Cartilage Acidic Protein a Novel Therapeutic Factor to Improve Skin Damage Repair?
Abstract
1. Introduction
2. Results
2.1. SJD.1 Cell Culture
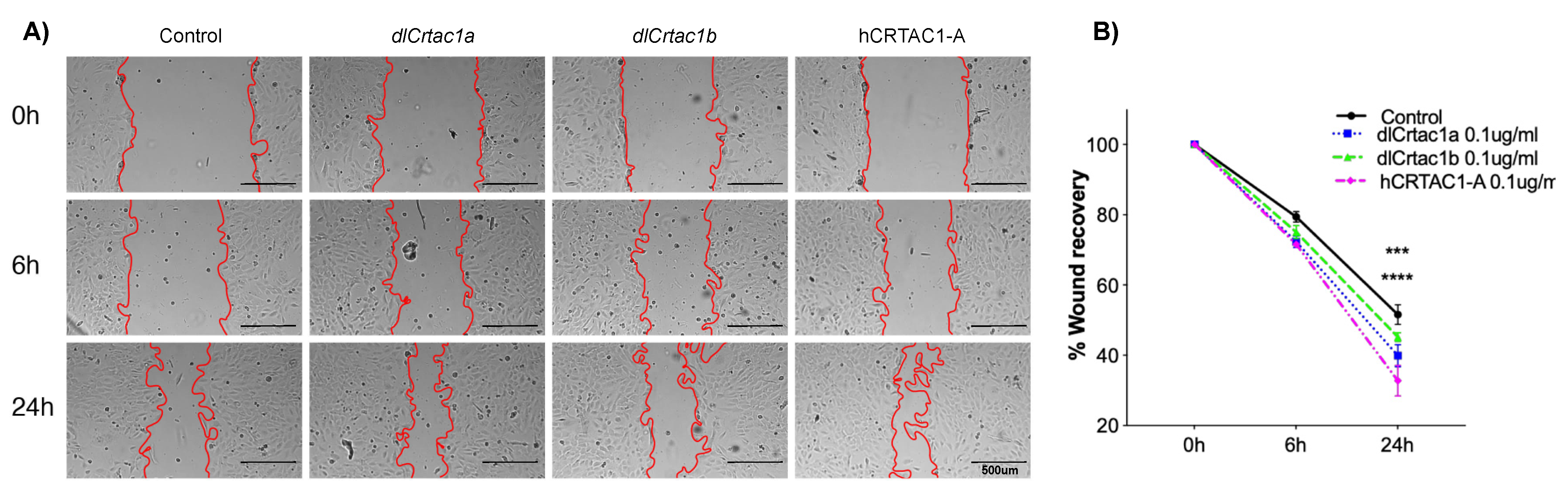
2.2. Classic Scratch Assay
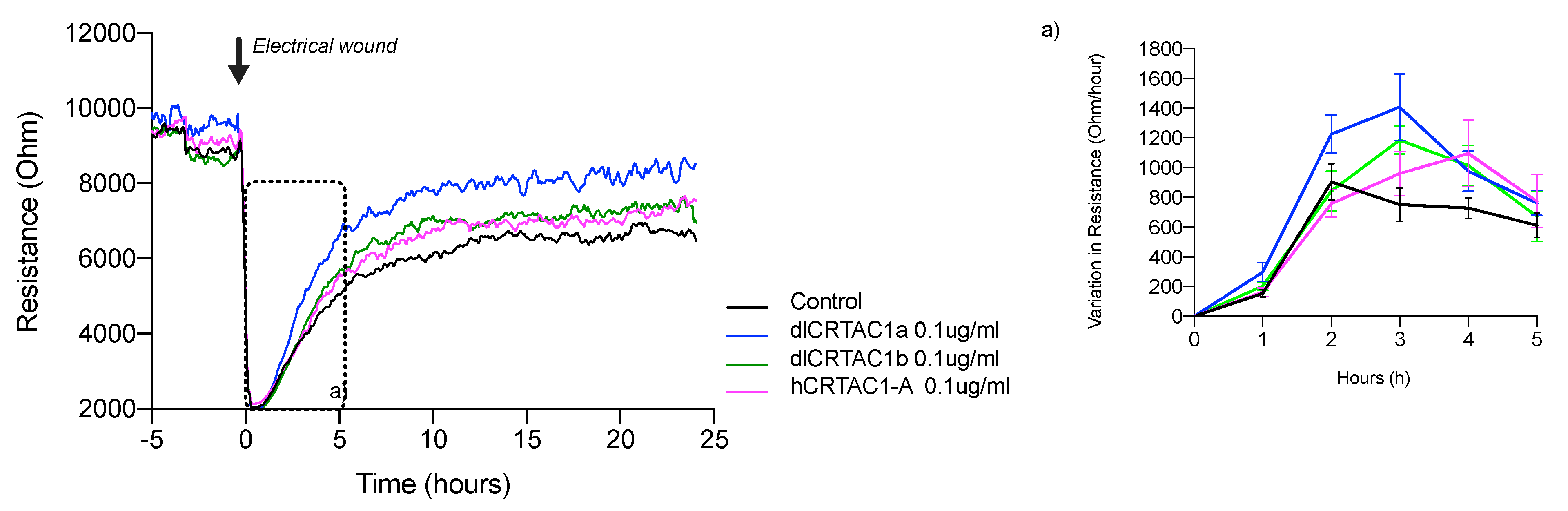
2.3. Electrical Cell Impedance System (ECIS) Analysis
2.4. Cell Cytoskeleton Structures during Scratch Closure
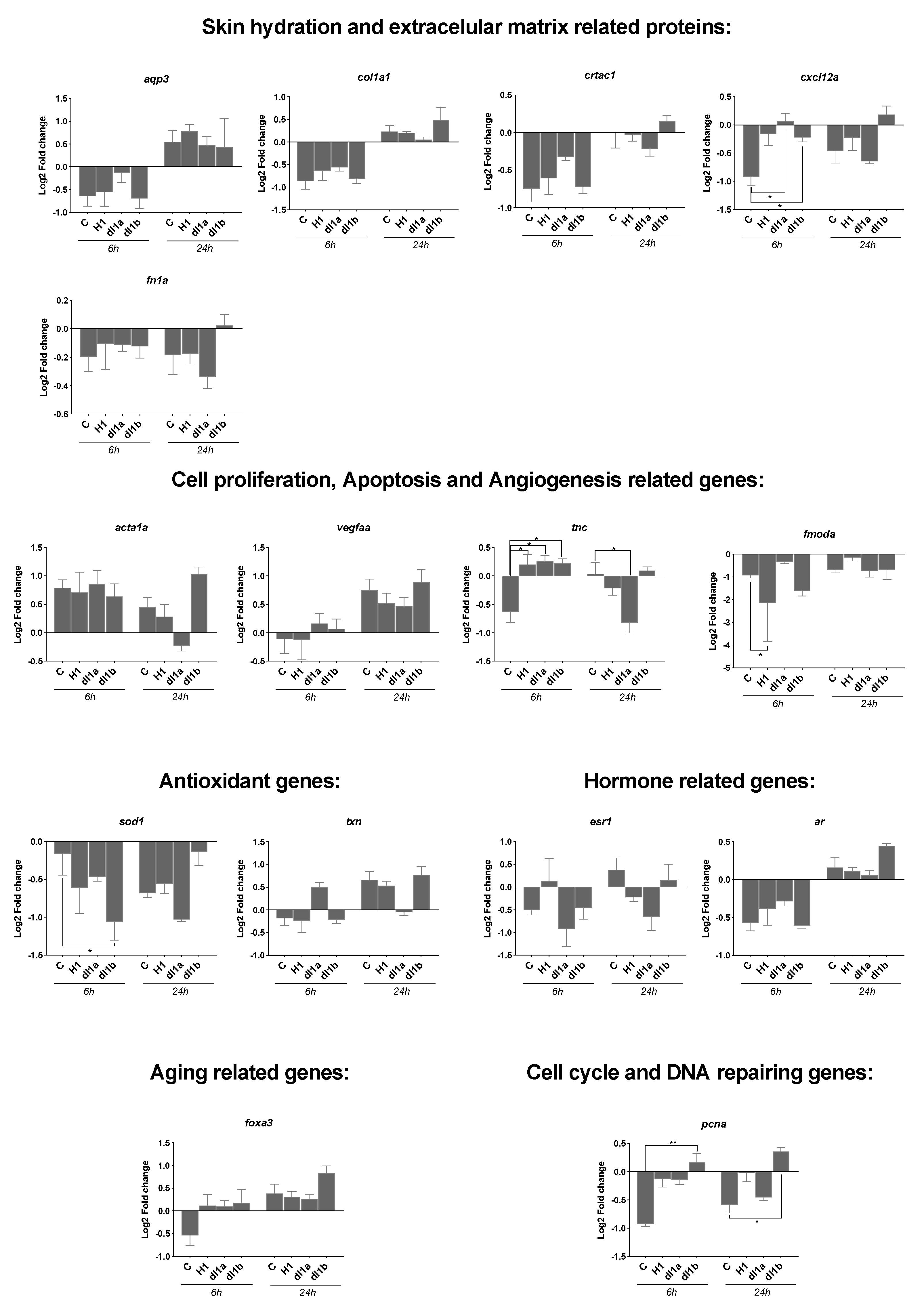
2.5. Gene Expression of Genes Associated with Wound Healing
2.5.1. Effect of Vertebrate CRTAC1 on Scratch Recovery/Cell Migration
2.5.2. Effect of Time on Gene Expression during Scratch Recovery/Cell Migration
3. Discussion
4. Materials and Methods
4.1. Culture of Zebrafish SJD.1 Primary Fibroblast Cells
4.2. Cell Growth Assay
4.3. Scratch Assay
4.4. Electric Cell-Substrate Impedance Sensing (ECIS) Assays
4.5. Cell Cytoskeleton Immunofluorescence Assay (IF)
4.6. RNA Extraction and cDNA Synthesis
4.7. Gene Expression by Quantitative PCR
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.L. Human Skin Is the Largest Epithelial Surface for Interaction with Microbes. J. Investig. Dermatol. 2017, 137, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.M.; Glatz, M.; Proksch, E. Optimal Support of Wound Healing: New Insights. Dermatology 2019, 236, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 8, 407–408, 410–412. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Griffin, M.F.; des Jardins-Park, H.E.; Mascharak, S.; Borrelli, M.R.; Longaker, M.T. Understanding the impact of fibroblast heterogeneity on skin fibrosis. DMM Dis. Model. Mech. 2020, 13, dmm044164. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Caplan, A.I. Chapter 4 Fibroblasts-A Diverse Population at the Center of It All. Int. Rev. Cell Mol. Biol. 2009, 276, 161–214. [Google Scholar]
- Ingerslev, H.C.; Ossum, C.G.; Lindenstrøm, T.; Engelbrecht Nielsen, M. Fibroblasts Express Immune Relevant Genes and Are Important Sentinel Cells during Tissue Damage in Rainbow Trout (Oncorhynchus mykiss). PLoS ONE 2010, 5, e9304. [Google Scholar] [CrossRef][Green Version]
- McAnulty, R.J. Fibroblasts and myofibroblasts: Their source, function and role in disease. Int. J. Biochem. Cell Biol. 2007, 39, 666–671. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef] [PubMed]
