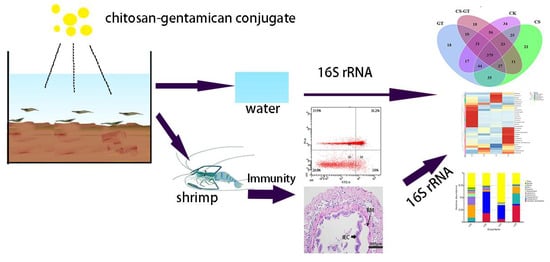
Effects of Chitosan–Gentamicin Conjugate Supplement on Non-Specific Immunity, Aquaculture Water, Intestinal Histology and Microbiota of Pacific White Shrimp (Litopenaeus vannamei)
Abstract
1. Introduction
2. Results and Discussion
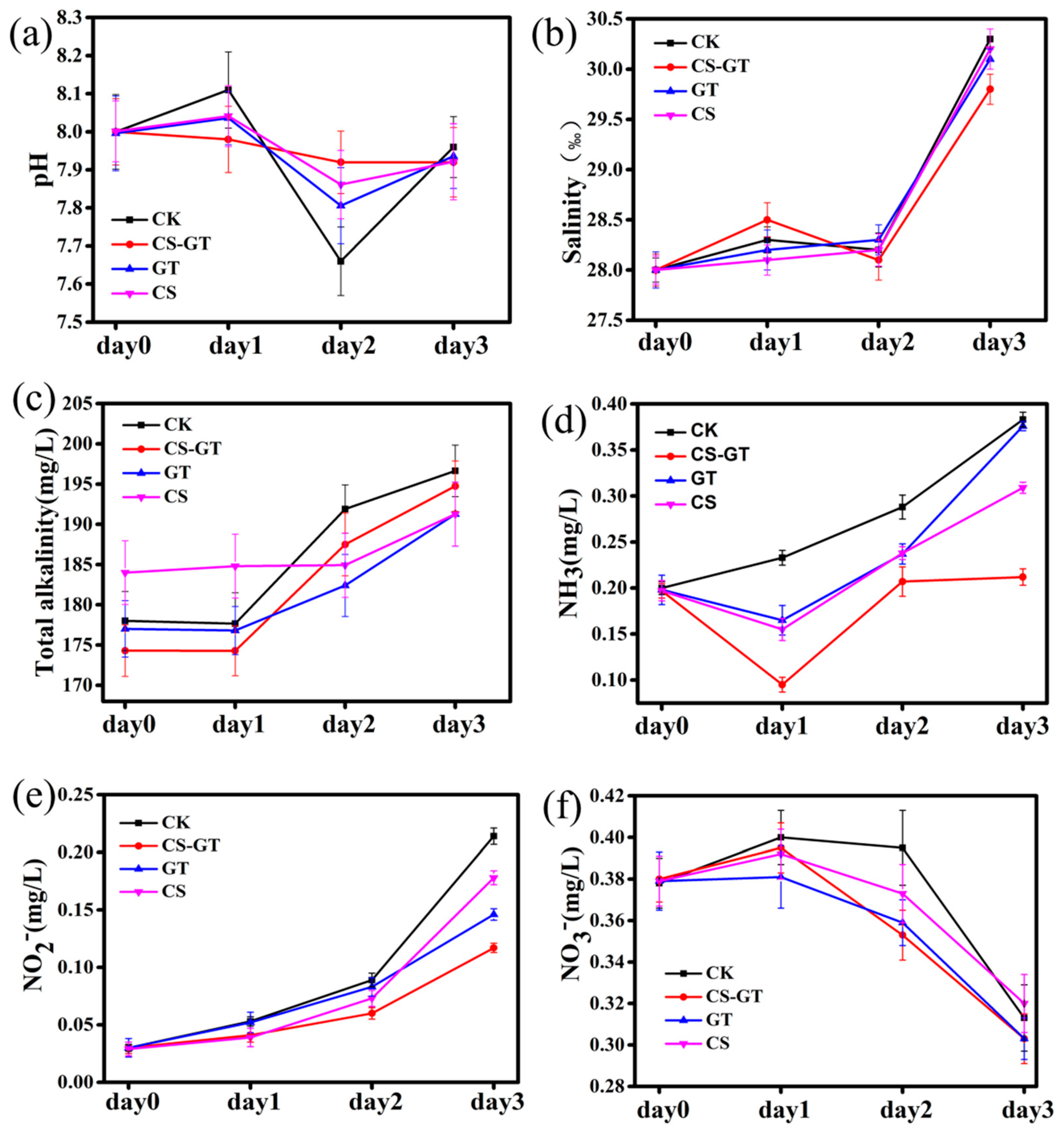
2.1. Water Physicochemical Quality
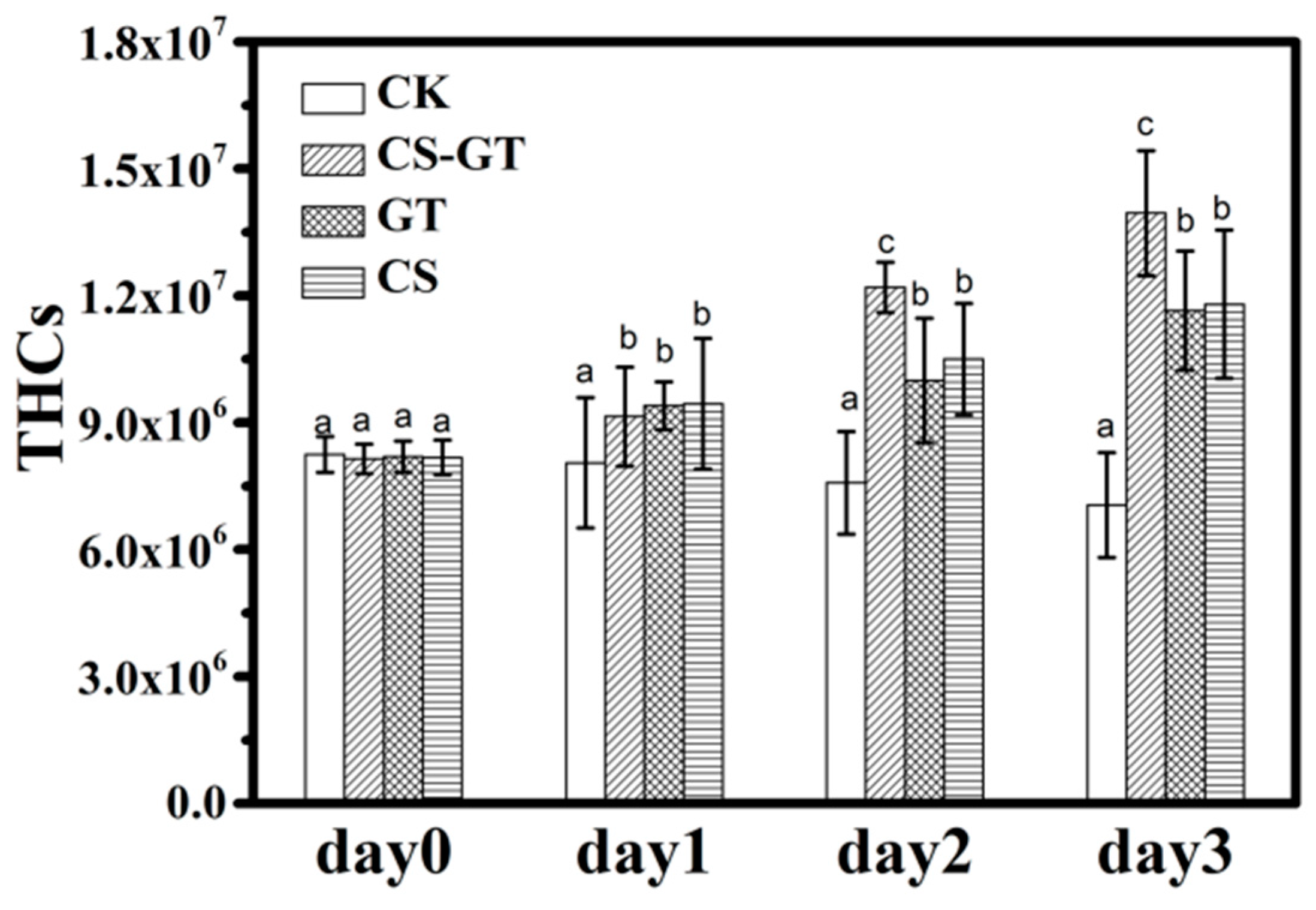
2.2. Hematological Profile
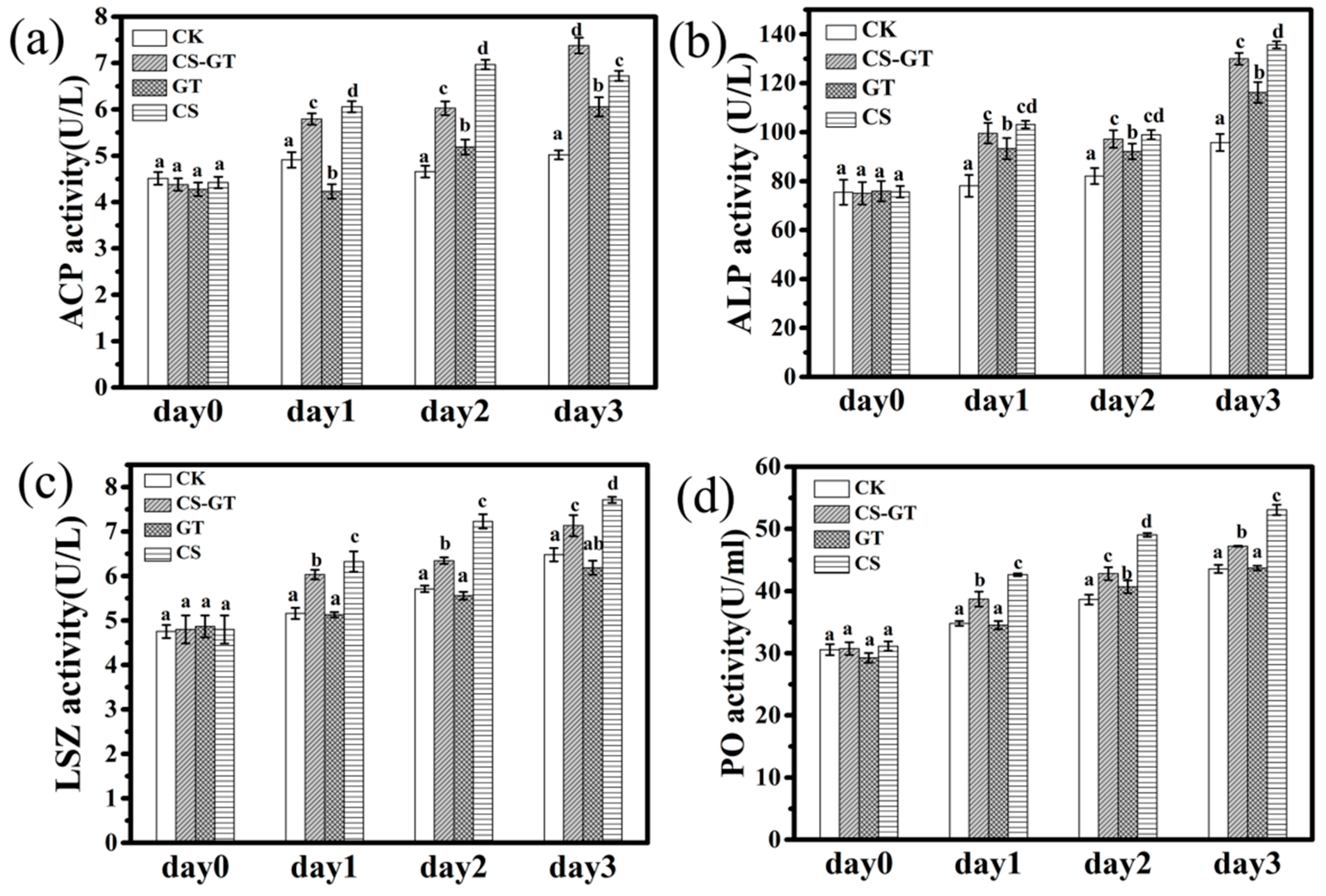
2.3. Nonspecific Immunity Parameters
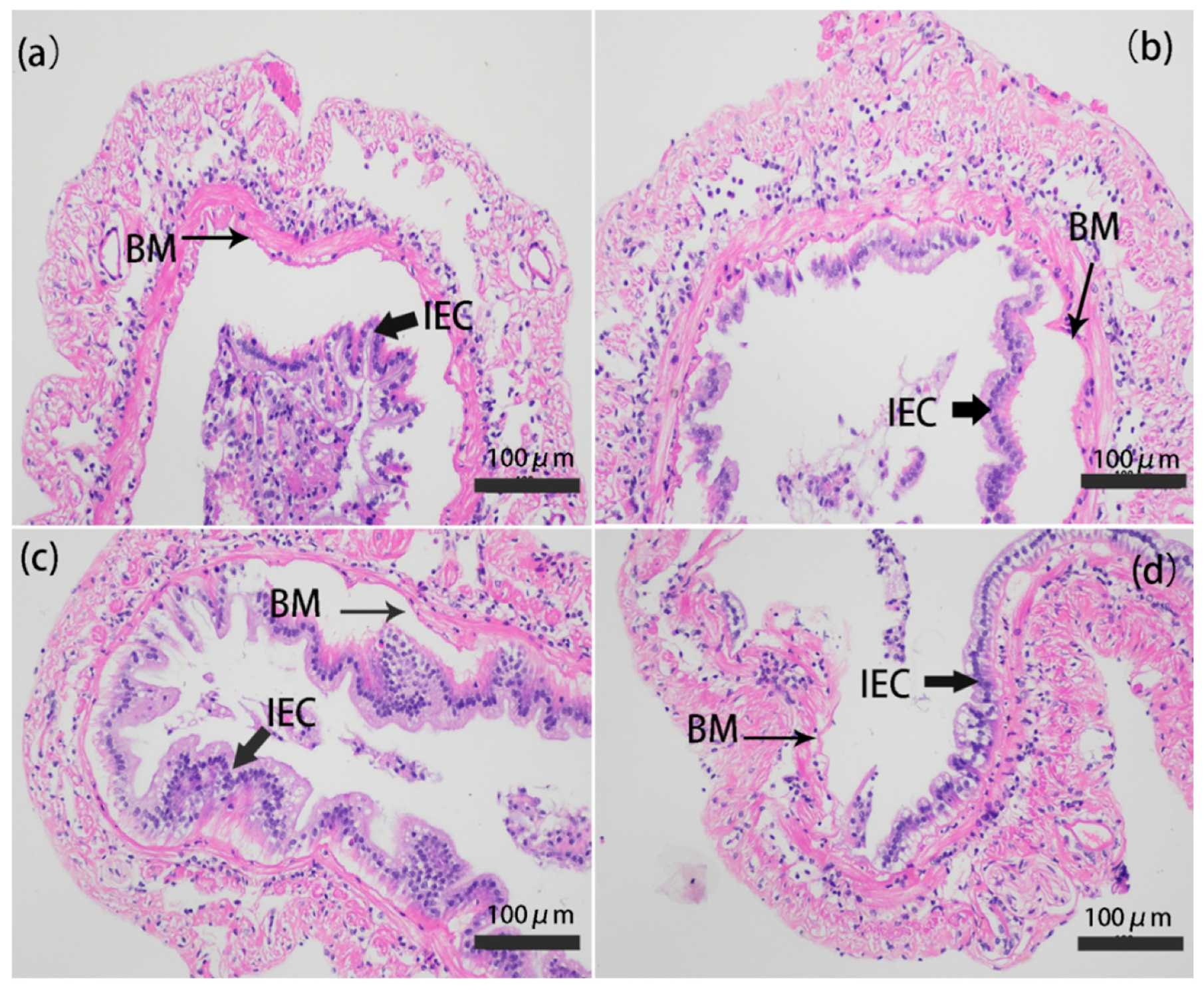
2.4. Intestinal Histology
2.5. Microbiota
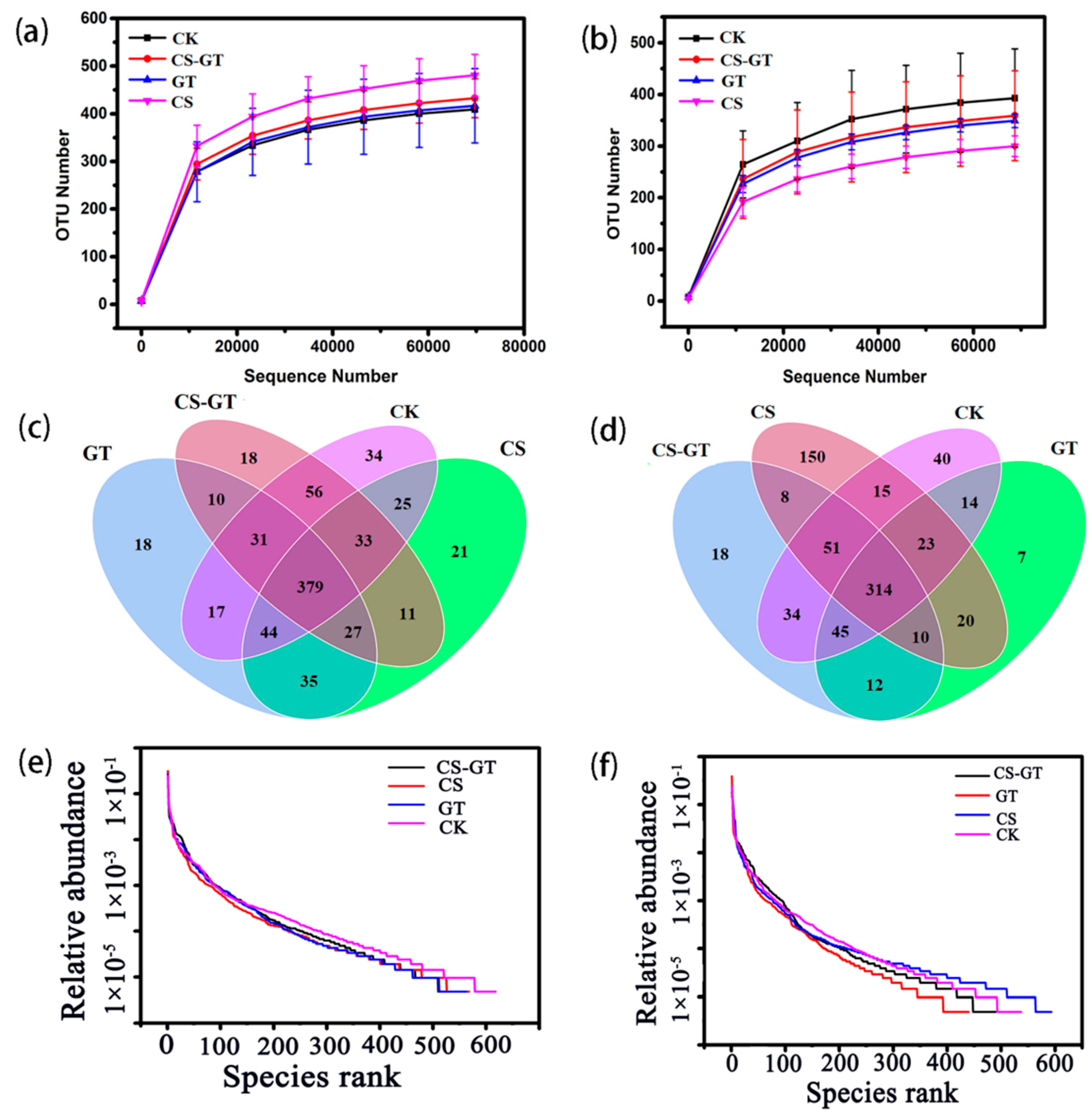
2.5.1. Alpha Diversity Analysis
2.5.2. Beta Diversity Analysis
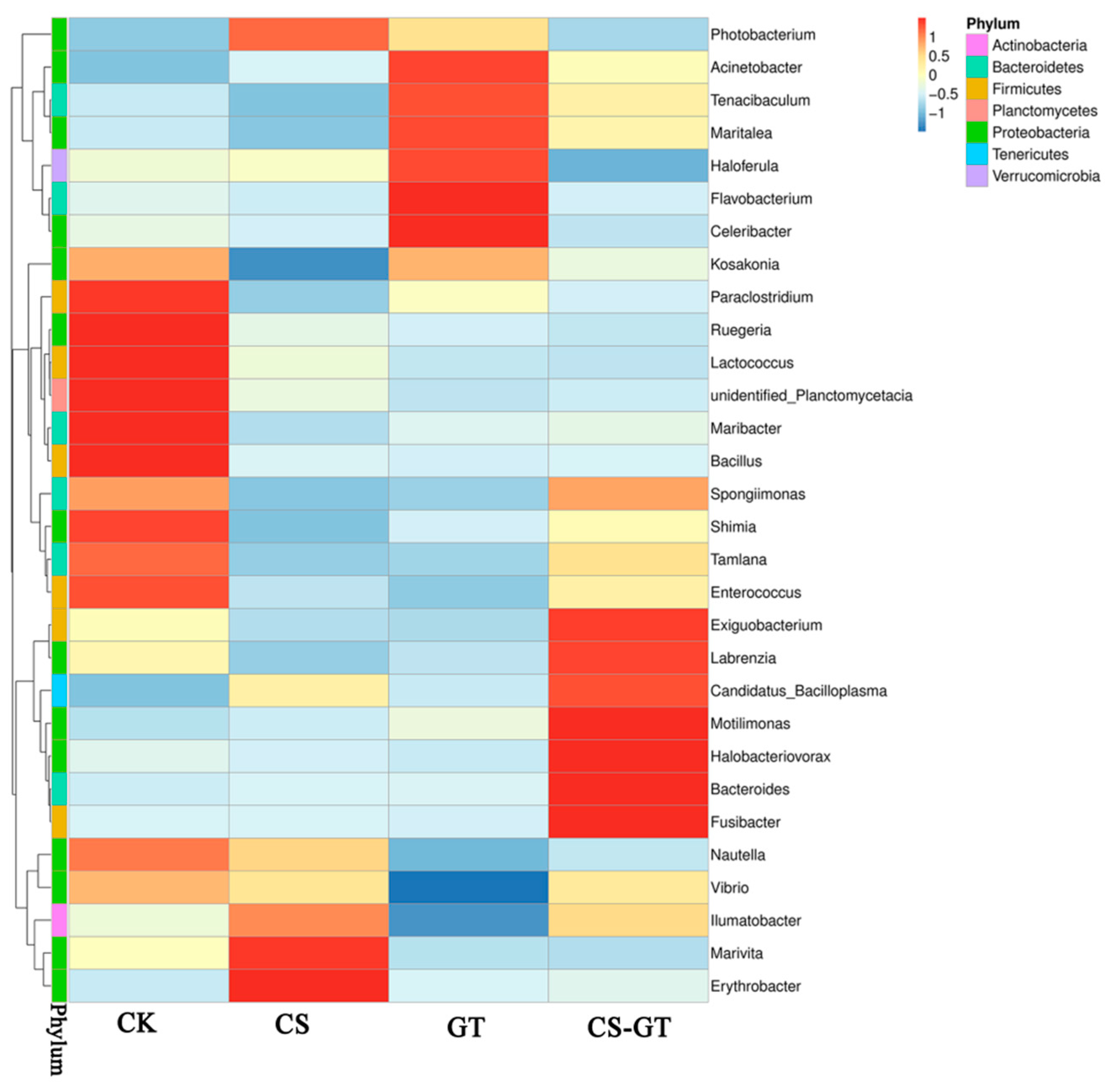
2.5.3. Microbial Composition and Changes
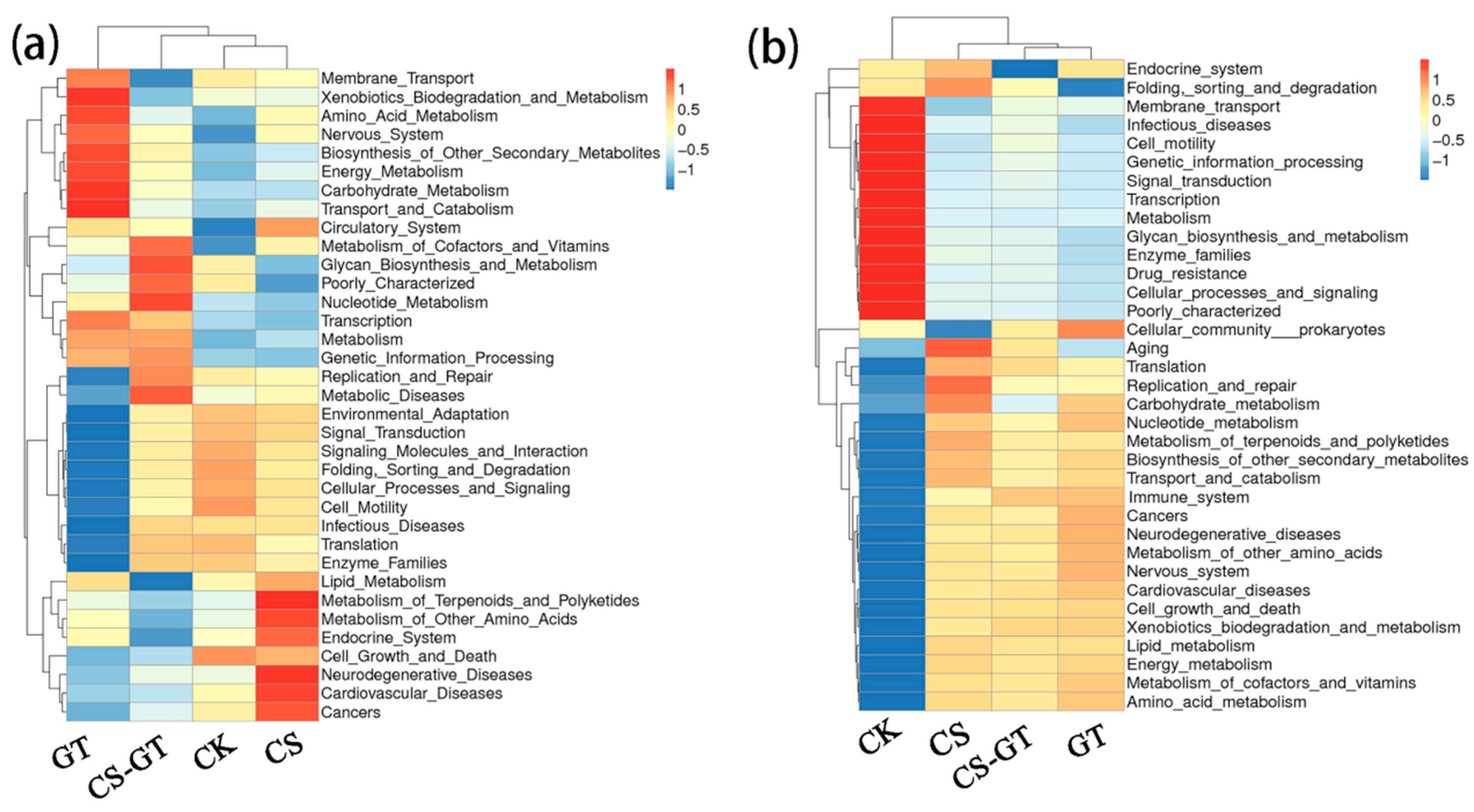
2.5.4. Microbial Function Prediction
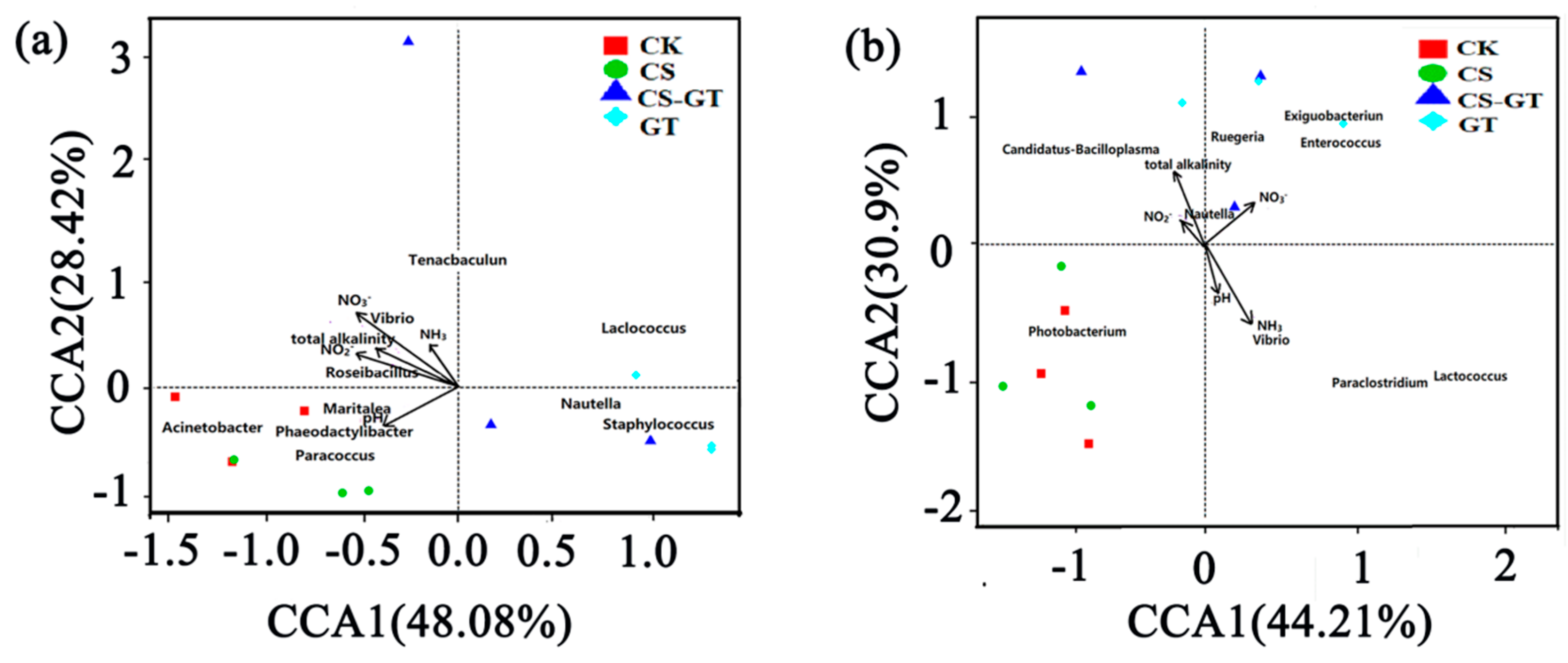
2.5.5. Correlation Analysis between Microbial Communities and Environmental Variables
3. Materials and Methods
3.1. CS-GT Synthesis
3.2. Experimental Design and Diets
3.3. Water Physicochemical Quality
3.4. Hematological Profile
3.4.1. Total Hemocyte Counts (THCs)
3.4.2. Hemocyte Apoptosis Analysis
3.5. Nonspecific Immunity Parameters
3.6. Intestinal Histology
3.7. Microbiota Analysis
3.7.1. DNA Extraction
3.7.2. 16S rRNA Gene Sequencing
3.7.3. Sequencing Data Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, W.; Xu, Y.; Huang, X.; Hu, X.; Xu, Y.; Su, H.; Li, Z.; Yang, K.; Wen, G.; Cao, Y. Addition of algicidal bacterium CZBC1 and molasses to inhibit cyanobacteria and improve microbial communities, water quality and shrimp performance in culture systems. Aquaculture 2019, 502, 303–311. [Google Scholar] [CrossRef]
- Alfiansah, Y.R.; Hassenruck, C.; Kunzmann, A.; Taslihan, A.; Harder, J.; Gardes, A. Bacterial abundance and community composition in pond water from shrimp aquaculture systems with different stocking densities. Front. Microbiol. 2018, 9, 2457. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Hou, D.; Liu, J.; Ji, P.; Weng, S.; He, J.; Huang, Z. Antibiotic supplement in feed can perturb the intestinal microbial composition and function in Pacific white shrimp. Appl. Microbiol. Biotechnol. 2019, 103, 3111–3122. [Google Scholar] [CrossRef] [PubMed]
