On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer
Abstract
1. Introduction
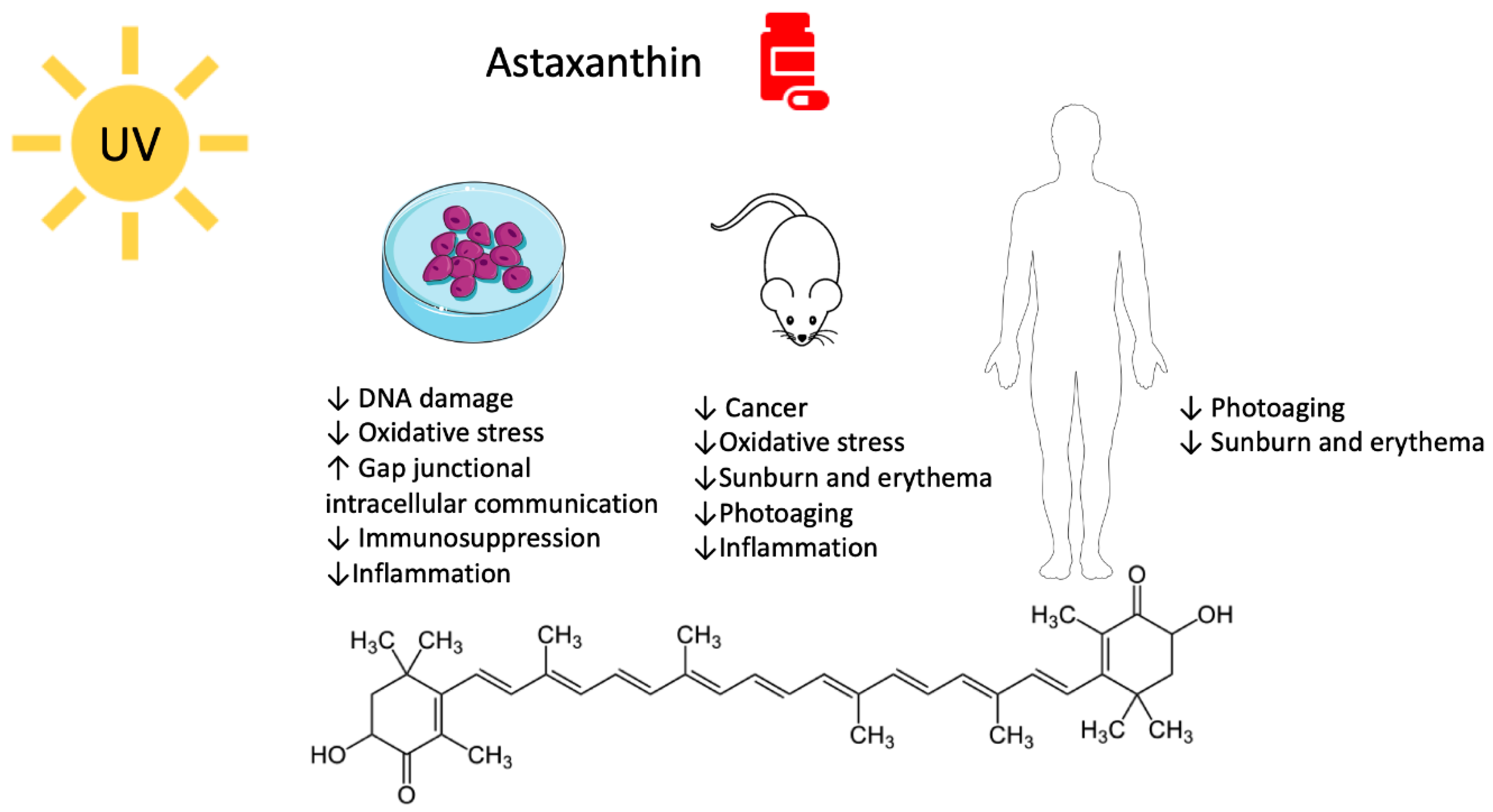
2. Astaxanthin
2.1. Astaxanthin and UV-Mediated Skin Cancer
2.2. Astaxanthin, Photodamage, and Photoaging
2.2.1. Pre-Clinical Studies
2.2.2. Clinical Studies
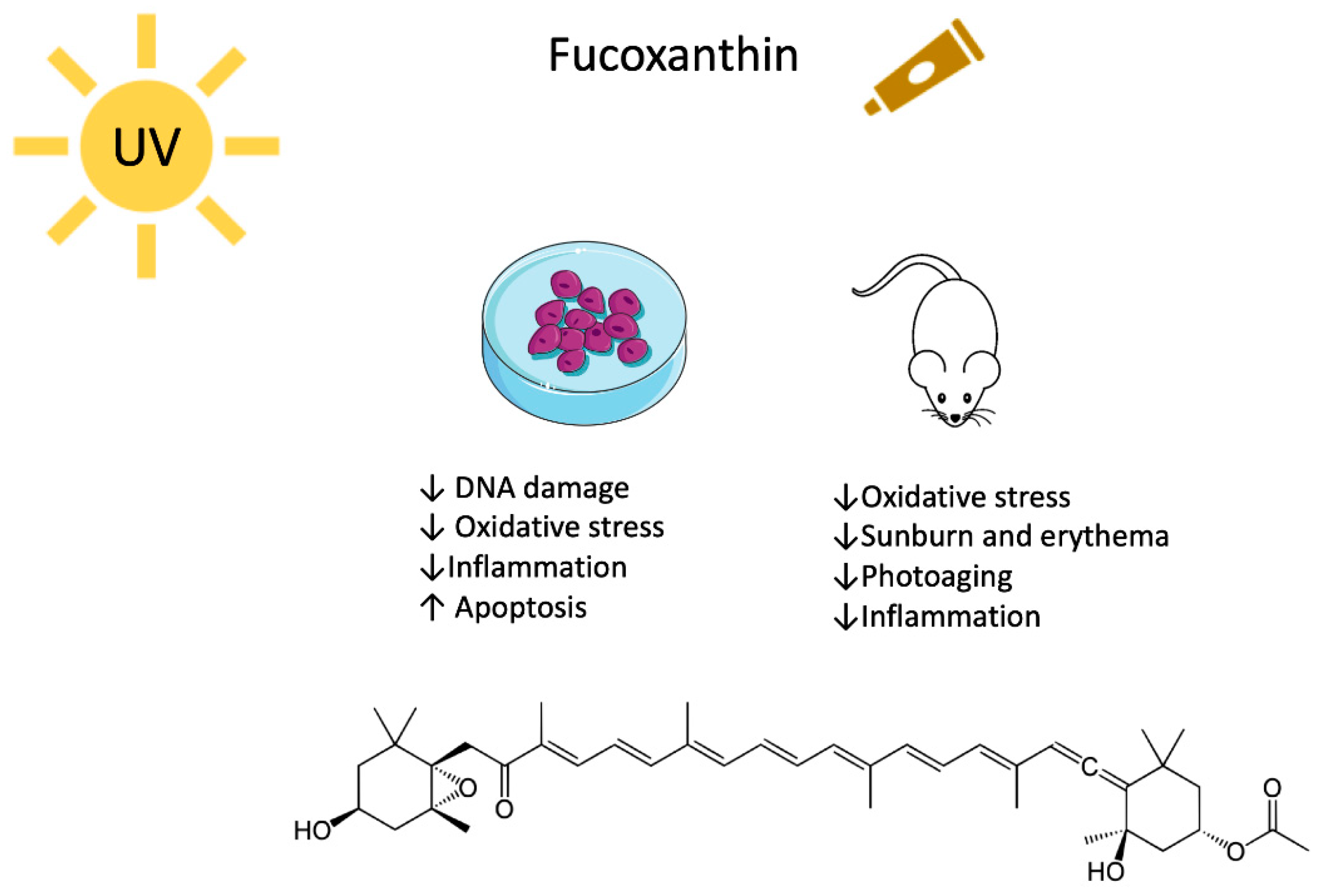
3. Fucoxanthin
3.1. Fucoxanthin and UV-Mediated Skin Cancer
3.2. Fucoxanthin, Photodamage and Photoaging
4. Current Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Solar UV Index: A Practical Guide. Available online: https://www.who.int/uv/publications/en/UVIGuide.pdf (accessed on 7 May 2020).
