Cold-Active β-Galactosidases: Insight into Cold Adaptation Mechanisms and Biotechnological Exploitation
Abstract
1. Introduction
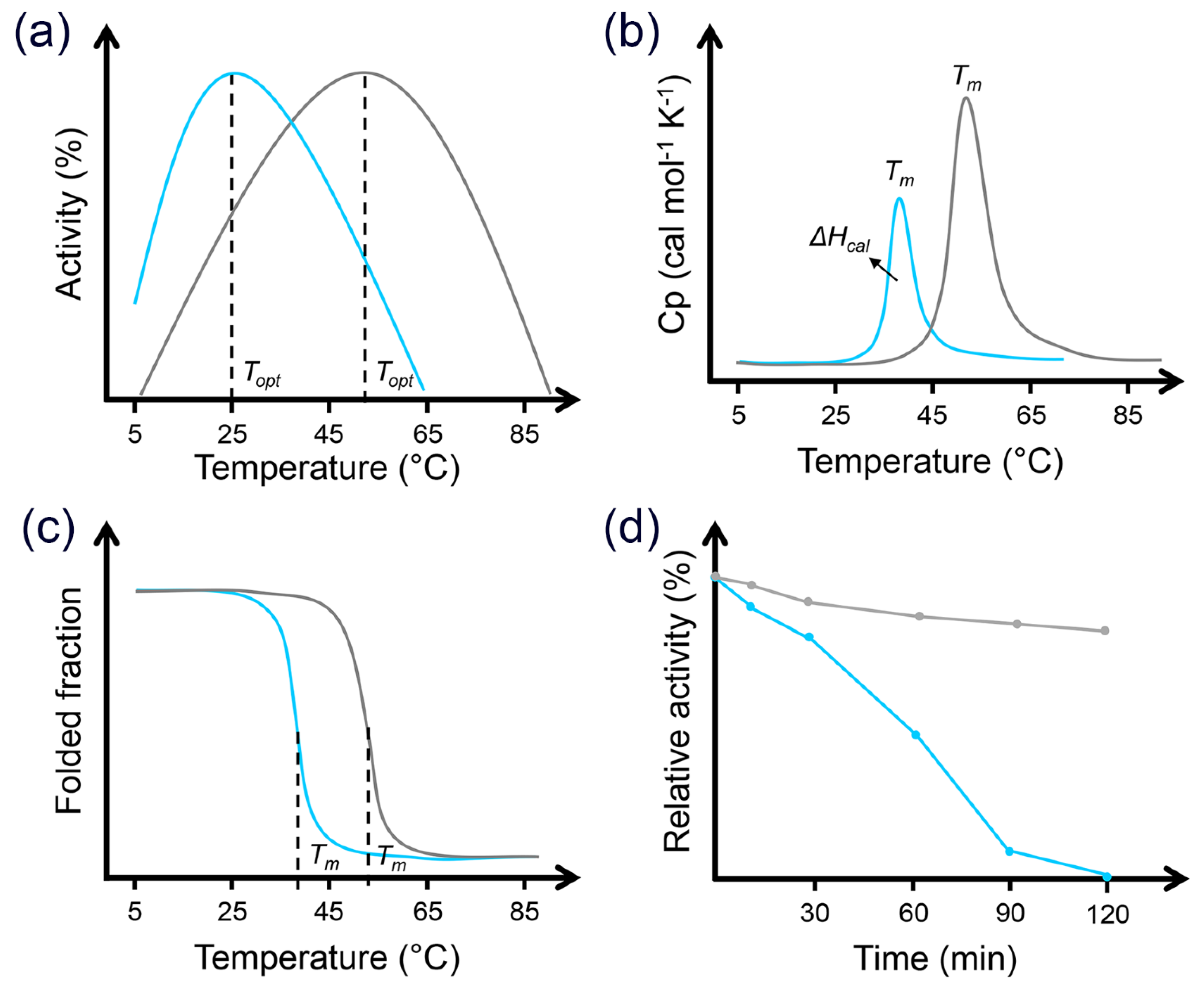
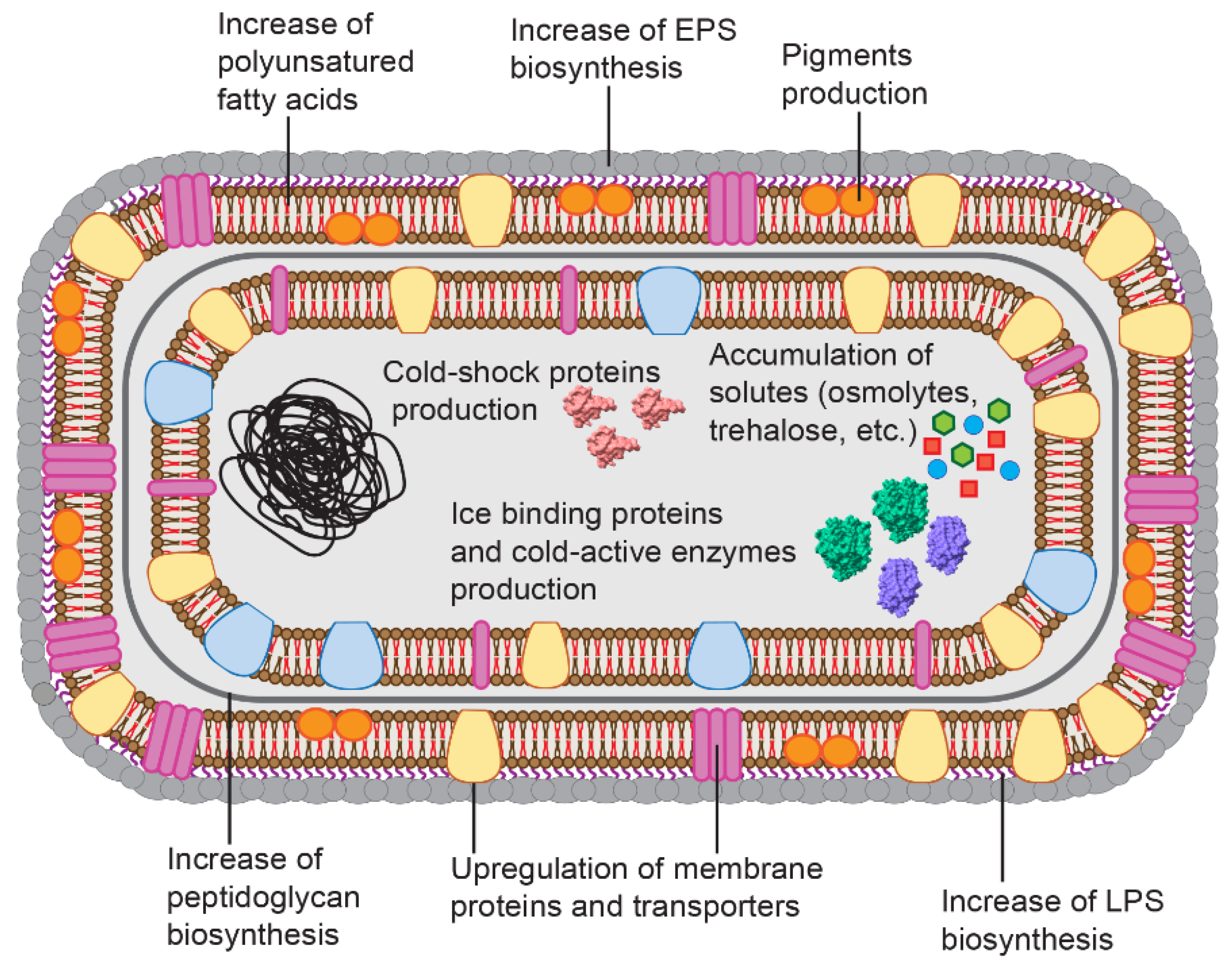
2. Mechanisms of Cold Adaptation
3. Sources of Cold-Active β-Galactosidases
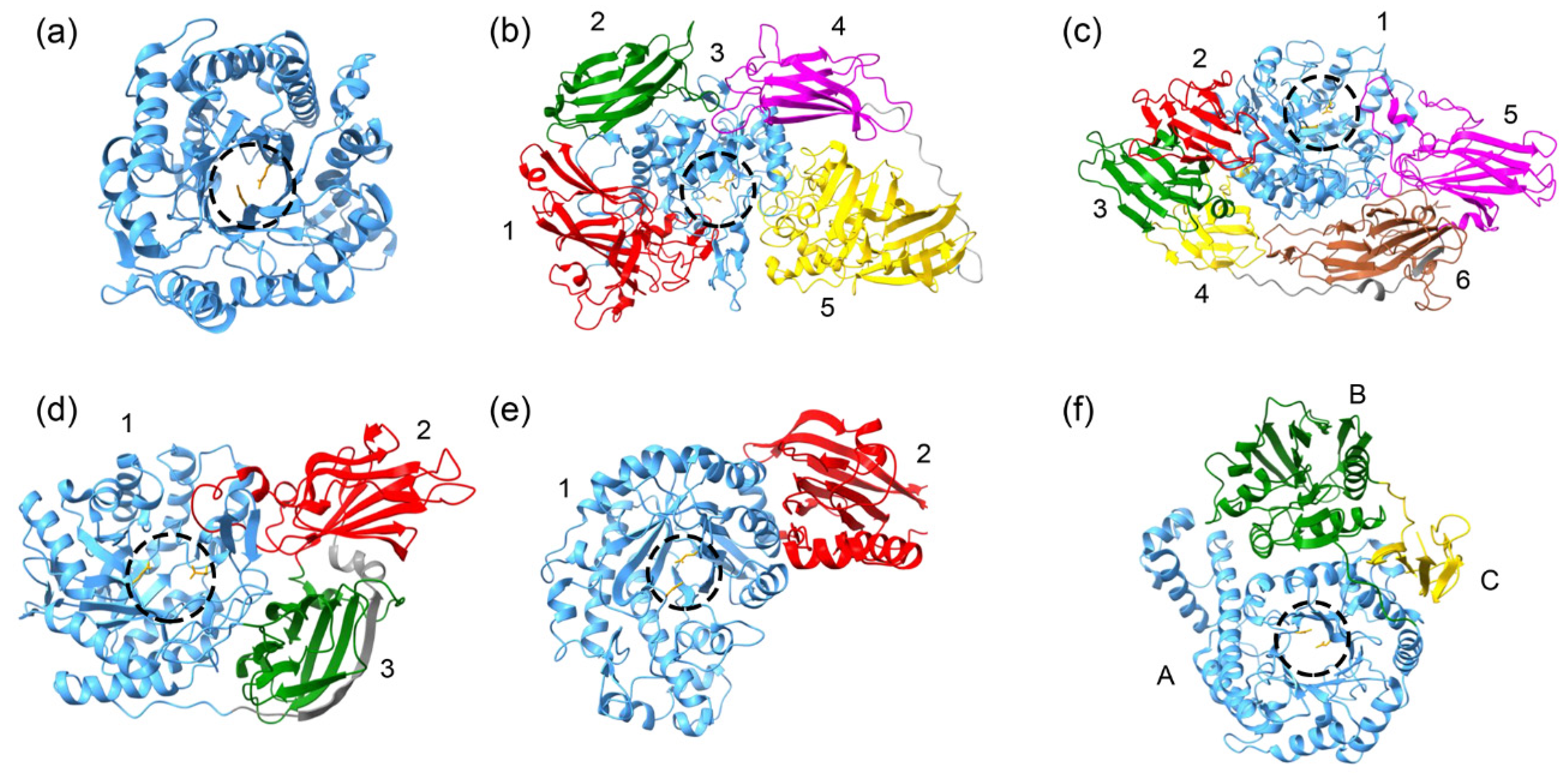
4. Classification, Structure and Activity of Cold-Active β-Galactosidases
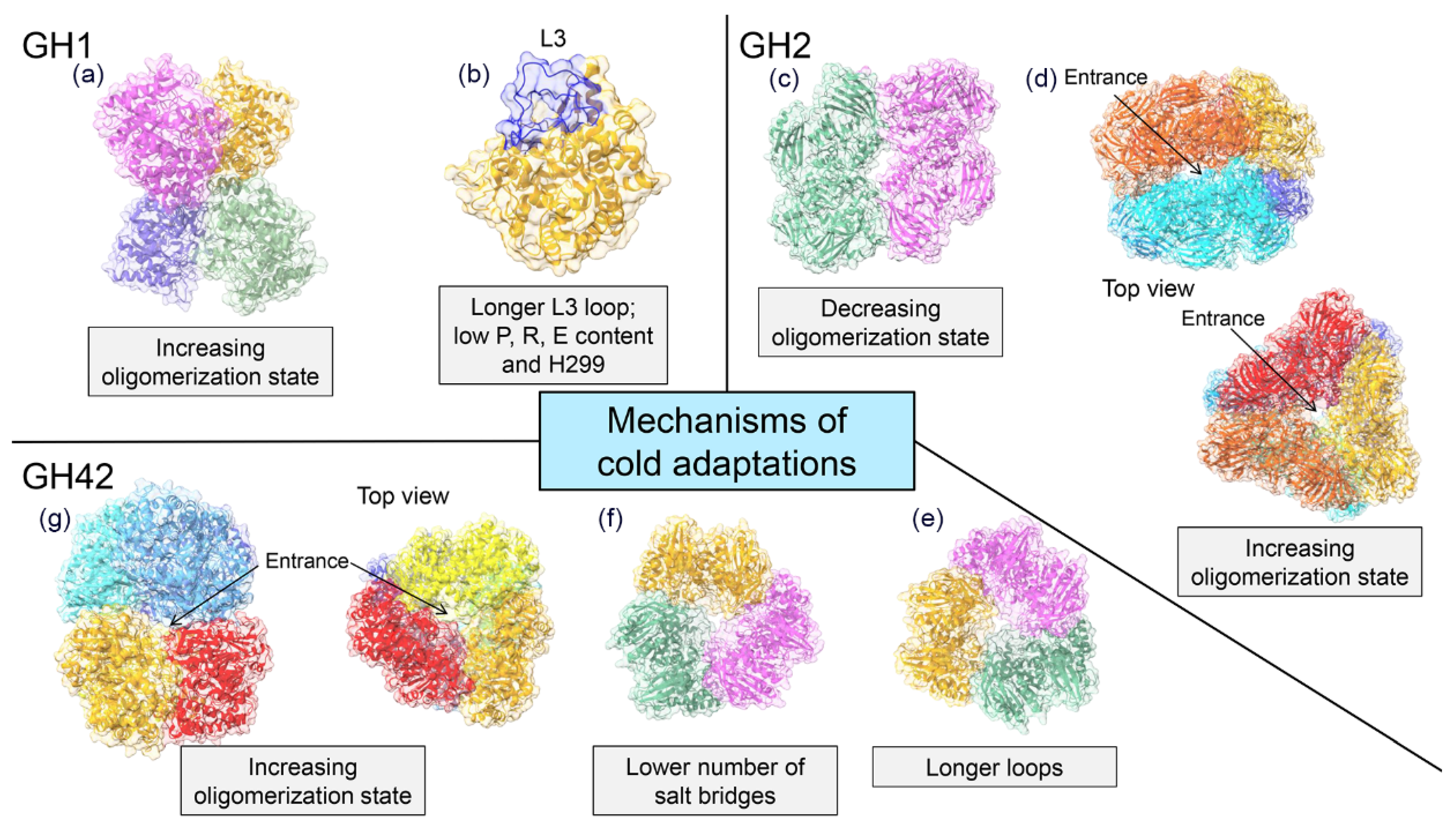
4.1. GH1 Family
4.2. GH2 Family
4.3. GH35 Family
4.4. GH42 Family
5. Industrial Applications of Cold-Active β-Galactosidases
5.1. Hydrolysis of Lactose in Milk
5.2. Hydrolysis of Lactose in Cheese Whey
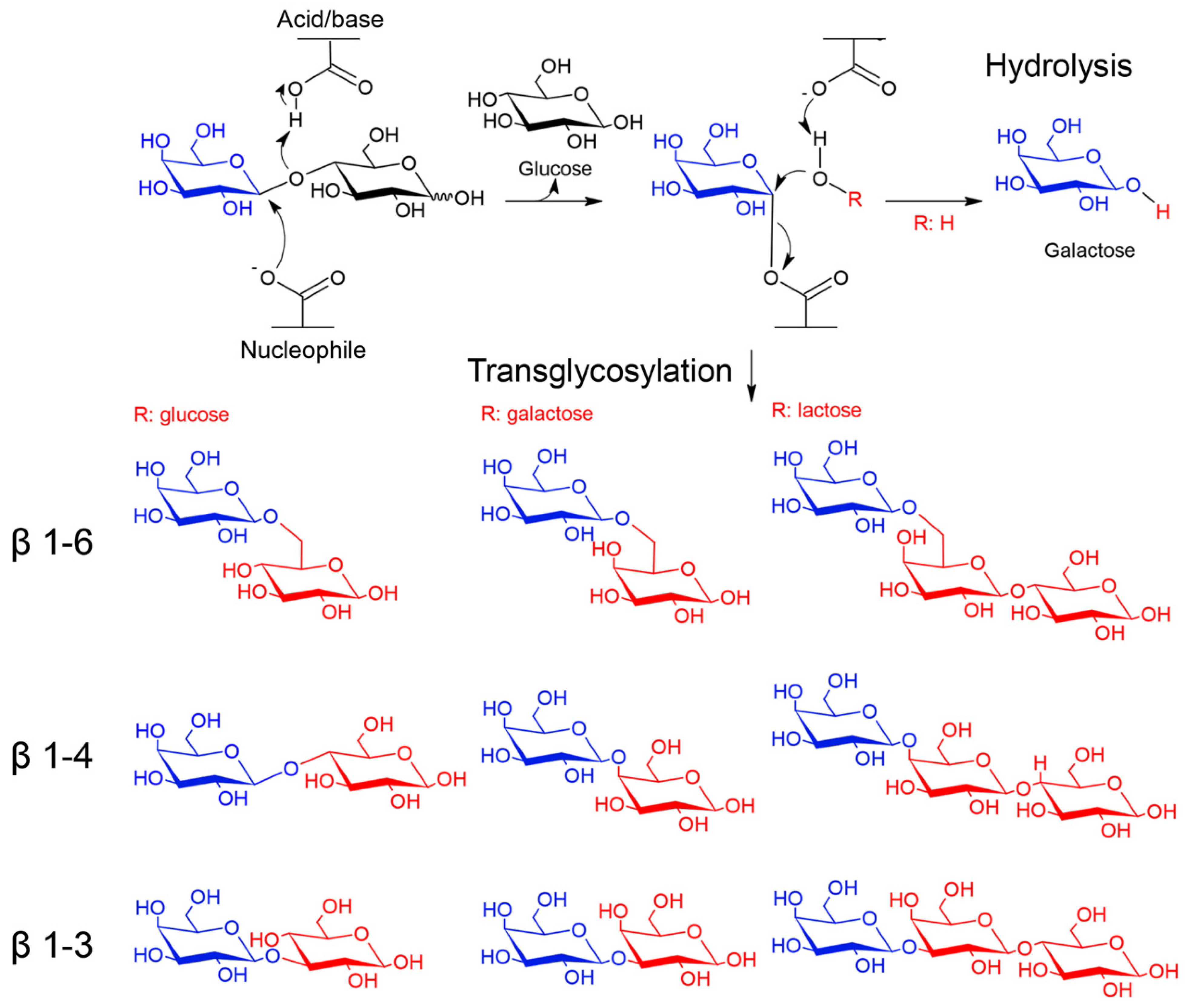
5.3. Synthesis of Oligosaccharides
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- De Maayer, P.; Anderson, D.; Cary, C.; Cowan, D.A. Some like it cold: Understanding the survival strategies of psychrophiles. Embo Rep. 2014, 15, 508–517. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, S.; Collins, T.; Marx, J.C.; Feller, G.; Gerday, C. Psychrophilic microorganisms: Challenges for life. Embo Rep. 2006, 7, 385–389. [Google Scholar] [CrossRef]
- Collins, T.; Margesin, R. Psychrophilic lifestyles: Mechanisms of adaptation and biotechnological tools. Appl. Microbiol. Biotechnol. 2019, 103, 2857–2871. [Google Scholar] [CrossRef]
- Bruno, S.; Coppola, D.; di Prisco, G.; Giordano, D.; Verde, C. Enzymes from Marine Polar Regions and Their Biotechnological Applications. Mar. Drugs 2019, 17, 544. [Google Scholar] [CrossRef]
- Vallesi, A.; Pucciarelli, S.; Buonanno, F.; Fontana, A.; Mangiagalli, M. Bioactive molecules from protists: Perspectives in biotechnology. Eur. J. Protistol. 2020, 75, 125720. [Google Scholar] [CrossRef]
- Vance, T.D.R.; Bayer-Giraldi, M.; Davies, P.L.; Mangiagalli, M. Ice-binding proteins and the ‘domain of unknown function’3494 family. Febs J. 2019, 286, 855–873. [Google Scholar] [CrossRef]
- Bar Dolev, M.; Braslavsky, I.; Davies, P.L. Ice-binding proteins and their function. Annu. Rev. Biochem. 2016, 85, 515–542. [Google Scholar] [CrossRef]
- Feller, G.; Gerday, C. Psychrophilic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol. 2003, 1, 200. [Google Scholar] [CrossRef]
- Feller, G. Protein stability and enzyme activity at extreme biological temperatures. J. Phys. Condens. Matter 2010, 22, 323101. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Ramírez-Sarmiento, C.A.; Zamora, R.A.; Parra, L.P. Discovery, molecular mechanisms, and industrial applications of cold-active enzymes. Front. Microbiol. 2016, 7, 1408. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, F.; Peralta, R.; Blamey, J.M. Cold and hot extremozymes: Industrial relevance and current trends. Front. Bioeng. Biotechnol. 2015, 3, 148. [Google Scholar] [CrossRef] [PubMed]
- Mangiagalli, M.; Brocca, S.; Orlando, M.; Lotti, M. The “cold revolution”. Present and future applications of cold-active enzymes and ice-binding proteins. New Biotechnol. 2020, 55, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Kataki, S.; Chatterjee, S.; Prasad, R.K.; Datta, S.; Vairale, M.G.; Sharma, S.; Dwivedi, S.K.; Gupta, D.K. Cold adaptation in bacteria with special focus on cellulase production and its potential application. J. Clean. Prod. 2020, 258, 120351. [Google Scholar] [CrossRef]
- Lu, L.; Guo, L.; Wang, K.; Liu, Y.; Xiao, M. β-Galactosidases: A great tool for synthesizing galactose-containing carbohydrates. Biotechnol. Adv. 2019, 39, 107465. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B.; Davies, G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef]
- Rappé, M.S.; Giovannoni, S.J. The uncultured microbial majority. Annu. Rev. Microbiol. 2003, 57, 369–394. [Google Scholar] [CrossRef]
- Lewis, W.H.; Tahon, G.; Geesink, P.; Sousa, D.Z.; Ettema, T.J.G. Innovations to culturing the uncultured microbial majority. Nat. Rev. Microbiol. 2020, 1–16. [Google Scholar] [CrossRef]
- Hansson, T.; Andersson, M.; Wehtje, E.; Adlercreutz, P. Influence of water activity on the competition between β-glycosidase-catalysed transglycosylation and hydrolysis in aqueous hexanol. Enzym. Microb. Technol. 2001, 29, 527–534. [Google Scholar] [CrossRef]
- Lonhienne, T.; Gerday, C.; Feller, G. Psychrophilic enzymes: Revisiting the thermodynamic parameters of activation may explain local flexibility. Biochim. Et Biophys. Acta (Bba)-Protein Struct. Mol. Enzymol. 2000, 1543, 1–10. [Google Scholar] [CrossRef]
- Georlette, D.; Blaise, V.; Collins, T.; D’Amico, S.; Gratia, E.; Hoyoux, A.; Marx, J.C.; Sonan, G.; Feller, G.; Gerday, C. Some like it cold: Biocatalysis at low temperatures. Fems Microbiol. Rev. 2004, 28, 25–42. [Google Scholar] [CrossRef]