- Amoah, K.; Huang, Q.C.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Dong, X.H. Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Dai, T.; Zhong, S.; Jin, M.; Sun, P.; Zhou, Q. Vibrio parahaemolyticus infection influenced trace element homeostasis, impaired antioxidant function, and induced inflammation response in Litopenaeus vannamei. Biol. Trace Elem. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Thadtapong, N.; Salinas, M.B.S.; Charoensawan, V.; Saksmerprome, V.; Chaturongakul, S. Genome Characterization and Comparison of Early Mortality Syndrome Causing Vibrio parahaemolyticus pirABvp− Mutant from Thailand With V. parahaemolyticus pirABvp+ AHPND Isolates. Front. Mar. Sci. 2020, 7, 290. [Google Scholar] [CrossRef]
- Rattanavichai, W.; Cheng, W. Effects of hot-water extract of banana (Musa acuminata) fruit’s peel on the antibacterial activity, and anti-hypothermal stress, immune responses and disease resistance of the giant freshwater prawn, Macrobrachium rosenbegii. Fish Shellfish Immunol. 2014, 39, 326–335. [Google Scholar] [CrossRef]
- Karnjana, K.; Soowannayan, C.; Wongprasert, K. Ethanolic extract of red seaweed Gracilaria fisheri and furanone eradicate Vibrio harveyi and Vibrio parahaemolyticus biofilms and ameliorate the bacterial infection in shrimp. Fish Shellfish Immunol. 2019, 88, 91–101. [Google Scholar] [CrossRef]
- Interaminense, J.A.; Vogeley, J.L.; Gouveia, C.K.; Portela, R.S.; Oliveira, J.P.; Silva, S.; Coimbra, M.R.M.; Peixoto, S.M.; Soares, R.B.; Buarque, D.S.; et al. Effects of dietary Bacillus subtilis and Shewanella algae in expression profile of immune-related genes from hemolymph of Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Fish Shellfish Immunol. 2019, 86, 253–259. [Google Scholar] [CrossRef]
- Liu, X.L.; Xi, Q.Y.; Yang, L.; Li, H.Y.; Jiang, Q.Y.; Shu, G.; Wang, S.B.; Gao, P.; Zhu, X.T.; Zhang, Y.L. The effect of dietary Panax ginseng polysaccharide extract on the immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2011, 30, 495–500. [Google Scholar] [CrossRef]
- Deng, B.; Wang, Z.P.; Tao, W.J.; Li, W.F.; Wang, C.; Wang, M.Q.; Ye, S.S.; Du, Y.J.; Wu, X.X.; Wu, D. Effects of polysaccharides from mycelia of Cordyceps sinensison growth performance, immunity and antioxidant indicators of the white shrimp, Litopenaeus vannamei. Aquac. Nutr. 2015, 21, 173–179. [Google Scholar] [CrossRef]