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Eller, M.S.; Ostrom, K.; Gilchrest, B.A. DNA damage enhances melanogenesis. Proc. Natl. Acad. Sci. USA 1996, 93, 1087–1092. [Google Scholar] [CrossRef]
- Damian, D.L.; Barnetson, R.S.; Halliday, G.M. Effects of low-dose ultraviolet radiation on in vivo human cutaneous recall responses. Aust. J. Dermatol. 2001, 42, 161–167. [Google Scholar] [CrossRef]
- Lyons, N.M.; O’Brien, N.M. Modulatory effects of an algal extract containing astaxanthin on UVA-irradiated cells in culture. J. Dermatol. Sci. 2002, 30, 73–84. [Google Scholar] [CrossRef]
- Ballard, R. The Astonishing Hidden World of the Deep Ocean. Available online: https://www.ted.com/talks/robert_ballard_the_astonishing_hidden_world_of_the_deep_ocean (accessed on 28 April 2020).
- Catanzaro, E.; Calcabrini, C.; Bishayee, A.; Fimognari, C. Antitumor Potential of Marine and Freshwater Lectins. Mar. Drugs 2019, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A. Natural antioxidants in foods. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 335–342. ISBN 978-0-12-227410-7. [Google Scholar]
- Hammond, B.R.; Renzi, L.M. Carotenoids. Adv. Nutr. Bethesda Md 2013, 4, 474–476. [Google Scholar] [CrossRef]
- Merhan, O. The biochemistry and antioxidant properties of carotenoids. In Carotenoids; IntechOpen: Rijeka, Croatia, 2017; pp. 51–66. [Google Scholar]
- Shimoda, H.; Tanaka, J.; Shan, S.-J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other Nutrients from Haematococcus pluvialis-Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef]
- Wong, S.K.; Ima-Nirwana, S.; Chin, K.-Y. Effects of astaxanthin on the protection of muscle health (Review). Exp. Ther. Med. 2020, 20, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Genç, Y.; Bardakci, H.; Yücel, Ç.; Karatoprak, G.Ş.; Küpeli Akkol, E.; Hakan Barak, T.; Sobarzo-Sánchez, E. Oxidative Stress and Marine Carotenoids: Application by Using Nanoformulations. Mar. Drugs 2020, 18, 423. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Tang, N.; Kord-Varkaneh, H.; Low, T.Y.; Tan, S.C.; Wu, X.; Zhu, Y. The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: A meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 161, 105113. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, C.; Wu, J. Astaxanthin in Liver Health and Disease: A Potential Therapeutic Agent. Drug Des. Devel. Ther. 2020, 14, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Mol. Basel Switz. 2020, 25, 3152. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Begum, S.; Cianci, M.; Durbeej, B.; Falklöf, O.; Hädener, A.; Helliwell, J.R.; Helliwell, M.; Regan, A.C.; Watt, C.I.F. On the origin and variation of colors in lobster carapace. Phys. Chem. Chem. Phys. 2015, 17, 16723–16732. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A. Molecular design of the photosystem II light-harvesting antenna: Photosynthesis and photoprotection. J. Exp. Bot. 2005, 56, 365–373. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Recent Process in Biotechnologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. ISBN 978-0-444-63784-0. [Google Scholar]
- Schoefs, B.; Rmiki, N.-E.; Rachadi, J.; Lemoine, Y. Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids. FEBS Lett. 2001, 500, 125–128. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Hydrolysis kinetics of astaxanthin esters and stability of astaxanthin of Haematococcus pluvialis during saponification. J. Agric. Food Chem. 1999, 47, 31–35. [Google Scholar] [CrossRef]
- Stewart, J.S.; Lignell, A.; Pettersson, A.; Elfving, E.; Soni, M.G. Safety assessment of Astaxanthin-rich microalgae biomass: Acute and subchronic toxicity studies in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 3030–3036. [Google Scholar] [CrossRef]
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef]
- Palozza, P.; Serini, S.; Di Nicuolo, F.; Piccioni, E.; Calviello, G. Prooxidant effects of beta-carotene in cultured cells. Mol. Aspects Med. 2003, 24, 353–362. [Google Scholar] [CrossRef]
