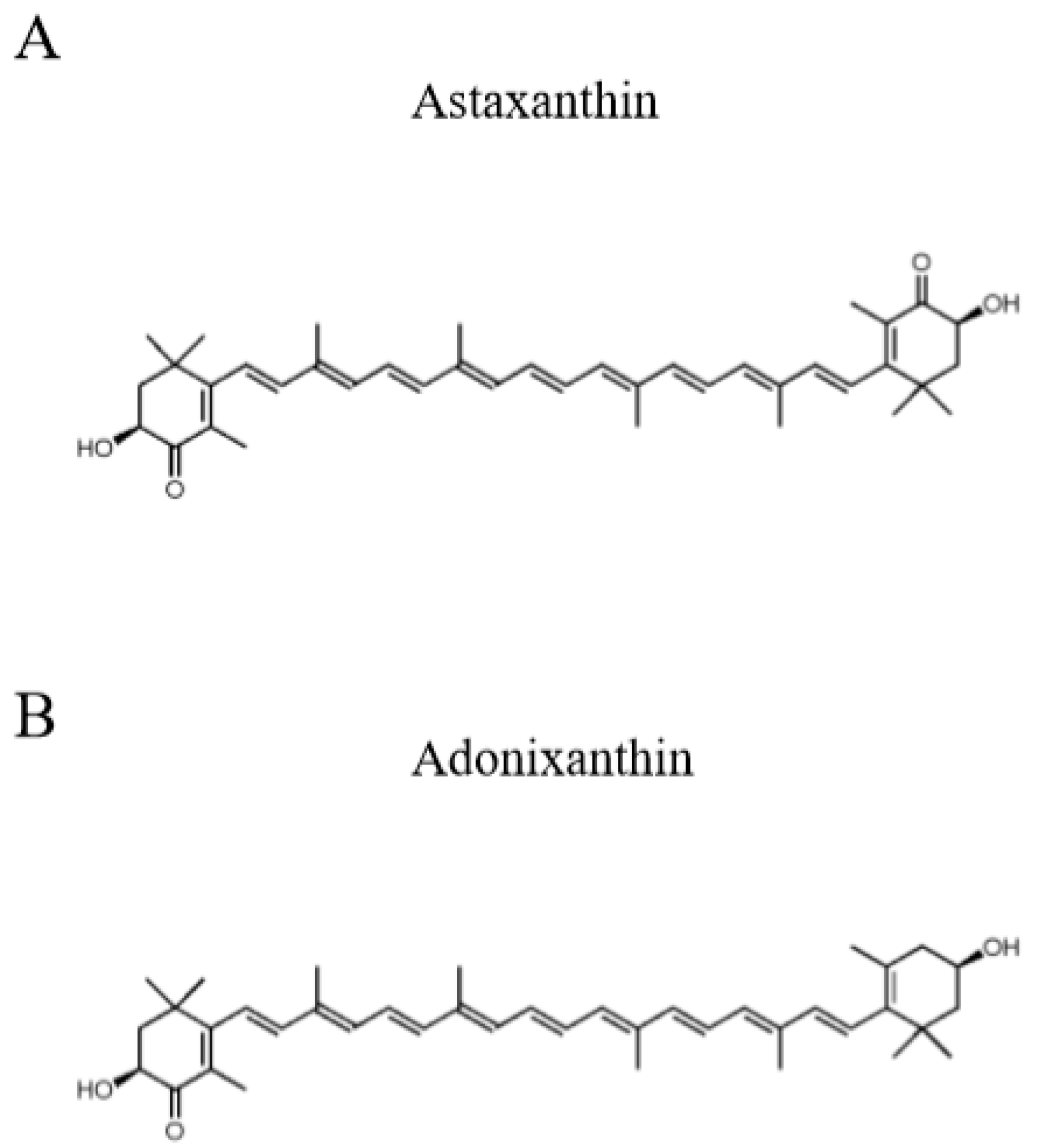
Antitumour Effects of Astaxanthin and Adonixanthin on Glioblastoma
Abstract
1. Introduction
2. Results
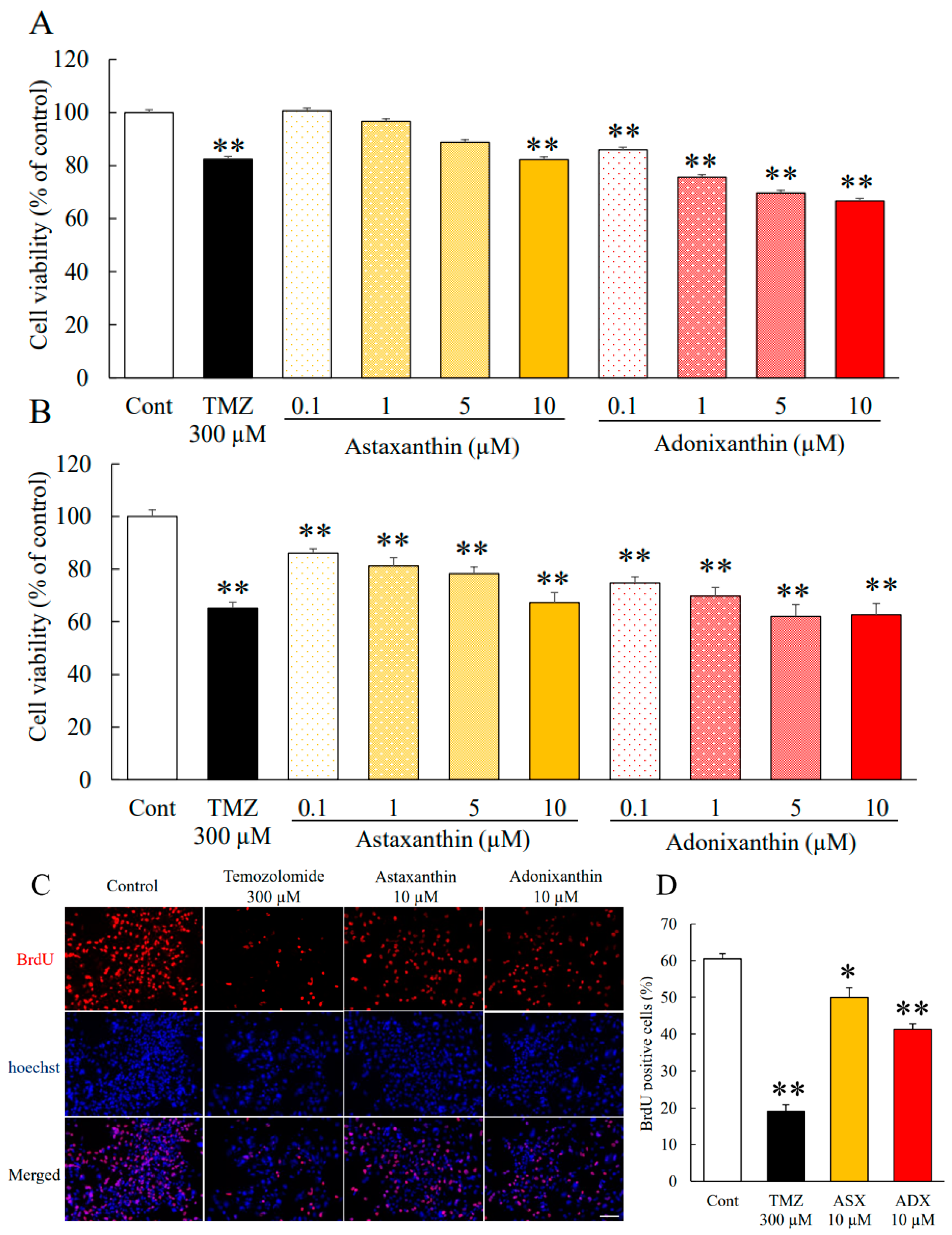
2.1. Astaxanthin and Adonixanthin Suppressed the Growth of Glioblastoma Cells
2.2. Astaxanthin and Adonixanthin Suppressed the Migration of Glioblastoma Cells
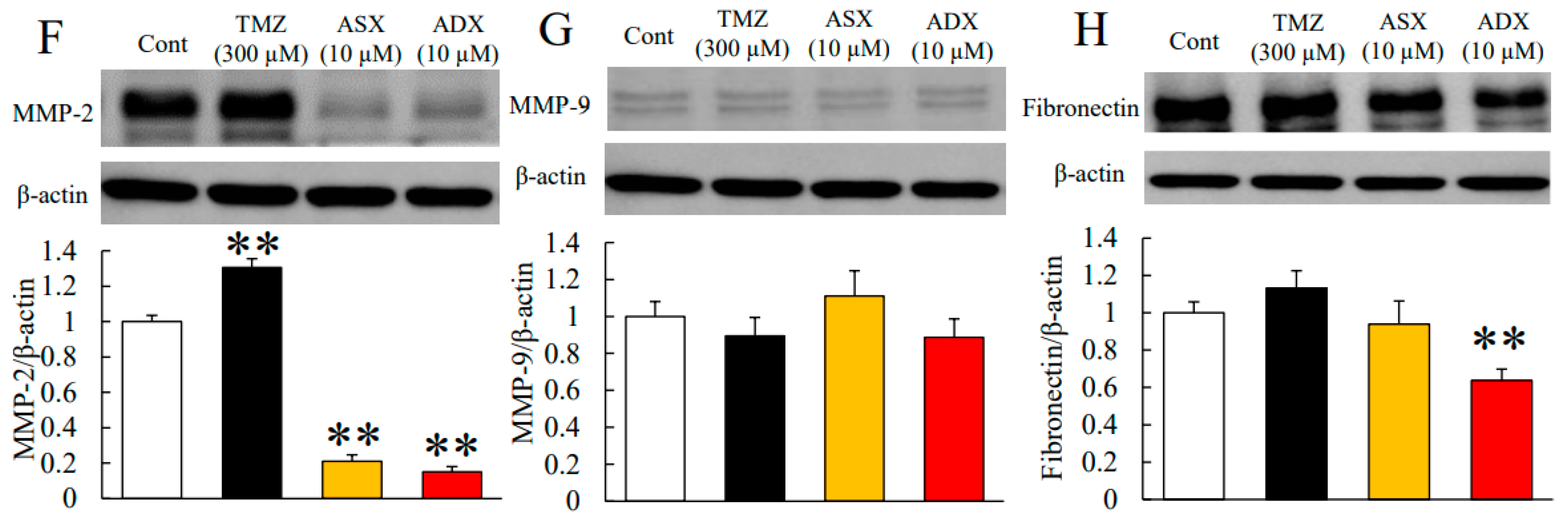
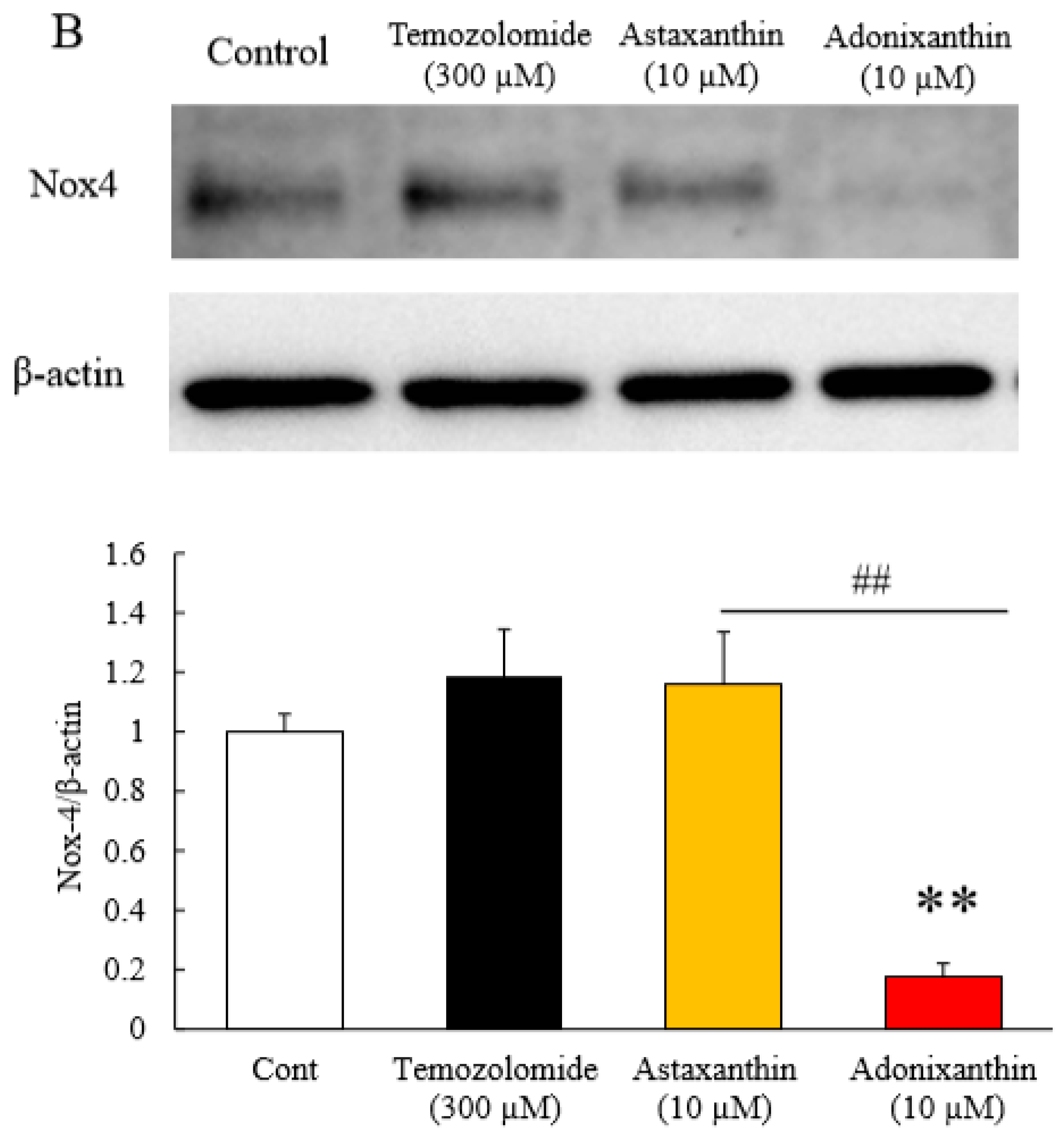
2.3. Astaxanthin and Adonixanthin Decreased the Expression of Some Proteins to Promote Cell Growth and Migration
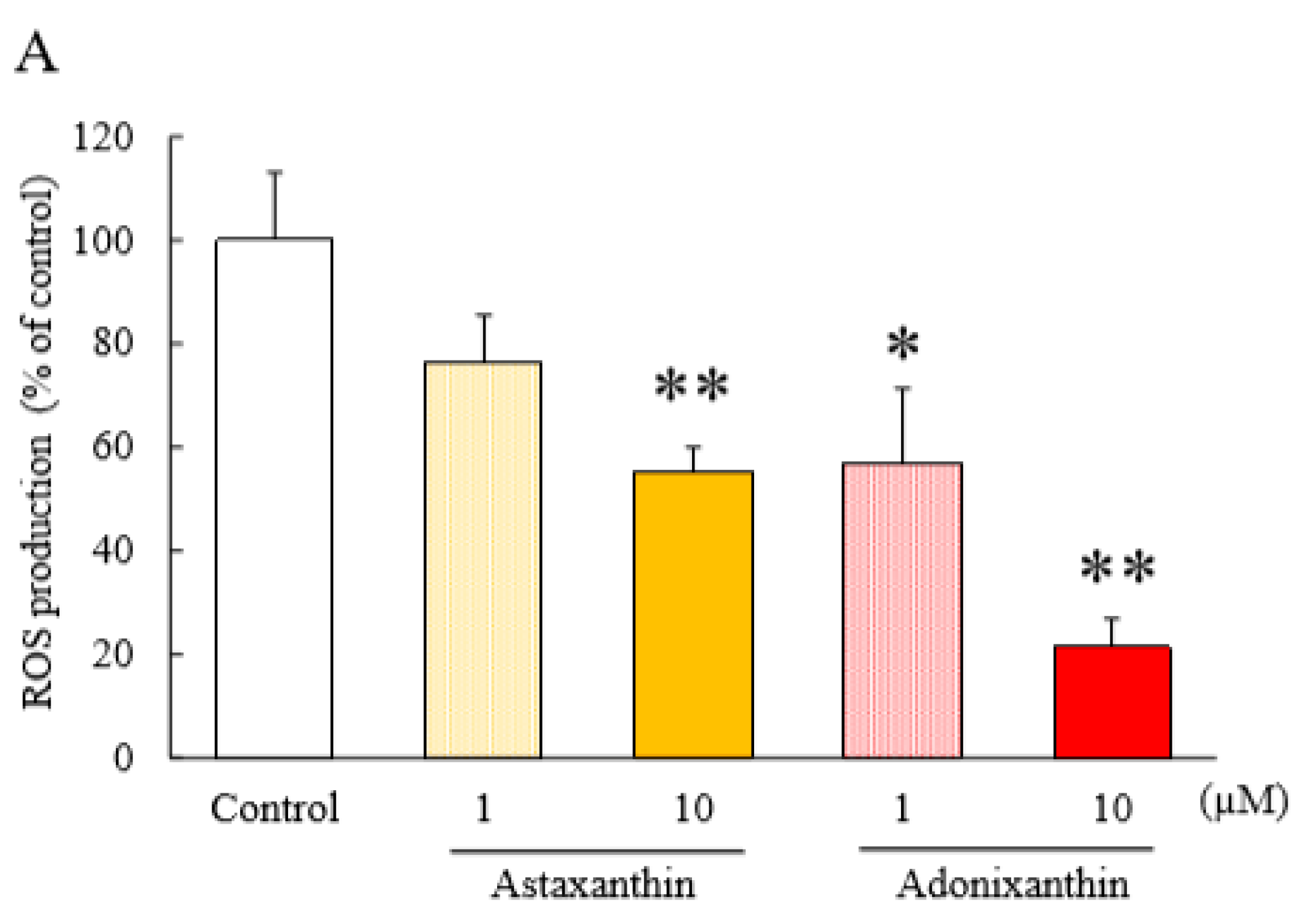
2.4. Astaxanthin and Adonixanthin Decreased the Production of Reactive Oxygen Species (ROS)
2.5. Concentrations of Astaxanthin and Adonixanthin in Murine Serum and Tissues
2.6. The Effects of Astaxanthin and Adonixanthin on Body Weight
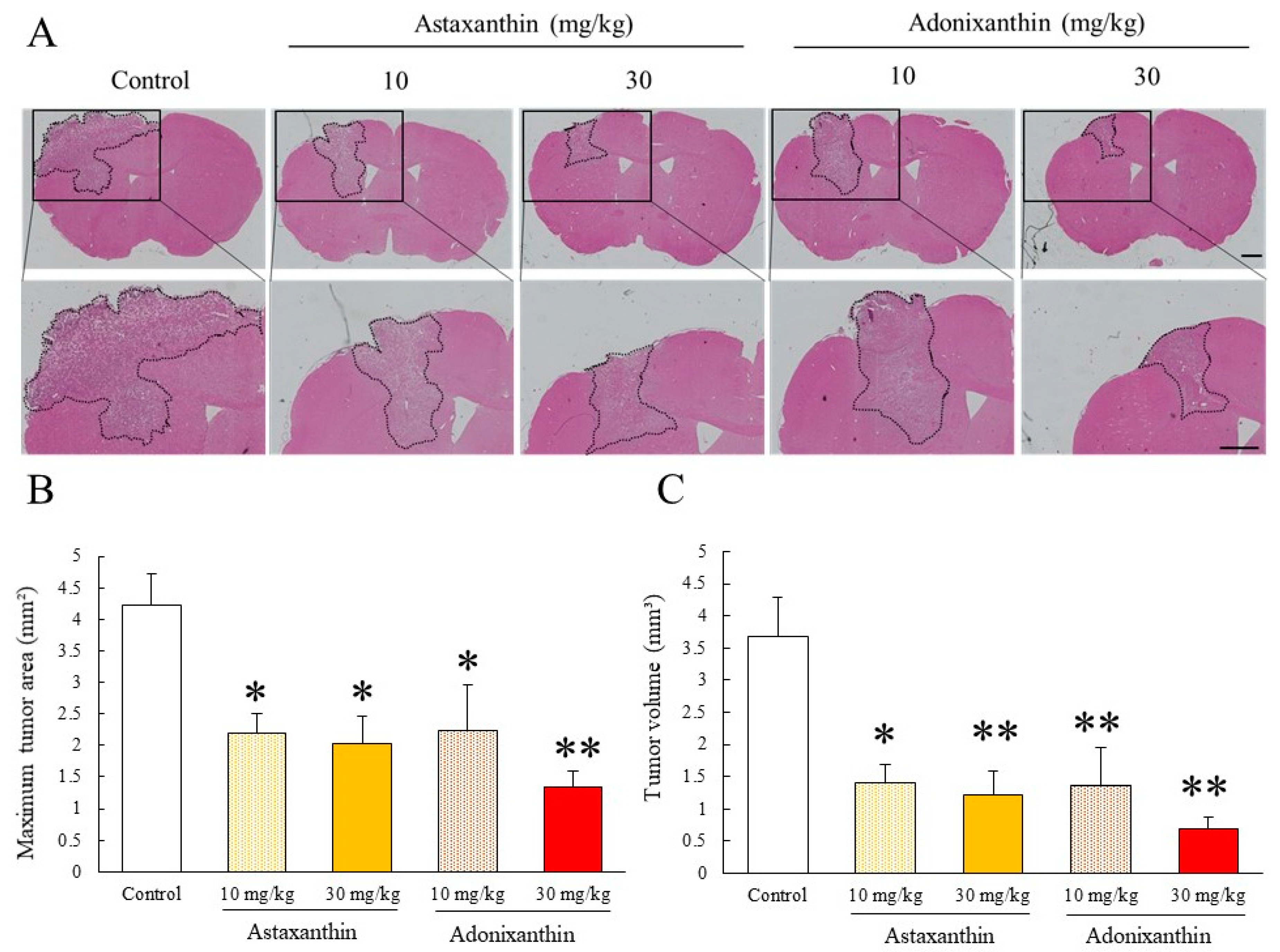
2.7. Astaxanthin and Adonixanthin Showed Antitumour Effects in a Murine Orthotopic Glioblastoma Model
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Line and Culture Condition
4.3. Cell Viability
4.4. BrdU (Bromodeoxyuridine) Cell Proliferation Assay
4.5. Cell Migration Assay
4.6. Immunoblotting
4.7. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction Analysis (qRT-PCR)
4.8. Cell Death Assay
4.9. Reactive Oxygen Species Assay
4.10. Animals
4.11. Murine orthotopic Glioblastoma Model
4.12. In Vivo Drug Treatment
4.13. Mouse Brain Analysis on In Vivo Glioblastoma Model
4.14. Collecting Blood and Tissues
4.15. Analysis of Astaxanthin and Adonixanthin