- Tello-Olea, M.; Rosales-Mendoza, S.; Campa-Cordova, A.I.; Palestino, G.; Luna-Gonzalez, A.; Reyes-Becerril, M.; Velazquez, E.; Hernandez-Adame, L.; Angulo, C. Gold nanoparticles (AuNP) exert immunostimulatory and protective effects in shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish Shellfish Immunol. 2019, 84, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Fierro-Coronado, J.A.; Angulo, C.; Rubio-Castro, A.; Luna-González, A.; Cáceres-Martínez, C.J.; Ruiz-Verdugo, C.A.; Álvarez-Ruíz, P.; Escamilla-Montes, R.; González-Ocampo, H.A.; Diarte-Plata, G. Dietary fulvic acid effects on survival and expression of immune-related genes in Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Aquac. Res. 2018, 49, 3218–3227. [Google Scholar] [CrossRef]
- Pereira, L.A.; da Silva Reis, L.; Batista, F.A.; Mendes, A.N.; Osajima, J.A.; Silva-Filho, E.C. Biological properties of chitosan derivatives associated with the ceftazidime drug. Carbohydr. Polym. 2019, 222, 115002. [Google Scholar] [CrossRef]
- Xing, K.; Xing, Y.; Liu, Y.; Zhang, Y.; Shen, X.; Li, X.; Miao, X.; Feng, Z.; Peng, X.; Qin, S. Fungicidal effect of chitosan via inducing membrane disturbance against Ceratocystis fimbriata. Carbohydr. Polym. 2018, 192, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Chen, J.C. The protective effect of chitin and chitosan against Vibrio alginolyticus in white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2005, 19, 191–204. [Google Scholar] [CrossRef]
- Kamali Najafabad, M.; Imanpoor, M.R.; Taghizadeh, V.; Alishahi, A. Effect of dietary chitosan on growth performance, hematological parameters, intestinal histology and stress resistance of Caspian kutum (Rutilus frisii kutum Kamenskii, 1901) fingerlings. Fish Physiol. Biochem. 2016, 42, 1063–1071. [Google Scholar] [CrossRef]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. Recent progress in biomedical applications of chitosan and its nanocomposites in aquaculture: A review. Res. Vet. Sci. 2019, 126, 68–82. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L. Effects of chitosan-supplemented diets on the growth performance, nonspecific immunity and health of loach fish (Misgurnus anguillicadatus). Carbohydr. Polym. 2019, 225, 115227. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Razek, N.A.; Abdel-Rahman, A.M. Immunostimulatory effect of dietary chitosan nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.). Fish Shellfish Immunol. 2019, 88, 254–258. [Google Scholar] [CrossRef]
- Khani Oushani, A.; Soltani, M.; Sheikhzadeh, N.; Shamsaie Mehrgan, M.; Rajabi Islami, H. Effects of dietary chitosan and nano-chitosan loaded clinoptilolite on growth and immune responses of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2020, 98, 210–217. [Google Scholar] [CrossRef]