- Lonhienne, T.; Zoidakis, J.; Vorgias, C.E.; Feller, G.; Gerday, C.; Bouriotis, V. Modular structure, local flexibility and cold-activity of a novel chitobiase from a psychrophilic Antarctic bacterium. J. Mol. Biol. 2001, 310, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.A.; Ramirez-Sarmiento, C.A.; Castro-Fernández, V.c.; Villalobos, P.; Maturana, P.; Herrera-Morande, A.; Komives, E.A.; Guixé, V. Tuning of Conformational Dynamics Through Evolution-Based Design Modulates the Catalytic Adaptability of an Extremophilic Kinase. ACS Catal. 2020, 10, 10847–10857. [Google Scholar] [CrossRef]
- Russell, N.J. Toward a molecular understanding of cold activity of enzymes from psychrophiles. Extremophiles 2000, 4, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Smalås, A.O.; Leiros, H.K.; Os, V.; Willassen, N.P. Cold adapted enzymes. Biotechnol. Annu. Rev. 2000, 6, 1–57. [Google Scholar]
- Gianese, G.; Bossa, F.; Pascarella, S. Comparative structural analysis of psychrophilic and meso-and thermophilic enzymes. Proteins Struct. Funct. Bioinform. 2002, 47, 236–249. [Google Scholar] [CrossRef]
- D’Amico, S.; Claverie, P.; Collins, T.; Georlette, D.; Gratia, E.; Hoyoux, A.; Meuwis, M.-A.; Feller, G.; Gerday, C. Molecular basis of cold adaptation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2002, 357, 917–925. [Google Scholar] [CrossRef]
- Pucci, F.; Rooman, M. Physical and molecular bases of protein thermal stability and cold adaptation. Curr. Opin. Struct. Biol. 2017, 42, 117–128. [Google Scholar] [CrossRef]
- Brocca, S.; Ferrari, C.; Barbiroli, A.; Pesce, A.; Lotti, M.; Nardini, M. A bacterial acyl aminoacyl peptidase couples flexibility and stability as a result of cold adaptation. FEBS J. 2016, 283, 4310–4324. [Google Scholar] [CrossRef]
- Mangiagalli, M.; Lapi, M.; Maione, S.; Orlando, M.; Brocca, S.; Pesce, A.; Barbiroli, A.; Camilloni, C.; Pucciarelli, S.; Lotti, M. The co-existence of cold activity and thermal stability in an Antarctic GH42 β-galactosidase relies on its hexameric quaternary arrangement. FEBS J. 2020. [Google Scholar] [CrossRef]
- Skalova, T.; Dohnalek, J.; Spiwok, V.; Lipovova, P.; Vondráčková, E.; Petrokova, H.; Dušková, J.; Strnad, H.; Kralova, B.; Hašek, J. Cold-active β-galactosidase from Arthrobacter sp. C2-2 forms compact 660 kDa hexamers: Crystal structure at 1.9 Å resolution. J. Mol. Biol. 2005, 353, 282–294. [Google Scholar] [CrossRef]
- Zanphorlin, L.M.; De Giuseppe, P.O.; Honorato, R.V.; Tonoli, C.C.C.; Fattori, J.; Crespim, E.; De Oliveira, P.S.L.; Ruller, R.; Murakami, M.T. Oligomerization as a strategy for cold adaptation: Structure and dynamics of the GH1 β-glucosidase from Exiguobacterium antarcticum B7. Sci. Rep. 2016, 6, 23776. [Google Scholar] [CrossRef] [PubMed]
- Pischedda, A.; Ramasamy, K.P.; Mangiagalli, M.; Chiappori, F.; Milanesi, L.; Miceli, C.; Pucciarelli, S.; Lotti, M. Antarctic marine ciliates under stress: Superoxide dismutases from the psychrophilic Euplotes focardii are cold-active yet heat tolerant enzymes. Sci. Rep. 2018, 8, 14721. [Google Scholar] [CrossRef] [PubMed]
- Merlino, A.; Krauss, I.R.; Castellano, I.; De Vendittis, E.; Rossi, B.; Conte, M.; Vergara, A.; Sica, F. Structure and flexibility in cold-adapted iron superoxide dismutases: The case of the enzyme isolated from Pseudoalteromonas haloplanktis. J. Struct. Biol. 2010, 172, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Kazuoka, T.; Soda, K. Paradoxical thermostable enzymes from psychrophile: Molecular characterization and potentiality for biotechnological application. J. Mol. Catal. B Enzym. 2003, 23, 65–70. [Google Scholar] [CrossRef]
- Sočan, J.; Purg, M.; Åqvist, J. Computer simulations explain the anomalous temperature optimum in a cold-adapted enzyme. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Chrast, L.; Tratsiak, K.; Planas-Iglesias, J.; Daniel, L.; Prudnikova, T.; Brezovsky, J.; Bednar, D.; Kuta Smatanova, I.; Chaloupkova, R.; Damborsky, J. Deciphering the Structural Basis of High Thermostability of Dehalogenase from Psychrophilic Bacterium Marinobacter sp. ELB17. Microorganisms 2019, 7, 498. [Google Scholar] [CrossRef]
- Novak, H.R.; Sayer, C.; Panning, J.; Littlechild, J.A. Characterisation of an l-haloacid dehalogenase from the marine psychrophile Psychromonas ingrahamii with potential industrial application. Mar. Biotechnol. 2013, 15, 695–705. [Google Scholar] [CrossRef]
- Fedøy, A.-E.; Yang, N.; Martinez, A.; Leiros, H.-K.S.; Steen, I.H. Structural and functional properties of isocitrate dehydrogenase from the psychrophilic bacterium Desulfotalea psychrophila reveal a cold-active enzyme with an unusual high thermal stability. J. Mol. Biol. 2007, 372, 130–149. [Google Scholar] [CrossRef]
- Brunialti, E.A.S.; Gatti-Lafranconi, P.; Lotti, M. Promiscuity, stability and cold adaptation of a newly isolated acylaminoacyl peptidase. Biochimie 2011, 93, 1543–1554. [Google Scholar] [CrossRef]
- Bujacz, A.; Rutkiewicz-Krotewicz, M.; Nowakowska-Sapota, K.; Turkiewicz, M. Crystal structure and enzymatic properties of a broad substrate-specificity psychrophilic aminotransferase from the Antarctic soil bacterium Psychrobacter sp. B6. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 632–645. [Google Scholar] [CrossRef]