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Friedman, H.S.; Kerby, T.; Calvert, H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 2000, 6, 2585–2597. [Google Scholar]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro. Oncol. 2014, 16, iv1–iv63. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.E. Beyond the blood: Brain barrier: The importance of central nervous system (CNS) pharmacokinetics for the treatment of CNS tumors, including diffuse intrinsic pontine glioma. Front. Oncol. 2018, 8, 239. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, M.; Fu, C.; Yu, Y.; Fu, A. Targeted therapy of intracranial glioma model mice with curcumin nanoliposomes. Int. J. Nanomed. 2018, 13, 1601–1610. [Google Scholar] [CrossRef]
- Clark, P.A.; Bhattacharya, S.; Elmayan, A.; Darjatmoko, S.R.; Thuro, B.A.; Yan, M.B.; Van Ginkel, P.R.; Polans, A.S.; Kuo, J.S. Resveratrol targeting of AKT and p53 in glioblastoma and glioblastoma stem-like cells to suppress growth and infiltration. J. Neurosurg. 2017, 126, 1448–1460. [Google Scholar] [CrossRef]
- Maoka, T.; Yasui, H.; Ohmori, A.; Tokuda, H.; Suzuki, N.; Osawa, A.; Shindo, K.; Ishibashi, T. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenic activities of adonirubin and adonixanthin. J. Oleo Sci. 2013, 62, 181–186. [Google Scholar] [CrossRef]
- Miki, W.; Yamaguchi, K.; Konosu, S. Comparison of carotenoids in the ovaries of marine fish and shellfish. Comp. Biochem. Physiol. Part B Biochem. 1982, 71, 7–11. [Google Scholar] [CrossRef]
- Nakajima, Y.; Inokuchi, Y.; Shimazawa, M.; Otsubo, K.; Ishibashi, T.; Hara, H. Astaxanthin, a dietary carotenoid, protects retinal cells against oxidative stress in-vitro and in mice in-vivo. J. Pharm. Pharmacol. 