- Beyazit, N.; Cakran, H.S.; Cabir, A.; Akiscan, Y.; Demetgul, C. Synthesis, characterization and antioxidant activity of chitosan Schiff base derivatives bearing (-)-gossypol. Carbohydr. Polym. 2020, 240, 116333. [Google Scholar] [CrossRef] [PubMed]
- Ghazaie, M.; Ghiaci, M.; Soleimanian-Zad, S.; Behzadi-Teshnizi, S. Preparing natural biocomposites of N-quaternary chitosan with antibacterial activity to reduce consumption of antibacterial drugs. J. Hazard. Mater. 2019, 371, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Hasanin, M.; Hesemann, P. Synthesis and antimicrobial properties of new chitosan derivatives containing guanidinium groups. Carbohydr. Polym. 2020, 241, 116363. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.F.G.; Attjioui, M.; Leitao, A.; Moerschbacher, B.M.; Cavalheiro, E.T.G. Characterization, solubility and biological activity of amphihilic biopolymeric Schiff bases synthesized using chitosans. Carbohydr. Polym. 2019, 220, 1–11. [Google Scholar] [CrossRef]
- Yan, T.; Li, C.; Ouyang, Q.; Zhang, D.; Zhong, Q.; Li, P.; Li, S.; Yang, Z.; Wang, T.; Zhao, Q. Synthesis of gentamicin-grafted-chitosan with improved solubility and antibacterial activity. React. Funct. Polym. 2019, 137, 38–45. [Google Scholar] [CrossRef]
- Yan, T.; Kong, S.; Ouyang, Q.; Li, C.; Hou, T.; Chen, Y.; Li, S. Chitosan-Gentamicin Conjugate Hydrogel Promoting Skin Scald Repair. Mar. Drugs 2020, 18, 233. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Zhang, D. Effects of microbial products and extra carbon on water quality and bacterial community in a fish polyculture system. Aquac. Res. 2019, 50, 1220–1229. [Google Scholar] [CrossRef]
- Cole, A.J.; Tulsankar, S.S.; Saunders, B.J.; Fotedar, R. Effects of pond age and a commercial substrate (the water cleanser™) on natural productivity, bacterial abundance, nutrient concentrations, and growth and survival of MARRON (CHERAX CAINII Austin, 2002) in semi-intensive pond culture. Aquaculture 2019, 502, 242–249. [Google Scholar] [CrossRef]
- Ray, A.J.; Seaborn, G.; Leffler, J.W.; Wilde, S.B.; Lawson, A.; Browdy, C.L. Characterization of microbial communities in minimal-exchange, intensive aquaculture systems and the effects of suspended solids management. Aquaculture 2010, 310, 130–138. [Google Scholar] [CrossRef]
- Chen, G.F.; Huang, J.; Song, X.L. General situation of the immunological capability of shrimp. J. Fish. China 2004, 28, 209–215. [Google Scholar]
