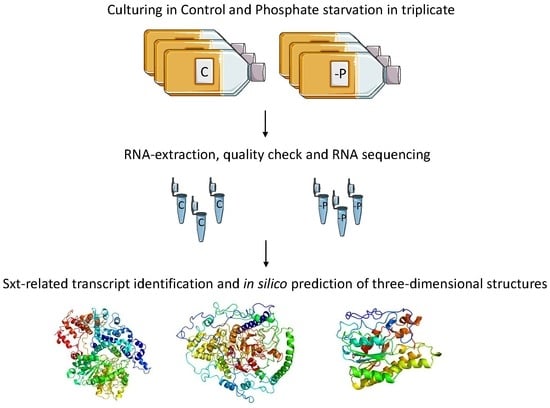
De novo Transcriptome of the Non-saxitoxin Producing Alexandrium tamutum Reveals New Insights on Harmful Dinoflagellates
Abstract
1. Introduction
2. Results and Discussion
2.1. Transcriptome Sequencing and De Novo Assembly
2.2. Functional Annotation
2.3. Differential Expression Analysis
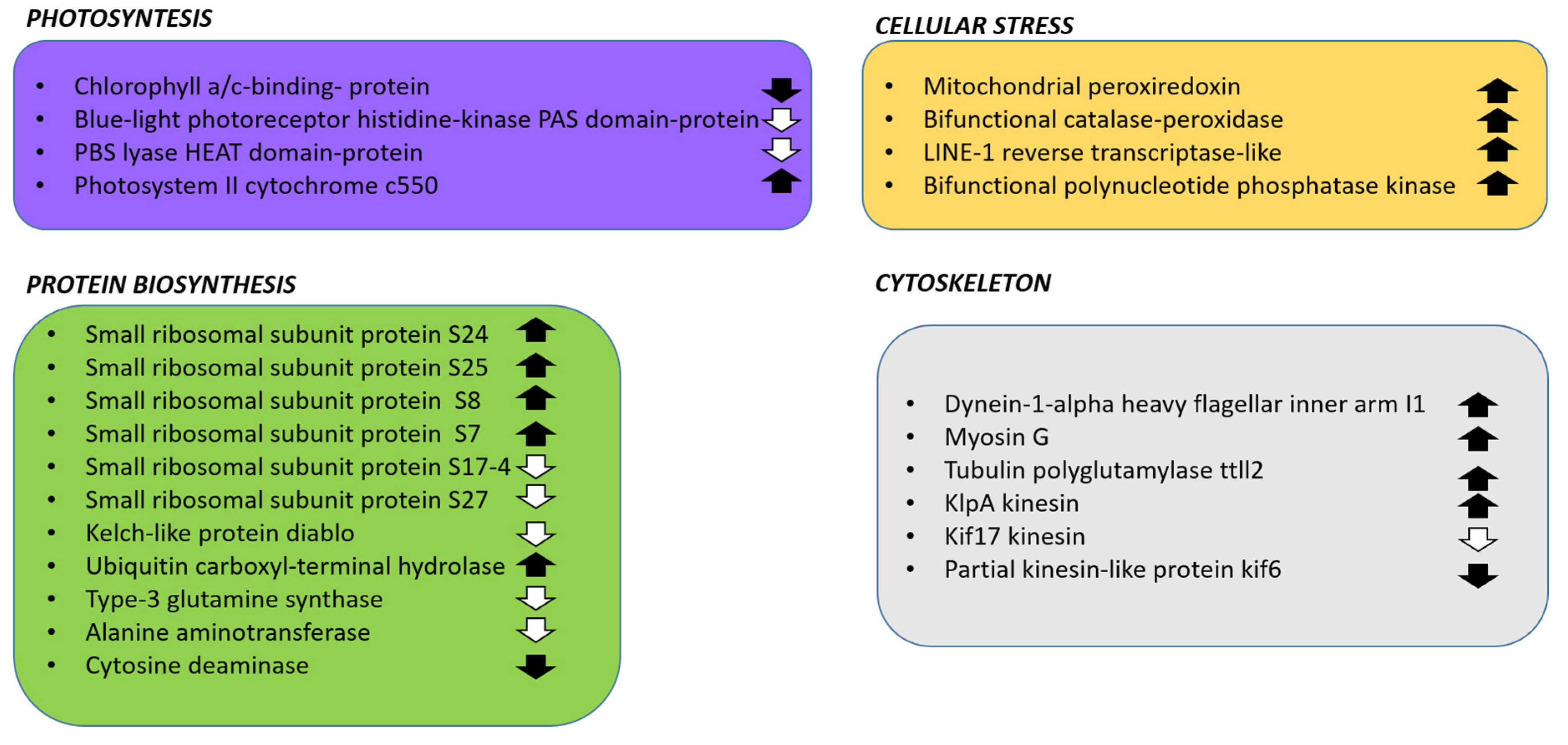
2.3.1. DEGs Involved in Photosynthesis
2.3.2. DEGs Involved in Protein Synthesis
2.3.3. DEGs Related to Cellular Stress
2.3.4. DEGs Related to Cytoskeleton
2.4. Sequences Coding Enzymes Involved in Toxin Synthesis
2.5. Structure Prediction of Proteins Encoded by sxt Genes
2.6. Other Toxin-Related Transcripts
3. Materials and Methods
3.1. Cell Culturing and Harvesting
3.2. RNA Extraction
3.3. Library Preparation and Sequencing
3.4. Transcriptome Assembly and Annotation
3.5. Transcriptome Expression Quantification and Differential Expression Analysis
3.6. In Silico Protein Modelling
3.7. Phylogenetic Tree
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Glibert, P.M.; Berdalet, E.; Burford, M.A.; Pitcher, G.C.; Zhou, M. Harmful Algal Blooms and the Importance of Understanding Their Ecology and Oceanography; Springer: Cham, Switzerland, 2018; pp. 9–25. [Google Scholar]
- Díaz, P.A.; Álvarez, G.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E.; et al. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Perspect. Phycol. 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Kudela, R.; Berdalet, E.; Enevoldsen, H.; Pitcher, G.; Raine, R.; Urban, E. GEOHAB–The Global Ecology and Oceanography of Harmful Algal Blooms Program: Motivation, Goals, and Legacy. Oceanography 2017, 30, 12–21. [Google Scholar] [CrossRef]
- Akbar, M.A.; Yusof, N.Y.M.; Tahir, N.I.; Ahmad, A.; Usup, G.; Sahrani, F.K.; Bunawan, H. Biosynthesis of saxitoxin in marine dinoflagellates: An omics perspective. Mar. Drugs 2020, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Ianora, A.; Guisande, C.; Fontana, A.; Romano, G.; Carotenuto, Y.; Esposito, F.; Turner, J.; d’Ippolito, G.; Miralto, A. Copepod egg production and hatching success is reduced by maternal diets of a non-neurotoxic strain of the dinoflagellate Alexandrium tamarense. Mar. Ecol. Prog. Ser. 2007, 280, 199–210. [Google Scholar] [CrossRef]
- Etheridge, S.M. Paralytic shellfish poisoning: Seafood safety and human health perspectives. Toxicon 2010, 56, 108–122. [Google Scholar] [CrossRef]
- Broadwater, M.H.; Van Dolah, F.M.; Fire, S.E. Vulnerabilities of Marine Mammals to Harmful Algal Blooms. In Harmful Algal Blooms; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 191–222. [Google Scholar]
- Cusick, K.D.; Sayler, G.S. An overview on the marine neurotoxin, saxitoxin: Genetics, moleculartargets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef]
- D’Agostino, P.; Moffitt, M.; Neilan, B. Current Knowledge of Paralytic Shellfish Toxin Biosynthesis, Molecular Detection and Evolution. In Toxins and Biologically Active Compounds from Microalgae, Volume 1; CRC Press: Boca Raton, FL, USA, 2014; pp. 251–280. [Google Scholar]