2008, 60, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, Y.; Oyagi, A.; Tsuruma, K.; Shimazawa, M.; Ishibashi, T.; Hara, H. The antianxiety-like effect of astaxanthin extracted from Paracoccus carotinifaciens. BioFactors 2011, 37, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Oyagi, A.; Takahira, D.; Tsuruma, K.; Shimazawa, M.; Ishibashi, T.; Hara, H. Protective Effects of Astaxanthin from Paracoccus carotinifaciens on Murine Gastric Ulcer Models. Phyther. Res. 2012, 26, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Shimazawa, M.; Nakanishi, T.; Ohno, Y.; Inoue, Y.; Tsuruma, K.; Ishibashi, T.; Hara, H. The protective effects of a dietary carotenoid, astaxanthin, against light-induced retinal damage. J. Pharmacol. Sci. 2013, 123, 209–218. [Google Scholar] [CrossRef]
- Otsuka, T.; Shimazawa, M.; Inoue, Y.; Nakano, Y.; Ojino, K.; Izawa, H.; Tsuruma, K.; Ishibashi, T.; Hara, H. Astaxanthin Protects Against Retinal Damage: Evidence from In Vivo and In Vitro Retinal Ischemia and Reperfusion Models. Curr. Eye Res. 2016, 41, 1465–1472. [Google Scholar] [CrossRef]
- Inoue, Y.; Shimazawa, M.; Nagano, R.; Kuse, Y.; Takahashi, K.; Tsuruma, K.; Hayashi, M.; Ishibashi, T.; Maoka, T.; Hara, H. Astaxanthin analogs, adonixanthin and lycopene, activate Nrf2 to prevent light-induced photoreceptor degeneration. J. Pharmacol. Sci. 2017, 134, 147–157. [Google Scholar] [CrossRef]
- Pan, L.; Zhou, Y.; Li, X.F.; Wan, Q.J.; Yu, L.H. Preventive treatment of astaxanthin provides neuroprotection through suppression of reactive oxygen species and activation of antioxidant defense pathway after stroke in rats. Brain Res. Bull. 2017, 130, 211–220. [Google Scholar] [CrossRef]