- Martin, H.-D.; Jäger, C.; Ruck, C.; Schmidt, M.; Walsh, R.; Paust, J. Anti- and Prooxidant Properties of Carotenoids. J. Für Prakt. Chem. 1999, 341, 302–308. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Maoka, T.; Yasui, H.; Ohmori, A.; Tokuda, H.; Suzuki, N.; Osawa, A.; Shindo, K.; Ishibashi, T. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenic activities of adonirubin and adonixanthin. J. Oleo Sci. 2013, 62, 181–186. [Google Scholar] [CrossRef]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. J. Clin. Ther. 2011, 16, 355–364. [Google Scholar]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta 2001, 1512, 251–258. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58D–68D. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ananthaswamy, H.N. Short-term and long-term cellular and molecular events following UV irradiation of skin: Implications for molecular medicine. Expert Rev. Mol. Med. 2002, 4, 1–22. [Google Scholar] [CrossRef]
- Tang, X.; Kim, A.L.; Feith, D.J.; Pegg, A.E.; Russo, J.; Zhang, H.; Aszterbaum, M.; Kopelovich, L.; Epstein, E.H.; Bickers, D.R.; et al. Ornithine decarboxylase is a target for chemoprevention of basal and squamous cell carcinomas in Ptch1+/- mice. J. Clin. Investig. 2004, 113, 867–875. [Google Scholar] [CrossRef]
- Smith, M.K.; Trempus, C.S.; Gilmour, S.K. Co-operation between follicular ornithine decarboxylase and v-Ha-ras induces spontaneous papillomas and malignant conversion in transgenic skin. Carcinogenesis 1998, 19, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Savouré, N.; Briand, G.; Amory-Touz, M.C.; Combre, A.; Maudet, M.; Nicol, M. Vitamin A status and metabolism of cutaneous polyamines in the hairless mouse after UV irradiation: Action of beta-carotene and astaxanthin. Int. J. Vitam. Nutr. Res. Int. Z. Vitam. Ernahrungsforschung J. Int. Vitaminol. Nutr. 1995, 65, 79–86. [Google Scholar]
- Yoshihisa, Y.; Rehman, M.U.; Shimizu, T. Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Exp. Dermatol. 2014, 23, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Wiraguna, A.A.G.P.; Pangkahila, W.; Astawa, I.N.M. Antioxidant properties of topical Caulerpa sp. extract on UVB-induced photoaging in mice. Dermatol. Rep. 2018, 10, 7597. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef]
- Poswig, A.; Wenk, J.; Brenneisen, P.; Wlaschek, M.; Hommel, C.; Quel, G.; Faisst, K.; Dissemond, J.; Krieg, T.; Scharffetter-Kochanek, K.; et al. Adaptive antioxidant response of manganese-superoxide dismutase following repetitive UVA irradiation. J. Investig. Dermatol. 1999, 112, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Meewes, C.; Brenneisen, P.; Wenk, J.; Kuhr, L.; Ma, W.; Alikoski, J.; Poswig, A.; Krieg, T.; Scharffetter-Kochanek, K. Adaptive antioxidant response protects dermal fibroblasts from UVA-induced phototoxicity. Free Radic. Biol. Med. 2001, 30, 238–247. [Google Scholar] [CrossRef]
- Fimognari, C.; Lenzi, M.; Ferruzzi, L.; Turrini, E.; Scartezzini, P.; Poli, F.; Gotti, R.; Guerrini, A.; Carulli, G.; Ottaviano, V.; et al. Mitochondrial pathway mediates the antileukemic effects of hemidesmus indicus, a promising botanical drug. PLoS ONE 2011, 6, e21544. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10 (Suppl. 1), S4. [Google Scholar] [CrossRef]
- Baum, A.; Cohen, L. Successful behavioral interventions to prevent cancer: The example of skin cancer. Annu. Rev. Public Health 1998, 19, 319–333. [Google Scholar] [CrossRef]
- Chintong, S.; Phatvej, W.; Rerk-Am, U.; Waiprib, Y.; Klaypradit, W. In vitro antioxidant, antityrosinase, and cytotoxic activities of Astaxanthin from Shrimp Waste. Antioxid. Basel Switz. 2019, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Cuello-López, J.; Fidalgo-Zapata, A.; López-Agudelo, L.; Vásquez-Trespalacios, E. Platelet-to-lymphocyte ratio as a predictive factor of complete pathologic response to neoadjuvant chemotherapy in breast cancer. PLoS ONE 2018, 13, e0207224. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.S.; Fernandes, P.C.; Silva, M.J.B.; Lima, V.C.; Fontes, W.; Freitas-Junior, R.; Eterovic, A.K.; Forget, P. The neutrophil-to-lymphocyte ratio: A narrative review. Ecancermedicalscience 2016, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Rangwala, S.; Tsai, K.Y. Roles of the immune system in skin cancer. Br. J. Dermatol. 2011, 165, 953–965. [Google Scholar] [CrossRef]
