Protein Recovery from Underutilised Marine Bioresources for Product Development with Nutraceutical and Pharmaceutical Bioactivities
Abstract
1. Introduction
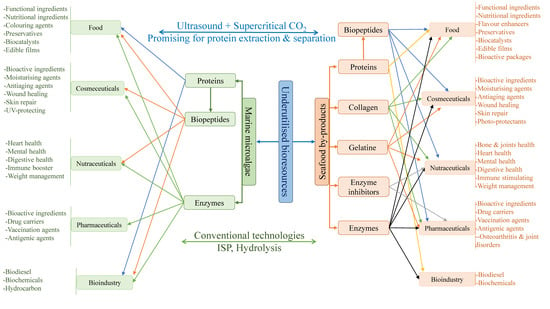
2. SPBs and Marine Microalgae as Advantageous Bioresources for Green and Sustainable Production of Proteins and Protein-Based Products
2.1. Inexpensive and Untapped SPBs for Recovery of Various Proteins and Protein Derivatives
2.1.1. Heads, Shells, and Frames for Recovery of Muscle Proteins, Carotenoproteins, and Their Hydrolysed Products
2.1.2. Skins, Scales, and Bones for Recovery of Collagens, Gelatines, and Their Hydrolysed Products
2.1.3. Viscera for Production of Intestinal Enzymes and Biopeptides
2.1.4. Fish Blood for Production of Protein Hydrolysates, Biopeptides, Bioactive Amino Acids (Taurine, GABA), Protease Inhibitors, and Cell-Culture Media
2.2. Marine Microalgae as an Advantageous Biomass for Green and Sustainable Production of Proteins and Enzymes
3. Nutritional Quality and Biological Activities of Marine-Derived Proteins and Their Derivatives
3.1. Nutritional Quality of Marine Proteins
3.2. Bioactivities of Marine-Derived Proteins
3.2.1. Antihypertensive
3.2.2. Antioxidant
3.2.3. Antidiabetic
3.2.4. Anticancer
3.2.5. Antimicrobial
4. Applications of Proteins and Protein-Based Products Recovered from SPBs and Marine Microalgae
4.1. Protein Concentrates and Protein-Derived Products
4.2. Fish Collagen and Gelatines
4.3. Bioactive Peptides and Active Amino Acids
4.4. Enzymes
5. Process Development for Production of Proteins and Protein-Based Products from SPBs and Marine Microalgae
5.1. Isoelectric Solubilisation/Precipitation (ISP) Process for Intact Protein Production
5.2. Hydrolysis Processes for Production of Protein Hydrolysates, Peptides, and Amino Acids
5.2.1. Chemical Hydrolysis (Using Acids or Alkaline Extraction of Protein Hydrolysates, Biopeptides, Collagen, Gelatine, and Enzymes from SPBs and Microalgae)
5.2.2. Autolytic Hydrolysis (Fermentation)
5.2.3. Enzymatic Hydrolysis
5.3. Economic Feasibility and Industrial Production of Protein-Based Products from Underutilised Marine Bioresources
5.4. Ultrasound and Supercritical Carbon Dioxide (SCrCO2) as Promising Extraction and Separation Technologies for Protein Recovery from Marine Bioresources
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-I-converting enzyme |
| DPP-IV | Dipeptidyl peptidase IV |
| FCG | Fish collagen and gelatine |
| FPH | Fish protein hydrolysate |
| ISP | Isoelectric solubilisation precipitation |
| MT | Million tonnes |
| MW | Molecular weight |
| PCs | Protein concentrates |
| PHs | Protein hydrolysates |
| SCrCO2 | Supercritical carbon dioxide |
| SHR | Spontaneously hypertensive rats |
| SPB | Seafood processing by-products |
| YTKPBs | Yellow tail kingfish processing by-products |
References
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M. Single cell protein: State-of-the-art, industrial landscape and patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Morlighem, J.-É.R.; Radis-Baptista, G. The place for enzymes and biologically active peptides from marine organisms for application in industrial and pharmaceutical biotechnology. Curr. Prot. Pept. Sci. 2019, 20, 334–355. [Google Scholar] [CrossRef]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and fractionation of microalgae-based protein products. Algal Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef]
- OECD. OECD-FAO Agricultural Outlook 2019–2028; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Soldo, B.; Šimat, V.; Vlahović, J.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. High Quality Oil Extracted from Sardine By-Products as an Alternative to Whole Sardines: Production and Refining. Eur. J. Lipid Sci. Technol. 2019, 121, 1800513. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Zotos, A. Fish processing by-products as a potential source of gelatin: A review. J. Aquat. Food Prod. Technol. 2016, 25, 65–92. [Google Scholar] [CrossRef]
- Šimat, V.; Vlahović, J.; Soldo, B.; Mekinić, I.G.; Čagalj, M.; Hamed, I.; Skroza, D. Production and characterization of crude oils from seafood processing by-products. Food Biosci. 2020, 33, 100484. [Google Scholar] [CrossRef]
- Sasidharan, A.; Venugopal, V. Proteins and co-products from seafood processing discards: Their recovery, functional properties and applications. Waste Biomass Valorization 2019, 1–17. [Google Scholar] [CrossRef]
- Bendif, E.M.; Probert, I.; Schroeder, D.C.; de Vargas, C. On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta). J. Appl. Phycol. 2013, 25, 1763–1776. [Google Scholar]
- Aime, F.; Concepcion, H. Marine microalgae as a potential source of single cell protein. Appl. Microbiol. Biotechnol. 1985, 1985, 110–113. [Google Scholar]
- Chen, Y.; Wang, C.; Xu, C. Nutritional evaluation of two marine microalgae as feedstock for aquafeed. Aquac. Res. 2020, 51, 946–956. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factories 2018, 17, 173. [Google Scholar] [CrossRef]
- Sitepu, E.K.; Heimann, K.; Raston, C.L.; Zhang, W. Critical evaluation of process parameters for direct biodiesel production from diverse feedstock. Renew. Sustain. Energy Rev. 2020, 123, 109762. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Zhang, W.; Barber, R.A.; Su, P.; He, S. Microwave-intensified enzymatic deproteinization of Australian rock lobster shells (Jasus edwardsii) for the efficient recovery of protein hydrolysate as food functional nutrients. Food Bioprocess. Technol. 2016, 9, 628–636. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Luo, X.; Su, P.; Balakrishnan, B.; Zhang, W. Highly efficient recovery of nutritional proteins from Australian Rock Lobster heads (Jasus edwardsii) by integrating ultrasonic extraction and chitosan co-precipitation. Innov. Food Sci. Emerg. Technol. 2020, 60, 102308. [Google Scholar] [CrossRef]
- Gates, K. Marine products for healthcare: Functional and bioactive nutraceutical compounds from the ocean. In Functional Foods and Nutraceuticals Series; Venugopal, V., Ed.; CRC Press: Boca Raton, FL, USA, 2009; Volume 19, pp. 48–54. [Google Scholar]
- Korhonen, H.; Pihlanto-Leppäla, A.; Rantamäki, P.; Tupasela, T. Impact of processing on bioactive proteins and peptides. Trends Food Sci. Technol. 1998, 9, 307–319. [Google Scholar] [CrossRef]
- Frøkjaer, S. Use of hydrosylates for protein supplementation. Food Technol. 1994, 48, 86–88. [Google Scholar]
- Nakai, S.; Modler, H.W. Food Proteins: Properties and Characterization; John Wiley & Sons: New York, NY, USA, 1996; p. 560. [Google Scholar]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Sathivel, S.; Bechtel, P.J. Properties of soluble protein powders from Alaska pollock (Theragra chalcogramma). Int. J. Food Sci. Technol. 2006, 41, 520–529. [Google Scholar] [CrossRef]
- Niki, H.; Deya, E.; Kato, T.; Igarashi, S. The process of producing active fish protein powder. Bull. Jap. Soc. Sci. Fish. 1982, 48, 999–1004. [Google Scholar] [CrossRef]
- Yoon, I.S.; Lee, H.J.; Kang, S.I.; Park, S.Y.; Kang, Y.M.; Kim, J.S.; Heu, M.S. Food functionality of protein isolates extracted from Yellowfin tuna (Thunnus albacares) roe using alkaline solubilization and acid precipitation process. Food Sci. Nutr. 2019, 7, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Prabhasankar, P. Marine foods as functional ingredients in bakery pasta products. Food Res. Int. 2010, 43, 1975–1980. [Google Scholar] [CrossRef]
- Vijaykrishnaraj, M.; Roopa, B.; Prabhasankar, P. Preparation of gluten free bread enriched with green mussel (Perna canaliculus) protein hydrolysates and characterization of peptides responsible for mussel flavour. Food Chem. 2016, 211, 715–725. [Google Scholar] [CrossRef]
- Tacon, A.G.; Metian, M. Fish matters: Importance of aquatic foods in human nutrition and global food supply. Rev. Fish. Sci. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- Wong, C.P.; Bray, T.M.; Khanna, S.K. Growth, bone health, and cognition: Nutritional evaluation of a sustainable ocean-based advance protein powder (APP). Ecol. Food Nutr. 2019, 58, 80–92. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Kent, G. Fish, Food, and Hunger: The Potential of Fisheries for Alleviating Malnutrition; Routledge: New York, NY, USA, 2019. [Google Scholar]
- Da Silva, C.P.; Bezerra, R.S.; dos Santos, A.C.O.; Messias, J.B.; de Castro, C.R.O.B.; Junior, L.B.C. Biological value of shrimp protein hydrolysate by-product produced by autolysis. LWT Food Sci. Technol. 2017, 80, 456–461. [Google Scholar] [CrossRef]
- Pires, C.; Batista, I.; Fradinho, P.; Costa, S. Utilization of alkaline-recovered proteins from cape hake by-products in the preparation of Frankfurter-type fish sausages. J. Aquat. Food Prod. Technol. 2009, 18, 170–190. [Google Scholar] [CrossRef]
- Harrison, R.W.; Stringer, T.; Prinyawiwatkul, W. Evaluating consumer preferences for aquacultural products: An application to the US crawfish industry. Aquac. Econ. Manag. 2001, 5, 337–349. [Google Scholar] [CrossRef]
- Taskaya, L.; Jaczynski, J. Flocculation-enhanced protein recovery from fish processing by-products by isoelectric solubilization/precipitation. LWT-Food Sci. Technol. 2009, 42, 570–575. [Google Scholar]
- Tahergorabi, R.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Functional food products made from fish protein isolate recovered with isoelectric solubilization/precipitation. Food Sci. Technol. 2012, 48, 89–95. [Google Scholar] [CrossRef]
- Wijaya, W.; Patel, A.R.; Setiowati, A.D.; Van der Meeren, P. Functional colloids from proteins and polysaccharides for food applications. Trends Food Sci. Technol. 2017, 68, 56–69. [Google Scholar] [CrossRef]
- Hu, X.; Cebe, P.; Weiss, A.S.; Omenetto, F.; Kaplan, D.L. Protein-based composite materials. Mater. Today 2012, 15, 208–215. [Google Scholar] [CrossRef]
- Kim, S.K.; Mendis, E. Bioactive compounds from marine processing byproducts–A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Kwon, J.Y.; Kim, S.-K. A novel angiotensin I converting enzyme inhibitory peptide from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. J. Agric. Food Chem. 2004, 52, 7842–7845. [Google Scholar] [CrossRef] [PubMed]
- Je, J.-Y.; Kim, S.-Y.; Kim, S.-K. Preparation and antioxidative activity of hoki frame protein hydrolysate using ultrafiltration membranes. Eur. Food Res. Technol. 2005, 221, 157–162. [Google Scholar] [CrossRef]
- Huang, S.-L.; Jao, C.-L.; Ho, K.-P.; Hsu, K.-C. Dipeptidyl-Peptidase IV Inhibitory Activity of Peptides Derived from Tuna Cooking Juice Hydrolysates, Peptides 2000. In Proceedings of the European Peptide Symposium 26th, Montpellier, France, 10–15 September 2000; Martinez, J., Fehrentz, J.-A., Eds.; Peptides: Montpellier, France, 2012; pp. 114–121. [Google Scholar]
- Vilas Boas, L.C.P.; de Lima, L.M.P.; Migliolo, L.; Mendes, G.d.S.; de Jesus, M.G.; Franco, O.L.; Silva, P.A. Linear antimicrobial peptides with activity against herpes simplex virus 1 and Aichi virus. Pept. Sci. 2017, 108, e22871. [Google Scholar] [CrossRef]
- Abdou, A.M.; Higashiguchi, S.; Horie, K.; Kim, M.; Hatta, H.; Yokogoshi, H. Relaxation and immunity enhancement effects of γ-Aminobutyric acid (GABA) administration in humans. Biofactors 2006, 26, 201–208. [Google Scholar] [CrossRef]
- Fugelli, K. Gamma-aminobutyric acid (GABA) in fish erythrocytes. Experientia 1970, 26, 361. [Google Scholar] [CrossRef]
- Nakajima, K.; Yoshie-Stark, Y.; Ogushi, M. Comparison of ACE inhibitory and DPPH radical scavenging activities of fish muscle hydrolysates. Food Chem. 2009, 114, 844–851. [Google Scholar] [CrossRef]
- Shao, A.; Hathcock, J.N. Risk assessment for the amino acids taurine, L-glutamine and L-arginine. Regul. Toxicol. Pharmacol. 2008, 50, 376–399. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Olivieri, G.; de Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.; Kleinegris, D.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Albers, E.; Undeland, I. pH-shift processing of Nannochloropsis oculata microalgal biomass to obtain a protein-enriched food or feed ingredient. Algal Res. 2015, 11, 95–102. [Google Scholar] [CrossRef]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Recent advances in the recovery techniques of plant-based proteins from agro-industrial by-products. Food Rev. Int. 2020, 1–22. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Vermuë, M.; Eppink, M.; Wijffels, R.; Van Den Berg, C. Multi-product microalgae biorefineries: From concept towards reality. Trends Biotechnol. 2018, 36, 216–227. [Google Scholar]
- Nguyen, T.T. Biorefinery Process Development for Recovery of Functional and Bioactive Compounds from Lobster Processing by-Products for Food and Nutraceutical Applications; Flinders University: Adelaide, Australia, 2017. [Google Scholar]
- Yan, N.; Chen, X. Don’t waste seafood waste. Nature 2015, 254, 155–157. [Google Scholar] [CrossRef]
- Denise, S.; Jason, B. Food Grade Astaxanthin from Lobster Shell Discards; Maine Agricultural Center: Orono, ME, USA, 2012. [Google Scholar]
- Arason, S.; Karlsdottir, M.; Valsdottir, T.; Slizyte, R.; Rustad, T.; Falch, E.; Eysturskard, J.; Jakobsen, G. Technical Report: Maximum Resource Utilisation-Value Added Fish by-Products; Nordic Innovation Centre: Oslo, Norway, 2010; p. 129. [Google Scholar]
- Fletcher, R. Don’t Bypass the Value of Aquaculture by-Products. Fish. Site 2018. Available online: https://thefishsite.com/articles/dont-bypass-the-value-of-aquaculture-by-products (accessed on 25 April 2020).