- Iwata, S.; Imai, T.; Shimazawa, M.; Ishibashi, T.; Hayashi, M. Protective e ff ects of the astaxanthin derivative, adonixanthin, on brain hemorrhagic injury. Brain Res. 2018, 1698, 130–138. [Google Scholar] [CrossRef]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.K.; Baba, A.B.; Nagini, S. Astaxanthin inhibits NF-κB and Wnt/β-catenin signaling pathways via inactivation of Erk/MAPK and PI3K/Akt to induce intrinsic apoptosis in a hamster model of oral cancer. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4433–4444. [Google Scholar] [CrossRef]
- Tanaka, T.; Morishita, Y.; Suzui, M.; Kojima, T.; Okumura, A.; Mori, H. Chemoprevention of mouse urinary bladder carcinogenesis by the naturally occurring carotenoid astaxanthin. Carcinogenesis 1994, 15, 15–19. [Google Scholar] [CrossRef]
- Nagendraprabhu, P.; Sudhandiran, G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Investig. N. Drugs 2011, 29, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.; Hu, L.; Zhao, L.; Huang, J. Carotenoids inhibit proliferation and regulate expression of peroxisome proliferators-activated receptor gamma (PPAR c) in K562 cancer cells. Arch. Biochem. Biophys. 2011, 512, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Ong, X.S.; Hang, J.Z.; Ang, M.W.; Iu, W.L.; Xin-bin, G.U. Astaxanthin Induces Mitochondria-Mediated Apoptosis in Rat Hepatocellular Carcinoma CBRH-7919 Cells. Biol. Pharm. Bull. 2011, 34, 839–844. [Google Scholar]
- Zhang, L.; Wang, H. Multiple mechanisms of anti-cancer effects exerted by astaxanthin. Mar. Drugs 2015, 13, 4310–4330. [Google Scholar] [CrossRef] [PubMed]
- Mccubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, W.T.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Stivala, F.; et al. Roles of the RAF/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2009, 1773, 1263–1284. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 2017, 9, 52. [Google Scholar]