- Togo, S.; Holz, O.; Liu, X.; Sugiura, H.; Kamio, K.; Wang, X.; Kawasaki, S.; Ann, Y.; Fredriksson, K.; Skold, C.M.; et al. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am. J. Respir. Crit. Care Med. 2008, 178, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Bartling, B.; Hofmann, H.S. Reduced proliferation capacity of lung cells in chronic obstructive pulmonary disease. Z. Gerontol. Geriatr. 2019, 52, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P.; Sage, E.H. Matricellular proteins: Extracellular modulators of cell function. Curr. Opin. Cell Biol. 2002, 14, 608–616. [Google Scholar] [CrossRef]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. The role of the extracellular matrix components in cutaneous wound healing. Biomed. Res. Int. 2014, 747584. [Google Scholar] [CrossRef]
- Golz, S.; Summer, H.; Geerts, A.; Brueggemeier, U.; Albrecht-Kuepper, B.; Klein, M.; Bruggemeier, U.; Albrecht-Kupper, B. CRTAC as a Biomarker, Therapeutic and Diagnostic Target. Patent WO2008046511, 24 April 2008. [Google Scholar]
- Ji, Y.; Rong, X.; Li, D.; Cai, L.; Rao, J.; Lu, Y. Inhibition of Cartilage Acidic Protein 1 Reduces Ultraviolet B Irradiation Induced-Apoptosis through P38 Mitogen-Activated Protein Kinase and Jun Amino-Terminal Kinase Pathways. Cell. Physiol. Biochem. 2016, 39, 2275–2286. [Google Scholar] [CrossRef]
- Sun, Y.; Rong, X.; Li, D.; Lu, Y.; Ji, Y. NF-κB/Cartilage Acidic Protein 1 Promotes Ultraviolet B Irradiation-Induced Apoptosis of Human Lens Epithelial Cells. DNA Cell Biol. 2020, 39, 513–521. [Google Scholar] [CrossRef]
- Steck, E.; Braun, J.; Pelttari, K.; Kadel, S.; Kalbacher, H.; Richter, W. Chondrocyte secreted CRTAC1: A glycosylated extracellular matrix molecule of human articular cartilage. Matrix Biol. 2007, 26, 30–41. [Google Scholar] [CrossRef]
- Jaillon, O.; Aury, J.M.; Brunet, F.; Petit, J.L.; Stange-Thomann, N.; Mauceli, E.; Bouneau, L.; Fischer, C.; Ozouf-Costaz, C.; Bernot, A.; et al. Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature 2004, 431, 946–957. [Google Scholar] [CrossRef]