- Nguyen, T.T.; Barber, R.A.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioresour. Bioprocess. 2017, 4, 1–19. [Google Scholar] [CrossRef]
- Šilovs, M. Fish processing by-products exploitation and innovative fish-based food production. Res. Rural Dev. 2018, 2, 210–215. [Google Scholar]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Chandrasekaran, M. Valorization of Food Processing by-Products; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Suresh, P.; Kudre, T.G.; Johny, L.C. Sustainable valorization of seafood processing by-product/discard. In Waste to Wealth; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Berlin, Germany, 2018; pp. 111–139. [Google Scholar]
- Pateiro, M.; Munekata, P.E.; Domínguez, R.; Wang, M.; Barba, F.J.; Bermúdez, R.; Lorenzo, J.M. Nutritional profiling and the value of processing by-products from gilthead sea bream (Sparus aurata). Mar. Drugs 2020, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Nigg, J.D.; Stine, J.J.; Bechtel, P.J. Nutritional and chemical composition of by-product fractions produced from wet reduction of individual red salmon (Oncorhynchus nerka) heads and viscera. J. Aquat. Food Prod. Technol. 2011, 20, 183–195. [Google Scholar] [CrossRef]
- Shruthy, R.; Preetha, R. Utilization of fish and shellfish byproducts from Marine food Industries: Benefits and challenges. In Technological Processes for Marine Foods, from Water to Fork: Bioactive Compounds, Industrial Applications, Genomics; Goyal, M., Suleria, H., Kirubanandan, S., Eds.; Routledge: Palm Bay, FL, USA, 2019; p. 271. [Google Scholar]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of fish skin collagen: An opportunity for valorizing fish industry byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Toppe, J.; Albrektsen, S.; Hope, B.; Aksnes, A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2007, 146, 395–401. [Google Scholar]
- Masood, Z.; Yasmeen, R.; Haider, M.S.; Tarar, O.M.; Hossain, M. Evaluations of crude protein and amino acid contents from the scales of four mullet species (Mugilidae) collected from Karachi fish harbour, Pakistan. Ind. J. Geo-Mar. Sci. 2015, 44, 724–731. [Google Scholar]
- Cardoso, C.; Nunes, M.L. Improved utilization of fish waste, discards, and by-products and low-value fish towards food and health products. In Utilization of Fish Waste; Galvez, R., Berge, J.-P., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 26–58. [Google Scholar]
- Hayes, M.; Gallagher, M. Processing and recovery of valuable components from pelagic blood-water waste streams: A review and recommendations. J. Clean. Prod. 2019, 215, 410–422. [Google Scholar] [CrossRef]
- Chernyavskikh, S.D.; Borodaeva, Z.A.; Borisovskiy, I.P.; Ostapenko, S.I.; Galtseva, O.A. Blood protein spectrum in representatives of the fish superclass. Eurasianj. Biosci. 2019, 13, 979–981. [Google Scholar]
- Rødde, R.H.; Einbu, A.; Vårum, K.M. A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalus borealis). Carbohydr. Polym. 2008, 71, 388–393. [Google Scholar] [CrossRef]
- Hossain, M.; Shikha, F.; Sharma, A. Waste management status of shrimp processing plants of south and south-west region of Bangladesh. J. Environ. Sci. Nat. Resour. 2018, 11, 73–81. [Google Scholar] [CrossRef]
- Trung, T.S.; Phuong, P.T.D. Bioactive compounds from by-products of shrimp processing industry in Vietnam. J. Food Drug Anal. 2012, 20, 194–197. [Google Scholar]
- Nguyen, T.T.; Zhang, W.; Barber, R.A.; Su, P.; He, S. Significant enrichment of polyunsaturated fatty acids (PUFAs) in the lipids extracted by supercritical CO2 from the livers of Australian rock lobsters (Jasus edwardsii). J. Agric. Food Chem. 2015, 63, 4621–4628. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.P.; Backeljau, T.; Chapelle, G. Shells from aquaculture: A valuable biomaterial, not a nuisance waste product. Rev. Aquac. 2019, 11, 42–57. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G. Molluscan shell proteins. Comptes Rendus Palevol 2004, 3, 469–492. [Google Scholar] [CrossRef]
- Huang, G.; Bi, X.; Zhang, B.; Qu, T.; Liu, B.; Fan, S.; Yu, D. Expression, purification, and functional activity of shell matrix protein pearlin from the pearl oyster Pinctada fucata. J. Shellfish Res. 2017, 36, 373–378. [Google Scholar] [CrossRef]
- Naik, A.; Hayes, M. Bioprocessing of mussel by-products for value added ingredients. Trends Food Sci. Technol. 2019, 92, 111–121. [Google Scholar] [CrossRef]
- Kumar, V.; Muzaddadi, A.U.; Mann, S.; Balakrishnan, R.; Bembem, K.; Kalnar, Y. Utilization of fish processing waste: A waste to wealth approach. Emerg. Post-Harvest Eng. Techological Interv. Enhancing Farmer’s Income 2018, 5, 127–131. [Google Scholar]
- Derby, C.D. Cephalopod ink: Production, chemistry, functions and applications. Mar. Drugs 2014, 12, 2700–2730. [Google Scholar] [CrossRef]
- Jose, J.; Krishnakumar, K.; Dineshkumar, B. Squid ink and its pharmacological activities. GSC Biol. Pharm. Sci. 2018, 2, 17–22. [Google Scholar]
- Sugiyama, M.; Hanabe, M. Utilization of Squid; CRC Press: New York, NY, USA, 1989; p. 251. [Google Scholar]
- Singh, A.; Mittal, A.; Benjakul, S. Full utilization of squid meat and its processing by-products: Revisit. Food Rev. Int. 2020, 1–25. [Google Scholar] [CrossRef]
- Amarowicz, R.; Synowiecki, J.; Shahidi, F. Chemical composition of shells from red (Strongylocentrotus franciscanus) and green (Strongylocentrotus droebachiensis) sea urchin. Food Chem. 2012, 133, 822–826. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef]
- Brotz, L.; Schiariti, A.; López-Martínez, J.; Álvarez-Tello, J.; Hsieh, Y.-H.P.; Jones, R.P.; Quiñones, J.; Dong, Z.; Morandini, A.C.; Preciado, M. Jellyfish fisheries in the Americas: Origin, state of the art, and perspectives on new fishing grounds. Rev. Fish. Biol. Fish. 2017, 27, 1–29. [Google Scholar] [CrossRef]
- Liu, C.; Li, S.; Kong, J.; Liu, Y.; Wang, T.; Xie, L.; Zhang, R. In-depth proteomic analysis of shell matrix proteins of Pinctada fucata. Sci. Rep. 2015, 5, 17269. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.H.; Martin, A.M.; Saker-Sampaiao, S.; Omar, S.; Goncalves, R.C. Studies on the enzymatic hydrolysis of Brazilian lobster (Panulirus spp.) processing wastes. J. Sci. Food Agric. 1995, 69, 61–65. [Google Scholar] [CrossRef]
- Bechtel, P.J.; Watson, M.A.; Lea, J.M.; Bett-Garber, K.L.; Bland, J.M. Properties of bone from catfish heads and frames. Food Sci. Nutr. 2019, 7, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Mace, O.J.; Morgan, E.L.; Affleck, J.A.; Lister, N.; Kellett, G.L. Calcium absorption by Cav1. 3 induces terminal web myosin II phosphorylation and apical GLUT2 insertion in rat intestine. J. Physiol. 2007, 580, 605–616. [Google Scholar] [CrossRef]
- Muralidharan, N.; Shakila, R.J.; Sukumar, D.; Jeyasekaran, G. Skin, bone and muscle collagen extraction from the trash fish, leather jacket (Odonus niger) and their characterization. J. Food Sci. Technol. 2013, 50, 1106–1113. [Google Scholar] [CrossRef]
- Xu, S.; Yang, H.; Shen, L.; Li, G. Purity and yield of collagen extracted from southern catfish (Silurus meridionalis Chen) skin through improved pretreatment methods. Int. J. Food Prop. 2017, 20, S141–S153. [Google Scholar] [CrossRef]
- Benjakul, S.; Oungbho, K.; Visessanguan, W.; Thiansilakul, Y.; Roytrakul, S. Characteristics of gelatin from the skins of bigeye snapper, Priacanthus tayenus and Priacanthus macracanthus. Food Chem. 2009, 116, 445–451. [Google Scholar] [CrossRef]
- Binsi, P.; Shamasundar, B.; Dileep, A.; Badii, F.; Howell, N. Rheological and functional properties of gelatin from the skin of Bigeye snapper (Priacanthus hamrur) fish: Influence of gelatin on the gel-forming ability of fish mince. Food Hydrocoll. 2009, 23, 132–145. [Google Scholar] [CrossRef]
- Sukkwai, S.; Kijroongrojana, K.; Benjakul, S. Extraction of gelatin from bigeye snapper (Priacanthus tayenus) skin for gelatin hydrolysate production. Int. Food Res. J. 2011, 18, 1129–1134. [Google Scholar]
- Aewsiri, T.; Benjakul, S.; Visessanguan, W. Functional properties of gelatin from cuttlefish (Sepia pharaonis) skin as affected by bleaching using hydrogen peroxide. Food Chem. 2009, 115, 243–249. [Google Scholar] [CrossRef]
- Nagai, T.; Araki, Y.; Suzuki, N. Collagen of the skin of ocellate puffer fish (Takifugu rubripes). Food Chem. 2002, 78, 173–177. [Google Scholar] [CrossRef]
- Kołodziejska, I.; Sikorski, Z.E.; Niecikowska, C. Parameters affecting the isolation of collagen from squid (Illex argentinus) skins. Food Chem. 1999, 66, 153–157. [Google Scholar] [CrossRef]
- Senaratne, L.; Park, P.-J.; Kim, S.-K. Isolation and characterization of collagen from brown backed toadfish (Lagocephalus gloveri) skin. Bioresour. Technol. 2006, 97, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Suzuki, N. Isolation of collagen from fish waste material—Skin, bone and fins. Food Chem. 2000, 68, 277–281. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Aewsiri, T.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Chemical compositions and functional properties of gelatin from pre-cooked tuna fin. Int. J. Food Sci. 2008, 43, 685–693. [Google Scholar] [CrossRef]
- Okazaki, E.; Osako, K. Isolation and characterization of acid-soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chem. 2014, 149, 264–270. [Google Scholar]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Ezquerra-Brauer, J.M.; Haard, N.F.; Ramirez-Olivas, R.; Olivas-Burrola, H.; Vela zquez-Sanchez, C.J. Influence of harvest season on the proteolytic activity of hepatopancreas and mantle tissues from jumbo squid (Doswicus gigas). J. Food Biochem. 2002, 26, 459–475. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals; Food and Agriculture Organization of the United Nation: Rome, Italy, 2018; p. 227. [Google Scholar]
- Kumar, N.S.; Nazeer, R.; Jaiganesh, R. Purification and Biochemical Characterization of Antioxidant Peptide from Horse Mackerel (Magalaspis Cordyla) Viscera Protein, Peptides 2000. In Proceedings of the European Peptide Symposium 26th, Montpellier, France, 10–15 September 2000; Peptides: Montpellier, France, 2011; pp. 1496–1501. [Google Scholar]
- Sriket, C. Proteases in fish and shellfish: Role on muscle softening and prevention. Int. Food Res. J. 2014, 21, 433. [Google Scholar]
- Shahidi, F.; Kamil, Y.J. Enzymes from fish and aquatic invertebrates and their application in the food industry. Trends Food Sci. Technol. 2001, 12, 435–464. [Google Scholar] [CrossRef]
- Raa, J. Biotechnology in Aquaculture and the Fish Processing Industry: A Success Story in Norway; Technomic Publishing: Lancaster, PA, USA, 1990; pp. 509–524. [Google Scholar]
- North, M. Prevention of unwanted proteolysis. In Proteolytic Enzymes: A Practical Approach; Beynon, R.J., Bond, J.S., Eds.; Oxford University Press: Oxford, UK, 1989; pp. 105–124. [Google Scholar]
- Bezerra, R.S.; Lins, E.J.; Alencar, R.B.; Paiva, P.M.; Chaves, M.E.; Coelho, L.C.; Carvalho, L.B., Jr. Alkaline proteinase from intestine of Nile tilapia (Oreochromis niloticus). Process Biochem. 2005, 40, 1829–1834. [Google Scholar] [CrossRef]
- Klomklao, S.; Kishimura, H.; Nonami, Y.; Benjakul, S. Biochemical properties of two isoforms of trypsin purified from the intestine of skipjack tuna (Katsuwonus pelamis). Food Chem. 2009, 115, 155–162. [Google Scholar] [CrossRef]
- Gildberg, A. Recovery of proteinases and protein hydrolysates from fish viscera. Bioresour. Technol. 1992, 39, 271–276. [Google Scholar] [CrossRef]
- Murthy, L.N.; Phadke, G.G.; Unnikrishnan, P.; Annamalai, J.; Joshy, C.G.; Zynudheen, A.A.; Ravishankar, C.N. Valorization of fish viscera for crude proteases production and its use in bioactive protein hydrolysate preparation. Waste Biomass Valoriz. 2018, 9, 1735–1746. [Google Scholar] [CrossRef]
- Ketnawa, S.; Benjakul, S.; Ling, T.C.; Martínez-Alvarez, O.; Rawdkuen, S. Enhanced recovery of alkaline protease from fish viscera by phase partitioning and its application. Chem. Cent. J. 2013, 7, 79. [Google Scholar] [CrossRef]
- Bezerra, R.; Santos, J.F.; Paiva, P.M.; Correia, M.T.; Coelho, L.C.; Vieira, V.L.; Carvalho JR, L.B. Partial purification and characterization of a thermostable trypsin from pyloric caeca of tambaqui (Colossoma macropomum). J. Food Biochem. 2001, 25, 199–210. [Google Scholar] [CrossRef]
- Khantaphant, S.; Benjakul, S. Purification and characterization of trypsin from the pyloric caeca of brownstripe red snapper (Lutjanus vitta). Food Chem. 2010, 120, 658–664. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Simpson, B.K. Trypsin from the pyloric caeca of bluefish (Pomatomus saltatrix). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 382–389. [Google Scholar] [CrossRef]
- Silva, J.F.; Espósito, T.S.; Marcuschi, M.; Ribeiro, K.; Cavalli, R.O.; Oliveira, V.; Bezerra, R.S. Purification and partial characterisation of a trypsin from the processing waste of the silver mojarra (Diapterus rhombeus). Food Chem. 2011, 129, 777–782. [Google Scholar] [CrossRef]
- Travis, J.; Salvesen, G. Human plasma proteinase inhibitors. Ann. Rev. Biochem. 1983, 52, 655–709. [Google Scholar] [CrossRef]
- Li, D.; Lin, H.; Kim, S. Effect of rainbow trout (Oncorhynchus mykiss) plasma protein on the gelation of Alaska pollock (Theragra chalcogramma) surimi. J. Food Sci. 2008, 73, C227–C234. [Google Scholar] [CrossRef]
- Li, D.K.; Lin, H.; Kim, S.M. Purification and characterization of a cysteine protease inhibitor from chum salmon (Oncorhynchus keta) plasma. J. Agric. Food Chem. 2008, 56, 106–111. [Google Scholar] [CrossRef]
- Fowler, M.R.; Park, J.W. Salmon blood plasma: Effective inhibitor of protease-laden Pacific whiting surimi and salmon mince. Food Chem. 2015, 176, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J. [54] α2-Macroglobulin. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1981; Volume 80, pp. 737–754. [Google Scholar]
- Simke, A. Fish Blood May Provide Biotech Researchers with an Ethical Alternative to Fetal Bovine Fluid. Forbes 2020. Available online: https://www.forbes.com/sites/ariellasimke/2020/02/24/could-fish-blood-replace-ethically-questionable-fetal-fluid/ (accessed on 25 May 2020).