- Wu, W.-S.; Wu, J.-R.; Hu, C.-T. Signal cross talks for sustained MAPK activation and cell migration: The potential role of reactive oxygen species. Cancer Metastasis Rev. 2008, 27, 303–314. [Google Scholar] [CrossRef]
- Jhou, B.; Song, T.; Lee, I.; Hu, M.; Yang, N. Lycopene Inhibits Human liver adenocarcinoma SK-Hep-1 Cells Metastasis by Down Regulation of NADPH Oxidase 4 Protein Expression. J. Agric. Food Chem. 2017, 65, 6893–6903. [Google Scholar] [CrossRef]
- Hayashi, M.; Ishibashi, T.; Maoka, T. Effect of astaxanthin rich extract derived from Paracoccus carotinifaciens on cognitive function in middle aged and older individuals. J. Clin. Biochem. Nutr. 2018, 62, 195–205. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, M.; Yang, W.; Sun, F.; Xu, C.; Yin, L.; Pu, Y. Inhibition of Glucose-6-Phosphate Dehydrogenase Could Enhance 1, 4-Benzoquinone-Induced Oxidative Damage in K562 Cells. Oxid. Med. Cell. Longev. 2016, 2016, 3912515. [Google Scholar] [CrossRef]
- Zhu, W.; Xue, Y.; Liang, C.; Zhang, R.; Zhang, Z.; Li, H.; Su, D.; Liang, X.; Zhang, Y.; Huang, Q.; et al. S100A16 promotes cell proliferation and metastasis via AKT and ERK cell signaling pathways in human prostate cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 12241–12250. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Liu, Y.; Zhu, S.; Peng, X.; Li, H.; Jiao, W.; Lin, P.; Zhang, Z.; Qiu, Y.; Jin, M.; et al. Co-targeting PI3K/Akt and MAPK/ERK pathways leads to an enhanced antitumor effect on human hypopharyngeal squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2019, 145, 2921–2936. [Google Scholar] [CrossRef]