- Zhai, Q.; Li, J. Effectiveness of traditional Chinese herbal medicine, San-Huang-San, in combination with enrofloxacin to treat AHPND-causing strain of Vibrio parahaemolyticus infection in Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Arojojoye, O.A.; Oyagbemi, A.A.; Afolabi, J.M. Toxicological assessment of heavy metal bioaccumulation and oxidative stress biomarkers in clarias gariepinus from Igbokoda River of South Western Nigeria. Bull. Environ. Contam. Toxicol. 2018, 100, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.J.; Liu, Q.Q.; Liao, S.; Fang, H.H.; Yin, P.; Xie, S.W.; Tian, L.X.; Liu, Y.J.; Niu, J. Effects of dietary mixed probiotics on growth, non-specific immunity, intestinal morphology and microbiota of juvenile pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 90, 456–465. [Google Scholar] [CrossRef]
- Couto, N.; Malys, N.; Gaskell, S.J.; Barber, J. Partition and turnover of glutathione reductase from saccharomyces cerevisiae: A proteomic approach. J. Proteome Res. 2013, 12, 2885–2894. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, J.; Dong, H.; Wang, Y.; Liu, Q.; Li, H. Oxidative stress response of the black tiger shrimp Penaeus monodon to Vibrio parahaemolyticus challenge. Fish Shellfish Immunol. 2015, 46, 354–365. [Google Scholar] [CrossRef]
- Yang, C.; Kong, J.; Wang, Q.; Liu, Q.; Tian, Y.; Luo, K. Heterosis of haemolymph analytes of two geographic populations in Chinese shrimp Fenneropenaeus chinensis. Fish Shellfish Immunol. 2007, 23, 62–70. [Google Scholar] [CrossRef]
- Feng, N.N.; Sun, Y.M.; Wen, R.; Zhang, C.S.; Fu-Hua, L.I. Analysis on the activity of immune related enzymes in survived Exopalaemon carinicauda from WSSV infection. Mar. Sci. 2014, 38, 75–79. [Google Scholar]
- Zhang, M.; Wang, L.; Guo, Z.Y.; Wang, B.J. Effect of lipopolysaccharide and Vibrio anguillarum on the activities of phosphatase, superoxide dismutase and the content of hemocyanin in the serum of Fenneropenaeus chinesis. Mar. Sci. 2004, 28, 25–30. [Google Scholar]
- Sotelo-Mundo, R.R.; Islas-Osuna, M.A.; De-La-Re-Vega, E.; Hernández-López, J.; Yepiz-Plascencia, G. cDNA cloning of the lysozyme of the white shrimp Penaeus vannamei. Fish Shellfish Immunol. 2003, 15, 325–331. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Jin, L.; Li, X. Effects of a probiotic mixture (Bacillus subtilis YBand Bacillus cereus YB on disease resistance and non-specific immunity of sea cucumber, Apostichopus japonicus (Selenka). Aquac. Res. 2015, 46, 3008–3019. [Google Scholar] [CrossRef]