- Anjos, L.; Morgado, I.; Guerreiro, M.; Cardoso, J.C.R.; Melo, E.P.; Power, D.M. Cartilage acidic protein 1, a new member of the beta-propeller protein family with amyloid propensity. Proteins Struct. Funct. Bioinforma. 2017, 85, 242–255. [Google Scholar] [CrossRef]
- Letsiou, S.; Félix, R.C.; Cardoso, J.C.R.; Anjos, L.; Mestre, A.L.; Gomes, H.L.; Power, D.M. Cartilage acidic protein 1 promotes increased cell viability, cell proliferation and energy metabolism in primary human dermal fibroblasts. Biochimie 2020, 171–172, 72–78. [Google Scholar] [CrossRef]
- Anjos, L.; Gomes, A.S.; Melo, E.P.; Canário, A.V.; Power, D.M. Cartilage Acidic Protein 2 a hyperthermostable, high affinity calcium-binding protein. Biochim. Biophys. Acta-Proteins Proteom. 2013, 1834, 642–650. [Google Scholar] [CrossRef]
- Paw, B.H.; Zon, L.I. Primary fibroblast cell culture. Methods Cell Biol. 1999, 59, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Rakus, K.; Adamek, M.; Mojżesz, M.; Podlasz, P.; Chmielewska-Krzesińska, M.; Naumowicz, K.; Kasica-Jarosz, N.; Kłak, K.; Rakers, S.; Way, K.; et al. Evaluation of zebrafish (Danio rerio) as an animal model for the viral infections of fish. J. Fish Dis. 2019, 42, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Cheuk, W.K.; Chan, K.M. Differential regulation of zebrafish metallothionein-II (zMT-II) gene transcription in ZFL and SJD cell lines by metal ions. Aquat. Toxicol. 2009, 91, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Power, D.M. Skin and scale regeneration after mechanical damage in a teleost. Mol. Immunol. 2018, 95, 73–82. [Google Scholar] [CrossRef]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive Assessment of Nile Tilapia Skin (Oreochromis niloticus) Collagen Hydrogels for Wound Dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef]
- Lima-Jr, E.M.; de Moraes Filho, M.O.; Costa, B.A.; Fechine, F.V.; de Moraes, M.E.A.; Silva-Junior, F.R.; Soares, M.F.A.d.N.; Rocha, M.B.S.; Leontsinis, C.M.P. Innovative treatment using tilapia skin as a xenograft for partial thickness burns after a gunpowder explosion. J. Surg. Case Rep. 2019, 2019, rjz181. [Google Scholar] [CrossRef]
- Gottrup, F.; Apelqvist, J.; Price, P. Outcomes in controlled and comparative studies on nonhealing wounds: Recommendations to improve the quality of evidence in wound management. J. Wound Care 2010, 19, 239–268. [Google Scholar] [CrossRef]