- Tadashi, M.; Haruko, T.; Miyashita, H.; Hiroko, Y. Marine microalgae. Adv. Biochem. Engin. Biotechnol. 2005, 2005, 165–188. [Google Scholar]
- Vigani, M.; Parisi, C.; Rodríguez-Cerezo, E.; Barbosa, M.J.; Sijtsma, L.; Ploeg, M.; Enzing, C. Food and feed products from micro-algae: Market opportunities and challenges for the EU. Trends Food Sci. Technol. 2015, 42, 81–92. [Google Scholar] [CrossRef]
- William, M.; Asaf, T. Micro Solutions for a Macro Problem: How Marine Algae Could Help Feed the World. Conversation 2017. Available online: https://theconversation.com/micro-solutions-for-a-macro-problem-how-marine-algae-could-help-feed-the-world-85702 (accessed on 15 May 2020).
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Velu, C.; Cirés, S.; Brinkman, D.L.; Heimann, K. Bioproduct potential of outdoor cultures of Tolypothrix sp.: Effect of carbon dioxide and metal-rich wastewater. Front. Bioeng. Biotechnol. 2020, 8, 1–18. [Google Scholar]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Blackwell Science: Oxford, UK, 2008; p. 588. [Google Scholar]
- Van Krimpen, M.; Bikker, P.; Van der Meer, I.; Van der Peet-Schwering, C.; Vereijken, J. Cultivation, Processing and Nutritional Aspects for Pigs and Poultry of European Protein Sources as Alternatives for Imported Soybean Products; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2013; p. 63. [Google Scholar]
- Hur, S.B.; Bae, J.H.; Youn, J.-Y.; Jo, M.J. KMMCC-Korea marine microalgae culture center: List of strains. Algae 2015, 30, 1–18. [Google Scholar] [CrossRef]
- Mogharabi, M.; Faramarzi, M.A. Are algae the future source of enzymes? Trends Pept. Prot. Sci. 2016, 1, 1–6. [Google Scholar]
- Brasil, B.D.S.A.F.; de Siqueira, F.G.; Salum, T.F.C.; Zanette, C.M.; Spier, M.R. Microalgae and cyanobacteria as enzyme biofactories. Algal Res. 2017, 25, 76–89. [Google Scholar] [CrossRef]
- Gong, Y.; Hu, H.; Gao, Y.; Xu, X.; Gao, H. Microalgae as platforms for production of recombinant proteins and valuable compounds: Progress and prospects. J. Ind. Microbiol. Biotechnol. 2011, 38, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.D. Spirulina: Production & Potential; Edisud: La Calade, France, 1996. [Google Scholar]
- Belay, A. The potential application of Spirulina (Arthrospira) as a nutritional and therapeutic supplement in health management. Am. Nutraceut. Assoc. 2002, 5, 27–48. [Google Scholar]
- Vingiani, G.M.; De Luca, P.; Ianora, A.; Dobson, A.D.; Lauritano, C. Microalgal enzymes with biotechnological applications. Mar. Drugs 2019, 17, 459. [Google Scholar] [CrossRef] [PubMed]
- Becker, E. Microalgae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Patelakis, S.J.; Whitney-Lalonde, C.G.; Garrison, L.L.; Wall, C.L.; MacQuarrie, S.P. Nutrient composition and protein quality of microalgae meals produced from the marine prymnesiophyte Pavlova sp. 459 mass-cultivated in enclosed photobioreactors for potential use in salmonid aquafeeds. J. Appl. Phycol. 2019, 32, 1–20. [Google Scholar]
- Tahergorabi, R.; Jaczynski, J. Seafood proteins and human health. In Fish and Fish Oil in Health and Disease Prevention; Raatz, S., Bibus, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 323–331. [Google Scholar]
- Peng, S.; Chen, C.; Shi, Z.; Wang, L. Amino acid and fatty acid composition of the muscle tissue of yellowfin tuna (Thunnus albacares) and bigeye tuna (Thunnus obesus). J. Food Nutr. Res. 2013, 1, 42–45. [Google Scholar]
- Leal, A.L.G.; de Castro, P.F.; de Lima, J.P.V.; de Souza Correia, E.; de Souza Bezerra, R. Use of shrimp protein hydrolysate in Nile tilapia (Oreochromis niloticus, L.) feeds. Aquac. Int. 2010, 18, 635–646. [Google Scholar]
- Barka, A.; Blecker, C. Microalgae as a potential source of single-cell proteins. A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 427–436. [Google Scholar]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Lim, A.S.; Jeong, H.J.; Kim, S.J.; Ok, J.H.; Lim, A.S.; Jeong, H.J.; Kim, S.J.; Ok, J.H. Amino acids profiles of six dinoflagellate species belonging to diverse families: Possible use as animal feeds in aquaculture. Algae 2018, 33, 279–290. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, C.; Hong, P.; Ji, H. Response surface methodology for autolysis parameters optimization of shrimp head and amino acids released during autolysis. Food Chem. 2008, 109, 176–183. [Google Scholar] [CrossRef]
- Niittynen, L.; Nurminen, M.-L.; Korpela, R.; Vapaatalo, H. Role of arginine, taurine 4 and homocysteine in cardiovascular diseases. Ann. Med. 1999, 31, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.; Mazza, G. Functional foods. In The Wiley Encyclopedia of Science and Technology, 2nd ed.; Francis, F., Ed.; Wiley: New York, NY, USA, 2000; Volume 2, pp. 1176–1182. [Google Scholar]
- Sidransky, H. Possible role of dietary proteins and amino acids in atherosclerosis. Ann. N. Y. Acad. Sci. 1990, 598, 464–481. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K. Sensory and receptor responses to umami: An overview of pioneering work. Am. J. Clin. Nutr. 2009, 90, 723S–727S. [Google Scholar] [CrossRef]
- Yamaguchi, S. Basic properties of umami and its effects on food flavor. Food Rev. Int. 1998, 14, 139–176. [Google Scholar] [CrossRef]
- Konasu, S.; Yamaguchi, K. Trimethylamine contents in fishery products. In Chemistry and Biochemistry of Marine Food Products; Hebard, C., Flick, G., Martin, R., Eds.; Avi Publishing Company: Westport, CT, USA, 1982; pp. 149–304. [Google Scholar]
- Guichard, E.; Salles, C. Retention and release of taste and aroma compounds from the food matrix during mastication and ingestion. In Flavor: From Food to Behaviors, Wellbeing and Health; Elisabeth, G., Christan, S., Andree, V., Patrick, E., Eds.; Woodhead Publishing: Cambridge, MA, USA, 2016; pp. 3–22. [Google Scholar]
- Imm, J.; Lee, C. Production of seafood flavor from red hake (Urophycis chuss) by enzymatic hydrolysis. J. Agric. Food Chem. 1999, 47, 2360–2366. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Bouglé, D.; Bouhallab, S. Dietary bioactive peptides: Human studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 335–343. [Google Scholar] [CrossRef]
- Jo, C.; Khan, F.F.; Khan, M.I.; Iqbal, J. Marine bioactive peptides: Types, structures, and physiological functions. Food Rev. Int. 2017, 33, 44–61. [Google Scholar] [CrossRef]
- Ishak, N.; Sarbon, N. A review of protein hydrolysates and bioactive peptides deriving from wastes generated by fish processing. Food Bioprocess. Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Lee, S.-H.; Qian, Z.-J.; Kim, S.-K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Du, L.; Fang, M.; Wu, H.; Xie, J.; Wu, Y.; Li, P.; Zhang, D.; Huang, Z.; Xia, Y.; Zhou, L. A novel angiotensin I-converting enzyme inhibitory peptide from Phascolosoma esculenta water-soluble protein hydrolysate. J. Function. Foods 2013, 5, 475–483. [Google Scholar] [CrossRef]
- Qian, Z.-J.; Heo, S.-J.; Oh, C.H.; Kang, D.-H.; Jeong, S.H.; Park, W.S.; Choi, I.-W.; Jeon, Y.-J.; Jung, W.-K. Angiotensin I-converting enzyme (ACE) inhibitory peptide isolated from biodiesel byproducts of marine microalgae, Nannochloropsis oculata. J. Biobased Mater. Bioenergy 2013, 7, 135–142. [Google Scholar] [CrossRef]
- He, H.-L.; Chen, X.-L.; Wu, H.; Sun, C.-Y.; Zhang, Y.-Z.; Zhou, B.-C. High throughput and rapid screening of marine protein hydrolysates enriched in peptides with angiotensin-I-converting enzyme inhibitory activity by capillary electrophoresis. Bioresour. Technol. 2007, 98, 3499–3505. [Google Scholar] [CrossRef] [PubMed]
- Pujiastuti, D.Y.; Amin, G.; Nur, M.; Alamsjah, M.A.; Hsu, J.-L. Marine organisms as potential sources of bioactive peptides that inhibit the activity of angiotensin I-converting enzyme: A review. Molecules 2019, 24, 2541. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure− activity relationship study of di-and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef]
- Wang, Q. Preparation of functional peanut oligopeptide and its biological activity. In Peanut Processing Characteristics and Quality Evaluation; Wang, Q., Ed.; Springer: Berlin, Germany, 2018; pp. 461–537. [Google Scholar]
- Zhou, D.Y.; Liu, Z.Y.; Zhao, J.; Xi, M.Z.; Fu, Y.H.; Zhang, T.; Ji, C.F.; Zhu, B.W. Antarctic krill (Euphausia superba) protein hydrolysates stimulate cholecystokinin release in STC-1 cells and its signaling mechanism. J. Food Process. Preserv. 2017, 41, e12903. [Google Scholar] [CrossRef]
- He, H.-L.; Liu, D.; Ma, C.-B. Review on the angiotensin-I-converting enzyme (ACE) inhibitor peptides from marine proteins. Appl. Biochem. Biotechnol. 2013, 169, 738–749. [Google Scholar] [CrossRef]
- Samarakoon, K.W.; Kwon, O.-N.; Ko, J.-Y.; Lee, J.-H.; Kang, M.-C.; Kim, D.; Lee, J.B.; Lee, J.-S.; Jeon, Y.-J. Purification and identification of novel angiotensin-I converting enzyme (ACE) inhibitory peptides from cultured marine microalgae (Nannochloropsis oculata) protein hydrolysate. J. Appl. Phycol. 2013, 25, 1595–1606. [Google Scholar] [CrossRef]
- Samarakoon, K.; Ko, S.-C.; You-Jin, J. Isolation and purification of angiotensin-I converting enzyme (ACE) inhibitory peptides from marine microalgae. In Proceedings of the International Conference on Fisheries & Marine Science (Marine Fish 2012), Negombo, Sri Lanka, 23 August 2012. [Google Scholar]
- Wu, H.; Xu, N.; Sun, X.; Yu, H.; Zhou, C. Hydrolysis and purification of ACE inhibitory peptides from the marine microalgae Isochrysis galbana. J. Appl. Phycol. 2015, 27, 351–361. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and identification of ACE inhibitory peptides from the marine macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef] [PubMed]
- Saito, T. Antihypertensive peptides derived from bovine casein and whey proteins. In Bioactive Components of Milk; Bösze, Z., Ed.; Springer: Berlin, Germany, 2008; pp. 295–317. [Google Scholar]
- Nakashima, Y.; Arihara, K.; Sasaki, A.; Mio, H.; Ishikawa, S.; Itoh, M. Antihypertensive activities of peptides derived from porcine skeletal muscle myosin in spontaneously hypertensive rats. J. Food Sci. 2002, 67, 434–437. [Google Scholar] [CrossRef]
- Stadnik, J.; Kęska, P. Meat and fermented meat products as a source of bioactive peptides. Acta Sci. Polon. Technol. Aliment. 2015, 14, 181–190. [Google Scholar] [CrossRef]
- Davalos, A.; Miguel, M.; Bartolome, B.; Lopez-Fandino, R. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Protect. 2004, 67, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Park, S.Y.; Je, J.-Y.; Ahn, C.-B. Protein hydrolysates and ultrafiltration fractions obtained from krill (Euphausia superba): Nutritional, functional, antioxidant, and ACE-Inhibitory characterization. J. Aquat. Food Prod. Technol. 2016, 25, 1266–1277. [Google Scholar] [CrossRef]
- Hai-Lun, H.; Xiu-Lan, C.; Cai-Yun, S.; Yu-Zhong, Z.; Bai-Cheng, Z. Analysis of novel angiotensin-I-converting enzyme inhibitory peptides from protease-hydrolyzed marine shrimp Acetes Chinensis. J. Pept. Sci. 2006, 12, 726–733. [Google Scholar] [CrossRef]
- Chizuru, S.; Satoshi, T.; Riho, T.; Saki, F.; Miyu, K.; Chikako, A.; Yoshitoshi, N. Isolation and identification of an angiotensin I-converting enzyme inhibitory peptide from pearl oyster (Pinctada fucata) shell protein hydrolysate. Process Biochem. 2019, 137–142. [Google Scholar]
- Noorani, K.P.M.; Nazeer, R. Enzymatic production of two tri-peptides on ACE-I Inhibition and antioxidant activities. Int. J. Pept. Res. Therapeut. 2020, 1–13. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Shi, Y.; Qiao, R.; Tang, W.; Sun, Z. Production of the angiotensin I converting enzyme inhibitory peptides and isolation of four novel peptides from jellyfish (Rhopilema esculentum) protein hydrolysate. J. Sci. Food Agric. 2016, 96, 3240–3248. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, B.; Dong, S.; Liu, Z.; Zhao, X.; Wang, J.; Zeng, M. A novel ACE Inhibitory Peptide Isolated from Acaudina Molpadioidea Hydrolysate, Peptides 2000. In Proceedings of the European Peptide Symposium 26th, Montpellier, France, 10–15 September 2000; Peptides: Montpellier, France, 2009; pp. 1028–1033. [Google Scholar]
- Balti, R.; Nedjar-Arroume, N.; Bougatef, A.; Guillochon, D.; Nasri, M. Three novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) using digestive proteases. Food Res. Int. 2010, 43, 1136–1143. [Google Scholar] [CrossRef]