- Angelaccio, S.; Florio, R.; Consalvi, V.; Festa, G.; Pascarella, S. Serine hydroxymethyltransferase from the cold adapted microorganism Psychromonas ingrahamii: A low temperature active enzyme with broad substrate specificity. Int. J. Mol. Sci. 2012, 13, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Nobeli, I.; Favia, A.D.; Thornton, J.M. Protein promiscuity and its implications for biotechnology. Nat. Biotechnol. 2009, 27, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Struvay, C.; Feller, G. Optimization to low temperature activity in psychrophilic enzymes. Int. J. Mol. Sci. 2012, 13, 11643–11665. [Google Scholar] [CrossRef] [PubMed]
- Feller, G. Psychrophilic enzymes: From folding to function and biotechnology. Scientifica 2013, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, X.; Xing, M. A New β-Galactosidase from the Antarctic Bacterium Alteromonas sp. ANT48 and Its Potential in Formation of Prebiotic Galacto-Oligosaccharides. Mar. Drugs 2019, 17, 599. [Google Scholar] [CrossRef] [PubMed]
- Hoyoux, A.; Jennes, I.; Dubois, P.; Genicot, S.; Dubail, F.; François, J.-M.; Baise, E.; Feller, G.; Gerday, C. Cold-adapted β-galactosidase from the Antarctic psychrophile Pseudoalteromonas haloplanktis. Appl. Environ. Microbiol. 2001, 67, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Turkiewicz, M.; Kur, J.; Białkowska, A.; Cieśliński, H.; Kalinowska, H.; Bielecki, S. Antarctic marine bacterium Pseudoalteromonas sp. 22b as a source of cold-adapted β-galactosidase. Biomol. Eng. 2003, 20, 317–324. [Google Scholar] [CrossRef]
- Ding, H.; Zeng, Q.; Zhou, L.; Yu, Y.; Chen, B. Biochemical and structural insights into a novel thermostable β-1, 3-galactosidase from Marinomonas sp. BSi20414. Mar. Drugs 2017, 15, 13. [Google Scholar] [CrossRef]
- Schmidt, M.; Stougaard, P. Identification, cloning and expression of a cold-active β-galactosidase from a novel Arctic bacterium, Alkalilactibacillus ikkense. Environ. Technol. 2010, 31, 1107–1114. [Google Scholar] [CrossRef]
- Wierzbicka-Woś, A.; Bartasun, P.; Cieśliński, H.; Kur, J. Cloning and characterization of a novel cold-active glycoside hydrolase family 1 enzyme with β-glucosidase, β-fucosidase and β-galactosidase activities. BMC Biotechnol. 2013, 13, 22. [Google Scholar] [CrossRef]
- Yao, C.; Sun, J.; Wang, W.; Zhuang, Z.; Liu, J.; Hao, J. A novel cold-adapted β-galactosidase from Alteromonas sp. ML117 cleaves milk lactose effectively at low temperature. Process Biochem. 2019, 82, 94–101. [Google Scholar] [CrossRef]
- Sun, J.; Yao, C.; Wang, W.; Zhuang, Z.; Liu, J.; Dai, F.; Hao, J. Cloning, Expression and Characterization of a Novel Cold-adapted β-galactosidase from the Deep-sea Bacterium Alteromonas sp. ML52. Mar. Drugs 2018, 16, 469. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, W.; Yao, C.; Dai, F.; Zhu, X.; Liu, J.; Hao, J. Overexpression and characterization of a novel cold-adapted and salt-tolerant GH1 β-glucosidase from the marine bacterium Alteromonas sp. L82. J. Microbiol. 2018, 56, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Trimbur, D.E.; Gutshall, K.R.; Prema, P.; Brenchley, J.E. Characterization of a psychrotrophic Arthrobacter gene and its cold-active beta-galactosidase. Appl. Environ. Microbiol. 1994, 60, 4544–4552. [Google Scholar] [CrossRef] [PubMed]
- Karasová-Lipovová, P.; Strnad, H.; Spiwok, V.; Malá, Š.; Králová, B.; Russell, N.J. The cloning, purification and characterisation of a cold-active β-galactosidase from the psychrotolerant Antarctic bacterium Arthrobacter sp. C2-2. Enzym. Microb. Technol. 2003, 33, 836–844. [Google Scholar]
- Białkowska, A.M.; Cieśliński, H.; Nowakowska, K.M.; Kur, J.; Turkiewicz, M. A new β-galactosidase with a low temperature optimum isolated from the Antarctic Arthrobacter sp. 20B: Gene cloning, purification and characterization. Arch. Microbiol. 2009, 191, 825. [Google Scholar]
- Coker, J.A.; Sheridan, P.P.; Loveland-Curtze, J.; Gutshall, K.R.; Auman, A.J.; Brenchley, J.E. Biochemical characterization of a β-galactosidase with a low temperature optimum obtained from an Antarctic Arthrobacter isolate. J. Bacteriol. 2003, 185, 5473–5482. [Google Scholar] [CrossRef]
- Nakagawa, T.; Fujimoto, Y.; Ikehata, R.; Miyaji, T.; Tomizuka, N. Purification and molecular characterization of cold-active β-galactosidase from Arthrobacter psychrolactophilus strain F2. Appl. Microbiol. Biotechnol. 2006, 72, 720. [Google Scholar] [CrossRef]
- Xu, K.; Tang, X.; Gai, Y.; Mehmood, M.; Xiao, X.; Wang, F. Molecular characterization of cold-inducible beta-galactosidase from Arthrobacter sp. ON14 isolated from Antarctica. J. Microbiol. Biotechnol. 2011, 21, 236–242. [Google Scholar] [CrossRef]
- Pawlak-Szukalska, A.; Wanarska, M.; Popinigis, A.T.; Kur, J. A novel cold-active β-d-galactosidase with transglycosylation activity from the Antarctic Arthrobacter sp. 32cB–Gene cloning, purification and characterization. Process Biochem. 2014, 49, 2122–2133. [Google Scholar] [CrossRef]