- Fiakos, G.; Kuang, Z.; Lo, E. Improved skin regeneration with acellular fish skin grafts. Eng. Regen. 2020, 1, 95–101. [Google Scholar] [CrossRef]
- Patel, M.; Lantis, J.C. Chronic Wound Care Management and Research Dovepress Fish skin acellular dermal matrix: Potential in the treatment of chronic wounds. Chronic Wound Care Manag. Res. 2019, 6, 59–70. [Google Scholar] [CrossRef]
- Rakers, S.; Gebert, M.; Uppalapati, S.; Meyer, W.; Maderson, P.; Sell, A.F.; Kruse, C.; Paus, R. “Fish matters”: The relevance of fish skin biology to investigative dermatology. Exp. Dermatol. 2010, 19, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Gemberling, M.; Bailey, T.J.; Hyde, D.R.; Poss, K.D. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013, 29, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult zebrafish as a model system for cutaneous wound-healing research. J. Investig. Dermatol. 2013, 133, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Abe, G.; Hayashi, T.; Yoshida, K.; Yoshida, T.; Kudoh, H.; Sakamoto, J.; Konishi, A.; Kamei, Y.; Takeuchi, T.; Tamura, K.; et al. Insights regarding skin regeneration in non-amniote vertebrates: Skin regeneration without scar formation and potential step-up to a higher level of regeneration. Semin. Cell Dev. Biol. 2020, 100, 109–121. [Google Scholar] [CrossRef]
- Sivalingam, N.; Seepoo, A.; Gani, T.; Selvam, S.; Sait, S. Zebrafish fin-derived fibroblast cell line: A model for in vitro wound healing. J. Fish Dis. 2019, 42, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Manneville, S. Microtubules in cell migration. Annu. Rev. Cell Dev. Biol. 2013, 29, 471–499. [Google Scholar] [CrossRef]
- Etienne-Manneville, S. Actin and microtubules in cell motility: Which one is in control? Traffic 2004, 5, 470–477. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Simpson, K.J.; Selfors, L.M.; Bui, J.; Reynolds, A.; Leake, D.; Khvorova, A.; Brugge, J.S. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat. Cell Biol. 2008, 10, 1027–1038. [Google Scholar] [CrossRef]