- Martens, H.; Tillmann, U.; Harju, K.; Dell’Aversano, C.; Tartaglione, L.; Krock, B. Toxin Variability Estimations of 68 Alexandrium ostenfeldii (Dinophyceae) Strains from The Netherlands Reveal a Novel Abundant Gymnodimine. Microorganisms 2017, 5, 29. [Google Scholar] [CrossRef]
- Touzet, N.; Franco, J.M.; Raine, R. Morphogenetic diversity and biotoxin composition of Alexandrium (Dinophyceae) in Irish coastal waters. Harmful Algae 2008, 7, 782–797. [Google Scholar] [CrossRef]
- Montresor, M.; John, U.; Beran, A.; Medlin, L.K. Alexandrium tamutum sp. nov. (Dinophyceae): A new nontoxic species in the genus Alexandrium. J. Phycol. 2004, 40, 398–411. [Google Scholar] [CrossRef]
- Collins, C.; Graham, J.; Brown, L.; Bresnan, E.; Lacaze, J.P.; Turrell, E.A. Identification and toxicity of Alexandrium tamarense (dinophyceae) in Scottish waters. J. Phycol. 2009, 45, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Fong, N.; Soon, K.; Hii, T.; Hie, L.; Teen, P.; Pin, C. First Record of Marine Dinoflagellate, Alexandrium tamutum (Dinophyceae ) from Malaysia. Malaysian J. Sci. 2013, 32, 81–88. [Google Scholar]
- Gu, H.; Zeng, N.; Liu, T.; Yang, W.; Müller, A.; Krock, B. Morphology, toxicity, and phylogeny of Alexandrium (Dinophyceae) species along the coast of China. Harmful Algae 2013, 27, 68–81. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Stüken, A.; Orr, R.J.S.; Kellmann, R.; Murray, S.A.; Neilan, B.A.; Jakobsen, K.S. Discovery of nuclear-encoded genes for the neurotoxin saxitoxin in dinoflagellates. PLoS ONE 2011, 6, e20096. [Google Scholar] [CrossRef]
- Hackett, J.D.; Wisecaver, J.H.; Brosnahan, M.L.; Kulis, D.M.; Anderson, D.M.; Bhattacharya, D.; Gerald Plumley, F.; Erdner, D.L. Evolution of saxitoxin synthesis in cyanobacteria and dinoflagellates. Mol. Biol. Evol. 2013, 30, 70–78. [Google Scholar] [CrossRef]
- Lauritano, C.; Ferrante, M.I.; Rogato, A. Marine Natural Products from Microalgae: An -Omics Overview. Mar. Drugs 2019, 17, 269. [Google Scholar] [CrossRef]
- Kellmann, R.; Stüken, A.; Orr, R.J.S.; Svendsen, H.M.; Jakobsen, K.S. Biosynthesis and Molecular Genetics of Polyketides in Marine Dinoflagellates. Mar. Drugs 2010, 8, 1011–1048. [Google Scholar] [CrossRef]
- Keeling, P.J.; Burki, F.; Wilcox, H.M.; Allam, B.; Allen, E.E.; Amaral-Zettler, L.A.; Armbrust, E.V.; Archibald, J.M.; Bharti, A.K.; Bell, C.J.; et al. The Marine Microbial Eukaryote Transcriptome Sequencing Project (MMETSP): Illuminating the Functional Diversity of Eukaryotic Life in the Oceans through Transcriptome Sequencing. PLoS Biol. 2014, 12, e1001889. [Google Scholar] [CrossRef]
- Murray, S.A.; Diwan, R.; Orr, R.J.S.; Kohli, G.S.; John, U. Gene duplication, loss and selection in the evolution of saxitoxin biosynthesis in alveolates. Mol. Phylogenet. Evol. 2015, 92, 165–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.F.; Lin, L.; Wang, D.Z. Comparative transcriptome analysis of a toxin-producing dinoflagellate Alexandrium catenella and its non-toxic mutant. Mar. Drugs 2014, 12, 5698–5718. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, U.; Kremp, A.; Tahvanainen, P.; Krock, B. Characterization of spirolide producing Alexandrium ostenfeldii (Dinophyceae) from the western Arctic. Harmful Algae 2014, 39, 259–270. [Google Scholar] [CrossRef]