- Newton, K.; Peters, R.; Raftos, D. Phenoloxidase and QX disease resistance in Sydney rock oysters (Saccostrea glomerata). Dev. Comp. Immunol. 2004, 28, 565–569. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Xiao, S.; Bu, X.; Lin, Z.; Qi, C.; Qin, J.G.; Chen, L. T-2 toxin in the diet suppresses growth and induces immunotoxicity in juvenile Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2020, 97, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, Z.; Zhou, C.; Huang, Z.; Tan, L.; Xun, P.; Huang, Q.; Lin, H.; Ye, C.; Wang, A. Effects of dietary dandelion extract on intestinal morphology, antioxidant status, immune function and physical barrier function of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2018, 73, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Li, H.; Yang, H.; Peng, C.; Peng, Z.; Lu, L. Shift in the microbial community composition of surface water and sediment along an urban river. Sci. Total Environ. 2018, 627, 600–612. [Google Scholar] [CrossRef]
- Cardona, E.; Gueguen, Y.; Magre, K.; Lorgeoux, B.; Piquemal, D.; Pierrat, F.; Noguier, F.; Saulnier, D. Bacterial community characterization of water and intestine of the shrimp Litopenaeus stylirostris in a biofloc system. BMC Microbiol. 2016, 16, 157. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Z.; Chen, M.; Qu, Y.; Li, J.; Zhou, A.; Xie, S.; Zeng, F.; Zou, J. Microbiota comparison of Pacific white shrimp intestine and sediment at freshwater and marine cultured environment. Sci. Total Environ. 2019, 657, 1194–1204. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, L.; Huang, F.; Gao, S.; Su, C.; Zhang, M.; He, Z. Metagenomic analysis of composition, function and cycling processes of microbial community in water, sediment and effluent of Litopenaeus vannamei farming environments under different culture modes. Aquaculture 2019, 506, 280–293. [Google Scholar] [CrossRef]
- Beyersmann, P.G.; Tomasch, J.; Son, K.; Stocker, R.; Goker, M.; Wagner-Dobler, I.; Simon, M.; Brinkhoff, T. Dual function of tropodithietic acid as antibiotic and signaling molecule in global gene regulation of the probiotic bacterium Phaeobacter inhibens. Sci. Rep. 2017, 7, 730. [Google Scholar] [CrossRef]