- Gutshall, K.R.; Trimbur, D.E.; Kasmir, J.J.; Brenchley, J.E. Analysis of a novel gene and beta-galactosidase isozyme from a psychrotrophic Arthrobacter isolate. J. Bacteriol. 1995, 177, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, P.; Wanarska, M.; Kur, J. A new cold-adapted β-D-galactosidase from the Antarctic Arthrobacter sp. 32c-gene cloning, overexpression, purification and properties. BMC Microbiol. 2009, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka-Woś, A.; Cieśliński, H.; Wanarska, M.; Kozłowska-Tylingo, K.; Hildebrandt, P.; Kur, J. A novel cold-active β-D-galactosidase from the Paracoccus sp. 32d-gene cloning, purification and characterization. Microb. Cell Factories 2011, 10, 108. [Google Scholar]
- Fan, Y.; Hua, X.; Zhang, Y.; Feng, Y.; Shen, Q.; Dong, J.; Zhao, W.; Zhang, W.; Jin, Z.; Yang, R. Cloning, expression and structural stability of a cold-adapted β-galactosidase from Rahnella sp. R3. Protein Expr. Purif. 2015, 115, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Karan, R.; Capes, M.D.; DasSarma, P.; DasSarma, S. Cloning, overexpression, purification, and characterization of a polyextremophilic β-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi. Bmc Biotechnol. 2013, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, G.; Yu, S.Q.; Zhang, C.T.; Liu, Y.H. A novel metagenome-derived β-galactosidase: Gene cloning, overexpression, purification and characterization. Appl. Microbiol. Biotechnol. 2010, 88, 155–165. [Google Scholar] [CrossRef]
- Wang, L.; Mou, Y.; Guan, B.; Hu, Y.; Zhang, Y.; Zeng, J.; Ni, Y. Genome sequence of the psychrophilic Cryobacterium sp. LW097 and characterization of its four novel cold-adapted β-galactosidases. Int. J. Biol. Macromol. 2020, 163, 2068–2083. [Google Scholar] [CrossRef]
- Coombs, J.; Brenchley, J.E. Characterization of Two New Glycosyl Hydrolases from the Lactic Acid Bacterium Carnobacterium piscicolaStrain BA. Appl. Environ. Microbiol. 2001, 67, 5094–5099. [Google Scholar] [CrossRef]
- Shipkowski, S.; Brenchley, J.E. Bioinformatic, genetic, and biochemical evidence that some glycoside hydrolase family 42 β-galactosidases are arabinogalactan type I oligomer hydrolases. Appl. Environ. Microbiol. 2006, 72, 7730–7738. [Google Scholar] [CrossRef]
- Cairns, J.R.K.; Esen, A. β-Glucosidases. Cell. Mol. Life Sci. 2010, 67, 3389–3405. [Google Scholar] [CrossRef]
- Crespim, E.; Zanphorlin, L.M.; de Souza, F.H.M.; Diogo, J.A.; Gazolla, A.C.; Machado, C.B.; Figueiredo, F.; Sousa, A.S.; Nobrega, F.; Pellizari, V.H. A novel cold-adapted and glucose-tolerant GH1 β-glucosidase from Exiguobacterium antarcticum B7. Int. J. Biol. Macromol. 2016, 82, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-X.; Miao, L.-L.; Liu, Y.; Liu, H.-C.; Liu, Z.-P. Gene cloning and characterization of a cold-adapted β-glucosidase belonging to glycosyl hydrolase family 1 from a psychrotolerant bacterium Micrococcus antarcticus. Enzym. Microb. Technol. 2011, 49, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Meng, C.; Wu, Y.; Xu, J.; Tang, X.; Zhang, X.; Xiao, Y.; Wang, X.; Fang, Z.; Fang, W. An unusual GH1 β-glucosidase from marine sediment with β-galactosidase and transglycosidation activities for superior galacto-oligosaccharide synthesis. Appl. Microbiol. Biotechnol. 2020, 104, 4927–4943. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Peng, R.; Xiong, A.; Fu, X.; Tian, Y.; Yao, Q. Expression and characterization of a cold-active and xylose-stimulated β-glucosidase from Marinomonas MWYL1 in Escherichia coli. Mol. Biol. Rep. 2012, 39, 2937–2943. [Google Scholar] [CrossRef]
- Miao, L.-L.; Hou, Y.-J.; Fan, H.-X.; Qu, J.; Qi, C.; Liu, Y.; Li, D.-F.; Liu, Z.-P. Molecular structural basis for the cold adaptedness of the psychrophilic β-glucosidase BglU in Micrococcus antarcticus. Appl. Environ. Microbiol. 2016, 82, 2021–2030. [Google Scholar] [CrossRef]
- Miao, L.-L.; Fan, H.-X.; Qu, J.; Liu, Y.; Liu, Z.-P. Specific amino acids responsible for the cold adaptedness of Micrococcus antarcticus β-glucosidase BglU. Appl. Microbiol. Biotechnol. 2017, 101, 2033–2041. [Google Scholar] [CrossRef]
- Maksimainen, M.; Hakulinen, N.; Kallio, J.M.; Timoharju, T.; Turunen, O.; Rouvinen, J. Crystal structures of Trichoderma reesei β-galactosidase reveal conformational changes in the active site. J. Struct. Biol. 2011, 174, 156–163. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, L.; Jiang, Y.-L.; Bai, X.-H.; Chu, J.; Li, Q.; Yu, G.; Liang, Q.-L.; Zhou, C.-Z.; Chen, Y. Structural insights into the substrate specificity of Streptococcus pneumoniae β (1, 3)-galactosidase BgaC. J. Biol. Chem. 2012, 287, 22910–22918. [Google Scholar] [CrossRef]
- Larsbrink, J.; Thompson, A.J.; Lundqvist, M.; Gardner, J.G.; Davies, G.J.; Brumer, H. A complex gene locus enables xyloglucan utilization in the model saprophyte C ellvibrio japonicus. Mol. Microbiol. 2014, 94, 418–433. [Google Scholar] [CrossRef]