- Hart, P.H.; Norval, M. Ultraviolet radiation-induced immunosuppression and its relevance for skin carcinogenesis. Photochem. Photobiol. Sci. 2018, 17, 1872–1884. [Google Scholar] [CrossRef]
- Chew, B.P.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Park, J.S. Dietary astaxanthin enhances immune response in dogs. Vet. Immunol. Immunopathol. 2011, 140, 199–206. [Google Scholar] [CrossRef]
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Chew, B.P. Astaxanthin stimulates cell-mediated and humoral immune responses in cats. Vet. Immunol. Immunopathol. 2011, 144, 455–461. [Google Scholar] [CrossRef]
- Okai, Y.; Higashi-Okai, K. Possible immunomodulating activities of carotenoids in in vitro cell culture experiments. Int. J. Immunopharmacol. 1996, 18, 753–758. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- Kurihara, H.; Koda, H.; Asami, S.; Kiso, Y.; Tanaka, T. Contribution of the antioxidative property of astaxanthin to its protective effect on the promotion of cancer metastasis in mice treated with restraint stress. Life Sci. 2002, 70, 2509–2520. [Google Scholar] [CrossRef]
- Jyonouchi, H.; Sun, S.; Iijima, K.; Gross, M.D. Antitumor activity of astaxanthin and its mode of action. Nutr. Cancer 2000, 36, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Krutovskikh, V.A.; Piccoli, C.; Yamasaki, H.; Yamasaki, H. Gap junction intercellular communication propagates cell death in cancerous cells. Oncogene 2002, 21, 1989–1999. [Google Scholar] [CrossRef]
- Mesnil, M.; Crespin, S.; Avanzo, J.-L.; Zaidan-Dagli, M.-L. Defective gap junctional intercellular communication in the carcinogenic process. Biochim. Biophys. Acta 2005, 1719, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. The role of carotenoids and retinoids in gap junctional communication. Int. J. Vitam. Nutr. Res. Int. Z. Vitam. Ernahrungsforschung J. Int. Vitaminol. Nutr. 1998, 68, 354–359. [Google Scholar]
- Daubrawa, F.; Sies, H.; Stahl, W. Astaxanthin diminishes gap junctional intercellular communication in primary human fibroblasts. J. Nutr. 2005, 135, 2507–2511. [Google Scholar] [CrossRef]
- Hix, L.M.; Frey, D.A.; McLaws, M.D.; Østerlie, M.; Lockwood, S.F.; Bertram, J.S. Inhibition of chemically-induced neoplastic transformation by a novel tetrasodium diphosphate astaxanthin derivative. Carcinogenesis 2005, 26, 1634–1641. [Google Scholar] [CrossRef]
- Zhang, L.X.; Acevedo, P.; Guo, H.; Bertram, J.S. Upregulation of gap junctional communication and connexin43 gene expression by carotenoids in human dermal fibroblasts but not in human keratinocytes. Mol. Carcinog. 1995, 12, 50–58. [Google Scholar] [CrossRef]
- Black, H.S. Radical interception by carotenoids and effects on UV carcinogenesis. Nutr. Cancer 1998, 31, 212–217. [Google Scholar] [CrossRef]
- Spiller, G.A.; Dewell, A. Safety of an Astaxanthin-rich Haematococcus pluvialis algal extract: A randomized clinical trial. J. Med. Food 2003, 6, 51–56. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, e05993. [Google Scholar] [CrossRef]
- Khalil, C.; Shebaby, W. UVB damage onset and progression 24 h post exposure in human-derived skin cells. Toxicol. Rep. 2017, 4, 441–449. [Google Scholar] [CrossRef]
- Hu, F.; Liu, W.; Yan, L.; Kong, F.; Wei, K. Optimization and characterization of poly(lactic-co-glycolic acid) nanoparticles loaded with astaxanthin and evaluation of anti-photodamage effect in vitro. R. Soc. Open Sci. 2019, 6, 191184. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.G.