- Amoah, K.; Huang, Q.C.; Dong, X.H.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Yang, Y.Z. Paenibacillus polymyxa improves the growth, immune and antioxidant activity, intestinal health, and disease resistance in Litopenaeus vannamei challenged with Vibrio parahaemolyticus. Aquaculture 2019, 734563. [Google Scholar] [CrossRef]
- Jang, W.J.; Lee, J.M.; Hasan, M.T.; Lee, B.J.; Lim, S.G.; Kong, I.S. Effects of probiotic supplementation of a plant-based protein diet on intestinal microbial diversity, digestive enzyme activity, intestinal structure, and immunity in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2019, 92, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Singh, C.; Arul, V. Inhibitory activity of probiotics Streptococcus phocae PI80 and Enterococcus faecium MC13 against Vibriosis in shrimp Penaeus monodon. World J. Microbiol. Biotechnol. 2009, 25, 697–703. [Google Scholar] [CrossRef]
- Sharifuzzaman, S.M.; Rahman, H.; Austin, D.A.; Austin, B. Properties of Probiotics Kocuria SM1 and Rhodococcus SM2 Isolated from Fish Guts. Probiotics Antimicrob. Proteins 2018, 10, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Santos, H.M.; Hu, S.-Y.; Sang, C.Y.; Yanuaria, C.A.S.; Lola, E.N.G.; Tayo, L.L.; Nitura, K.M.A.; Liu, C.H.; Chuang, K.P. LpxD gene knockout elicits protection to Litopenaeus vannamei, white shrimp, against Vibrio parahaemolyticus infection. Aquac. Int. 2019, 27, 1383–1393. [Google Scholar] [CrossRef]
- Huynh, T.G.; Hu, S.Y.; Chiu, C.S.; Truong, Q.P.; Liu, C.H. Bacterial population in intestines of white shrimp, Litopenaeus vannamei fed a synbiotic containing Lactobacillus plantarum and galactooligosaccharide. Aquac. Res. 2019, 50, 807–817. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Liu, M.; Wang, B.; Jiang, K.; Ma, S.; Li, Q. The intestinal microbial diversity in Chinese shrimp (Fenneropenaeus chinensis) as determined by PCR–DGGE and clone library analyses. Aquaculture 2011, 317, 32–36. [Google Scholar] [CrossRef]
- Koo, H.; Hakim, J.A.; Morrow, C.D.; Eipers, P.G.; Davila, A.; Andersen, D.T.; Bej, A.K. Comparison of two bioinformatics tools used to characterize the microbial diversity and predictive functional attributes of microbial mats from Lake Obersee, Antarctica. J. Microbiol. Methods 2017, 140, 15–22. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Vijayakumar, S.; Mukherjee, A.; Chandrasekaran, N.; Thomas, J. Antioxidant and antibacterial activity of Chaetomorpha antennina against shrimp pathogen Vibrio parahaemolyticus. Aquaculture 2014, 433, 467–475. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada, Bulletin: Ottawa, ON, USA, 1972; p. 167. [Google Scholar]