- Hidaka, M.; Fushinobu, S.; Ohtsu, N.; Motoshima, H.; Matsuzawa, H.; Shoun, H.; Wakagi, T. Trimeric crystal structure of the glycoside hydrolase family 42 β-galactosidase from Thermus thermophilus A4 and the structure of its complex with galactose. J. Mol. Biol. 2002, 322, 79–91. [Google Scholar] [CrossRef]
- Rutkiewicz, M.; Bujacz, A.; Bujacz, G. Structural features of cold-adapted dimeric GH2 β-D-galactosidase from Arthrobacter sp. 32cB. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2019, 1867, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yi, J.; Hua, X.; Feng, Y.; Yang, R.; Zhang, Y. Structure analysis of a glycosides hydrolase family 42 cold-adapted β-galactosidase from Rahnella sp. R3. RSC Adv. 2016, 6, 37362–37369. [Google Scholar] [CrossRef]
- Karan, R.; Mathew, S.; Muhammad, R.; Bautista, D.B.; Vogler, M.; Eppinger, J.; Oliva, R.; Cavallo, L.; Arold, S.T.; Rueping, M. Understanding High-Salt and Cold Adaptation of a Polyextremophilic Enzyme. Microorganisms 2020, 8, 1594. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, R.H.; Zhang, X.J.; DuBose, R.F.; Matthews, B.W. Three-dimensional structure of β-galactosidase from E. coli. Nature 1994, 369, 761. [Google Scholar] [CrossRef] [PubMed]
- Makowski, K.; Białkowska, A.; Szczęsna-Antczak, M.; Kalinowska, H.; Kur, J.; Cieśliński, H.; Turkiewicz, M. Immobilized preparation of cold-adapted and halotolerant Antarctic β-galactosidase as a highly stable catalyst in lactose hydrolysis. FEMS Microbiol. Ecol. 2007, 59, 535–542. [Google Scholar] [CrossRef]
- Rutkiewicz-Krotewicz, M.; Pietrzyk-Brzezinska, A.J.; Sekula, B.; Cieśliński, H.; Wierzbicka-Woś, A.; Kur, J.; Bujacz, A. Structural studies of a cold-adapted dimeric β-D-galactosidase from Paracoccus sp. 32d. Acta Crystallogr. Sect. D Struct. Biol. 2016, 72, 1049–1061. [Google Scholar] [CrossRef]
- Zinin, A.I.; Eneyskaya, E.V.; Shabalin, K.A.; Kulminskaya, A.A.; Shishlyannikov, S.M.; Neustroev, K.N. 1-O-Acetyl-β-d-galactopyranose: A novel substrate for the transglycosylation reaction catalyzed by the β-galactosidase from Penicillium sp. Carbohydr. Res. 2002, 337, 635–642. [Google Scholar] [CrossRef]
- Gamauf, C.; Marchetti, M.; Kallio, J.; Puranen, T.; Vehmaanperä, J.; Allmaier, G.; Kubicek, C.P.; Seiboth, B. Characterization of the bga1-encoded glycoside hydrolase family 35 β-galactosidase of Hypocrea jecorina with galacto-β-d-galactanase activity. FEBS J. 2007, 274, 1691–1700. [Google Scholar] [CrossRef]
- Tanthanuch, W.; Chantarangsee, M.; Maneesan, J.; Ketudat-Cairns, J. Genomic and expression analysis of glycosyl hydrolase family 35 genes from rice (Oryza sativa L.). BMC Plant. Biol. 2008, 8, 84. [Google Scholar] [CrossRef]
- Gutshall, K.; Wang, K.; Brenchley, J.E. A novel Arthrobacter beta-galactosidase with homology to eucaryotic beta-galactosidases. J. Bacteriol. 1997, 179, 3064–3067. [Google Scholar] [CrossRef]
- Coombs, J.M.; Brenchley, J.E. Biochemical and phylogenetic analyses of a cold-active β-galactosidase from the lactic acid bacterium Carnobacterium piscicola BA. Appl. Environ. Microbiol. 1999, 65, 5443–5450. [Google Scholar] [CrossRef] [PubMed]
- Maksimainen, M.M.; Lampio, A.; Mertanen, M.; Turunen, O.; Rouvinen, J. The crystal structure of acidic β-galactosidase from Aspergillus oryzae. Int. J. Biol. Macromol. 2013, 60, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.L.; Nagem, R.A.P.; Neustroev, K.N.; Arand, M.; Adamska, M.; Eneyskaya, E.V.; Kulminskaya, A.A.; Garratt, R.C.; Golubev, A.M.; Polikarpov, I. Crystal structures of β-galactosidase from Penicillium sp. and its complex with galactose. J. Mol. Biol. 2004, 343, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Ohto, U.; Usui, K.; Ochi, T.; Yuki, K.; Satow, Y.; Shimizu, T. Crystal Structure of Human β-Galactosidase Structural Basis of gm1 Gangliosidosis and Morquio b Diseases. J. Biol. Chem. 2012, 287, 1801–1812. [Google Scholar] [CrossRef]
- Henze, M.; You, D.-J.; Kamerke, C.; Hoffmann, N.; Angkawidjaja, C.; Ernst, S.; Pietruszka, J.; Kanaya, S.; Elling, L. Rational design of a glycosynthase by the crystal structure of β-galactosidase from Bacillus circulans (BgaC) and its use for the synthesis of N-acetyllactosamine type 1 glycan structures. J. Biotechnol. 2014, 191, 78–85. [Google Scholar] [CrossRef]
- Sheridan, P.P.; Brenchley, J.E. Characterization of a Salt-Tolerant Family 42 β-Galactosidase from a Psychrophilic Antarctic Planococcus Isolate. Appl. Environ. Microbiol. 2000, 66, 2438–2444. [Google Scholar] [CrossRef]
- Hu, J.M.; Li, H.; Cao, L.X.; Wu, P.C.; Zhang, C.T.; Sang, S.L.; Zhang, X.Y.; Chen, M.J.; Lu, J.Q.; Liu, Y.H. Molecular cloning and characterization of the gene encoding cold-active β-galactosidase from a psychrotrophic and halotolerant Planococcus sp. L4. J. Agric. Food Chem. 2007, 55, 2217–2224. [Google Scholar] [CrossRef]
