d (7.8) | 33′ | 135.0, C | |
| 34 | 135.0, C | 34′ | 150.2, C | ||
| 35 | 150.2, C | 34′-OH | 12.50, brs | ||
| 35-OH | 12.50, brs | 35′ | 136.6, C | ||
| 36 | 136.6, C | 36′ | 121.8, CH | 7.15, s | |
| 37 | 121.8, CH | 7.15, s | 37′ | 121.8, CH | 7.15, s |
| 38 | 121.8, CH | 7.15, s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Ma, S.; Khan, S.; Zhu, H.; Zhang, B.; Zhang, S.; Jiao, R. Zhaoshumycins A and B, Two Unprecedented Antimycin-Type Depsipeptides Produced by the Marine-Derived Streptomyces sp. ITBB-ZKa6. Mar. Drugs 2021, 19, 624. https://doi.org/10.3390/md19110624
Guo Z, Ma S, Khan S, Zhu H, Zhang B, Zhang S, Jiao R. Zhaoshumycins A and B, Two Unprecedented Antimycin-Type Depsipeptides Produced by the Marine-Derived Streptomyces sp. ITBB-ZKa6. Marine Drugs. 2021; 19(11):624. https://doi.org/10.3390/md19110624
Chicago/Turabian StyleGuo, Zhikai, Shiying Ma, Salman Khan, Hongjie Zhu, Bo Zhang, Shiqing Zhang, and Ruihua Jiao. 2021. "Zhaoshumycins A and B, Two Unprecedented Antimycin-Type Depsipeptides Produced by the Marine-Derived Streptomyces sp. ITBB-ZKa6" Marine Drugs 19, no. 11: 624. https://doi.org/10.3390/md19110624
APA StyleGuo, Z., Ma, S., Khan, S., Zhu, H., Zhang, B., Zhang, S., & Jiao, R. (2021). Zhaoshumycins A and B, Two Unprecedented Antimycin-Type Depsipeptides Produced by the Marine-Derived Streptomyces sp. ITBB-ZKa6. Marine Drugs, 19(11), 624. https://doi.org/10.3390/md19110624