- Redruello, B.; Louro, B.; Anjos, L.; Silva, N.; Greenwell, R.S.; Canario, A.V.; Power, D.M. CRTAC1 homolog proteins are conserved from cyanobacteria to man and secreted by the teleost fish pituitary gland. Gene 2010, 456, 1–14. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; Plastino, J. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 2014, 94, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Case, L.B.; Waterman, C.M. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat. Cell Biol. 2015, 17, 955–963. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Small, J.V.; Rinnerthaler, G.; Hinssen, H. Organization of actin meshworks in cultured cells: The leading edge. Cold Spring Harb. Symp. Quant. Biol. 1982, 46 Pt 2, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Valluru, M.; Staton, C.A.; Reed, M.W.R.; Brown, N.J. Transforming growth factor-β and endoglin signaling orchestrate wound healing. Front. Physiol. 2011, 2, 89. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, S.L.; Rajapaksha, D.C.; Nikapitiya, C.; Oh, C.; Lee, K.A.; Kang, D.H.; De Zoysa, M. Spirulina maxima derived marine pectin promotes the in vitro and in vivo regeneration and wound healing in zebrafish. Fish Shellfish Immunol. 2020, 107, 414–425. [Google Scholar] [CrossRef]
- Broughton, G., 2nd; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef]
- Abkowitz, J.L.; Robinson, A.E.; Kale, S.; Long, M.W.; Chen, J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood 2003, 102, 1249–1253. [Google Scholar] [CrossRef]
- Nogare, D.D.; Somers, K.; Rao, S.; Matsuda, M.; Reichman-Fried, M.; Raz, E.; Chitnis, A.B. Leading and trailing cells cooperate in collective migration of the zebrafish posterior lateral line primordium. Development 2014, 141, 3188–3196. [Google Scholar] [CrossRef]
- Meyen, D.; Tarbashevich, K.; Banisch, T.U.; Wittwer, C.; Reichman-Fried, M.; Maugis, B.; Grimaldi, C.; Messerschmidt, E.M.; Raz, E. Dynamic filopodia are required for chemokine-dependent intracellular polarization during guided cell migration in vivo. Elife 2015, 4, e05279. [Google Scholar] [CrossRef]
- Neelathi, U.M.; Nogare, D.D.; Chitnis, A.B. Cxcl12a induces snail1b expression to initiate collective migration and sequential Fgf-dependent neuromast formation in the zebrafish posterior lateral line primordium. Dev. 2018, 145, dev162453. [Google Scholar] [CrossRef]