- Maksimainen, M.; Paavilainen, S.; Hakulinen, N.; Rouvinen, J. Structural analysis, enzymatic characterization, and catalytic mechanisms of β-galactosidase from Bacillus circulans sp. alkalophilus. FEBS J. 2012, 279, 1788–1798. [Google Scholar] [CrossRef]
- Solomon, H.V.; Tabachnikov, O.; Lansky, S.; Salama, R.; Feinberg, H.; Shoham, Y.; Shoham, G. Structure–function relationships in Gan42B, an intracellular GH42 β-galactosidase from Geobacillus stearothermophilus. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 2433–2448. [Google Scholar] [CrossRef]
- Viborg, A.H.; Fredslund, F.; Katayama, T.; Nielsen, S.K.; Svensson, B.; Kitaoka, M.; Lo Leggio, L.; Abou Hachem, M. A β1-6/β1-3 galactosidase from B ifidobacterium animalis subsp. lactis B l-04 gives insight into sub-specificities of β-galactoside catabolism within B ifidobacterium. Mol. Microbiol. 2014, 94, 1024–1040. [Google Scholar] [CrossRef]
- Xavier, J.R.; Ramana, K.V.; Sharma, R.K. β-galactosidase: Biotechnological applications in food processing. J. Food Biochem. 2018, 42, e12564. [Google Scholar] [CrossRef]
- Husain, Q. β Galactosidases and their potential applications: A review. Crit. Rev. Biotechnol. 2010, 30, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Dekker, P.J.T.; Koenders, D.; Bruins, M.J. Lactose-free dairy products: Market developments, production, nutrition and health benefits. Nutrients 2019, 11, 551. [Google Scholar] [CrossRef] [PubMed]
- Nivetha, A.; Mohanasrinivasan, V. Mini Review on Role of β-Galactosidase in Lactose Intolerance. In Proceedings of the OP Conference Series: Materials Science and Engineering, Vellore, Tamil Nadu, India, 2–3 May 2017; p. 022046. [Google Scholar]
- Horner, T.W.; Dunn, M.L.; Eggett, D.L.; Ogden, L.V. β-Galactosidase activity of commercial lactase samples in raw and pasteurized milk at refrigerated temperatures. J. Dairy Sci. 2011, 94, 3242–3249. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Geueke, B.; Delgado, O.; Coleman, J.; Hatti-Kaul, R. β-Galactosidase from a cold-adapted bacterium: Purification, characterization and application for lactose hydrolysis. Appl. Microbiol. Biotechnol. 2002, 58, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-X.; Gao, Y.; Hu, B.; Lu, X.-L.; Liu, X.-Y.; Jiao, B.-H. A novel cold-adapted β-galactosidase isolated from Halomonas sp. S62: Gene cloning, purification and enzymatic characterization. World J. Microbiol. Biotechnol. 2013, 29, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Vincent, V.; Aghajari, N.; Pollet, N.; Boisson, A.; Boudebbouze, S.; Haser, R.; Maguin, E.; Rhimi, M. The acid tolerant and cold-active β-galactosidase from Lactococcus lactis strain is an attractive biocatalyst for lactose hydrolysis. Antonie Leeuwenhoek 2013, 103, 701–712. [Google Scholar] [CrossRef]
- Siso, M.I.G. The biotechnological utilization of cheese whey: A review. Bioresour. Technol. 1996, 57, 1–11. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—from ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Marques Monteiro Amaro, T.M.; Rosa, F.; Comi, G.; Iacumin, L. Prospects for the use of whey for polyhydroxyalkanoate (PHA) production. Front. Microbiol. 2019, 10, 992. [Google Scholar] [CrossRef]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Potential of various archae-and eubacterial strains as industrial polyhydroxyalkanoate producers from whey. Macromol. Biosci. 2007, 7, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Dutra Rosolen, M.; Gennari, A.; Volpato, G.; Volken de Souza, C.F. Lactose hydrolysis in milk and dairy whey using microbial β-galactosidases. Enzym. Res. 2015, 1–8. [Google Scholar] [CrossRef]
- Bertelsen, H.; Eriknauer, K.; Bøttcher, K.; Christensen, H.J.S.; Stougaard, P.; Hansen, O.C.; Jørgensen, F. Process for MANUFACTURING of Tagatose. U.S. Patent 5002612A, 26 March 1991. [Google Scholar]
- Szczodrak, J. Hydrolysis of lactose in whey permeate by immobilized β-galactosidase from Kluyveromyces fragilis. J. Mol. Catal. B Enzym. 2000, 10, 631–637. [Google Scholar] [CrossRef]
- Van De Voorde, I.; Goiris, K.; Syryn, E.; Van den Bussche, C.; Aerts, G. Evaluation of the cold-active Pseudoalteromonas haloplanktis β-galactosidase enzyme for lactose hydrolysis in whey permeate as primary step of d-tagatose production. Process Biochem. 2014, 49, 2134–2140. [Google Scholar] [CrossRef]
- Huerta, L.M.; Vera, C.; Guerrero, C.; Wilson, L.; Illanes, A. Synthesis of galacto-oligosaccharides at very high lactose concentrations with immobilized β-galactosidases from Aspergillus oryzae. Process Biochem. 2011, 46, 245–252. [Google Scholar] [CrossRef]
- Martins, G.N.G.; Ureta, M.M.; Tymczyszyn, E.E.; Castilho, P.; Gomez-Zavaglia, A. Technological aspects of the production of fructo and galacto-oligosaccharides. Enzymatic synthesis and hydrolysis. Front. Nutr. 2019, 6, 78. [Google Scholar] [CrossRef]
- Petzelbauer, I.; Splechtna, B.; Nidetzky, B. Development of an ultrahigh-temperature process for the enzymatic hydrolysis of lactose. III. Utilization of two thermostable β-glycosidases in a continuous ultrafiltration membrane reactor and galacto-oligosaccharide formation under steady-state conditions. Biotechnol. Bioeng. 2002, 77, 394–404. [Google Scholar]
