Combined Anticancer Effect of Sulfated Laminaran from the Brown Alga Alaria angusta and Polyhydroxysteroid Glycosides from the Starfish Protoreaster lincki on 3D Colorectal Carcinoma HCT 116 Cell Line
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation, Modification of Laminaran from A. angusta, and Structural Characteristics of Sulfated Laminaran
2.2. The Polyhydroxysteroid Glycosides from the Starfish P. lincki
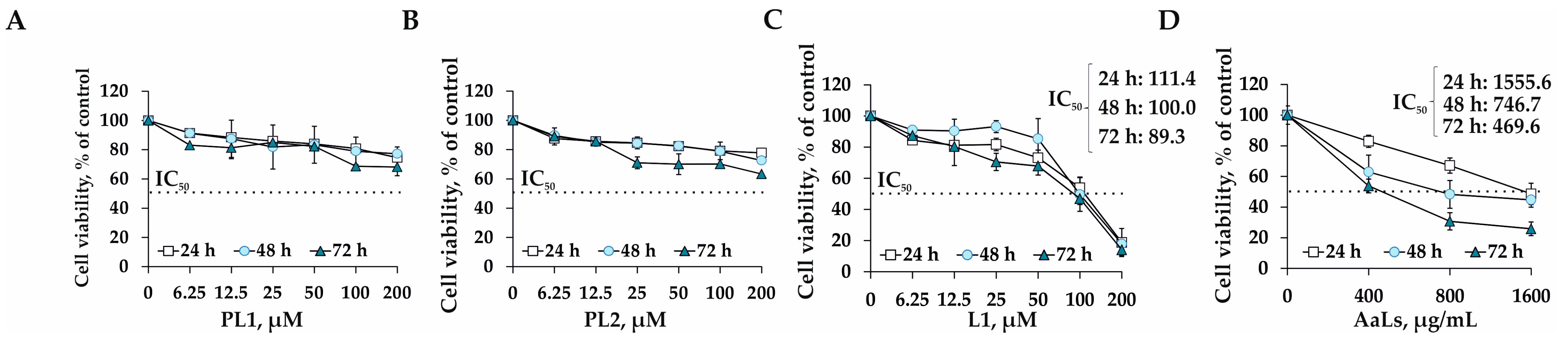
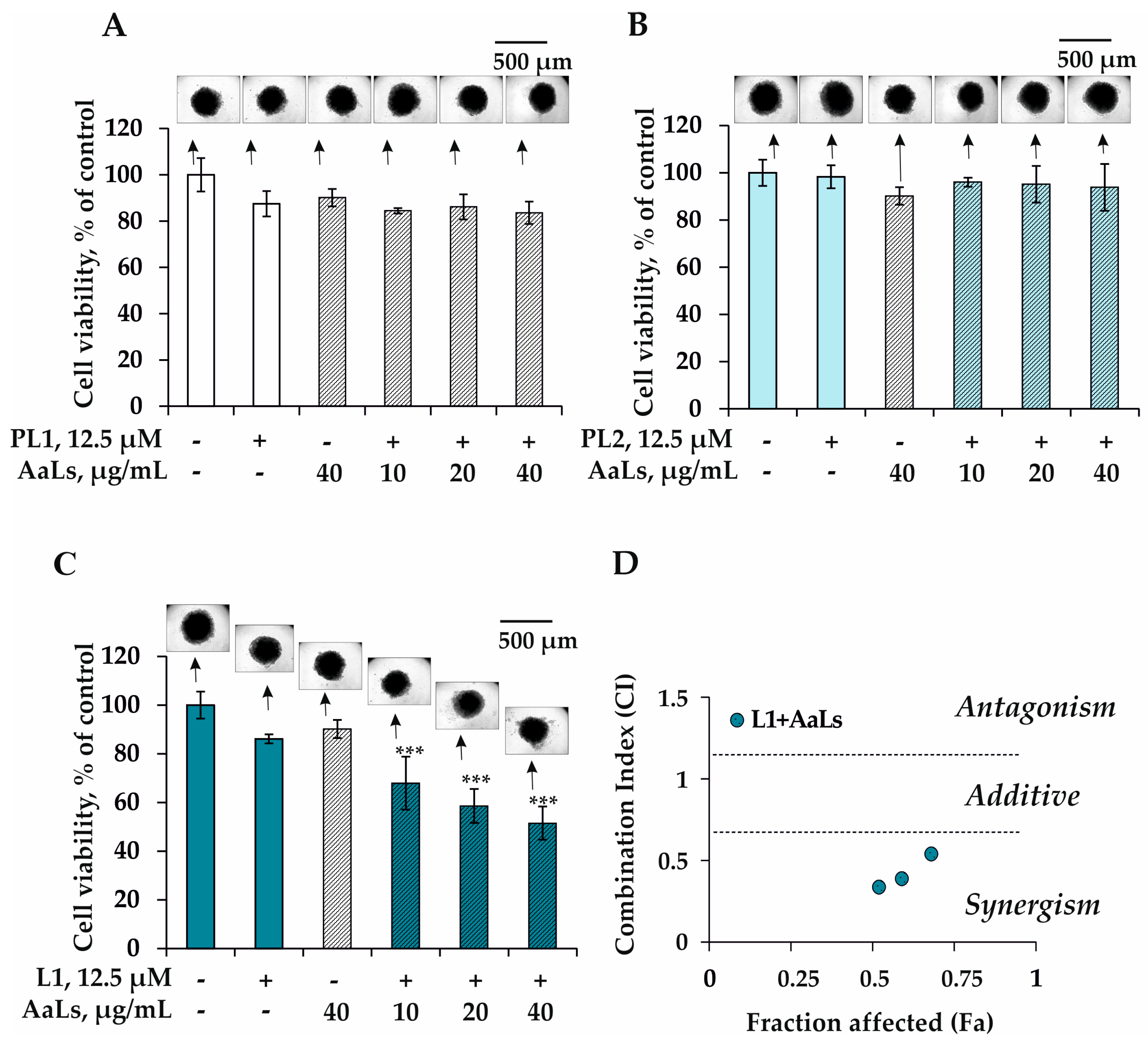
2.3. The Individual and Combined Effects of Sulfated Laminaran and Polyxydroxysteroid Glycosides on Cell Viability and Proliferation of 3D HCT 116 Spheroids
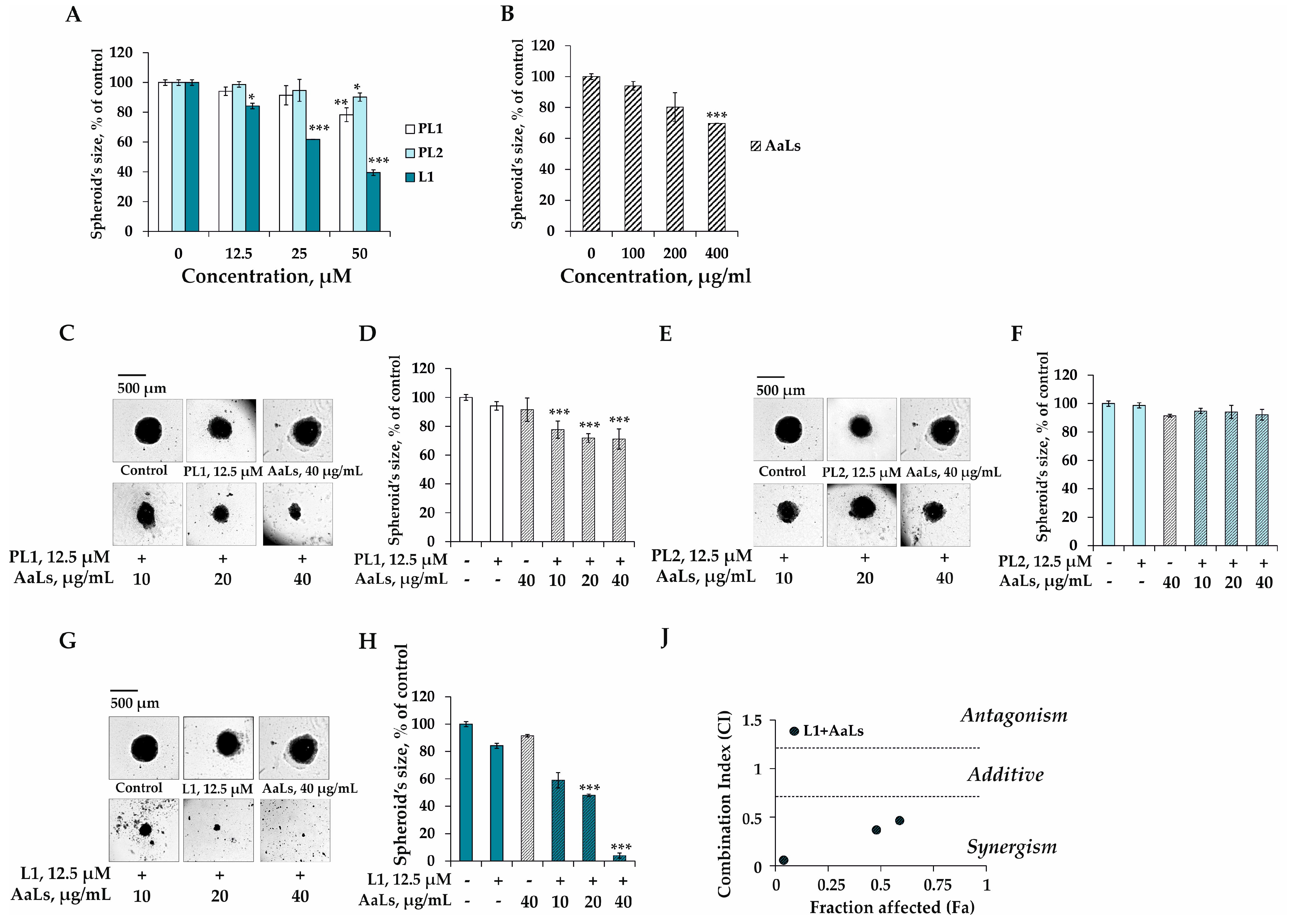
2.4. The Individual and Combined Effects of Sulfated Laminaran and Polyhydroxysteroid Glycosides on Colony Formation of 3D HCT 116 Spheroids
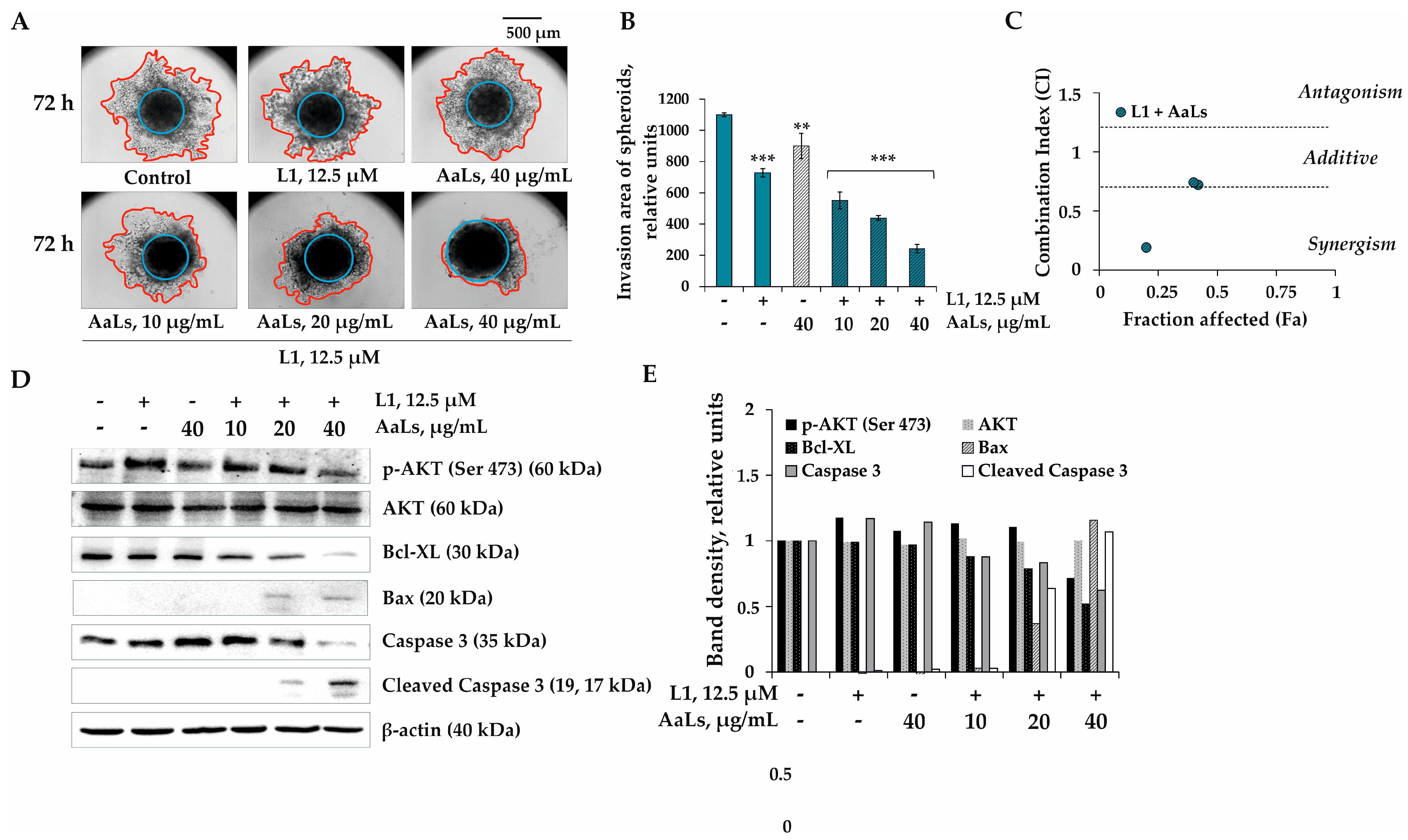
2.5. The Individual and Combined Effects of Sulfated Laminaran and Polyhydroxysteroid Glycosides on Invasion of 3D HCT 116 Spheroids
3. Materials and Methods
3.1. Laminaran and Its Sulfated Derivative
3.1.1. Brown Alga
3.1.2. Isolation of Laminaran from A. angusta
3.1.3. The Sulfation of Laminaran from A. angusta
3.1.4. The Structural Characteristics of Sulfated Laminaran
3.2. Polyhydroxysteroid Glycosides from the Starfish P. lincki
3.2.1. Starfish
3.2.2. Polyhydroxysteroid Glycosides Isolation
3.3. Preparation of Compounds for Investigations of In Vitro Anticancer Activity
3.4. In Vitro Anticancer Activity
3.4.1. Cell Lines and Culture Conditions
3.4.2. Formation of Spheroids (3D Cell Culture) by Liquid Overlay Technique (LOT)
3.4.3. Determination of Cytostatic Activity by MTS Method
3.4.4. Determination of Colony-Inhibiting Activity by Soft Agar Assay