- Chen, S.; Birk, D.E. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013, 280, 2120–2137. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; James, A.W.; Li, C.; Jiang, W.; Wang, J.Z.; Chang, G.X.; Lee, K.S.; Chen, F.; Berthiaume, E.A.; Chen, Y.; et al. Fibromodulin reduces scar formation in adult cutaneous wounds by eliciting a fetal-like phenotype. Signal Transduct. Target. Ther. 2017, 2, 17050. [Google Scholar] [CrossRef] [PubMed]
- Wegener, J.; Keese, C.R.; Giaever, I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp. Cell Res. 2000, 259, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with image. J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]

| Name | Sequence (5′-3′) | Annealing Temperature (°C) | Eff. (%) | R2 |
|---|---|---|---|---|
| Superoxide dismutase 1 | GCCGTTTGTGTGCTTAAAGG CCTGGAGTAAGGCCAGTAAT | 60 | 99.4 | 0.99 |
| Thioredoxin | CCTGACTATTCTAATGTGGTC GCTTCTTCCCGTTCTTGTAG | 60 | 93.2 | 0.99 |
| Vascular endothelial growth factor Aa | AGCTGCTGGTAGACATCATC TTCGAGCGCCTCATCATTAC | 62 | 104.9 | 1.00 |
| Actin alpha1, skeletal muscle a | CCACGATGTACCCTGGTATT GCCGATCCAGACTGAGTATT | 62 | 104.1 | 1.00 |
| Tenascin C | CCTGGGACTGAATATGGAATG GAAGGTCTTTGGGAGGATCA | 62 | 101.7 | 0.99 |
| Forkhead box A3 | AGTCCAATTCGGGCAAAG CGTTTCTATGGCAGGAAGAG | 62 | 106.8 | 1.00 |
| Chemokine (C-X-C motif) ligand 12a | CAACACAGTCCCACAGAGAA GGGTTAATGCACACCTCCTT | 60 | 96.3 | 0.99 |
| Proliferating cell nuclear antigen | GACTCCTCTCATGTGTCTCT GCGTCAGCATTGTCTTCA | 62 | 90.1 | 0.99 |
| Collagen, type I, alpha 1a | CCTCCCAGAACATTACATACC CCCTCTGCTCTGATCTCAAT | 60 | 98.3 | 0.99 |
| Fibronectin 1a | ACCTCAGGTGCCTCCTATAA AGCTCCTGGAACGCTATTTC | 62 | 106.6 | 1.00 |
| Fibromodulin a | ACCTTCGTCTCAACCACAATA TCAGCCCAACACCAATATCC | 62 | 105.4 | 0.99 |
| Aquaporin 3a | CTTCACAGCCAGGGATTATTG CTTTCTTATCTCGTGCCTCTC | 60 | 98.7 | 0.99 |
| Cartilage acidic protein 1a | CGGGAGCCACAATAACAGAT GAGCCCTGGTTGACCTTAAA | 60 | 105.3 | 0.99 |
| Estrogen receptor1 | TACGCCTCTGGATATCATTAC TGGTCGCTGGACAAACATAG | 60 | 93.2 | 0.99 |
| Androgen receptor 1 | CTCCTCCTGTTTAGCGTCAT GTTGGTCTTCCTGCCATAGT | 60 | 93.3 | 0.99 |
| Ribosomal protein S18 | TGACGGAAGGGCACCACCAG AATCGCTCCACCAACTAAGAACGC | 58 | 96.9 | 0.99 |