| Samples | Group | Richness Estimate | Diversity Estimate | |||
|---|---|---|---|---|---|---|
| Chao1 | ACE | Shannon | Simpson | Good’s Coverage | ||
| water | CK | 475.84 ± 25.31 a | 478.52 ± 45.45 a | 5.27 ± 0.27 a | 0.79 ± 0.01 a | 0.9995 ± 0.0018 a |
| CS | 461.95 ± 15.54 a | 432.83 ± 44.36 a | 5.19 + 0.14 a | 0.83 ± 0.02 a | 0.9995 ± 0.0004 a | |
| CS-GT | 435.39 ± 91.77 a | 411.55 ± 75.57 a | 4.72 ± 0.57 a | 0.88 ± 0.04 b | 0.9995 ± 0.0004 a | |
| GT | 417.15 ± 5.68 a | 404.70 ± 10.55 a | 4.17 ± 0.22 b | 0.92 ± 0.03 b | 0.9995 ± 0.0002 a | |
| gut | CK | 346.18 ± 90.64 a | 348.52 ± 88.57 a | 4.51 ± 2.57 a | 0.78 ± 0.14 a | 0.9997 ± 0.0007 a |
| CS | 378.42 ± 98.21 a | 382.83 ± 96.31 a | 4.81 ± 0.32 a | 0.72 ± 0.03 a | 0.9997 ± 0.0003 a | |
| CS-GT | 324.53 ± 0.53 a | 339.37 ± 13.50 a | 4.63 ± 0.56 a | 0.81 ± 0.05 a | 0.9997 ± 0.0005 a | |
| GT | 287.03 ± 23.05 a | 291.53 ± 21.06 a | 4.18 ± 1.33 a | 0.88 ± 0.17 a | 0.9997 ± 0.0004 a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, F.; Li, C.; Hou, T.; Wen, C.; Kong, S.; Ma, D.; Sun, C.; Li, S. Effects of Chitosan–Gentamicin Conjugate Supplement on Non-Specific Immunity, Aquaculture Water, Intestinal Histology and Microbiota of Pacific White Shrimp (Litopenaeus vannamei). Mar. Drugs 2020, 18, 419. https://doi.org/10.3390/md18080419
Liang F, Li C, Hou T, Wen C, Kong S, Ma D, Sun C, Li S. Effects of Chitosan–Gentamicin Conjugate Supplement on Non-Specific Immunity, Aquaculture Water, Intestinal Histology and Microbiota of Pacific White Shrimp (Litopenaeus vannamei). Marine Drugs. 2020; 18(8):419. https://doi.org/10.3390/md18080419
Chicago/Turabian StyleLiang, Fengyan, Chengpeng Li, Tingting Hou, Chongqing Wen, Songzhi Kong, Dong Ma, Chengbo Sun, and Sidong Li. 2020. "Effects of Chitosan–Gentamicin Conjugate Supplement on Non-Specific Immunity, Aquaculture Water, Intestinal Histology and Microbiota of Pacific White Shrimp (Litopenaeus vannamei)" Marine Drugs 18, no. 8: 419. https://doi.org/10.3390/md18080419
APA StyleLiang, F., Li, C., Hou, T., Wen, C., Kong, S., Ma, D., Sun, C., & Li, S. (2020). Effects of Chitosan–Gentamicin Conjugate Supplement on Non-Specific Immunity, Aquaculture Water, Intestinal Histology and Microbiota of Pacific White Shrimp (Litopenaeus vannamei). Marine Drugs, 18(8), 419. https://doi.org/10.3390/md18080419