- Harju, K.; Koskela, H.; Kremp, A.; Suikkanen, S.; De La Iglesia, P.; Miles, C.O.; Krock, B.; Vanninen, P. Identification of gymnodimine D and presence of gymnodimine variants in the dinoflagellate Alexandrium ostenfeldii from the Baltic Sea. Toxicon 2016, 112, 68–76. [Google Scholar] [CrossRef]
- Zurhelle, C.; Nieva, J.; Tillmann, U.; Harder, T.; Krock, B.; Tebben, J. Identification of novel gymnodimines and spirolides from the marine dinoflagellate Alexandrium ostenfeldii. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Caruana, A.M.N.; Amzil, Z. Microalgae and toxins. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 263–305. [Google Scholar]
- Harris, C.M.; Reece, K.S.; Stec, D.F.; Scott, G.P.; Jones, W.M.; Hobbs, P.L.M.; Harris, T.M. The toxin goniodomin, produced by Alexandrium spp., is identical to goniodomin A. Harmful Algae 2020, 92, 101707. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Z.; Kong, L.; Holmes, M.J. Dinoflagellate Alexandrium leei (Dinophyceae) from Singapore coastal waters produces a water-soluble ichthyotoxin. Mar. Biol. 2007, 150, 541–549. [Google Scholar] [CrossRef]
- Frangópulos, M.; Guisande, C.; DeBlas, E.; Maneiro, I. Toxin production and competitive abilities under phosphorus limitation of Alexandrium species. Harmful Algae 2004, 3, 131–139. [Google Scholar] [CrossRef]
- Yang, I.; Beszteri, S.; Tillmann, U.; Cembella, A.; John, U. Growth- and nutrient-dependent gene expression in the toxigenic marine dinoflagellate Alexandrium minutum. Harmful Algae 2011, 12, 55–69. [Google Scholar] [CrossRef]
- Sampedro, N.; Franco, J.M.; Zapata, M.; Riobó, P.; Garcés, E.; Penna, A.; Caillaud, A.; Diogène, J.; Cacho, E.; Camp, J. The toxicity and intraspecific variability of Alexandrium andersonii balech. Harmful Algae 2013, 25, 26–38. [Google Scholar] [CrossRef]
- Parra, G.; Bradnam, K.; Korf, I.; Bateman, A. CEGMA: A pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics 2007, 23, 1061–1067. [Google Scholar] [CrossRef]
- Smit, A.F.; Hubley, R.; Green, P. RepeatMasker Home Page. Available online: http://www.repeatmasker.org/ (accessed on 25 June 2020).
- CDC4-Cell division control protein 4-Saccharomyces cerevisiae (strain ATCC 204508/S288c) (Baker’s yeast)-CDC4 gene & protein. Available online: https://www.uniprot.org/uniprot/P07834 (accessed on 18 June 2020).
- IMPDH-Inosine-5’-monophosphate dehydrogenase-Homo sapiens (Human)-IMPDH gene & protein. Available online: https://www.uniprot.org/uniprot/B4DNJ7 (accessed on 18 June 2020).
- GBP1-Guanylate-binding protein 1 precursor-Homo sapiens (Human)-GBP1 gene & protein. Available online: https://www.uniprot.org/uniprot/P32455 (accessed on 18 June 2020).
- PRMT7-Protein arginine N-methyltransferase 7-Homo sapiens (Human)-PRMT7 gene & protein. Available online: https://www.uniprot.org/uniprot/Q9NVM4 (accessed on 18 June 2020).
- PBS lyase HEAT-like repeat (IPR004155)-InterPro entry-InterPro. Available online: https://www.ebi.ac.uk/interpro/entry/InterPro/IPR004155/ (accessed on 18 June 2020).