3.4.5. Determination of Anti-Invasive Activity by 3D Spheroids Invasion Assay
3.4.6. Western Blotting Assay
3.4.7. Combination Index (CI) Calculation
3.4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baselga, J.; Bhardwaj, N.; Cantley, L.C.; DeMatteo, R.; DuBois, R.N.; Foti, M.; Gapstur, S.M.; Hahn, W.C.; Helman, L.J.; Jensen, R.A.; et al. AACR Cancer Progress Report 2015. Clin. Cancer Res. 2015, 21, S1–S128. [Google Scholar] [CrossRef] [Green Version]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal carcinoma: A general overview and future perspectives in colorectal cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretzsch, E.; Bösch, F.; Neumann, J.; Ganschow, P.; Bazhin, A.; Guba, M.; Werner, J.; Angele, M. Mechanisms of metastasis in colorectal cancer and metastatic organotropism: Hematogenous versus peritoneal spread. J. Oncol. 2019, 2019, 7407190. [Google Scholar] [CrossRef]
- Piawah, S.; Venook, A.P. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 2019, 125, 4139–4147. [Google Scholar] [CrossRef]
- Chen, S.; Lahav, G. Two is better than one; toward a rational design of combinatorial therapy. Curr. Opin. Struct. Biol. 2016, 41, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Bayat, M.R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [Green Version]
- Yap, T.A.; Omlin, A.; de Bono, J.S. Development of therapeutic combinations targeting major cancer signaling pathways. J. Clin. Oncol. 2013, 31, 1592–1605. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Belik, A.A.; Kusaykin, M.I.; Malyarenko, O.S.; Zvyagintseva, T.N.; Ermakova, S.P. Laminarans and 1,3-β-D-glucanases. Int. J. Biol. Macromol. 2020, 163, 1010–1025. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Song, G.; Lee, J.