| Elongation factor 1 alpha | GTCCGTTCTTAGAGATACCA GACACAGAGACTTCATCAAG | 58 | 98.5 | 1.00 |
| 0 h vs. 6 h | 0 h vs. 24 h | 6 h vs. 24 h | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | C | H1 | dl1a | dl1b | C | H1 | dl1a | dl1b | C | H1 | dl1a | dl1b | |
| Gene | |||||||||||||
| aqp3 | - | - | - | - | - | - | - | - | - | * ↑ | - | - | |
| col1a1a | *↓ | - | - | * ↓ | - | - | * ↑ | - | **** ↑ | ** ↑ | - | **** ↑ | |
| cxcl12a | - | - | - | - | - | - | - | * ↑ | - | - | - | - | |
| crtac1a | - | - | - | - | - | - | - | * ↑ | * ↑ | - | - | - | |
| fn1a | - | - | - | - | - | - | - | - | - | - | - | - | |
| vegfaa | - | - | - | - | * ↑ | - | - | * ↑ | * ↑ | - | - | - | |
| tnc | - | - | - | - | - | - | - | - | - | - | ** ↓ | - | |
| acta1a | - | * ↑ | - | - | - | - | - | - | - | * ↑ | ** ↓ | - | |
| fmoda | - | *** ↓ | - | ** ↓ | - | - | - | - | - | **** ↑ | - | - | |
| txn | - | - | - | - | - | - | - | - | - | - | - | - | |
| sod1 | - | - | - | ** ↓ | * ↓ | - | ** ↓ | - | - | - | - | * ↑ | |
| esr1 | - | - | - | - | - | - | - | - | - | - | - | - | |
| ar | - | - | - | - | - | - | - | ** ↑ | ** ↑ | - | - | **** ↑ | |
| foxa3 | - | - | - | - | ** ↑ | * ↑ | * ↑ | *** ↑ | ** ↑ | - | - | - | |
| pcna | - | - | - | * ↑ | - | - | - | ** ↑ | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Félix, R.C.; Anjos, L.; Costa, R.A.; Letsiou, S.; Power, D.M. Cartilage Acidic Protein a Novel Therapeutic Factor to Improve Skin Damage Repair? Mar. Drugs 2021, 19, 541. https://doi.org/10.3390/md19100541
Félix RC, Anjos L, Costa RA, Letsiou S, Power DM. Cartilage Acidic Protein a Novel Therapeutic Factor to Improve Skin Damage Repair? Marine Drugs. 2021; 19(10):541. https://doi.org/10.3390/md19100541
Chicago/Turabian StyleFélix, Rute Castelo, Liliana Anjos, Rita Alves Costa, Sophia Letsiou, and Deborah Mary Power. 2021. "Cartilage Acidic Protein a Novel Therapeutic Factor to Improve Skin Damage Repair?" Marine Drugs 19, no. 10: 541. https://doi.org/10.3390/md19100541
APA StyleFélix, R. C., Anjos, L., Costa, R. A., Letsiou, S., & Power, D. M. (2021). Cartilage Acidic Protein a Novel Therapeutic Factor to Improve Skin Damage Repair? Marine Drugs, 19(10), 541. https://doi.org/10.3390/md19100541