- Bi, T.; Zhu, A.; Yang, X.; Qiao, H.; Tang, J.; Liu, Y.; Lv, R. Metformin synergistically enhances antitumor activity of cisplatin in gallbladder cancer via the PI3K/AKT/ERK pathway. Cytotechnology 2018, 70, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Meco, D.; Servidei, T.; Lamorte, G.; Binda, E.; Arena, V.; Riccardi, R. Ependymoma stem cells are highly sensitive to temozolomide in vitro and in orthotopic models. Neuro. Oncol. 2014, 16, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Shono, T.; Yokoyama, N.; Uesaka, T.; Kuroda, J.; Takeya, R.; Yamasaki, T.; Amano, T.; Mizoguchi, M.; Suzuki, S.O.; Niiro, H.; et al. Enhanced expression of NADPH oxidase Nox4 in human gliomas and its roles in cell proliferation and survival. Int. J. Cancer 2008, 123, 787–792. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, J.; Yang, L.; Gu, C.; Xue, C. Thermal stability and oral absorbability of astaxanthin esters from Haematococcus pluvialis in Balb/c mice. J. Sci. Food Agric. 2019, 99, 3662–3671. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Liu, R.; Zhu, H.; Zhang, L.; Tsao, R. Bioaccessibility, Cellular Uptake, and Transport of Astaxanthin Isomers and their Antioxidative Effects in Human Intestinal Epithelial Caco-2 Cells. J. Agric. Food Chem. 2017, 65, 10223–10232. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Robel, S.; Watkins, S.; Sontheimer, H. A neurocentric perspective on glioma invasion. Nat. Rev. Neurosci. 2014, 15, 455–465. [Google Scholar] [CrossRef]
- Gao, X.; Yue, Q.; Liu, Y.; Fan, D.; Fan, K.; Li, S.; Qian, J.; Han, L.; Fang, F.; Xu, F.; et al. Image-guided chemotherapy with specifically tuned blood brain barrier permeability in glioma margins. Theranostics 2018, 8, 3126–3137. [Google Scholar] [CrossRef]