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta BBA Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Grandjean-Laquerriere, A.; Gangloff, S.C.; Le Naour, R.; Trentesaux, C.; Hornebeck, W.; Guenounou, M. Relative contribution of NF-kappaB and AP-1 in the modulation by curcumin and pyrrolidine dithiocarbamate of the UVB-induced cytokine expression by keratinocytes. Cytokine 2002, 18, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Abe, R.; Yoshihisa, Y.; Makino, T.; Matsunaga, K.; Nishihira, J.; Shimizu, H.; Shimizu, T. Deficient deletion of apoptotic cells by macrophage migration inhibitory factor (MIF) overexpression accelerates photocarcinogenesis. Carcinogenesis 2009, 30, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Joseph, J.; Feix, J.; Hogg, N.; Kalyanaraman, B. Nitration and oxidation of a hydrophobic tyrosine probe by peroxynitrite in membranes: comparison with nitration and oxidation of tyrosine by peroxynitrite in aqueous solution. Biochemistry 2001, 40, 7675–7686. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of Astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.S.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef]
- Imokawa, G.; Nakajima, H.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging II: Over-expression of neprilysin plays an essential role. Int. J. Mol. Sci. 2015, 16, 7776–7795. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A.; Yaar, M. Ageing and photoageing of the skin: Observations at the cellular and molecular level. Br. J. Dermatol. 1992, 127 (Suppl. 41), 25–30. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A. A review of skin ageing and its medical therapy. Br. J. Dermatol. 1996, 135, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Terazawa, S.; Niwano, T.; Yamamoto, Y.; Imokawa, G. The inhibitory effects of anti-oxidants on ultraviolet-induced up-regulation of the wrinkling-inducing enzyme neutral endopeptidase in human fibroblasts. PLoS ONE 2016, 11, e0161580. [Google Scholar] [CrossRef]
- Redmond, R.W.; Kochevar, I.E. Spatially resolved cellular responses to singlet oxygen. Photochem. Photobiol. 2006, 82, 1178–1186. [Google Scholar] [CrossRef]
- Hama, S.; Takahashi, K.; Inai, Y.; Shiota, K.; Sakamoto, R.; Yamada, A.; Tsuchiya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective effects of topical application of a poorly soluble antioxidant astaxanthin liposomal formulation on ultraviolet-induced skin damage. J. Pharm. Sci. 2012, 101, 2909–2916. [Google Scholar] [CrossRef]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive effect of dietary astaxanthin on UVA-induced skin photoaging in hairless mice. PLoS ONE 2017, 12, e0171178. [Google Scholar] [CrossRef]
- Nauroy, P.; Nyström, A. Kallikreins: Essential epidermal messengers for regulation of the skin microenvironment during homeostasis, repair and disease. Matrix Biol. Plus 2020, 6–7, 100019. [Google Scholar] [CrossRef]
- Elias, P.M.; Williams, M.L.; Choi, E.-H.; Feingold, K.R. Role of cholesterol sulfate in epidermal structure and function: Lessons from X-linked ichthyosis. Biochim. Biophys. Acta 2014, 1841, 353–361. [Google Scholar] [CrossRef]
- Nakahigashi, K.; Kabashima, K.; Ikoma, A.; Verkman, A.S.; Miyachi, Y.; Hara-Chikuma, M. Upregulation of aquaporin-3 is involved in keratinocyte proliferation and epidermal hyperplasia. J. Investig. Dermatol. 2011, 131, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Matsumoto, T.; Takuwa, M.; Saeed Ebrahim Shaiku Ali, M.; Hirabashi, T.; Kondo, H.; Fujino, H. Protective effects of astaxanthin supplementation against ultraviolet-induced photoaging in hairless mice. Biomedicines 2020, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Xu, Y.; Yu, Z.; Wang, X.; Cai, Y.; Zheng, H.; Li, W.; Zhang, W. Protective effect of fat extract on UVB-induced photoaging in vitro and in vivo. Oxid. Med. Cell. Longev. 2019, 2019, e6146942. [Google Scholar] [CrossRef]
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=201.327 (accessed on 12 July 2020).
- Ito, N.; Seki, S.; Ueda, F. The protective role of Astaxanthin for UV-induced skin deterioration in healthy people-a randomized, double-blind, placebo-controlled trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Grafico della variazione annuale dell’indice UV massimo annuale (valore di analisi). Available online: https://www.data.jma.go.jp/gmd/env/uvhp/link_uvindex_month54.html (accessed on 9 May 2020).