- Park, H.-Y.; Kim, H.-J.; Lee, J.-K.; Kim, D.; Oh, D.-K. Galactooligosaccharide production by a thermostable β-galactosidase from Sulfolobus solfataricus. World J. Microbiol. Biotechnol. 2008, 24, 1553–1558. [Google Scholar] [CrossRef]
- Ding, H.; Zhou, L.; Zeng, Q.; Yu, Y.; Chen, B. Heterologous expression of a thermostable β-1, 3-galactosidase and its potential in synthesis of galactooligosaccharides. Mar. Drugs 2018, 16, 415. [Google Scholar] [CrossRef]
- Makowski, K.; Białkowska, A.; Olczak, J.; Kur, J.; Turkiewicz, M. Antarctic, cold-adapted β-galactosidase of Pseudoalteromonas sp. 22b as an effective tool for alkyl galactopyranosides synthesis. Enzym. Microb. Technol. 2009, 44, 59–64. [Google Scholar] [CrossRef]
- Ismail, A.; Soultani, S.; Ghoul, M. Enzymatic-catalyzed synthesis of alkylglycosides in monophasic and biphasic systems. I. The transglycosylation reaction. J. Biotechnol. 1999, 69, 135–143. [Google Scholar] [CrossRef]
- Guerrero, C.; Vera, C.; Plou, F.; Illanes, A. Influence of reaction conditions on the selectivity of the synthesis of lactulose with microbial β-galactosidases. J. Mol. Catal. B Enzym. 2011, 72, 206–212. [Google Scholar] [CrossRef]
- Dong, Q.; Yan, X.; Zheng, M.; Yang, Z. Characterization of an extremely thermostable but cold-adaptive β-galactosidase from the hyperthermophilic archaeon Pyrococcus furiosus for use as a recombinant aggregation for batch lactose degradation at high temperature. J. Biosci. Bioeng. 2014, 117, 706–710. [Google Scholar] [CrossRef]
- Johnson, C.M. Differential scanning calorimetry as a tool for protein folding and stability. Arch. Biochem. Biophys. 2013, 531, 100–109. [Google Scholar] [CrossRef] [PubMed]
- King, L.; Lehrer, S.S. Thermal unfolding of myosin rod and light meromyosin: Circular dichroism and tryptophan fluorescence studies. Biochemistry 1989, 28, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Streicher, W.W.; Makhatadze, G.I. Unfolding thermodynamics of Trp-cage, a 20 residue miniprotein, studied by differential scanning calorimetry and circular dichroism spectroscopy. Biochemistry 2007, 46, 2876–2880. [Google Scholar] [CrossRef] [PubMed]
| Source | Topt (°C) | Cold Activity 1 (%) | Residual Activity (%) | Substrate Specificity 2 | References |
|---|---|---|---|---|---|
| Alteromonas sp. L82 | 40 | 9.4 (4 °C) | ~10 (3 h at 40 °C) | Cellobiose (100%) Lactobiose (3.6%) p-NP-β-d-glucopyranoside (100%) o-NP-β-d-glucopyranoside (120.7%) p-NP-β-d-cellobioside (8%) p-NP-β-d-galactopyranoside (9.2%) o-NP-β-d-galactopyranoside (13%) p-NP-β-d-xylopyranoside (0.9%) | [53] |
| Baltic sea sediment | 40–45 | 10 (5 °C) | 0 (30 min at 40 °C) | p-NP-β-d-glucopyranoside (130%) p-NP-β-d-fucopyranoside (133%) p-NP-β-d-galactopyranoside (100%) o-NP-β-d-galactopyranoside (82%) p-NP-β-d-cellobioside (59%) p-NP-β-d-xylopyranoside (4%) | [50] |
| Exiguobacterium antarcticum B7 | 30 | 25 (5 °C) | ~20 (72 h at 30 °C) | p-NP-β-d-glucopyranoside (100%) p-NP-β-d-cellobioside (50.9%) p-NP-β-d-galactopyranoside (2.3%) p-NP-α-d-glucopyranoside (1.2%) p-NP-β-d-mannopyranoside (0.5%) | [71] |
| Micrococcus antarcticus | 25 | 27 (0 °C) | ~20 (60 min at 35 °C) | p-NP-β-d-glucopyranoside (100%) p-NP-β-d-galactopyranoside (32.2%) | [72] |
| Marinomonassp. MWYL1 | 40 | 20 (5 °C) | ~75 (60 min at 40 °C) | p-NP-β-d-glucopyranoside (100%) p-NP-β-d-galactopyranoside (26.5%) | [74] |
| Source | Topt (°C) | Cold Activity 1 (%) | Residual Activity (%) | Substrate Specificity 2 | References |
|---|---|---|---|---|---|
| Alkalilactibacillus ikkense | 30 | ~60 (0 °C) | ~ 20 (5 h at 30 °C) | p-NP-β-d-galactopyranoside (100%) p-NP-β-d- fucopyranoside (4%) | [49] |
| Alteromonas sp. ANT48 | 50 | ~30 (0 °C) | ~20 (3 h at 60 °C) | o-NP-β-d-galactopyranoside (100%) p-NP-β-d-galactopyranoside (14%) | [45] |
| Alteromonassp.ML117 | 30–35 | ~20 (5 °C) | 0 (1 h at 30 °C) | o-NP-β-d-galactopyranoside (100%) p-NP-β-d-galactopyranoside (31%) | [51] |
| Alteromonassp. ML52 | 35 | ~20 (5 °C) | ~10 (1 h at 35 °C) | o-NP-β-d-galactopyranoside (100%) p-NP-β-d-galactopyranoside (12.8%) | [52] |
| Arthrobacter psychrolactophilus F2 | 10 | ~90 (0 °C) | ~20 (2 h at 35 °C) | o-NP-β-d-galactopyranoside | [58] |
| Arthrobacter sp. ON14 | 15 | ~30 (0 °C) | ~40 (2 h at 40 °C) | o-NP-β-d-galactopyranoside | [59] |
| Arthrobacter sp. 20B | 25 | ~30 (0 °C) | ~30 (1 h at 45 °C) | p-NP-β-d-galactopyranoside | [56] |
| Arthrobacter sp. 32cB | 28 | ~30 (5 °C) | ~10 (8 h at 35 °C) | p-NP-β-d-galactopyranoside (100%) p-NP-β-d-fucopyranoside (4%) | [60] |
| Arthrobacter sp. B7 | 40 | ~25 (10 °C) | ~50 (2 h at 40 °C) | p-NP-β-d-galactopyranoside (100%) p-NP-β-d-galuronide (4%) | [54] |
| Arthrobacter sp. C2–2 | 40 | ~15 (5 °C) | ~0 (1 h at 45 °C) | o-NP-β-d-galactopyranoside | [55] |