- Hiller, R.G.; Wrench, P.M.; Sharples, F.P. The light-harvesting chlorophyll a-c-binding protein of dinoflagellates: A putative polyprotein. FEBS Lett. 1995, 363, 175–178. [Google Scholar] [CrossRef]
- Taylor, B.L.; Zhulin, I.B. PAS Domains: Internal Sensors of Oxygen, Redox Potential, and Light. Microbiol. Mol. Biol. Rev. 1999, 63, 479–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.H.; Deng, M.G.; Zheng, M.; Zhou, M.; Parbel, A.; Storf, M.; Meyer, M.; Strohmann, B.; Scheer, H. Novel activity of a phycobiliprotein lyase: Both the attachment of phycocyanobilin and the isomerization to phycoviolobilin are catalyzed by the proteins PecE and PecF encoded by the phycoerythrocyanin operon. FEBS Lett. 2000, 469, 9–13. [Google Scholar] [CrossRef]
- Alipanah, L.; Winge, P.; Rohloff, J.; Najafi, J.; Brembu, T.; Bones, A.M. Molecular adaptations to phosphorus deprivation and comparison with nitrogen deprivation responses in the diatom Phaeodactylum tricornutum. PLoS ONE 2018, 13, e0193335. [Google Scholar] [CrossRef]
- Theodorou, M.E.; Elrifi, I.R.; Turpin, D.H.; Plaxton, W.C. Effects of phosphorus limitation on respiratory metabolism in the green alga Selenastrum minutum. Plant Physiol. 1991, 95, 1089–1095. [Google Scholar] [CrossRef]
- Woo, J.; MacPherson, C.R.; Liu, J.; Wang, H.; Kiba, T.; Hannah, M.A.; Wang, X.J.; Bajic, V.B.; Chua, N.H. The response and recovery of the Arabidopsis thaliana transcriptome to phosphate starvation. BMC Plant Biol. 2012, 12, 62. [Google Scholar] [CrossRef]
- Roncel, M.; Kirilovsky, D.; Guerrero, F.; Serrano, A.; Ortega, J.M. Photosynthetic cytochrome c550. In Proceedings of the Biochimica et Biophysica Acta-Bioenergetics; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1817, pp. 1152–1163. [Google Scholar]
- Guo, J.; Wilken, S.; Jimenez, V.; Choi, C.J.; Ansong, C.; Dannebaum, R.; Sudek, L.; Milner, D.S.; Bachy, C.; Reistetter, E.N.; et al. Specialized proteomic responses and an ancient photoprotection mechanism sustain marine green algal growth during phosphate limitation. Nat. Microbiol. 2018, 3, 781–790. [Google Scholar] [CrossRef]
- Morey, J.S.; Monroe, E.A.; Kinney, A.L.; Beal, M.; Johnson, J.G.; Hitchcock, G.L.; Van Dolah, F.M. Transcriptomic response of the red tide dinoflagellate, Karenia brevis, to nitrogen and phosphorus depletion and addition. BMC Genom. 2011, 12, 1–18. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, J.; Fang, L.; Zhang, X.; Chow, Y.; Liu, J. De novo transcriptome profiling uncovers a drastic downregulation of photosynthesis upon nitrogen deprivation in the nonmodel green alga Botryosphaerella sudeticus. BMC Genom. 2013, 14, 1–18. [Google Scholar] [CrossRef]
- Corteggiani Carpinelli, E.; Telatin, A.; Vitulo, N.; Forcato, C.; D’Angelo, M.; Schiavon, R.; Vezzi, A.; Giacometti, G.M.; Morosinotto, T.; Valle, G. Chromosome scale genome assembly and transcriptome profiling of Nannochloropsis gaditana in nitrogen depletion. Mol. Plant 2014, 7, 323–335. [Google Scholar] [CrossRef]
- De Las Heras-Rubio, A.; Perucho, L.; Paciucci, R.; Vilardell, J.; Lleonart, M.E. Ribosomal proteins as novel players in tumorigenesis. Cancer Metastasis Rev. 2014, 33, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liao, W.J.; Liao, J.M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef] [PubMed]
- dbo-Kelch-like protein diablo-Drosophila virilis (Fruit fly)-dbo gene & protein. Available online: https://www.uniprot.org/uniprot/B4LIG6 (accessed on 18 June 2020).
- Larsen, C.N.; Price, J.S.; Wilkinson, K.D. Substrate binding and catalysis by ubiquitin C-terminal hydrolases: Identification of two active site residues. Biochemistry 1996, 35, 6735–6744. [Google Scholar] [CrossRef] [PubMed]
- Cirulis, J.T.; Scott, J.A.; Ross, G.M. Management of oxidative stress by microalgae. Can. J. Physiol. Pharmacol. 2013, 91, 15–21. [Google Scholar] [CrossRef]
- Cox, A.G.; Winterbourn, C.C.; Hampton, M.B. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 2010, 425, 313–325. [Google Scholar] [CrossRef]
- Mukai, K.; Shimasaki, Y.; Qiu, X.; Kato-Unoki, Y.; Chen, K.; Khanam, M.R.M.; Oshima, Y. Effects of light and hydrogen peroxide on gene expression of newly identified antioxidant enzymes in the harmful algal bloom species Chattonella marina. Eur. J. Phycol. 2019, 54, 1–11. [Google Scholar] [CrossRef]
- Glaesener, A.G.; Merchant, S.S.; Blaby-Haas, C.E. Iron economy in Chlamydomonas reinhardtii. Front. Plant Sci. 2013, 4, 337. [Google Scholar] [CrossRef]
- Varnado, C.L.; Hertwig, K.M.; Thomas, R.; Roberts, J.K.; Goodwin, D.C. Properties of a novel periplasmic catalase-peroxidase from Escherichia coli O157:H7. Arch. Biochem. Biophys. 2004, 421, 166–174. [Google Scholar] [CrossRef]
- Guo, R.; Ki, J.S. Characterization of a novel catalase-peroxidase (KATG) gene from the dinoflagellate Prorocentrum minimum. J. Phycol. 2013, 49, 1011–1016. [Google Scholar]
- Sciamanna, I.; De Luca, C.; Spadafora, C. The reverse transcriptase encoded by LINE-1 retrotransposons in the genesis, progression, and therapy of cancer. Front. Chem. 2016, 4, 6. [Google Scholar] [CrossRef]
- Chénais, B.; Caruso, A.; Hiard, S.; Casse, N. The impact of transposable elements on eukaryotic genomes: From genome size increase to genetic adaptation to stressful environments. Gene 2012, 509, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Pnkp-Bifunctional polynucleotide phosphatase/kinase-Mus musculus (Mouse)-Pnkp gene & protein. Available online: https://www.uniprot.org/uniprot/Q9JLV6 (accessed on 18 June 2020).
- Dynein heavy chain (IPR026983)-InterPro entry-InterPro. Available online: https://www.ebi.ac.uk/interpro/entry/InterPro/IPR026983/ (accessed on 18 June 2020).