- Blanchette, M.; Tremblay, L.; Lepage, M.; Fortin, D. Impact of drug size on brain tumor and brain parenchyma delivery after a blood-brain barrier disruption. J. Cereb. Blood Flow Metab. 2014, 34, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, J. Expression in Escherichia coZi and properties of the carotene ketolase from Haematococcus pluvialis. FEMS Microbiol. Lett. 1996, 140, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.C.E. Dietary effects on carotenoid composition in the marine harpacticoid copepod Nitokra lacustris. J. Plankton Res. 2007, 29, i73–i83. [Google Scholar] [CrossRef]
- Tsuji, S.; Ohno, Y.; Nakamura, S.; Yamada, T.; Noda, Y. Temozolomide has anti-tumor e ff ects through the phosphorylation of cPLA 2 on glioblastoma cells. Brain Res. 2019, 1723, 146396. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Zhang, F.; Hu, X.-T.; Chen, J.; Tang, R.-X.; Zheng, K.-Y.; Song, Y.-J. Depression can be prevented by astaxanthin through inhibition of hippocampal inflammation in diabetic mice. Brain Res. 2017, 1657, 262–268. [Google Scholar] [CrossRef]
- Duan, J.; Gao, Y.; Zhang, X.; Wang, X.; Wang, B.; Meng, X. International Immunopharmacology CD30 ligand de fi ciency accelerates glioma progression by promoting the formation of tumor immune microenvironment. Int. Immunopharmacol. 2019, 71, 350–360. [Google Scholar] [CrossRef]
- Maoka, T. Structural studies of carotenoids in plants, animals, and food products. In Carotenoids; Wiley: Hoboken, NJ, USA, 2016; pp. 103–129. [Google Scholar]


| Astaxanthin | Adonixanthin | |||
|---|---|---|---|---|
| Compounds | Pre-treatment | 4 h After the Final Oral Administration | Pre-treatment | 4 h after the Final Oral Administration |