| Arthrobacter sp. SB | 18 | ~50 (0 °C) | ~50 (2 h at 40 °C) | o-NP-β-d-galactopyranoside | [57] |
| Flavobacterium sp. 4214 | 42 | ~10 (15 °C) | ~35 (2 h at 40 °C) | o-NP-β-d-galactopyranoside (100%) p-NP-β-d-fucopyranoside (39%) | [63] |
| Pseudoalteromonas haloplanktis TAE 79 | 45 | ~18 (7 °C) | 0 (1 h at 45 °C) | o-NP-β-d-galactopyranoside | [46] |
| Pseudoalteromonas sp. 22b | 40 | ~10 (0 °C) | ~90 (1 h at 40 °C) | o-NP-β-d-galactopyranoside (100%) p-NP-β-d-galuctoronide (1.5%) | [47] |
| Source | Topt (°C) | Cold Activity 1 (%) | Residual Activity (%) | Substrate Specificity 2 | References |
|---|---|---|---|---|---|
| Arthrobacter sp. 32cB | 50 | ~18 (0 °C) | N.A. | p-NP-β-d-galactopyranoside (100%) p-NP-β-d-glucopyranoside (1.4%) | [62] |
| Arthrobacter sp. B7 | 50 | ~50 (4 °C) | ~0 (15 min at 50 °C) | o-NP-β-d-galactopyranoside Lactose | [61] |
| Carnobacterium maltaromaticum | 30 | ~10 (0 °C) | ~10 (30 min at 35°C) | o-NP-β-d-galactopyranoside (100%) p-NP-β-d- fucopyranoside (10.1%) | [91] |
| Cryobacterium sp. LW097 (Bgal322) | 25 | ~60 (5 °C) | ~32 (12 h at 35 °C) | o-NP-β-d-galactopyranoside (100%) p-NP-β-d-galactopyranoside (69%)Lactose (5%) Galactobiose (100%) Lactulose (11%) Allolactose (44%) | [67] |
| Cryobacterium sp. LW097 (Bgal435) | 30 | ~60 (5 °C) | ~13 (12 h at 35 °C) | o-NP-β-d-galactopyranoside (100%) p-NP-β-d-glucopyranoside (34%)Lactose (34%) Galactobiose (6%) Lactulose (8%) Allolactose (100%) | [67] |
| Cryobacterium sp. LW097 (Bgal2567) | 35 | ~40 (5 °C) | ~14 (12 h at 35 °C) | o-NP-β-d-galactopyranoside (100%) p-NP-β-d-galactopyranoside (126%) Lactose 44%) Galactobiose (90%) Lactulose (97%) Allolactose (100%) | [67] |
| Halorubrum lacusprofundi | 50 | ~10 (<10 °C) | N.A. | o-NP-β-d-galactopyranoside | [65] |
| Marinomonassp. BSi20414 | 60 | ~10 (10 °C) | 76 (6 h at 40 °C) | p-NP-β-d-galactopyranoside | [48] |
| Marinomonassp. ef1 | 55 | 23 (5 °C) | 25 (4 days at 50 °C) | o-NP-β-d-galactopyranoside | [29] |
| Planococcus sp. L4 | 20 | ~30 (0 °C) | 20 (1 h at 40 °C) | o-NP-β-d-galactopyranoside | [97] |
| Planococcus sp. SOS orange | 40 | ~10 (0 °C) | 30 (2 h at 40 °C) | o-NP-β-d-galactopyranoside (100%) p-NP-β-d-fucopyranoside (6.1%) | [96] |
| Rahnella sp. R3 | 35 | 27 (4 °C) | ~90 (2 h at 35 °C) | o-NP-β-d-galactopyranoside (100%) Lactose | [64] |
| Topsoil of Daqing oil field | 40 | ~10 (0 °C) | ~17 (1 h at 40 °C) | o-NP-β-d-galactopyranoside (100%) p-NP-β-d-glucoronide (49.5%) p-NP-β-d-arabinoside (52.8%) p-NP-β-d-mannoside (61.3%) | [66] |
| Source | T (°C) | Time (h) | Amount of Enzyme | Hydrolysis Yield (%) | References |
|---|---|---|---|---|---|
| Alteromonassp. ML52 | 4 | 24 | 44.5 U | 90 | [52] |
| Arthrobacter psychrolactophilus F2 | 10 | 24 | 10 U/mL | 80 | [58] |
| Arthrobacter sp. ON14 | 4 | 8 | 5.08 U/mL | 100 | [59] |
| Arthrobacter sp. 32cB | 10 | 24 | 2 U/mL | 90 | [60] |
| Arthrobacter sp. SB | 2.5 | 7.5 | N.A. | 80 | [57] |
| Aspergillus oryzae (EYL) | 2 | 24 | 0.1% (w/v) | 85.23 | [105] |
| Cryobacterium sp. LW097 (Bgal322) | 4 | 48 | 5 U/mL | 8.4 | [67] |
| Cryobacterium sp. LW097 (Bgal435) | 4 | 48 | 5 U/mL | 5.1 | [67] |
| Cryobacterium sp. LW097 (Bgal436) | 4 | 48 | 5 U/mL | N.A. | [67] |
| Cryobacterium sp. LW097 (Bgal2567) | 4 | 48 | 5 U/mL | 7.8 | [67] |
| Halomonas sp. S62 | 7 | 24 | 0.15 U/10 µL | 60 | [107] |
| Kluyveromyces fragilis (LYL) | 2 | 24 | 0.1% (w/v) | 82.96 | [105] |
| Kluyveromyces lactis (DYL) | 2 | 24 | 0.1% (w/v) | 99.08 | [105] |
| Kluyveromyces lactis (VYL) | 2 | 24 | 0.8% (w/v) | 98.59 | [105] |
| Paracoccus sp. 32d | 10 | 11 | 1 U/mL | 91 | [63] |
| Planococcus sp. L4 | 5 | 1 | 2.5 ug | 36 | [97] |
| Pseudoalteromonas haloplanktis TAE 79 | 4 | 50 min | 1.3 U | 33 | [46] |
| Pseudoalteromonas sp. 79b | 10 | 8 | 1U | 40 | [106] |
| Pseudoalteromonas sp. 22b | 4 | 48 | 30 U | 65 | [85] |
| Topsoil of Daqing oil field | 4 | 1 | 1 U | 4.2 | [66] |
| Lactococcus lactis | 4 | 9 | 0.14 U | 98 | [108] |
| Immobilized | |||||
| Pseudoalteromonas sp. 22b | 4 | 24 | 30 U per g of lactose | 93 | [85] |
| Pseudoalteromonas sp. 79b | 10 | 8 | 1U | 40 | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangiagalli, M.; Lotti, M. Cold-Active β-Galactosidases: Insight into Cold Adaptation Mechanisms and Biotechnological Exploitation. Mar. Drugs 2021, 19, 43. https://doi.org/10.3390/md19010043
Mangiagalli M, Lotti M. Cold-Active β-Galactosidases: Insight into Cold Adaptation Mechanisms and Biotechnological Exploitation. Marine Drugs. 2021; 19(1):43. https://doi.org/10.3390/md19010043
Chicago/Turabian StyleMangiagalli, Marco, and Marina Lotti. 2021. "Cold-Active β-Galactosidases: Insight into Cold Adaptation Mechanisms and Biotechnological Exploitation" Marine Drugs 19, no. 1: 43. https://doi.org/10.3390/md19010043
APA StyleMangiagalli, M., & Lotti, M. (2021). Cold-Active β-Galactosidases: Insight into Cold Adaptation Mechanisms and Biotechnological Exploitation. Marine Drugs, 19(1), 43. https://doi.org/10.3390/md19010043