- Shi, X.; Lin, X.; Li, L.; Li, M.; Palenik, B.; Lin, S. Transcriptomic and microRNAomic profiling reveals multi-faceted mechanisms to cope with phosphate stress in a dinoflagellate. ISME J. 2017, 11, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.A.; Spudich, J.A. The myosin superfamily at a glance. J. Cell Sci. 2012, 125, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Tubulin-tyrosine ligase/Tubulin polyglutamylase (IPR004344)-InterPro entry-InterPro. Available online: https://www.ebi.ac.uk/interpro/entry/InterPro/IPR004344/ (accessed on 18 June 2020).
- Popchock, A.R.; Tseng, K.F.; Wang, P.; Karplus, P.A.; Xiang, X.; Qiu, W. The mitotic kinesin-14 KlpA contains a context-dependent directionality switch. Nat. Commun. 2017, 8, 13999. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liang, Y.; Meng, D.; Wang, L.; Pan, J. Microtubule-Depolymerizing Kinesins in the Regulation of Assembly, Disassembly, and Length of Cilia and Flagella. Int. Rev. Cell Mol. Biol. 2015, 317, 241–265. [Google Scholar] [PubMed]
- KIF6 Gene-GeneCards|KIF6 Protein|KIF6 Antibody. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=KIF6 (accessed on 18 June 2020).
- Kellmann, R.; Mihali, T.K.; Young, J.J.; Pickford, R.; Pomati, F.; Neilan, B.A. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 2008, 74, 4044–4053. [Google Scholar] [CrossRef]
- Mihali, T.K.; Kellmann, R.; Neilan, B.A. Characterisation of the paralytic shellfish toxin biosynthesis gene clusters in Anabaena circinalis AWQC131C and Aphanizomenon sp. NH-5. BMC Biochem. 2009, 10, 8. [Google Scholar] [CrossRef]
- Verma, A.; Barua, A.; Ruvindy, R.; Savela, H.; Ajani, P.A.; Murray, S.A. The Genetic Basis of Toxin Biosynthesis in Dinoflagellates. Microorganisms 2019, 7, 222. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.F.; Lin, L.; Wang, D.Z. Whole transcriptomic analysis provides insights into molecular mechanisms for toxin biosynthesis in a toxic dinoflagellate Alexandrium catenella (ACHK-T). Toxins (Basel) 2017, 9, 213. [Google Scholar] [CrossRef]
- Meng, F.Q.; Song, J.T.; Zhou, J.; Cai, Z.H. Transcriptomic Profile and Sexual Reproduction-Relevant Genes of Alexandrium minutum in Response to Nutritional Deficiency. Front. Microbiol. 2019, 10, 2629. [Google Scholar] [CrossRef]
- Tatters, A.O.; Van Wagoner, R.M.; Wright, J.L.C.; Tomas, C.R. Regulation of spiroimine neurotoxins and hemolytic activity in laboratory cultures of the dinoflagellate Alexandrium peruvianum (Balech & Mendiola) Balech & Tangen. Harmful Algae 2012, 19, 160–168. [Google Scholar]
- Han, M.; Lee, H.; Anderson, D.M.; Kim, B. Paralytic shellfish toxin production by the dinoflagellate Alexandrium pacificum (Chinhae Bay, Korea) in axenic, nutrient-limited chemostat cultures and nutrient-enriched batch cultures. Mar. Pollut. Bull. 2016, 104, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Ransom Hardison, D.; Sunda, W.G.; Wayne Litaker, R.; Shea, D.; Tester, P.A. Nitrogen limitation increases brevetoxins in Karenia brevis (dinophyceae): Implications for bloom toxicity. J. Phycol. 2012, 48, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Boyer, G.L.; Sullivan, J.J.; Andersen, R.J.; Harrison, P.J.; Taylor, F.J.R. Effects of nutrient limitation on toxin production and composition in the marine dinoflagellate Protogonyaulax tamarensis. Mar. Biol. 1987, 96, 123–128. [Google Scholar] [CrossRef]
- Macintyre, H.L.; Stutes, A.L.; Smith, W.L.; Dorsey, C.P.; Annabraham; Dickey, R.W. Environmental correlates of community composition and toxicity during a bloom of Pseudo-nitzschia spp. in the northern Gulf of Mexico. J. Plankton Res. 2011, 33, 273–295. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Lukowski, A.L.; Mallik, L.; Hinze, M.E.; Carlson, B.M.; Ellinwood, D.C.; Pyser, J.B.; Koutmos, M.; Narayan, A.R.H. Substrate Promiscuity of a Paralytic Shellfish Toxin Amidinotransferase. ACS Chem. Biol. 2020, 15, 626–631. [Google Scholar] [CrossRef]
- Penning, T.M. The aldo-keto reductases (AKRs): Overview. Chem. Biol. Interact. 2015, 234, 236–246. [Google Scholar] [CrossRef]
- Ellis, E.M.; Slattery, C.M.; Hayes, J.D. Characterization of the rat aflatoxin B1 aldehyde reductase gene, AKR7A1. Structure and chromosomal localization of AKR7A1 as well as identification of antioxidant response elements in the gene promoter. Carcinogenesis 2003, 24, 727–737. [Google Scholar] [CrossRef][Green Version]
- NGA_0683200-Aldehyde reductase i-Nannochloropsis gaditana (strain CCMP526) (Green microalga)-NGA_0683200 gene & protein. Available online: https://www.uniprot.org/uniprot/K8Z9U8 (accessed on 26 June 2020).
- Gamma-glutamyl cyclotransferase-like (IPR013024)-InterPro entry-InterPro. Available online: https://www.ebi.ac.uk/interpro/entry/InterPro/IPR013024/ (accessed on 26 June 2020).
- COCSUDRAFT_18395-Gamma-glutamylcyclotransferase-Coccomyxa subellipsoidea (strain C-169) (Green microalga)-COCSUDRAFT_18395 gene & protein. Available online: https://www.uniprot.org/uniprot/I0YPY5 (accessed on 26 June 2020).