- Yamashita, E.Y. The effects of a dietary supplement containing Astaxanthin on skin condition. Carot. Sci. 2006, 10, 6. [Google Scholar]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous Astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Cho, H.H.; Cho, S.; Lee, S.-R.; Shin, M.-H.; Chung, J.H. Supplementating with dietary astaxanthin combined with collagen hydrolysate improves facial elasticity and decreases matrix metalloproteinase-1 and -12 expression: A comparative study with placebo. J. Med. Food 2014, 17, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; De Deyn, M.L.Z.Q.; Loke, W.; Foo, N.X.; Chan, H.W.; Yeo, W.S. Effects of Astaxanthin supplementation on skin health: A systematic review of clinical studies. J. Diet. Suppl. 2020, 1–14. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A promising medicinal and nutritional ingredient. Evid. Based Complement. Altern. Med. ECAM 2015, 2015, 723515. [Google Scholar] [CrossRef] [PubMed]
- Sachindra, N.M.; Sato, E.; Maeda, H.; Hosokawa, M.; Niwano, Y.; Kohno, M.; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem. 2007, 55, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Luna, A.; Ávila-Román, J.; González-Rodríguez, M.L.; Cózar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Fucoxanthin-containing cream prevents epidermal hyperplasia and UVB-induced skin erythema in mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, M.; Fukunaga, A.; Washio, K.; Taguchi, K.; Oda, Y.; Ogura, K.; Nishigori, C. Anti-Inflammatory role of langerhans cells and apoptotic keratinocytes in ultraviolet-B-Induced cutaneous inflammation. J. Immunol. Baltim. Md 1950 2017, 199, 2937–2947. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin and Rosmarinic acid combination has anti-Inflammatory effects through regulation of NLRP3 inflammasome in UVB-exposed HaCaT keratinocytes. Mar. Drugs 2019, 17, 451. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Apetoh, L.; Tesniere, A.; Aymeric, L.; Ma, Y.; Ortiz, C.; Vermaelen, K.; Panaretakis, T.; Mignot, G.; Ullrich, E.; et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β–dependent adaptive immunity against tumors. Nat. Med. 2009. [Google Scholar] [CrossRef]
- Leerach, N.; Yakaew, S.; Phimnuan, P.; Soimee, W.; Nakyai, W.; Luangbudnark, W.; Viyoch, J. Effect of Thai banana (Musa AA group) in reducing accumulation of oxidation end products in UVB-irradiated mouse skin. J. Photochem. Photobiol. B 2017, 168, 50–58. [Google Scholar] [CrossRef]
- Tavares, R.S.N.; Kawakami, C.M.; de Pereira, K.C.; do Amaral, G.T.; Benevenuto, C.G.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Gaspar, L.R. Fucoxanthin for topical administration, a phototoxic vs. photoprotective potential in a tiered strategy assessed by In vitro methods. Antioxidants 2020, 9, 328. [Google Scholar] [CrossRef]
- El-Abaseri, T.B.; Putta, S.; Hansen, L.A. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis 2006, 27, 225–231. [Google Scholar] [CrossRef]
- Heo, S.-J.; Jeon, Y.-J. Protective effect of Fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, M.; Hoefnagel, J.; Westendorp, R.; Vermeer, B.-J.; Bouwes Bavinck, J.N. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment. Cell Res. 2004, 17, 225–229. [Google Scholar] [CrossRef]
- Bastonini, E.; Kovacs, D.; Picardo, M. Skin pigmentation and pigmentary disorders: Focus on epidermal/dermal cross-talk. Ann. Dermatol. 2016, 28, 279–289. [Google Scholar] [CrossRef]
- Matsui, M.; Tanaka, K.; Higashiguchi, N.; Okawa, H.; Yamada, Y.; Tanaka, K.; Taira, S.; Aoyama, T.; Takanishi, M.; Natsume, C.; et al. Protective and therapeutic effects of fucoxanthin against sunburn caused by UV irradiation. J. Pharmacol. Sci. 2016, 132, 55–64. [Google Scholar] [CrossRef]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H.I. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Urikura, I.; Sugawara, T.; Hirata, T. Protective effect of Fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci. Biotechnol. Biochem. 2011, 75, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Kajiya, K.; Ishiwata, M.; Hong, Y.-K.; Miyakawa, T.; Detmar, M. Ultraviolet B-induced skin angiogenesis is associated with a switch in the balance of vascular endothelial growth factor and thrombospondin-1 expression. J. Investig. Dermatol. 2004, 122, 201–208. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, Y.K.; Eun, H.C.; Cho, K.H.; Chung, J.H. All-trans retinoic acid antagonizes UV-induced VEGF production and angiogenesis via the inhibition of ERK activation in human skin keratinocytes. J. Investig. Dermatol. 2006, 126, 2697–2706. [Google Scholar] [CrossRef]