- Alemán, A.; Gómez-Guillén, M.; Montero, P. Identification of ACE-inhibitory peptides from squid skin collagen after in vitro gastrointestinal digestion. Food Res. Int. 2013, 54, 790–795. [Google Scholar] [CrossRef]
- Himaya, S.; Ngo, D.-H.; Ryu, B.; Kim, S.-K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012, 132, 1872–1882. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; Alashi, M.A.; Aluko, R.E.; FitzGerald, R.J. Peptide identification in a salmon gelatin hydrolysate with antihypertensive, dipeptidyl peptidase IV inhibitory and antioxidant activities. Food Res. Int. 2017, 100, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Mora, L.; Hussey, K.; Aluko, R.E. Boarfish protein recovery using the pH-shift process and generation of protein hydrolysates with ACE-I and antihypertensive bioactivities in spontaneously hypertensive rats. Innov. Food Sci. Emerg. Technol. 2016, 37, 253–260. [Google Scholar] [CrossRef]
- Salampessy, J.; Reddy, N.; Phillips, M.; Kailasapathy, K. Isolation and characterization of nutraceutically potential ACE-Inhibitory peptides from leatherjacket (Meuchenia sp.) protein hydrolysates. LWT-Food Sci. Technol. 2017, 80, 430–436. [Google Scholar]
- Ko, J.-Y.; Kang, N.; Lee, J.-H.; Kim, J.-S.; Kim, W.-S.; Park, S.-J.; Kim, Y.-T.; Jeon, Y.-J. Angiotensin I-converting enzyme inhibitory peptides from an enzymatic hydrolysate of flounder fish (Paralichthys olivaceus) muscle as a potent anti-hypertensive agent. Process Biochem. 2016, 51, 535–541. [Google Scholar] [CrossRef]
- Kim, H.-J.; Park, K.-H.; Shin, J.-H.; Lee, J.-S.; Heu, M.-S.; Lee, D.-H.; Kim, J.-S. Antioxidant and ACE inhibiting activities of the rockfish Sebastes hubbsi skin gelatin hydrolysates produced by sequential two-step enzymatic hydrolysis. Fish. Aquat. Sci. 2011, 14, 1–10. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Ryu, B.; Kim, S.-K. Active peptides from skate (Okamejei kenojei) skin gelatin diminish angiotensin-I converting enzyme activity and intracellular free radical-mediated oxidation. Food Chem. 2014, 143, 246–255. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, J.-Y.; Jung, W.-K.; Park, P.-J.; Kim, S.-K. Isolation of angiotensin I converting enzyme (ACE) inhibitor from fermented oyster sauce, Crassostrea gigas. Food Chem. 2005, 90, 809–814. [Google Scholar] [CrossRef]
- Alemán, A.; Pérez-Santín, E.; Bordenave-Juchereau, S.; Arnaudin, I.; Gómez-Guillén, M.; Montero, P. Squid gelatin hydrolysates with antihypertensive, anticancer and antioxidant activity. Food Res. Int. 2011, 44, 1044–1051. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kang, N.; Kim, E.-A.; Kang, M.C.; Lee, S.-H.; Kang, S.-M.; Lee, J.-B.; Jeon, B.-T.; Kim, S.-K.; Park, S.-J. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem. 2012, 47, 2005–2011. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chen, G.-W.; Yeh, C.H.; Song, H.; Tsai, J.-S. Purification and identification of angiotensin I-converting enzyme inhibitory peptides and the antihypertensive effect of Chlorella sorokiniana protein hydrolysates. Nutrients 2018, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Chiozzi, R.Z.; Laganà, A. Peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in Tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586. [Google Scholar] [CrossRef] [PubMed]
- Tejano, L.A.; Peralta, J.P.; Yap, E.E.S.; Chang, Y.W. Bioactivities of enzymatic protein hydrolysates derived from Chlorella sorokiniana. Food Sci. Nutr. 2019, 7, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.; Stormo, S.K.; Dragnes, B.T.; Elvevoll, E.O. Losses of taurine, creatine, glycine and alanine from cod (Gadus morhua L.) fillet during processing. J. Food Comp. Anal. 2007, 20, 396–402. [Google Scholar]
- Lourenco, R.; Camilo, M. Taurine: A conditionally essential amino acid in humans? An overview in health and disease. Nutr. Hosp. 2002, 17, 262–270. [Google Scholar] [PubMed]
- Militante, J.; Lombardini, J. Treatment of hypertension with oral taurine: Experimental and clinical studies. Amino Acids 2002, 23, 381–393. [Google Scholar] [CrossRef]
- Zhang, M.; Bi, L.; Fang, J.; Su, X.; Da, G.; Kuwamori, T.; Kagamimori, S. Beneficial effects of taurine on serum lipids in overweight or obese non-diabetic subjects. Amino Acids 2004, 26, 267–271. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef]
- Tsai, J.; Lin, Y.; Pan, B.; Chen, T. Antihypertensive peptides and γ-aminobutyric acid from prozyme 6 facilitated lactic acid bacteria fermentation of soymilk. Process Biochem. 2006, 41, 1282–1288. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Byun, H.-G.; Kim, S.-K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-J.; Jung, W.-K.; Byun, H.-G.; Kim, S.-K. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 2008, 99, 3365–3371. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Jung, W.-K.; Je, J.-Y.; Kim, S.-K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Yahia, D.A.; Madani, S.; Prost, E.; Prost, J.; Bouchenak, M.; Belleville, J. Tissue antioxidant status differs in spontaneously hypertensive rats fed fish protein or casein. J. Nutr. 2003, 133, 479–482. [Google Scholar] [CrossRef]
- Girard, A.; Prost, J.L.; Ait-Yahia, D.; Bouchenak, M.; Belleville, J. Fish protein improves the total antioxidant status of streptozotocin-induced diabetes in spontaneously hypertensive rat. Med. Sci. Monit. 2004, 10, 397–404. [Google Scholar]
- Jensen, I.-J.; Walquist, M.; Liaset, B.; Elvevoll, E.O.; Eilertsen, K.-E. Dietary intake of cod and scallop reduces atherosclerotic burden in female apolipoprotein E-deficient mice fed a Western-type high fat diet for 13 weeks. Nutr. Metabol. 2016, 13, 1–11. [Google Scholar] [CrossRef]
- Parra, D.; Bandarra, N.M.; Kiely, M.; Thorsdottir, I.; Martínez, J.A. Impact of fish intake on oxidative stress when included into a moderate energy-restricted program to treat obesity. Eur. J. Nutr. 2007, 46, 460–467. [Google Scholar] [CrossRef]
- Seth, A.; Mahoney, R.R. Iron chelation by digests of insoluble chicken muscle protein: The role of histidine residues. J. Sci. Food Agric. 2001, 81, 183–187. [Google Scholar] [CrossRef]
- Kang, K.-H.; Qian, Z.-J.; Ryu, B.; Kim, S.-K. Characterization of growth and protein contents from microalgae Navicula incerta with the investigation of antioxidant activity of enzymatic hydrolysates. Food Sci. Biotechnol. 2011, 20, 183–191. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Batista, I.; Pires, C.; Bandarra, N.M.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Antioxidant activity of protein hydrolysates obtained from discarded Mediterranean fish species. Food Res. Int. 2014, 65, 469–476. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, M.; Benjakul, S.; Ehsani, A.; Li, J.; Wu, F.; Yang, N.; Xu, B.; Jin, Z.; Xu, X. Antioxidant and cryoprotective effects of a tetrapeptide isolated from Amur sturgeon skin gelatin. J. Funct. Foods 2014, 7, 609–620. [Google Scholar] [CrossRef]
- Je, J.-Y.; Qian, Z.-J.; Byun, H.-G.; Kim, S.-K. Purification and characterisation of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B.; Hu, F.-Y.; Wang, Y.-M.; Zhang, B.; Deng, S.-G.; Wu, C.-W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Song, R.; Wei, R.; Zhang, B.; Yang, Z.; Wang, D. Antioxidant and antiproliferative activities of heated sterilized pepsin hydrolysate derived from half-fin anchovy (Setipinna taty). Mar. Drugs 2011, 9, 1142–1156. [Google Scholar] [CrossRef]
- Senphan, T.; Benjakul, S. Antioxidative activities of hydrolysates from seabass skin prepared using protease from hepatopancreas of Pacific white shrimp. J. Funct. Foods 2014, 6, 147–156. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Benjakul, S.; O’callaghan, Y.; O’Keeffe, M.; FitzGerald, R.; O’Brien, N. Purification and identification of antioxidant peptides from gelatin hydrolysates of unicorn leatherjacket skin. Ital. J. Food Sci. 2016, 29. [Google Scholar]
- Kim, S.M. Antioxidant and anticancer activities of enzymatic hydrolysates of solitary tunicate (Styela clava). Food Sci. Biotechnol. 2011, 20, 1075. [Google Scholar]
- Kang, K.H.; Qian, Z.J.; Ryu, B.; Karadeniz, F.; Kim, D.; Kim, S.K. Antioxidant peptides from protein hydrolysate of microalgae Navicula incerta and their protective effects in HepG2/CYP2E1 cells induced by ethanol. Phytother. Res. 2012, 26, 1555–1563. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kim, D.; Jeon, Y.-J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef]
- Stack, J.; Gouic, A.V.L.; Tobin, P.R.; Guihéneuf, F.; Stengel, D.B.; FitzGerald, R.J. Protein extraction and bioactive hydrolysate generation from two microalgae, Porphyridium purpureum and Phaeodactylum tricornutum. J. Food Bioact. 2018, 1, 153–165. [Google Scholar] [CrossRef]
- Ryu, B.; Kang, K.-H.; Ngo, D.-H.; Qian, Z.-J.; Kim, S.-K. Statistical optimization of microalgae Pavlova lutheri cultivation conditions and its fermentation conditions by yeast, Candida rugopelliculosa. Bioresour. Technol. 2012, 107, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, V.; Weisnagel, S.J.; Marois, J.; Bergeron, J.; Julien, P.; Gougeon, R.; Tchernof, A.; Holub, B.J.; Jacques, H. Dietary cod protein reduces plasma C-reactive protein in insulin-resistant men and women. J. Nutr. 2008, 138, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-F.; Li, G.-Z.; Peng, H.-B.; Zhang, F.; Chen, Y.; Li, Y. Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Appl. Physiol. Nutr. Metabol. 2010, 35, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, F.; Mizushige, T.; Uozumi, K.; Hayamizu, K.; Han, L.; Tsuji, T.; Kishida, T. Fish protein intake induces fast-muscle hypertrophy and reduces liver lipids and serum glucose levels in rats. Biosci. Biotechnol. Biochem. 2015, 79, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nasri, R.; Abdelhedi, O.; Jemil, I.; Daoued, I.; Hamden, K.; Kallel, C.; Elfeki, A.; Lamri-Senhadji, M.; Boualga, A.; Nasri, M.; et al. Ameliorating effects of goby fish protein hydrolysates on high-fat-high-fructose diet-induced hyperglycemia, oxidative stress and deterioration of kidney function in rats. Chem. Biol. Interact. 2015, 242, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ktari, N.; Nasri, R.; Mnafgui, K.; Hamden, K.; Belguith, O.; Boudaouara, T.; Feki, A.E.; Nasri, M. Antioxidative and ACE inhibitory activities of protein hydrolysates from zebra blenny (Salaria basilisca) in alloxan-induced diabetic rats. Process Biochem. 2014, 49, 890–897. [Google Scholar] [CrossRef]
- Khaled, H.B.; Ghlissi, Z.; Chtourou, Y.; Hakim, A.; Ktari, N.; Fatma, M.A.; Barkia, A.; Sahnoun, Z.; Nasri, M. Effect of protein hydrolysates from sardinelle (Sardinella aurita) on the oxidative status and blood lipid profile of cholesterol-fed rats. Food Res. Int. 2012, 45, 60–68. [Google Scholar] [CrossRef]
- Ou, Y.; Lin, L.; Pan, Q.; Yang, X.; Cheng, X.C. Preventive effect of phycocyanin from Spirulina platensis on alloxan-injured mice. Environ. Toxicol. Pharmacol. 2012, 34, 721–726. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Prospects for the management of type 2 diabetes using food protein-derived peptides with dipeptidyl peptidase IV (DPP-IV) inhibitory activity. Curr. Opin. Food Sci. 2016, 8, 19–24. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Zhu, S.-S.; He, M.-J.; Luo, F.; Fu, C.-Z.; Zou, T.-B. Marine peptides as potential agents for the management of type 2 diabetes mellitus-A prospect. Mar. Drugs 2017, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lammi, C.; Boschin, G.; Arnoldi, A.; Aiello, G. Recent advances in microalgae peptides: Cardiovascular health benefits and analysis. J. Agric. Food Chem. 2019, 67, 11825–11838. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.; Sirois, M.; Tamigneaux, É. Evaluation of the in vitro biological activity of protein hydrolysates of the edible red alga, Palmaria palmata (dulse) harvested from the Gaspe coast and cultivated in tanks. J. Appl. Phycol. 2016, 28, 3101–3115. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. An in silico model to predict the potential of dietary proteins as sources of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Food Chem. 2014, 165, 489–498. [Google Scholar] [CrossRef]
- Zhu, C.-F.; Peng, H.-B.; Liu, G.-Q.; Zhang, F.; Li, Y. Beneficial effects of oligopeptides from marine salmon skin in a rat model of type 2 diabetes. Nutrition 2010, 26, 1014–1020. [Google Scholar] [CrossRef]