- Gardner, R.G.; Shearer, A.G.; Hampton, R.Y. In vivo action of the HRD ubiquitin ligase complex: Mechanisms of endoplasmic reticulum quality control and sterol regulation. Mol. Cell. Biol. 2001, 21, 4276–4291. [Google Scholar] [CrossRef]
- Farrow, S.C.; Facchini, P.J. Functional diversity of 2-oxoglutarate/Fe(II)-dependent dioxygenases in plant metabolism. Front. Plant Sci. 2014, 5, 524. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.Q.; Hausinger, R.P. Amazing Diversity in Biochemical Roles of Fe(II)/2-Oxoglutarate Oxygenases. Trends Biochem. Sci. 2018, 43, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Koski, M.K.; Hieta, R.; Böllner, C.; Kivirikko, K.I.; Myllyharju, J.; Wierenga, R.K. The active site of an algal prolyl 4-hydroxylase has a large structural plasticity. J. Biol. Chem. 2007, 282, 37112–37123. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J. Amphidinolides and Its Related Macrolides from Marine Dinoflagellates. J. Antibiot. (Tokyo) 2008, 61, 271–284. [Google Scholar] [CrossRef]
- Zhang, J.; Du, L.; Liu, F.; Xu, F.; Hu, B.; Venturi, V.; Qian, G. Involvement of both PKS and NRPS in antibacterial activity in Lysobacter enzymogenes OH11. FEMS Microbiol. Lett. 2014, 355, 170–176. [Google Scholar] [CrossRef]
- Lauritano, C.; De Luca, D.; Ferrarini, A.; Avanzato, C.; Minio, A.; Esposito, F.; Ianora, A. De novo transcriptome of the cosmopolitan dinoflagellate Amphidinium carterae to identify enzymes with biotechnological potential. Sci. Rep. 2017, 7, 11701. [Google Scholar] [CrossRef]
- Nielsen, M.R.; Sondergaard, T.E.; Giese, H.; Sørensen, J.L. Advances in linking polyketides and non-ribosomal peptides to their biosynthetic gene clusters in Fusarium. Curr. Genet. 2019, 65, 1263–1280. [Google Scholar] [CrossRef]
- Gallo, A.; Ferrara, M.; Perrone, G. Phylogenetic study of polyketide synthases and nonribosomal peptide synthetases involved in the biosynthesis of mycotoxins. Toxins (Basel) 2013, 5, 717–742. [Google Scholar] [CrossRef]
- Monroe, E.A.; Van Dolah, F.M. The Toxic Dinoflagellate Karenia brevis Encodes Novel Type I-like Polyketide Synthases Containing Discrete Catalytic Domains. Protist 2008, 159, 471–482. [Google Scholar] [CrossRef]
- Pawlowiez, R.; Morey, J.S.; Darius, H.T.; Chinain, M.; Van Dolah, F.M. Transcriptome sequencing reveals single domain Type I-like polyketide synthases in the toxic dinoflagellate Gambierdiscus polynesiensis. Harmful Algae 2014, 36, 29–37. [Google Scholar] [CrossRef]
- Van Dolah, F.M.; Kohli, G.S.; Morey, J.S.; Murray, S.A. Both modular and single-domain Type I polyketide synthases are expressed in the brevetoxin-producing dinoflagellate, Karenia brevis (Dinophyceae). J. Phycol. 2017, 53, 1325–1339. [Google Scholar] [CrossRef] [PubMed]
- De Luca, D.; Lauritano, C. In silico identification of type III PKS chalcone and stilbene synthase homologs in marine photosynthetic organisms. Biology (Basel) 2020, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; De Luca, D.; Amoroso, M.; Benfatto, S.; Maestri, S.; Racioppi, C.; Esposito, F.; Ianora, A. New molecular insights on the response of the green alga Tetraselmis suecica to nitrogen starvation. Sci. Rep. 2019, 9, 3336. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.M.; Rödelsperger, C.; Eichholz, K.; Tillmann, U.; Cembella, A.; McGaughran, A.; John, U. Transcriptomic characterisation and genomic glimps into the toxigenic dinoflagellate Azadinium spinosum, with emphasis on polykeitde synthase genes. BMC Genom. 2015, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; Campbell, K.; John, U.; Smith, K.F.; Fraga, S.; Rhodes, L.L.; Murray, S.A. Role of Modular Polyketide Synthases in the Production of Polyether Ladder Compounds in Ciguatoxin-Producing Gambierdiscus polynesiensis and G. excentricus (Dinophyceae). J. Eukaryot. Microbiol. 2017, 64, 691–706. [Google Scholar] [CrossRef] [PubMed]
- López-Legentil, S.; Song, B.; DeTure, M.; Baden, D.G. Characterization and localization of a hybrid non-ribosomal peptide synthetase and polyketide synthase gene from the toxic dinoflagellate Karenia brevis. Mar. Biotechnol. 2010, 12, 32–41. [Google Scholar] [CrossRef]
- Vingiani, G.M.; De Luca, P.; Ianora, A.; Dobson, A.D.W.; Lauritano, C. Microalgal Enzymes with Biotechnological Applications. Mar. Drugs 2019, 17, 459. [Google Scholar] [CrossRef]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. MEDIA FOR THE CULTURE OF OCEANIC ULTRAPHYTOPLANKTON. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Elagoz, A.M.; Ambrosino, L.; Lauritano, C. De novo transcriptome of the diatom Cylindrotheca closterium identifies genes involved in the metabolism of anti-inflammatory compounds. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Babraham Bioinformatics-FastQC A Quality Control tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 18 June 2020).