| trans-Astaxanthin | N.D. | 6.04 ± 1.16 | ||
| cis-Astaxanthin | N.D. | 13.4 ± 3.78 | ||
| trans-Adonixanthin | N.D. | 34.7 ± 3.00 | ||
| cis-Adonixanthin | N.D. | 35.02 ± 5.01 | ||
| Brain Tissues | Astaxanthin | Adonixanthin | ||
|---|---|---|---|---|
| trans | cis | trans | cis | |
| Cerebral cortex | 5.22 ± 0.87 | 3.62 ± 1.56 | 3.24 ± 0.44 * | N.D. |
| Cerebellum | 5.06 ± 1.85 | 2.43 ± 1.58 | 2.85 ± 1.05 | N.D. |
| Striatum | 11.37 ± 3.14 | 3.76 ± 2.06 | 2.90 ± 1.87 | N.D. |
| Hippocampus | 12.28 ± 1.07 * | 2.61 ± 1.33 | 3.63 ± 2.10 | N.D. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, S.; Nakamura, S.; Maoka, T.; Yamada, T.; Imai, T.; Ohba, T.; Yako, T.; Hayashi, M.; Endo, K.; Saio, M.; et al. Antitumour Effects of Astaxanthin and Adonixanthin on Glioblastoma. Mar. Drugs 2020, 18, 474. https://doi.org/10.3390/md18090474
Tsuji S, Nakamura S, Maoka T, Yamada T, Imai T, Ohba T, Yako T, Hayashi M, Endo K, Saio M, et al. Antitumour Effects of Astaxanthin and Adonixanthin on Glioblastoma. Marine Drugs. 2020; 18(9):474. https://doi.org/10.3390/md18090474
Chicago/Turabian StyleTsuji, Shohei, Shinsuke Nakamura, Takashi Maoka, Tetsuya Yamada, Takahiko Imai, Takuya Ohba, Tomohiro Yako, Masahiro Hayashi, Ken Endo, Masanao Saio, and et al. 2020. "Antitumour Effects of Astaxanthin and Adonixanthin on Glioblastoma" Marine Drugs 18, no. 9: 474. https://doi.org/10.3390/md18090474
APA StyleTsuji, S., Nakamura, S., Maoka, T., Yamada, T., Imai, T., Ohba, T., Yako, T., Hayashi, M., Endo, K., Saio, M., Hara, H., & Shimazawa, M. (2020). Antitumour Effects of Astaxanthin and Adonixanthin on Glioblastoma. Marine Drugs, 18(9), 474. https://doi.org/10.3390/md18090474