- Li-Chan, E.C.; Hunag, S.-L.; Jao, C.-L.; Ho, K.-P.; Hsu, K.-C. Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef]
- La Rochelle, H.D.; Courois, E.; Cudennec, B.; Fouchereau-Peron, M.; Ravallec-Ple, R. Fish Protein Hydrolysate Having a Satietogenic Activity, Nutraceutical and Pharmacological Compositions Comprising Such a Hydrolysate and Method for Obtaining Same. U.S. Patent Application 12/866,878, 17 February 2011. [Google Scholar]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.; FitzGerald, R.J. Blue whiting (Micromesistius poutassou) muscle protein hydrolysate with in vitro and in vivo antidiabetic properties. J. Funct. Foods 2018, 40, 137–145. [Google Scholar] [CrossRef]
- Roomi, M.W.; Shanker, N.; Niedzwiecki, A.; Rath, M. Induction of apoptosis in the human prostate cancer cell line DU-145 by a novel micronutrient formulation. Open J. Apoptosis 2015, 4, 11. [Google Scholar] [CrossRef][Green Version]
- Guo-Fang, D.; Huang, F.-F.; Zui-Su, Y.; Di, Y.; Yong-Fang, Y. Anticancer activity of an oligopeptide isolated from hydrolysates of Sepia ink. Chin. J. Nat. Med. 2011, 9, 151–155. [Google Scholar]
- Le Gouic, A.; Harnedy, P.; FitzGerald, R. Bioactive peptides from fish protein by-products. In Bioactive Molecules in Food; Mérillon, J., Ramawat, K., Eds.; Springer: Berlin, Germany, 2018; pp. 355–388. [Google Scholar]
- Pan, X.; Zhao, Y.-Q.; Hu, F.-Y.; Chi, C.-F.; Wang, B. Anticancer activity of a hexapeptide from skate (Raja porosa) cartilage protein hydrolysate in HeLa Cells. Mar. Drugs 2016, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-F.; Hu, F.-Y.; Wang, B.; Li, T.; Ding, G.-F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wei, R.-B.; Luo, H.-Y.; Yang, Z.-S. Isolation and identification of an antiproliferative peptide derived from heated products of peptic hydrolysates of half-fin anchovy (Setipinna taty). J. Funct. Foods 2014, 10, 104–111. [Google Scholar] [CrossRef]
- Darvish, M.; Jalili, H.; Ranaei-Siadat, S.-O.; Sedighi, M. Potential cytotoxic effects of peptide fractions from Dunaliella salina protein hydrolyzed by gastric proteases. J. Aquat. Food Prod. Technol. 2018, 27, 165–175. [Google Scholar] [CrossRef]
- Otani, H.; Suzuki, H. Isolation and characterization of cytotoxic small peptides, α-casecidins, from bovine αs1-casein digested with bovine trypsin. Anim. Sci. J. 2003, 74, 427–435. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Li-Chan, E.C.Y.; Jao, C.-L. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011, 126, 617–622. [Google Scholar] [CrossRef]
- Yang, J.-I.; Tang, J.-Y.; Liu, Y.-S.; Wang, H.-R.; Lee, S.-Y.; Yen, C.-Y.; Chang, H.-W. Roe protein hydrolysates of giant grouper (Epinephelus lanceolatus) inhibit cell proliferation of oral cancer cells involving apoptosis and oxidative stress. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Picot, L.; Bordenave, S.; Didelot, S.; Fruitier-Arnaudin, I.; Sannier, F.; Thorkelsson, G.; Bergé, J.; Guérard, F.; Chabeaud, A.; Piot, J. Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines. Process Biochem. 2006, 41, 1217–1222. [Google Scholar] [CrossRef]
- Kannan, A.; hettiarachchy, N.S.; Marshall, M.; Raghavan, S.; Kristinsson, H. Shrimp shell peptide hydrolysates inhibit human cancer cell proliferation. J. Scifood Agric. 2011, 91, 1920–1924. [Google Scholar] [CrossRef]
- Wang, Y.-K.; He, H.-L.; Wang, G.-F.; Wu, H.; Zhou, B.-C.; Chen, X.-L.; Zhang, Y.-Z. Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar. Drugs 2010, 8, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-K.; Kim, Y.-S.; Hwang, J.-W.; Lee, J.S.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Purification and characterization of a novel anticancer peptide derived from Ruditapes philippinarum. Process Biochem. 2013, 48, 1086–1090. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X. Separation, antitumor activities, and encapsulation of polypeptide from Chlorella pyrenoidosa. Biotechnol. Prog. 2013, 29, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.C.; Fenical, W. Antibacterials from the sea. Chem. A Eur. J. 2010, 16, 12512–12525. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Liu, Z.; Zhao, Y.; Dong, S. Antimicrobial activities of marine protein and peptides. In Marine Proteins Peptides: Biological Activities Applications; Kim, S.K., Ed.; Wiley-Blackwell: Chichester, UK, 2013; pp. 369–383. [Google Scholar]
- Smith, V.J.; Desbois, A.P.; Dyrynda, E.A. Conventional and unconventional antimicrobials from fish, marine invertebrates and microalgae. Mar. Drugs 2010, 8, 1213–1262. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.-E.; Turgeon, S.L. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Campoverde, C.; Milne, D.J.; Estévez, A.; Duncan, N.; Secombes, C.J.; Andree, K.B. Ontogeny and modulation after PAMPs stimulation of β-defensin, hepcidin, and piscidin antimicrobial peptides in meagre (Argyrosomus regius). Fish. Shellfish Immunol. 2017, 69, 200–210. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, R.; Mora, L.; Toldrá, F.; Nasri, M.; Jridi, M. Collagenous proteins from black-barred halfbeak skin as a source of gelatin and bioactive peptides. Food Hydrocoll. 2017, 70, 123–133. [Google Scholar] [CrossRef]
- Abuine, R.; Rathnayake, A.U.; Byun, H.-G. Biological activity of peptides purified from fish skin hydrolysates. Fish. Aquat. Sci. 2019, 22, 10. [Google Scholar] [CrossRef]
- Aissaoui, N.; Chobert, J.-M.; Haertlé, T.; Marzouki, M.N.; Abidi, F. Purification and biochemical characterization of a neutral serine protease from Trichoderma harzianum: Use in antibacterial peptide production from a fish by-product hydrolysate. Appl. Biochem. Biotechnol. 2017, 182, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, F.; Wong, G.; Román, T.; Cárdenas, C.; Alvárez, C.; Schmitt, P.; Albericio, F.; Rojas, V. Identification of antimicrobial peptides from the microalgae Tetraselmis suecica (Kylin) Butcher and bactericidal activity improvement. Mar. Drugs 2019, 17, 453. [Google Scholar] [CrossRef] [PubMed]
- Kralovec, J.; Metera, K.; Kumar, J.; Watson, L.; Girouard, G.; Guan, Y.; Carr, R.; Barrow, C.; Ewart, H. Immunostimulatory principles from Chlorella pyrenoidosa—Part 1: Isolation and biological assessment in vitro. Phytomedicine 2007, 14, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Shaviklo, A.R. Development of fish protein powder as an ingredient for food applications: A review. J. Food Sci. Technol. 2015, 52, 648–661. [Google Scholar] [CrossRef]
- Park, J.W. Surimi and Surimi Seafood, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; p. 960. [Google Scholar]
- Kobayashi, Y.; Park, J.W. Optimal blending of differently refined fish proteins based on their functional properties. J. Food Process. Preserv. 2018, 42, e13346. [Google Scholar] [CrossRef]
- Venugopal, V. Seafood Processing: Adding Value through Quick Freezing, Retortable Packaging and Cook-Chilling; CRC Press: Boca Raton, FL, USA, 2005; p. 504. [Google Scholar]
- Shaviklo, G.R.; Olafsdottir, A.; Sveinsdottir, K.; Thorkelsson, G.; Rafipour, F. Quality characteristics and consumer acceptance of a high fish protein puffed corn-fish snack. J. Food Sci. Technol. 2011, 48, 668–676. [Google Scholar] [CrossRef]
- Adeleke, R.; Odedeji, J. Acceptability studies on bread fortified with tilapia fish flour. Pak. J. Nutr. 2010, 9, 531–534. [Google Scholar] [CrossRef]
- Ibrahim, S. Evaluation of production and quality of salt-biscuits supplemented with fish protein concentrate. World J. Dairy Food Sci. 2009, 4, 28–31. [Google Scholar]
- Huda, N.; Abdullah, A.; Babji, A.S. Substitution of Tapioca Flour with Surimi Powder in Traditional Crackers (Keropok Palembang). In Proceedings of the 16th Scientific Conference Nutrition Society, Kuala Lumpu, Malaysia, 10–11 April 2001; NutriScence: Kuala Lumpu, Malaysia; pp. 24–25.
- Shaviklo, A.R.; Dehkordi, A.K.; Zangeneh, P. Interactions and effects of the seasoning mixture containing fish protein powder/omega-3 fish oil on children’s liking and stability of extruded corn snacks using a mixture design approach. J. Food Process. Preserv. 2014, 38, 1097–1105. [Google Scholar] [CrossRef]
- Supawong, S.; Park, J.W.; Thawornchinsombut, S. Fat blocking roles of fish proteins in fried fish cake. LWT-Food Sci. Technol. 2018, 97, 462–468. [Google Scholar]
- Hashim, P.; Ridzwan, M.M.; Bakar, J.; Hashim, M.D. Collagen in food and beverage industries. Int. Food Res. J. 2015, 22, 1. [Google Scholar]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine collagen: An emerging player in biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Pal, G.K.; Suresh, P. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Jafarpour, A.; Undeland, I. Sequential extraction of gel-forming proteins, collagen and collagen hydrolysate from gutted silver carp (Hypophthalmichthys molitrix), a biorefinery approach. Food Chem. 2018, 242, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Antoniewski, M.; Barringer, S. Meat shelf-life and extension using collagen/gelatin coatings: A review. Crit. Rev. Food Sci. Nutr. 2010, 50, 644–653. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.E.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Gelatin alternatives for the food industry: Recent developments, challenges and prospects. Trends Food Sci. Technol. 2008, 19, 644–656. [Google Scholar] [CrossRef]
- Bilek, S.E.; Bayram, S.K. Fruit juice drink production containing hydrolyzed collagen. J. Funct. Foods 2015, 14, 562–569. [Google Scholar] [CrossRef]
- Czajka, A.; Kania, E.M.; Genovese, L.; Corbo, A.; Merone, G.; Luci, C.; Sibilla, S. Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing. Nutr. Res. 2018, 57, 97–108. [Google Scholar] [CrossRef]
- Asserin, J.; Lati, E.; Shioya, T.; Prawitt, J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015, 14, 291–301. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J. Food Sci. 2009, 74, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Marine collagens. In Biological Materials of Marine Origin; Ehrlich, H., Ed.; Springer: Berlin, Germany, 2015; pp. 321–341. [Google Scholar]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharma. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of fish collagen as a scaffold for regenerative medicine. Biomed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Natural preservatives for extending the shelf-life of seafood: A revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; De la Caba, K. Development of active gelatin films by means of valorisation of food processing waste: A review. Food Hydrocoll. 2017, 68, 192–198. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Suthiluk, P.; Kamhangwong, D.; Benjakul, S. Mechanical, physico-chemical, and antimicrobial properties of gelatin-based film incorporated with catechin-lysozyme. Chem. Cent. J. 2012, 6, 131. [Google Scholar] [CrossRef]
- Nuanmano, S.; Prodpran, T.; Benjakul, S. Potential use of gelatin hydrolysate as plasticizer in fish myofibrillar protein film. Food Hydrocoll. 2015, 47, 61–68. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. Characterization of protein hydrolysates from blue whiting (Micromesistius poutassou) and their application in beverage fortification. Food Chem. 2018, 245, 698–706. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on the safety of ‘sardine peptide product’. EFSA J. 2010, 8, 1684. [Google Scholar] [CrossRef]
- Lupo, M.P.; Cole, A.L. Cosmeceutical peptides. Dermatol. Ther. 2007, 20, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Hou, H.; Li, B.; Zhang, Z.; Xue, C.; Yu, G.; Wang, J.; Bao, Y.; Bu, L.; Sun, J.; Peng, Z. Moisture absorption and retention properties, and activity in alleviating skin photodamage of collagen polypeptide from marine fish skin. Food Chem. 2012, 135, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, N.; Kawada, C.; Nomura, Y. Optimization of dose of collagen hydrolysate to prevent UVB-irradiated skin damage. Biosci. Biotechnol. Biochem. 2016, 80, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, Y.; Zhuang, Y. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis niloticus) gelatin peptides. J. Funct. Foods 2013, 5, 154–162. [Google Scholar] [CrossRef]
- Just, V. Bovine Collagen vs. Marine Collagen. Available online: https://www.justvitamins.co.uk/blog/bovine-collagen-vs-marine-collagen/# (accessed on 7 June 2020).