- Leinonen, R.; Sugawara, H.; Shumway, M. The sequence read archive. Nucleic Acids Res. 2011, 39, D19–D21. [Google Scholar] [CrossRef]
- BBMap download|SourceForge.net. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 26 June 2020).
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S. Blast2GO: A Comprehensive Suite for Functional Analysis in Plant Genomics. Int. J. Plant Genom. 2008, 2008, 1–12. [Google Scholar] [CrossRef]
- Roberts, A.; Pachter, L. Streaming fragment assignment for real-time analysis of sequencing experiments. Nat. Methods 2013, 10, 71–73. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Leng, N.; Dawson, J.A.; Thomson, J.A.; Ruotti, V.; Rissman, A.I.; Smits, B.M.G.; Haag, J.D.; Gould, M.N.; Stewart, R.M.; Kendziorski, C. EBSeq: An empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 2013, 29, 1035–1043. [Google Scholar] [CrossRef]
- Papadopoulos, J.S.; Agarwala, R. COBALT: Constraint-based alignment tool for multiple protein sequences. Bioinformatics 2007, 23, 1073–1079. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A Simple, Fast, and Accurate Algorithm to Estimate Large Phylogenies by Maximum Likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Lefort, V.; Longueville, J.-E.; Gascuel, O. SMS: Smart Model Selection in PhyML | Molecular Biology and Evolution | Oxford Academic. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, M.; Gascuel, O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst. Biol. 2006, 55, 539–552. [Google Scholar] [CrossRef] [PubMed]
- FigTree. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 26 June 2020).
- Martínez Andrade, K.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Costantini, M.; Coppola, D.; Lauritano, C.; Núñez Pons, L.; Ruocco, N.; di Prisco, G.; Ianora, A.; Verde, C. Biotechnological Applications of Bioactive Peptides From Marine Sources. In Advances in Microbial Physiology; Academic Press: Cambridge, MA, USA, 2018; Volume 73, pp. 171–220. [Google Scholar]
- Brillatz, T.; Lauritano, C.; Jacmin, M.; Khamma, S.; Marcourt, L.; Righi, D.; Romano, G.; Esposito, F.; Ianora, A.; Queiroz, E.F.; et al. Zebrafish-based identification of the antiseizure nucleoside inosine from the marine diatom Skeletonema marinoi. PLoS ONE 2018, 13, e0196195. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with immunomodulatory activities. Mar. Drugs 2020, 18, 2. [Google Scholar] [CrossRef]
- Galasso, C.; Nuzzo, G.; Brunet, C.; Ianora, A.; Sardo, A.; Fontana, A.; Sansone, C. The marine dinoflagellate Alexandrium minutum activates a mitophagic pathway in human lung cancer cells. Mar. Drugs 2018, 16, 502. [Google Scholar] [CrossRef]
- Sansone, C.; Nuzzo, G.; Galasso, C.; Casotti, R.; Fontana, A.; Romano, G.; Ianora, A. The Marine Dinoflagellate Alexandrium andersonii Induces Cell Death in Lung and Colorectal Tumor Cell Lines. Mar. Biotechnol. 2018, 20, 343–352. [Google Scholar] [CrossRef]


| Number of Genes | 216,911 |
|---|---|
| Number of transcripts | 288,380 |
| Percent GC content | 65.31 |
| Contig N50 | 1204 |
| Median contig length | 474 |
| Average contig length | 778.28 |
| Number of proteins | 179,258 |
| Number of complete proteins | 26,230 |
| Number of partial proteins | 153,028 |
| Gene | Encoded Protein | Original Sequence Code in Hackett, 2013 | Sequence ID and Internal BLAST Results | NCBI BLAST Best Match and Accession Number |
|---|---|---|---|---|
| sxtA | Phosphopantetheine attachment site (ACP in PKS) | sxtA, N terminus Contig93306 | >TR16074|c0_g1_i1 Length = 3230 Score = 402 bits (203), Expect = e-110 Identities = 443/523 (84%) Strand = Plus/Minus | Alexandrium catenella strain CS319 sxtA-like (sxtA) gene, partial sequence; KM100452.1 |
| sxtA | Aspartate aminotransferase | sxtA, C terminus Aspartate aminotransferase Contig106704 | >TR2168|c0_g2_i2 Length = 1724 Score = 371 bits (187), Expect = e-101 Identities = 352/407 (86%) Strand = Plus/Plus | Aminobacter sp. MSH1 chromosome, complete genome; CP026265.1 |
| sxtG | Amidinotransferase | sxtG, Amidinotransferase Contig22175 | >TR24523|c0_g4_i1 Length = 3296 Score = 1292 bits (652), Expect = 0.0 Identities = 1483/1760 (84%) Strand = Plus/Plus | Ensifer adhaerens strain Casida A plasmid pCasidaAA, complete sequence; CP015881.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig1416 | >TR101104|c3_g5_i1 Length = 1221 Score = 712 bits (359), Expect = 0.0 Identities = 704/819 (85%) Strand = Plus/Minus | Polyangium brachysporum strain DSM 7029, complete genome; CP011371.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig22159 | >TR119378|c0_g1_i1 Length = 1982 Score = 696 bits (351), Expect = 0.0 Identities = 648/747 (86%) Strand = Plus/Minus | Sandaracinus amylolyticus strain DSM 53668, complete genome; CP011125.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig22852 | >TR140478|c0_g2_i1 Length = 1469 Score = 381 bits (192), Expect = e-104 Identities = 381/444 (85%) Strand = Plus/Minus | Emiliania huxleyi CCMP1516 hypothetical protein mRNA; XM_005787014.