-Y.; Hong, T.; Chang, M.-J.; Lim, W. Laminarin-derived from brown algae suppresses the growth of ovarian cancer cells via mitochondrial dysfunction and ER stress. Mar. Drugs 2020, 18, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.K.; Kim, I.H.; Kim, J.; Nam, T.J. Induction of apoptosis by laminarin, regulating the insulin-like growth factor-IR signaling pathways in HT-29 human colon cells. Int. J. Mol. Med. 2012, 30, 734–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.B.; Ji, C.F.; Zhang, H. Laminarin induces apoptosis of human colon cancer LOVO cells through a mitochondrial pathway. Molecules 2012, 17, 9947–9960. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.F.; Ji, Y.B.; Meng, D.Y. Sulfated modification and anti-tumor activity of laminarin. Exp. Ther. Med. 2013, 6, 1259–1264. [Google Scholar] [CrossRef] [Green Version]
- Malyarenko, O.S.; Usoltseva, R.V.; Shevchenko, N.M.; Isakov, V.V.; Zvyagintseva, T.N.; Ermakova, S.P. In vitro anticancer activity of the laminarans from Far Eastern brown seaweeds and their sulfated derivatives. J. Appl. Phycol. 2017, 29, 543–553. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Usoltseva, R.V.; Silchenko, A.S.; Ermakova, S.P. Aminated laminaran from brown alga Saccharina cichorioides: Synthesis, structure, anticancer, and radiosensitizing potential in vitro. Carbohydr. Polym. 2020, 250, 117007. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, O.S.; Usoltseva, R.V.; Zvyagintseva, T.N.; Ermakova. Laminaran from brown alga Dictyota dichotoma and its sulfated derivative as radioprotectors and radiosensitizers in melanoma therapy. Carbohydr. Polym. 2019, 206, 539–547. [Google Scholar] [CrossRef]