- Allard, R.; Malak, N.A.; Huc, A. Collagen Product Containing Collagen of Marine Origin with a Low Odor and Preferably with Improved Mechanical Properties, and Its Use in the Form of Cosmetic or Pharmaceutical Compositions or Products. U.S. Patent No. 6,660,280, 9 December 2003. [Google Scholar]
- Bello, A.E.; Oesser, S. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: A review of the literature. Curr. Med. Res. Opin. 2006, 22, 2221–2232. [Google Scholar] [CrossRef]
- Vellard, M. The enzyme as drug: Application of enzymes as pharmaceuticals. Curr. Opin. Biotechnol. 2003, 14, 444–450. [Google Scholar] [CrossRef]
- Kim, S.-K.; Dewapriya, P. Enzymes from fish processing waste materials and their commercial applications. In Seafood Processing by-Products. Trends and Applications; Kim, S.-K., Ed.; Springer Science+Business Media: New York, NY, USA, 2014; pp. 183–196. [Google Scholar]
- An, H.; Visessanguan, W. Recovery of enzymes from seafood-processing wastes. In Seafood Enzymes: Utilisation and Influence on Postharvest Seafood Quality; Haard, N.F., Simpson, B.K., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 641–664. [Google Scholar]
- Kandasamy, N.; Velmurugan, P.; Sundarvel, A.; Raghava, R.J.; Bangaru, C.; Palanisamy, T. Eco-benign enzymatic dehairing of goatskins utilizing a protease from a Pseudomonas fluorescens species isolated from fish visceral waste. J. Clean. Prod. 2012, 25, 27–33. [Google Scholar] [CrossRef]
- Klomklao, S.; Kishimura, H.; Yabe, M.; Benjakul, S. Purification and characterization of two pepsins from the stomach of pectoral rattail (Coryphaenoides pectoralis). Com. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 147, 682–689. [Google Scholar] [CrossRef]
- Gudmundsdóttir, Á.; Pálsdóttir, H.M. Atlantic cod trypsins: From basic research to practical applications. Mar. Biotechnol. 2005, 7, 77–88. [Google Scholar] [CrossRef]
- Paul, J. Isolation and characterization of a Chlamydomonas L-asparaginase. Biochem. J. 1982, 203, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminezhad, A.; Rasoul-Amini, S.; Ghoshoon, M.B.; Ghasemi, Y. Chlorella vulgaris, a novel microalgal source for L-asparaginase production. Biocat. Agric. Biotechnol. 2014, 3, 214–217. [Google Scholar] [CrossRef]
- Batool, T.; Makky, E.A.; Jalal, M.; Yusoff, M.M. A comprehensive review on L-asparaginase and its applications. Appl. Biochem. Biotechnol. 2016, 178, 900–923. [Google Scholar] [CrossRef] [PubMed]
- Bafana, A.; Dutt, S.; Kumar, S.; Ahuja, P.S. Superoxide dismutase: An industrial perspective. Crit. Rev. Biotechnol. 2011, 31, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Undeland, I.; Kelleher, S.D.; Hultin, H.O. Recovery of functional proteins from herring (Clupea harengus) light muscle by an acid or alkaline solubilization process. J. Agric. Food Chem. 2002, 50, 7371–7379. [Google Scholar] [CrossRef]
- Hernández, D.; Molinuevo-Salces, B.; Riaño, B.; Larrán-García, A.M.; Tomás-Almenar, C.; García-González, M.C. Recovery of protein concentrates from microalgal biomass grown in manure for fish feed and valorization of the by-products through anaerobic digestion. Front. Sustain. Food Syst. 2018, 2, 28. [Google Scholar] [CrossRef]
- Ursu, A.-V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef]
- Shavandi, A.; Hu, Z.; Teh, S.; Zhao, J.; Carne, A.; Bekhit, A.; Bekhit, A.E.-D.A. Antioxidant and functional properties of protein hydrolysates obtained from squid pen chitosan extraction effluent. Food Chem. 2017, 227, 194–201. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.; Jantawat, P.; Sanguandeekul, R. Recovery and characterization of proteins precipitated from surimi wash-water. LWT-Food Sci. Technol. 2009, 42, 599–605. [Google Scholar]
- Kennedy, J.F.; Paterson, M.; Taylor, D.W.; Silva, M.P. Recovery of proteins from whey using chitosan as a coagulant. In Biotechnology and Bioactive Polymer; Gebelein, C.G., Caraher, C.E., Eds.; Springer Science+Business Media: New York, NY, USA, 1994; pp. 55–69. [Google Scholar]
- Wibowo, S.; Velazquez, G.; Savant, V.; Torres, J.A. Effect of chitosan type on protein and water recovery efficiency from surimi wash water treated with chitosan–alginate complexes. Bioresour. Technol. 2007, 98, 539–545. [Google Scholar] [CrossRef]
- Holland, C.; Shahbaz, M. The utilization of chitosan in mussel protein recovery. Ir. J. Food Sci. Technol. 1985, 9, 107–114. [Google Scholar]
- Trang, S.T. Innovation of Fishery by-Products in Vietnam. In Proceedings of the FFTC-KU Joint Seminar on Improved Utilization of Fishery by-Products as Potential Nutraceuticals and Functional Foods, Bangkok, Thailand, 25–29 October 2010; Food and Fertilizer Technology Centre for the Asian and Pacific Region: Bangkok, Thailand; pp. 76–84.
- Chomnawang, C.; Yongsawatdigul, J. Protein recovery of tilapia frame by-products by pH-shift method. J. Aquat. Food Prod. Technol. 2013, 22, 112–120. [Google Scholar] [CrossRef]
- Ba, F.; Ursu, A.V.; Laroche, C.; Djelveh, G. Haematococcus pluvialis soluble proteins: Extraction, characterization, concentration/fractionation and emulsifying properties. Bioresour. Technol. 2016, 200, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.S.; Van Leeuwen, J.; Safi, C.; Sijtsma, L.; Eppink, M.H.; Wijffels, R.H.; van den Berg, C. Selective and energy efficient extraction of functional proteins from microalgae for food applications. Bioresour. Technol. 2018, 268, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Song, K.-M.; Jung, S.K.; Kim, Y.H.; Kim, Y.E.; Lee, N.H. Development of industrial ultrasound system for mass production of collagen and biochemical characteristics of extracted collagen. Food Bioprod. Process. 2018, 110, 96–103. [Google Scholar] [CrossRef]
- Mireles DeWitt, C.; Nabors, R.; Kleinholz, C. Pilot plant scale production of protein from catfish treated by acid solubilization/isoelectric precipitation. J. Food Sci. 2007, 72, 351–355. [Google Scholar] [CrossRef]
- Matak, K.E.; Tahergorabi, R.; Jaczynski, J. A review: Protein isolates recovered by isoelectric solubilization/precipitation processing from muscle food by-products as a component of nutraceutical foods. Food Res. Int. 2015, 77, 697–703. [Google Scholar] [CrossRef]
- Tahergorabi, R.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Isoelectric solubilization/precipitation as a means to recover protein isolate from striped bass (Morone saxatilis) and its physicochemical properties in a nutraceutical seafood product. J. Agric. Food Chem. 2012, 60, 5979–5987. [Google Scholar] [CrossRef]
- Gehring, C.; Gigliotti, J.; Moritz, J.; Tou, J.; Jaczynski, J. Functional and nutritional characteristics of proteins and lipids recovered by isoelectric processing of fish by-products and low-value fish: A review. Food Chem. 2011, 124, 422–431. [Google Scholar] [CrossRef]
- Benelhadj, S.; Gharsallaoui, A.; Degraeve, P.; Attia, H.; Ghorbel, D. Effect of pH on the functional properties of Arthrospira (Spirulina) platensis protein isolate. Food Chem. 2016, 194, 1056–1063. [Google Scholar] [CrossRef]
- Teuling, E.; Schrama, J.W.; Gruppen, H.; Wierenga, P.A. Characterizing emulsion properties of microalgal and cyanobacterial protein isolates. Algal Res. 2019, 39, 101471. [Google Scholar] [CrossRef]
- Marmon, S.K.; Liljelind, P.; Undeland, I. Removal of lipids, dioxins, and polychlorinated biphenyls during production of protein isolates from Baltic herring (Clupea harengus) using pH-shift processes. J. Agric. Food Chem. 2009, 57, 7819–7825. [Google Scholar] [CrossRef]
- Petrova, I.; Tolstorebrov, I.; Eikevik, T.M. Production of fish protein hydrolysates step by step: Technological aspects, equipment used, major energy costs and methods of their minimizing. Int. Aquat. Res. 2018, 10, 223–241. [Google Scholar] [CrossRef]
- Pasupuleti, V.K.; Braun, S. State of the art manufacturing of protein hydrolysates. In Protein Hydrolysates in Biotechnology; Pasupuleti, V., Demain, A., Eds.; Springer: Berlin, Germany, 2008; pp. 11–32. [Google Scholar]
- Lahl, W.J.; Braun, S.D. Enzymatic production of protein hydrolysates for food use. Food Technol. 1994, 48, 68–71. [Google Scholar]
- Howieson, J.; Choo, K. New Opportunities for Seafood Processing Waste; FRDC: Canberra, Australia, 2017; p. 22.
- Owen, J.; Mendoza, I. Enzymatically hydrolyzed and bacterially fermented fishery product. J. Food Technol. 1985, 20, 273–293. [Google Scholar] [CrossRef]
- Gildberg, A.; Espejo-Hermes, J.; Magno-Orejana, F. Acceleration of autolysis during fish sauce fermentation by adding acid and reducing the salt content. J. Sci. Food Agric. 1984, 35, 1363–1369. [Google Scholar] [CrossRef]
- Raa, J.; Gildberg, A.; Olley, J.N. Fish silage: A review. Crit. Rev. Food Sci. Nutr. 1982, 16, 383–419. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, H.J.; Heydenrych, C.M. The production of naturally fermented fish silage using various lactobacilli and different carbohydrate sources. J. Sci. Food Agric. 1985, 36, 1093–1103. [Google Scholar] [CrossRef]
- Arruda, L.F.d.; Borghesi, R.; Oetterer, M. Use of fish waste as silage: A review. Braz. Arch. Biol. Technol. 2007, 50, 879–886. [Google Scholar] [CrossRef]
- Hale, M.B. Making Fish Protein Concentrate by Enzymatic Hydrolysis; A Status Report on Research and Some Processes and Products Studied by NMFS; National Oceanographic and Atmospheric Administration: Washington, DC, USA, 1972; p. 44.
- Marti-Quijal, F.J.; Remize, F.; Meca, G.; Ferrer, E.; Ruiz, M.-J.; Barba, F.J. Fermentation in fish and by-products processing: An overview of current research and future prospects. Curr. Opini. Food Sci. 2020, 31, 9–16. [Google Scholar] [CrossRef]
- Onodenalore, A.C.; Shahidi, F. Protein dispersions and hydrolysates from shark (Isurus oxyrinchus). J. Aquat. Food Prod. Technol. 1996, 5, 43–59. [Google Scholar] [CrossRef]
- Välimaa, A.-L.; Mäkinen, S.; Mattila, P.; Marnila, P.; Pihlanto, A.; Mäki, M.; Hiidenhovi, J. Fish and fish side streams are valuable sources of high-value components. Food Qual. Saf. 2019, 3, 209–226. [Google Scholar]
- Shahidi, F.; Han, X.-Q.; Synowiecki, J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem. 1995, 53, 285–293. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Kinetics of the hydrolysis of Atlantic salmon (Salmo salar) muscle proteins by alkaline proteases and a visceral serine protease mixture. Process Biochem. 2000, 36, 131–139. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Bryl, P.; Carbonneau, M.-É. Characterization of enzymatic hydrolyzed snow crab (Chionoecetes opilio) by-product fractions: A source of high-valued biomolecules. Bioresour. Technol. 2009, 100, 3332–3342. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.; Thibodeau, J.; Bonnet, C.; Bryl, P.; Carbonneau, M.-E. Evidence of anti-proliferative activities in blue mussel (Mytilus edulis) by-products. Mar. Drugs 2013, 11, 975–990. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, J.; Liu, J.; Wu, J.; Zhang, Q. Technology for enzymolysis of jellyfish brain protein by bromelain. Agric. Sci. Technol. 2013, 14, 1486. [Google Scholar]
- Yan, M.; Tao, H.; Qin, S. Effect of enzyme type on the antioxidant activities and functional properties of enzymatic hydrolysates from sea cucumber (Cucumaria frondosa) viscera. J. Aquat. Food Prod. Technol. 2016, 25, 940–952. [Google Scholar] [CrossRef]
- Ran, X.G.; Wang, L.Y. Use of ultrasonic and pepsin treatment in tandem for collagen extraction from meat industry by-products. J. Sci. Food Agric. 2014, 94, 585–590. [Google Scholar] [CrossRef]
- Marciniak, A.; Suwal, S.; Naderi, N.; Pouliot, Y.; Doyen, A. Enhancing enzymatic hydrolysis of food proteins and production of bioactive peptides using high hydrostatic pressure technology. Trends Food Sci. Technol. 2018, 80, 187–198. [Google Scholar] [CrossRef]
- Andler, S.M.; Goddard, J.M. Transforming food waste: How immobilized enzymes can valorize waste streams into revenue streams. NPJ Sci. Food 2018, 2, 1–11. [Google Scholar] [CrossRef]
- De la Fuente, B.; Tornos, A.; Príncep, A.; Lorenzo, J.M.; Pateiro, M.; Berrada, H.; Barba, F.J.; Ruiz, M.-J.; Martí-Quijal, F.J. Scaling-up processes: Patents and commercial applications. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 92, pp. 187–223. [Google Scholar]
- He, S.; Franco, C.M.; Zhang, W. Economic feasibility analysis of the industrial production of fish protein hydrolysates using conceptual process simulation software. J. Bioprocess. Biotech. 2015, 5, 1. [Google Scholar]
- Marnis; Syahrul; Fitri; Mardayulis. Valuation of economic utilization of fish processing waste patin (Pangasius hypopthalmus) as an added value for fish processing industry players in the district Kampar, Riau. Int. J. Econ. Financ. 2016, 8, 104–116. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Chatziantoniou, S.E.; Zotos, A. Co-extraction of gelatin and lipids from Yellowfin tuna (Thunnus albacares) skins: Physicochemical characterization, process simulation and economic analysis. J. Food Process. Pres. 2015, 39, 2361–2370. [Google Scholar] [CrossRef]
- Nugraha, A.; Hardyastuti, S.; Mulyo, J.H. Financial feasibility of Sijuk shrimp paste business in Sungai Padang village, Sijuk District, Belitung Regency. Agro Ekon. 2017, 28, 142–156. [Google Scholar] [CrossRef]
- Asiedu, A.; Ben, S.; Resurreccion, E.; Kumar, S. Techno-economic analysis of protein concentrate produced by flash hydrolysis of microalgae. Environ. Prog. Sustain. 2018, 37, 881–890. [Google Scholar] [CrossRef]
- Torres-Acosta, M.A.; Ruiz-Ruiz, F.; Aguilar-Yáñez, J.M.; Benavides, J.; Rito-Palomares, M. Economic analysis of pilot-scale production of B-phycoerythrin. Biotechnol. Prog. 2016, 32, 1472–1479. [Google Scholar] [CrossRef]
- SAMPI. SAMPI—Organically Certified Fish Hydrolysate. Available online: http://www.sampi.com.au/ (accessed on 16 July 2020).
- Chen, X.-L.; Peng, M.; Li, J.; Tang, B.-L.; Shao, X.; Zhao, F.; Liu, C.; Zhang, X.-Y.; Li, P.-Y.; Shi, M. Preparation and functional evaluation of collagen oligopeptide-rich hydrolysate from fish skin with the serine collagenolytic protease from Pseudoalteromonas sp. SM9913. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Beal, C.M.; Hebner, R.E.; Webber, M.E.; Ruoff, R.S.; Seibert, A.F. The energy return on investment for algal biocrude: Results for a research production facility. Bioenergy Res. 2012, 5, 341–362. [Google Scholar] [CrossRef]
- Agyei, D.; Ongkudon, C.M.; Wei, C.Y.; Chan, A.S.; Danquah, M.K. Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprod. Process. 2016, 98, 244–256. [Google Scholar] [CrossRef]
- Catherine, N. Innovation in Processing and Product Development is Identifying New Opportunities to Increase the Value of Waste in the Seafood Sector. Available online: https://www.fishfiles.com.au/media/fish-magazine/FISH-Vol-24-3/New-value-from-seafood (accessed on 16 July 2020).