- Yano, K.; Oura, H.; Detmar, M. Targeted overexpression of the angiogenesis inhibitor thrombospondin-1 in the epidermis of transgenic mice prevents ultraviolet-B-induced angiogenesis and cutaneous photo-damage. J. Investig. Dermatol. 2002, 118, 800–805. [Google Scholar] [CrossRef]
- Lorigo, M.; Cairrao, E. Antioxidants as stabilizers of UV filters: An example for the UV-B filter octylmethoxycinnamate. Biomed. Dermatol. 2019, 3, 11. [Google Scholar] [CrossRef]
- Balić, A.; Mokos, M. Do we utilize our knowledge of the skin protective effects of carotenoids enough? Antioxid. Basel Switz. 2019, 8, 259. [Google Scholar] [CrossRef]
- Tuong, W.; Armstrong, A.W. Effect of appearance-based education compared with health-based education on sunscreen use and knowledge: A randomized controlled trial. J. Am. Acad. Dermatol. 2014, 70, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Mahler, H.I.M.; Kulik, J.A.; Harrell, J.; Correa, A.; Gibbons, F.X.; Gerrard, M. Effects of UV photographs, photoaging information, and use of sunless tanning lotion on sun protection behaviors. Arch. Dermatol. 2005, 141, 373–380. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific Opinion on the substantiation of health claims related to astaxanthin and maintenance of joints, tendons, and connective tissue (ID 1918, 1978, 3142), protection of DNA, proteins and lipids from oxidative damage (ID 1449, 3141), maintenance of visual acuity (ID 1448), maintenance of blood cholesterol concentrations and maintenance of low plasma concentrations of C-reactive protein (ID 1450) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1–17. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Natural Astaxanthin Extract Reduces DNA Oxidation. Available online: https://patents.google.com/patent/WO2005011712A1/en2005 (accessed on 22 October 2020).
- Mercke Odeberg, J.; Lignell, A.; Pettersson, A.; Höglund, P. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharm. Sci. 2003, 19, 299–304. [Google Scholar] [CrossRef]
- Ambati, R.R.; Siew Moi, P.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.D.; Kang, H.E.; Yang, S.H.; Lee, M.G.; Shin, W.G. Pharmacokinetics and first-pass metabolism of astaxanthin in rats. Br. J. Nutr. 2011, 105, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, Y.; Mizuno, M.; Yoshida, M.; Nishitani, Y.; Azuma, T.; Komoto, A.; Maoka, T.; Tanino, Y.; Kanazawa, K. Pharmacokinetics of fucoxanthinol in human plasma after the oral administration of kombu extract. Br. J. Nutr. 2012, 107, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Iio, K.; Okada, Y.; Ishikura, M. Single and 13-week oral toxicity study of fucoxanthin oil from microalgae in rats. Shokuhin Eiseigaku zasshi J. Food Hyg. Soc. Jpn. 2011, 52, 183–189. [Google Scholar] [CrossRef]
- Spagolla Napoleão Tavares, R.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Schäfer-Korting, M.; Marx, U.; Gaspar, L.R.; Zoschke, C. Skin irritation testing beyond tissue viability: Fucoxanthin effects on inflammation, homeostasis, and metabolism. Pharmaceutics 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- FDA. Overview of Safety and Regulatory Issues Related to So-Called Tanning Pills. Available online: https://www.fda.gov/cosmetics/cosmetic-products/tanning-pills (accessed on 10 June 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catanzaro, E.; Bishayee, A.; Fimognari, C. On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer. Mar. Drugs 2020, 18, 544. https://doi.org/10.3390/md18110544
Catanzaro E, Bishayee A, Fimognari C. On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer. Marine Drugs. 2020; 18(11):544. https://doi.org/10.3390/md18110544
Chicago/Turabian StyleCatanzaro, Elena, Anupam Bishayee, and Carmela Fimognari. 2020. "On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer" Marine Drugs 18, no. 11: 544. https://doi.org/10.3390/md18110544
APA StyleCatanzaro, E., Bishayee, A., & Fimognari, C. (2020). On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer. Marine Drugs, 18(11), 544. https://doi.org/10.3390/md18110544