- Stonik, V.A.; Ivanchina, N.V.; Kicha, A.A. New polar steroids from starfish. Nat. Prod. Commun. 2008, 3, 1587–1610. [Google Scholar] [CrossRef] [Green Version]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef]
- Gomes, A.R.; Freitas, A.C.; Rocha-Santos, T.A.P.; Duarte, A.C. Bioactive compounds derived from echinoderms. RCS Adv. 2014, 4, 29365–29382. [Google Scholar] [CrossRef]
- Ivanchina, N.V.; Kicha, A.A.; Malyarenko, T.V.; Stonik, V.A. Advances in Natural Products Discovery; Gomes, A.R., Rocha-Santos, T., Duarte, A., Eds.; Nova Science Publishers: New York, NY, USA, 2017; Volume 6. [Google Scholar]
- Malyarenko, T.V.; Kicha, A.A.; Kalinovsky, A.I.; Ivanchina, N.V.; Popov, R.S.; Pislyagin, E.A.; Menchinskaya, E.A.; Padmakumarb, K.P.; Stonik, V.A. Four new steroidal glycosides, protolinckiosides A–D, from the starfish Protoreaster lincki. Chem. Biodivers. 2016, 13, 998–1007. [Google Scholar] [CrossRef]
- Usoltseva, R.V.; Shevchenko, N.M.; Malyarenko, O.S.; Ishina, I.A.; Ivannikova, S.I.; Ermakova, S.P. Structure and anticancer activity of native and modified polysaccharides from brown alga Dictyota dichotoma. Carbohydr. Polym. 2018, 180, 21–28. [Google Scholar] [CrossRef]
- Usoltseva (Menshova), R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Zvyagintseva, T.N.; Ermakova, S.P. The comparison of structure and anticancer activity in vitro of polysaccharides from brown algae Alaria marginata and A. angusta. Carbohydr. Polym. 2016, 153, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, N.M.; Anastyuk, S.D.; Gerasimenko, N.I.; Dmitrenok, P.S.; Isakov, V.V.; Zvyagintseva, T.N. Polysaccharide and lipid composition of the brown seaweed Laminaria gurjanovae. Russ. J. Bioorgan. Chem. 2007, 38, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Ermakova, S.P.; Malyarenko (Vishchuk), O.S.; Isakov, V.V.; Zvyagintseva, T.N. Structural elucidation of polysaccharide fractions from the brown alga Coccophora langsdorfii and in vitro investigation of their anticancer activity. Carbohydr. Polym. 2016, 135, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Surits, V.V.; Silchenko, A.S.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Ermakova, S.P. Polysaccharides from brown algae Sargassum duplicatum: The structure and anticancer activity in vitro. Carbohydr. Polym. 2017, 175, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, W.; Wang, J.; Ren, S.; Song, N.; Duan, D.; Zhang, Q. Characterization of laminaran and a highly sulfated polysaccharide from Sargassum fusiforme. Carbohydr. Res. 2014, 385, 58–64. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [Green Version]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. AKT as a therapeutic target for cancer. Cancer. Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef] [Green Version]
- Engelman, J.A. Targeting PI3K signaling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer. 2009, 9, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.H.; Cheng, T.C.; Leu, Y.L.; Chuang, K.H.; Tzou, S.C.; Chen, C.S. Discovery of Akt kinase inhibitors through structure-based virtual screening and their evaluation as potential anticancer agents. Int. J. Mol. Sci. 2015, 16, 3202–3212. [Google Scholar] [CrossRef] [PubMed]
- Los, M.; Maddika, S.; Erb, B.; Schulze-Osthoff, K. Switching Akt: From survival signaling to deadly response. BioEssays 2009, 31, 492–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AAT Bioquest. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 10 November 2020).