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrasonic. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Majid, I.; Nayik, G.A.; Nanda, V. Ultrasonication and food technology: A review. Cogent Food Agric. 2015, 1, 107–122. [Google Scholar] [CrossRef]
- Lebovka, N.; Vorobiev, E.; Chemat, F. Enhancing Extraction Processes in the Food Industry; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Tian, J.; Wang, Y.; Zhu, Z.; Zeng, Q.; Xin, M. Recovery of tilapia (Oreochromis niloticus) protein isolate by high-intensity ultrasound-aided alkaline isoelectric solubilization/precipitation process. Food Bioprocess. Technol. 2015, 8, 758–769. [Google Scholar] [CrossRef]
- Álvarez, C.; Lélu, P.; Lynch, S.A.; Tiwari, B.K. Optimised protein recovery from mackerel whole fish by using sequential acid/alkaline isoelectric solubilization precipitation (ISP) extraction assisted by ultrasound. LWT-Food Sci. Technol. 2018, 88, 210–216. [Google Scholar]
- Álvarez, C.; Tiwari, B.K. Ultrasound assisted extraction of proteins from fish processing by-products. In Institute of Food Technologist; AEP Colloids: Chicago, IL, USA, 2015. [Google Scholar]
- Kim, H.K.; Kim, Y.H.; Kim, Y.J.; Park, H.J.; Lee, N.H. Effects of ultrasonic treatment on collagen extraction from skins of the sea bass Lateolabrax japonicus. Fish. Sci. 2012, 78, 485–490. [Google Scholar] [CrossRef]
- Vernes, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of ultrasound for green extraction of proteins from spirulina: Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef]
- Li, D.; Mu, C.; Cai, S.; Lin, W. Ultrasonic irradiation in the enzymatic extraction of collagen. Ultrason. Sonochem. 2009, 16, 605–609. [Google Scholar] [CrossRef]
- Waghmare, A.G.; Salve, M.K.; LeBlanc, J.G.; Arya, S.S. Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresourc. Bioprocess. 2016, 3, 16. [Google Scholar] [CrossRef]
- Hirata, G.A.M.; Bernardo, A.; Miranda, E.A. Crystallization of porcine insulin with carbon dioxide as acidifying agent. Powder Technol. 2010, 197, 54–57. [Google Scholar] [CrossRef]
- Nakamura, K.; Hoshino, T.; Ariyama, H. Adsorption of carbon dioxide on proteins in the supercritical region. Agric. Biol. Chem. 1991, 55, 2341–2347. [Google Scholar]
- Chaitanya, V.; Senapati, S. Self-assembled reverse micelles in supercritical CO2 entrap protein in native state. J. Am. Chem. Soc. 2008, 130, 1866–1870. [Google Scholar] [CrossRef]
- Winters, M.A.; Knutson, B.L.; Debenedetti, P.G.; Sparks, H.G.; Przybycien, T.M.; Stevenson, C.L.; Prestrelski, S.J. Precipitation of proteins in supercritical carbon dioxide. J. Pharm. Sci. 1996, 85, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, P.; Ooi, E.; Nikolov, Z. Off-flavor removal from soy-protein isolate by using liquid and supercritical carbon dioxide. J. Am. Oil Chem. Soc. 1995, 72, 1107–1115. [Google Scholar] [CrossRef]
- Bonnaillie, L.M.; Qi, P.; Wickham, E.; Tomasula, P.M. Enrichment and purification of casein glycomacropeptide from whey protein isolate using supercritical carbon dioxide processing and membrane ultrafiltration. Foods 2014, 3, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Khorshid, N.; Hossain, M.M.; Farid, M. Precipitation of food protein using high pressure carbon dioxide. J. Food Eng. 2007, 79, 1214–1220. [Google Scholar] [CrossRef]
- Yver, A.L.; Bonnaillie, L.M.; Yee, W.; McAloon, A.; Tomasula, P.M. Fractionation of whey protein isolate with supercritical carbon dioxide—Process modeling and cost estimation. Int. J. Mol. Sci. 2012, 13, 240–259. [Google Scholar] [CrossRef]
- Lima, J.; Seixas, F.; Coimbra, J.; Pimentel, T.; Barão, C.; Cardozo-Filho, L. Continuous fractionation of whey protein isolates by using supercritical carbon dioxide. J. CO2 Utiliz. 2019, 30, 112–122. [Google Scholar] [CrossRef]
- Park, J.Y.; Back, S.S.; Chun, B.S. Protein properties of mackerel viscera extracted by supercritical carbon dioxide. Environ. Biol. 2008, 29, 443–448. [Google Scholar]
- Kang, K.-Y.; Ahn, D.-H.; Jung, S.-M.; Kim, D.-H.; Chun, B.-S. Separation of protein and fatty acids from tuna viscera using supercritical carbon dioxide. Biotechno. Bioprocess. Eng. 2005, 10, 315–321. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Leng, X.; Liao, X.; Hu, X. Acceleration of precipitation formation in peach juice induced by high-pressure carbon dioxide. J. Agric. Food Chem. 2010, 58, 9605–9610. [Google Scholar] [CrossRef]
- Sarkari, M.; Darrat, I.; Knutson, B.L. CO2 and fluorinated solvent-based technologies for protein microparticle precipitation from aqueous solutions. Biotechnol. Prog. 2003, 19, 448–454. [Google Scholar] [CrossRef]
- Olano, A.; Calvo, M.M.; Troyano, E.; Amigo, L. Changes in the fractions of carbohydrates and whey proteins during heat treatment of milk acidified with carbon dioxide. J. Dairy Res. 1992, 59, 95–99. [Google Scholar] [CrossRef]
- Tomasula, P.; Boswell, R.; Dupre, N. Buffer properties of milk treated with high pressure carbon dioxide. Milchwissenschaft 1999, 54, 667–670. [Google Scholar]
- Tomasula, P.; Boswell, R. Measurement of the solubility of carbon dioxide in milk at high pressures. J. Supercrit. Fluids 1999, 16, 21–26. [Google Scholar] [CrossRef]
- Moshashaée, S.; Bisrat, M.; Forbes, R.T.; Quinn, é.Á.; Nyqvist, H.; York, P. Supercritical fluid processing of proteins: Lysozyme precipitation from aqueous solution. J. Pharm. Pharmacol. 2003, 55, 185–192. [Google Scholar] [CrossRef]
- Hofland, G.; Berkhoff, M.; Witkamp, G.; Van der Wielen, L. Dynamics of precipitation of casein with carbon dioxide. Int. Dairy J. 2003, 13, 685–697. [Google Scholar] [CrossRef]
- Hofland, G.W.; de Rijke, A.; Thiering, R.; van der Wielen, L.A.; Witkamp, G.-J. Isoelectric precipitation of soybean protein using carbon dioxide as a volatile acid. J. Chromatogr. B Biomed. Sci. Appl. 2000, 743, 357–368. [Google Scholar] [CrossRef]
- Hofland, G.W.; van Es, M.; van der Wielen, L.A.; Witkamp, G.-J. Isoelectric precipitation of casein using high-pressure CO2. Ind. Eng. Chem. Res. 1999, 38, 4919–4927. [Google Scholar] [CrossRef]
- Perry, R.; DW, G. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill: New York, NY, USA, 2007; p. 2700. [Google Scholar]
- Bonnaillie, L.M.; Tomasula, P.M. Kinetics, aggregation behavior and optimization of the fractionation of whey protein isolate with hydrochloric acid. Food Bioprod. Process. 2012, 90, 737–747. [Google Scholar] [CrossRef]
- Bonnaillie, L.M.; Tomasula, P.M. Fractionation of whey protein isolate with supercritical carbon dioxide to produce enriched α-lactalbumin and β-lactoglobulin food ingredients. J. Agric. Food Chem. 2012, 60, 5257–5266. [Google Scholar] [CrossRef]
- Zhong, Q.; Jin, M. Enhanced functionalities of whey proteins treated with supercritical carbon dioxide. J. Dairy Sci. 2008, 91, 490–499. [Google Scholar] [CrossRef]
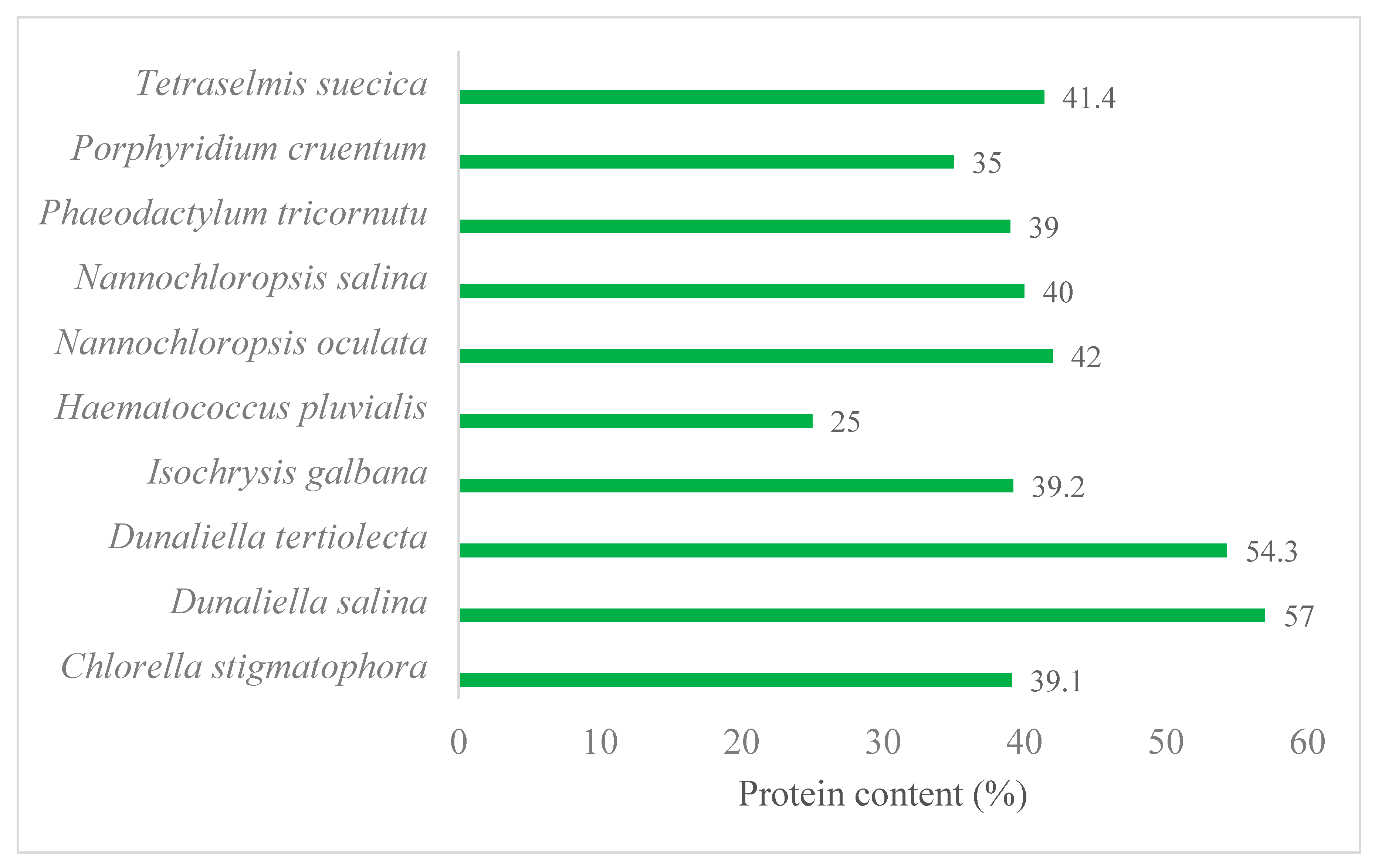
| Marine Groups | Typical Species | By-Product Types | Ratio of By-Products (%) of Total Weight | Protein Contents (%) | Type of Proteins or Protein-Derived Products | References |
|---|---|---|---|---|---|---|
| Finfish | Pollock, cod, hake, haddock, salmon, tuna, herring, mackerel, and among many others | Heads | 15–20 | 11.9–12.9 a | Proteins, protein hydrolysates, biopeptides | [63,64,65,66] |
| Frames | 10–15 | 11.5–17.5 a | Collagen, gelatine, protein hydrolysates, biopeptides | [63,65,67] | ||
| Skins and fins | 1–3 | 24.8–27.0 a | Collagen, gelatine, protein hydrolysates, biopeptides | [63,65,68] | ||
| Bones | 9–15 | 36.3–56.8 b | Collagen, gelatine, protein hydrolysates, biopeptides | [69,70] | ||
| Scales | 3–5 | 41–81 b | Ichthylepidin and collagen, biopeptides | [69,71] | ||
| Viscera (livers, roes, and milts) | 15–20 | 12.9–14.8 a | Enzymes, protein hydrolysates, peptides, biopeptides | [63,65,66,67,72] | ||
| Blood | 2–7 | 0.8–5.7 a | Plasma proteins, active amino acids, enzyme inhibitors | [73,74] | ||
| Crustacean | Krill, shrimp, crap, crayfish, lobster | Shells, tails | 15 | 29–40 b | Shell proteins, caroteno-proteins | [18,75,76] |
| Heads | 25 | 43.5–54.4 b | Shell and meat proteins | [19,76,77] | ||
| Viscera (livers, roes) | 5 | 41 b | Enzymes, protein hydrolysates, peptides, biopeptides | [60,78] | ||
| Mollusc | Oyster, mussel, clam, scallop | Shells | 75–80 | 1–5 b | Bioactive peptides | [79,80,81,82] |
| Body parts and organs | 58.7 b | Enzyme, protein hydrolysate, biopeptide, flavour | [82,83] | |||
| Cuttlefish, Squid, Octopus | Ink bags, organs, and non-edible portions | 25–44.3 | 5–22 a | Enzymes, bioactive peptides, food flavours, taurine | [84,85,86,87] | |
| Coelenterate and echinoderm | Sea urchin | Shells, viscera | 40.7–77.9 | 4.1–5.0 b | Bioactive proteins for self-assembly of skeletal structure | [88] |
| Sea cucumber | viscera | 4.5 a | Enzymes, protein hydrolysate, bioactive peptides | [89] | ||
| Jelly fish | 3–7 a | Protein hydrolysate, bioactive peptides, collagen, gelatine | [83,90] |
| Anti-Hypertensive Assays | Peptide Names or Sequences | Efficiency (IC50, EC50) (μM) | Types of SPBs, Marine Species | Enzymes, Production Conditions | References |
|---|---|---|---|---|---|
| GGPAGPAV GPVA PP GF | 673.2 445.6 1912.4 178.1 | Trimming of Atlantic salmon (Salmo salar) | Corolase PP | [195] | |
| Phe-Gly-Ala-Ser-Thr-Arg-Gly-Ala | 14.7 | Frames of Alaska pollock (Theragra chalcogramma) | Pepsin | [42] | |
| GDLGKTTTSNWSPP | 11.3 | Frame of bluefin tuna (Thunnus thynnus) | Pepsin | [168] | |
| - | Observed at 4.8 μM 85.8% ACE inhibition | Mince of Boarfish (Capros aper) | Protease AP | [196] | |
| EPLYV DPHI AER EQIDNLQ WDDME | 118 48.7 420 270 31.6 | Mince of Leatherjacket (Meuschenia sp.) | Papain Bromelain Flavourzyme 500 L | [197] | |
| SBP6h (40 mg/kg) | MEVFVP VSQLTR | 79 μM, 44.3 mmHg 105 μM, 34.3 mmHg | Mince of Olive flounder (Paralichthys olivaceus) | Pepsin | [198] |
| - | 3.9 | Skin gelatine of Rockfish (Sebastes hubbsi) | Flavourzyme | [199] | |
| MVGSAPGVL LGPLGHQ | 3.1 4.2 | Skin gelatine of skate (Okamejei kenojei) | Alcalase 2.4 L | [200] | |
| - | 0.4 | Oysters (Crassostrea gigas) | Fermentation with 25% NaCl at 20 °C for 6 months | [201] | |
| ACE inhibitor | - | 1.6 | Gelatine of giant squid (Dosidicus gigas) | Alcalase | [202] |
| Nitric oxide production No cytotoxicity on HUVECs | GMNNLTP (Gly-Met-Asn-Asn-Leu-Thr-Pro MW 728 Da) LEQ (Leu-Glu-Gln, MW 369 Da) | 123–173 | Nannochloropsis oculata | Pepsin, trypsin, αchymotrypsin, papain, alcalase, and neutrase | [177] |
| Spontaneously hypertensive rats (SHRs) | VEGY (Val–Glu–Gly–Tyr, MW 467.2 Da) | 128.4 | Chlorella ellipsoidea | Protamex, Kojizyme, Neutrase, Flavourzyme, Alcalase, trypsin, α-chymotrypsin, pepsin and papain | [203] |
| WV (Trp-Val) VW (Val-Trp) IW (Ile-Trp) LW (Leu-Trp) | 307.6 0.6 0.5 1.1 | Chlorella sorokiniana | Protease N, pepsin, pancreatin | [204] | |
| GPDRPKFLGPF WYGPDRPKFL | 5.73, EC50 0.82, EC50 | Tetradesmus obliquus | Alcalase | [205] | |
| ACE-inhibitory | Peptides < 5 kDa | Observed at 4.8 μM 30.8–37.8% | Chlorella sorokiniana | Pepsin, bromelain, and thermolysin | [206] |
| Antioxidant Assays | Peptide Names or Sequences | Efficiency (IC50, EC50, TE) (μM) | Types of SPBs, Marine Species | Enzymes, Production Conditions | References |
|---|---|---|---|---|---|
| ORAC | GGPAGPAV GPVA PP GF | 5.5 9.5 12.5 19.7 | Trimming of Atlantic salmon (Salmo salar) | Corolase PP | [195] |
| DPPH ABTS Hydroxyl | PAGT | 25.8 EC50 0.04 EC50 4.3 EC50 | Skin gelatine of Amur sturgeon (Acipenser schrenckii) | Alcalase 2.4 L | [226] |
| DPPH Hydroxyl Superoxide | APTBP | 3.5 EC50 1.0 EC50 2.9 EC50 | Backbone of bluefin tuna (Thunnus thynnus) | Pepsin | [227] |
| DPPH Hydroxyl Oxygen | FIGP | 0.6 EC50 0.4 EC50 1.5 EC50 | Skin of bluefin leatherjacket (Navodon septentrionalis) | Papain | [228] |
| DPPH | - | 0.02 EC50 | Half-fin anchovy (Setipinna taty) | Pepsin | [229] |
| DPPH Hydroxyl Alkyl Superoxide | - | Observed at 19.2 μM 45.8% 94.7% 64.8% 67.8% | Skin gelatin of rockfish (Sebastes hubbsi) | Flavourzyme | [199] |
| DPPH ABTS FRAP DPPH ABTS FRAP | - | 6.8 μmol TE/g dw 65.5 μmol TE/g dw 2.6 μmol TE/g dw 6.3 μmol TE/g dw 59.4 μmol TE/g dw 2.7 μmol TE/g dw | Skin of seabass (Lates calcarifer) | Alcalase 2.4 L Protease from hepatopancreas of Pacific white shrimp | [230] |
| ABTS | EPGPVG LPGPAG LDGPVG EGPLG | 1.25 μmol TE/g peptide 1.22 μmol TE/g peptide 1.36 μmol TE/g peptide 4.95 μmol TE/g peptide | Skin of unicorn leatherjacket (Aluterus monoceros) | Glycyl endopeptidase from papaya | [231] |
| DPPH ABTS | 9.6 0.04 | Shrimp (Penaeus monodon and Penaeus indicus) | Alcalase | ||
| DPPH Oxygen radical 2,2-azino-bis(3-ethylbenzthiazoline)-6-Sulfonic acid cation | 2.4 μM, IC50 497.4 μmol TE/mg 48.4 μmol TE/mg 110.4 μmol TE/mg | Krill (Euphausia superba) | Pepsin | [186] | |
| ABTS DPPH | F2 3.6 kDa | 0.05 1.1 | Solitary Tunicate (Styela clava) | Alcalase 2.4 L FG, Thermoase PC10F, pepsin | [232] |
| Hydroxyl Superoxide DPPH | Enzymatic hydrolysates | 102−196 μg/mL | Navicula incerta | Alcalase, pronase-E, α-chymotrypsin, neutrase, papain, pepsin, and trypsin | [222] |
| NIPP-1 (Pro-Gly-Trp-Asn-Gln-Trp-Phe-Leu) 1.171 kDa NIPP-2 (Val-Glu-Val-Leu-Pro-Pro-Ala-Glu-Leu) 1.108 kDa | Navicula incerta | Alcalase, α-chymotrypsin, neutrase, papain, pepsin, pronase-E and trypsin | [233] | ||
| ABTS DPPH | WPRGYFL (MW 937 Da) SDWDRF (MW 824 Da) | 4.70, EC50 14.0, EC50 | Tetradesmus obliquus | Alcalase | [205] |
| Peroxyl DPPH Hydroxyl | LNGDVW | 0.02 mM 0.92 mM 1.42 mM | Chlorella ellipsoidea | Papain, trypsin, pepsin and a-chymotrypsin | [234] |
| ORAC FRAP ORAC FRAP | - | 14.0 μmol TE/g dw 478.9 μmol TE/g dw 15.0 μmol TE/g dw 155.7 μmol TE/g dw | Porphyridium purpureum Phaeodactylum tricornutum | Alcalase 2.4 L and Flavourzyme 500 L | [235] |
| Hydroxyl radical | MPGPLSPL (793.01 Da) | Pavlova lutheri | Proteolytic yeast Candidia rugopelliculosa | [236] | |
| DPPH | Peptides < 5 kDa | Observed at 4.8 μM 46.9–50.9% | Chlorella sorokiniana | Pepsin, bromelain, and thermolysin | [206] |
| Antidiabetic Assays | Peptide Names or Sequences | Efficiency (IC50, EC50) (μM) | Types of SPBs, Marine Species | Enzymes, Production Conditions | References |
|---|---|---|---|---|---|
| PGVGGPLGPIGPCYE CAYQWQRPVDRIR PACGGFWISGRPG | 116.1 78.0 96.4 | Longtail tuna (Thunnus tonggol) | Protease XXIII | [44] | |
| GGPAGPAV GPVA PP GF | 8139.1 264.7 4343.5 1547.2 | Trimming of Atlantic salmon (Salmo salar) | Corolase PP | [195] | |
| GPAE GPGA | 49.6 41.9 | Skin of Atlantic salmon (Salmo salar) | Flavourzyme | [250] | |
| AP VR | 0.02 0.07 | Skin collagen of Atlantic salmon (Salmo salar) | Alcalase 2.4 L, papain | [202] | |
| - | 1.0% hydrolysate 122 pM CKK release | Mince of Blue whiting (Micromesistius poutassou) | Endopeptidase | [251] | |
| - | 7.2 | Mince of Blue whiting (Micromesistius poutassou) | Alcalase 2.4 L Flavourzyme 500 L | [252] | |
| DPP-IV inhibitory | - | 10.9 12.9 | Porphyridium purpureum and Phaeodactylum tricornutum | Alcalase 2.4 L and Flavourzyme 500 L | [235] |
| Anticancer Assays | Peptide Names or Sequences | Efficiency (IC50, EC50) (μM) | Types of SPBs, Marine Species | Enzymes, Production Conditions | References |
|---|---|---|---|---|---|
| MCF-7 | LPHVLTPEAGAT PTAEGGVYMVT | 8.1 8.8 | Dark muscle byproduct of longtail tuna (Thunnus tonggol) | Papain Protease XXIII | [262] |
| DU-145 cell | - | 200 | Half-fin anchovy (Setipinna taty) | Pepsin | [229] |
| Ca9-22 | - | 4.1 | Roe of Rohu (Labeo rohita) | Protease N | [263] |
| MCF-7/6 MDAMB-231 | Free amino acids, peptides with ~7 kDa | Exhibited cell growth inhibition | Blue whiting, cod, plaice, and salmon | Alcalase and protamex | [264] |
| Caco2 (Human colon) HepG2 (Human liver) | Fraction < 10 kDa Fraction 10–30 kDa Fraction > 30 kDa | Significantly inhibited the growth of both colon and liver cancer cells by 60%. <10 kDa fraction from shrimp shells (FL) inhibited growth of liver cancer cells alone by 55%, compared to controls | Shrimp shell | Cryotin enzyme | [265] |
| Female BALB/c mice with transplanted sarcoma S180 cells | MW < 3 kDa | Significantly inhibited the growth of transplanted sarcoma S180 cells in mice | Oyster (Crassostrea gigas) | Protease from Bacillus sp. SM98011 | [266] |
| PC-3 DU-145 H-1299 HeLa | BCP-A (Trp-Pro-Pro), 398.4 Da | Cytotoxicity in a dose-dependent manner. Significantly changed the morphologies of the PC-3 cells. The percentage of early stage apoptotic PC-3 cells increased from 11.22 to 22.78% when they were treated with Trp-ProPro concentrations of 5 and 15 mg/mL, respectively, for 24 h. | Blood of clam (Tegillarca granosa) | Neutrase | [257] |
| PC-3 (prostate) A549 (lung) MDA-MB-231 (breast) Normal liver cells | Ala-Val-LeuVal-Asp-Lys-Gln-Cys-Pro-Asp | Lethal concentration (LC) 6.2, LC50 6.5, LC50 7.6, LC50 No cytotoxicity | Ruditapes philippinarum | α-Chymotrypsin | [267] |
| DU-145 cells | N Gln-Pro-Lys, MW 343.4 Da | Sepia Ink | Trypsin | [254] | |
| MCF-7 (human breast carcinoma) U87 (glioma) | 0.6 0.48 | Gelatine of giant squid (Dosidicus gigas) | Esperase | [202] | |
| AGS, DLD-1 HeLa | F2, 3.6 kDa | 2.8 5.6 4.3 | Solitary Tunicate (Styela clava) | Alcalase 2.4 L FG, Thermoase PC10F, pepsin | [232] |
| HepG2 cells | Polypeptide CPAP | 426 μg/mL | Chlorella pyrenoidosa | Papain, trypsin, and alcalase | [268] |
| Navicula incerta | Alcalase, α-chymotrypsin, neutrase, papain, pepsin, pronase-E and trypsin | [233] | |||
| SW480 (Colon cancer cell lines) | Peptides < 3 kDa | 0.8 | Dunaliella salina | Trypsin and chymotrypsin | [260] |
| Antimicrobial Assays | Peptide Names or Sequences | Efficiency (IC50, EC50) (μM) | Types of SPBs, Marine Species | Enzymes, Production Conditions | References |
|---|---|---|---|---|---|
| Bacillus cereus Bacillus subtilis Staphylococcus aureus Salmonella sp. Listeria innocua Escherichia coli | FPIGMGHGSRPA | 2.9 2.0 2.4 3.5 2.4 3.8 | Viscera of Small red scorpionfish (Scorpaena notata) | Crude enzyme from Trichoderma harzianum | [277] |
| Antibacterial | Gelatine (BG) Gelatine hydrolysate | Skin of black-barred halfbeak (Hemiramphus far) | Purafect | [275] | |
| AQ-3001, AQ-3002, AQ3369, AQ-3370, AQ-3371, and AQ-3372 | Tetraselmis suecica | Acid extracts | [278] | ||
| Escherichia coli Staphylococcus aureus | Protein hydrolysate 63 kDa | 59.4% 42.9% | Dunaliella salina | Trypsin and chymotrypsin | [260] |
| HSV-1 | 83 μg, EC50 | Winter flounder (Pleuronectes americanus) | Synthetic peptide | [45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Heimann, K.; Zhang, W. Protein Recovery from Underutilised Marine Bioresources for Product Development with Nutraceutical and Pharmaceutical Bioactivities. Mar. Drugs 2020, 18, 391. https://doi.org/10.3390/md18080391
Nguyen TT, Heimann K, Zhang W. Protein Recovery from Underutilised Marine Bioresources for Product Development with Nutraceutical and Pharmaceutical Bioactivities. Marine Drugs. 2020; 18(8):391. https://doi.org/10.3390/md18080391
Chicago/Turabian StyleNguyen, Trung T., Kirsten Heimann, and Wei Zhang. 2020. "Protein Recovery from Underutilised Marine Bioresources for Product Development with Nutraceutical and Pharmaceutical Bioactivities" Marine Drugs 18, no. 8: 391. https://doi.org/10.3390/md18080391
APA StyleNguyen, T. T., Heimann, K., & Zhang, W. (2020). Protein Recovery from Underutilised Marine Bioresources for Product Development with Nutraceutical and Pharmaceutical Bioactivities. Marine Drugs, 18(8), 391. https://doi.org/10.3390/md18080391