- Berens, E.B.; Holy, J.M.; Riegel, A.T.; Wellstein, A. A cancer cell spheroid assay to assess invasion in a 3d setting. J. Vis. Exp. 2015, 20, 53409. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Sun, H.; Wang, Z.; Ermakova, S.P.; Xiao, J.; Lu, T.; Xue, P.; Zvyagintseva, T.N.; Xiong, H.; Shao, C.; et al. PDZ-binding kinase/T-LAK cell-originated protein kinase is a target of the fucoidan from brown alga Fucus evanescens in the prevention of EGF-induced neoplastic cell transformation and colon cancer growth. Oncotarget 2016, 7, 18763–18773. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]


| Polysaccharides from A. angusta | Yield, % | Mw, kDa | Content, % ** | |
|---|---|---|---|---|
| Carbohydrates | SO3Na− | |||
| AaL | 0.8 * | 5–6 | 98 | 0 |
| AaLs | 89 ** | 10–12 | 51 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malyarenko, O.S.; Malyarenko, T.V.; Usoltseva, R.V.; Surits, V.V.; Kicha, A.A.; Ivanchina, N.V.; Ermakova, S.P. Combined Anticancer Effect of Sulfated Laminaran from the Brown Alga Alaria angusta and Polyhydroxysteroid Glycosides from the Starfish Protoreaster lincki on 3D Colorectal Carcinoma HCT 116 Cell Line. Mar. Drugs 2021, 19, 540. https://doi.org/10.3390/md19100540
Malyarenko OS, Malyarenko TV, Usoltseva RV, Surits VV, Kicha AA, Ivanchina NV, Ermakova SP. Combined Anticancer Effect of Sulfated Laminaran from the Brown Alga Alaria angusta and Polyhydroxysteroid Glycosides from the Starfish Protoreaster lincki on 3D Colorectal Carcinoma HCT 116 Cell Line. Marine Drugs. 2021; 19(10):540. https://doi.org/10.3390/md19100540
Chicago/Turabian StyleMalyarenko, Olesya S., Timofey V. Malyarenko, Roza V. Usoltseva, Valerii V. Surits, Alla A. Kicha, Natalia V. Ivanchina, and Svetlana P. Ermakova. 2021. "Combined Anticancer Effect of Sulfated Laminaran from the Brown Alga Alaria angusta and Polyhydroxysteroid Glycosides from the Starfish Protoreaster lincki on 3D Colorectal Carcinoma HCT 116 Cell Line" Marine Drugs 19, no. 10: 540. https://doi.org/10.3390/md19100540
APA StyleMalyarenko, O. S., Malyarenko, T. V., Usoltseva, R. V., Surits, V. V., Kicha, A. A., Ivanchina, N. V., & Ermakova, S. P. (2021). Combined Anticancer Effect of Sulfated Laminaran from the Brown Alga Alaria angusta and Polyhydroxysteroid Glycosides from the Starfish Protoreaster lincki on 3D Colorectal Carcinoma HCT 116 Cell Line. Marine Drugs, 19(10), 540. https://doi.org/10.3390/md19100540