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig22852 | >TR140478|c0_g1_i1 Length = 1457 Score = 381 bits (192), Expect = e-104 Identities = 381/444 (85%) Strand = Plus/Minus | Emiliania huxleyi CCMP1516 hypothetical protein mRNA; XM_005787014.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig24779 | >TR3419|c0_g1_i1 Length = 1019 Score = 559 bits (282), Expect = e-158 Identities = 585/686 (85%) Strand = Plus/Minus | Emiliania huxleyi CCMP1516 hypothetical protein partial mRNA; XM_005768652.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig31067 | >TR32351|c0_g1_i1 Length = 691 Score = 394 bits (199), Expect = e-108 Identities = 415/487 (85%) Strand = Plus/Minus | Stigmatella aurantiaca DW4/3-1, complete genome; CP002271.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig34277 | >TR63446|c0_g4_i1 Length = 1023 Score = 533 bits (269), Expect = e-150 Identities = 551/645 (85%) Strand = Plus/Plus | Caulobacter mirabilis strain FWC 38 chromosome, complete genome; CP024201.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig34756 | >TR70482|c0_g1_i1 Length = 1181 Score = 486 bits (245), Expect = e-136 Identities = 587/701 (83%) Strand = Plus/Minus | Anopheles gambiae str. PEST AGAP008667-RA (AgaP_AGAP008667), partial mRNA; XM_314766.4 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig44170 | >TR142607|c0_g1_i1 Length = 1418 Score = 593 bits (299), Expect = e-168 Identities = 638/751 (84%) Strand = Plus/Minus | Chromobacterium vaccinii strain XC0014 chromosome, complete genome; CP022344.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig44865 | >TR84807|c0_g1_i1 Length = 1081 Score = 422 bits (213), Expect = e-117 Identities = 450/529 (85%) Strand = Plus/Minus | Phenylobacterium zucineum HLK1, complete genome; CP000747.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig64321 | >TR80533|c0_g3_i1 Length = 989 Score = 472 bits (238), Expect = e-131 Identities = 490/574 (85%) Strand = Plus/Plus | Bradyrhizobium diazoefficiens DNA, complete genome, strain: NK6; AP014685.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig64321 | >TR80533|c0_g2_i1 Length = 916 Score = 472 bits (238), Expect = e-131 Identities = 490/574 (85%) Strand = Plus/Plus | Bradyrhizobium diazoefficiens DNA, complete genome, strain: NK6; AP014685.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig86383 | >TR142098|c0_g1_i2 Length = 1193 Score = 448 bits (226), Expect = e-124 Identities = 427/494 (86%) Strand = Plus/Minus | PREDICTED: Aegilops tauschii subsp. tauschii momilactone A synthase-like (LOC109773470), mRNA; XM_020332162.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig86383 | >TR142098|c0_g1_i3 Length = 936 Score = 424 bits (214), Expect = e-117 Identities = 424/494 (85%) Strand = Plus/Minus | PREDICTED: Aegilops tauschii subsp. tauschii momilactone A synthase-like (LOC109773470), mRNA; XM_020332162.1 |
| sxtU | Short-chain alcohol dehydrogenase | sxtU, Short-chain alcohol dehydrogenase Contig97277 | >TR33997|c0_g1_i3 Length = 1012 Score = 555 bits (280), Expect = e-157 Identities = 616/728 (84%) Strand = Plus/Plus | Stenotrophomonas maltophilia strain AA1, complete genome; CP018756.1 |
| A. tamutum sxt Putative Protein | Template (PDB Code) | Confidence | % id | CDD Search Output |
|---|---|---|---|---|
| sxtA N-Terminus; TR16074|c0_g1_i1 | Methyltransferase (6B3A) | 100% | 24% | Phosphopantetheine attachment site |
| sxtG Amidinotransferase; TR24523|c0_g4_i1 | Arginine deaminase (1RXX) | 100% | 32% | Aminotransferase superfamily; Arginine deiminase |
| sxtU Short-chain alcohol dehydrogenase; TR101104|c3_g5_i1 | Glucose dehydrogenase (1GEE) | 100% | 46% | Rossmann-fold NAD(P)(+)-binding proteins |
| Transcript Automatic Annotation | Transcript ID | CDD Search Output | Potential Function |
|---|---|---|---|
| aflatoxin b1 aldehyde reductase member 2 | TR29768|c0_g1_i1 | Aldo-keto reductase (AKR) | Metabolites detoxification; carbon metabolism |
| aflatoxin b1 aldehyde reductase member 4-like | TR93512|c0_g1_i1 | Aldo-keto reductase (AKR) | Metabolites detoxification; carbon metabolism |
| gliotoxin biosynthesis protein | TR57386|c0_g1_i1 | GGCT-like domain | Metabolites biosynthesis; glutathione homeostasis |
| “toxicos en levadura” | TR119771|c0_g1_i1 | HRD ubiquitin ligase complex | Ubiquitination; triggered immunity |
| “toxicos en levadura” | TR119771|c0_g2_i1 | HRD ubiquitin ligase complex | Ubiquitination; triggered immunity |
| toxin biosynthesis protein | TR47414|c1_g1_i2 | 2OG-Fe(II) oxygenase superfamily | Toxins/Metabolites production |
| toxin biosynthesis protein | TR120505|c0_g1_i1 | 2OG-Fe(II) oxygenase superfamily | Toxins/Metabolites production |
| toxin biosynthesis protein | TR120505|c0_g1_i2 | 2OG-Fe(II) oxygenase superfamily | Toxins/Metabolites production |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vingiani, G.M.; Štālberga, D.; De Luca, P.; Ianora, A.; De Luca, D.; Lauritano, C. De novo Transcriptome of the Non-saxitoxin Producing Alexandrium tamutum Reveals New Insights on Harmful Dinoflagellates. Mar. Drugs 2020, 18, 386. https://doi.org/10.3390/md18080386
Vingiani GM, Štālberga D, De Luca P, Ianora A, De Luca D, Lauritano C. De novo Transcriptome of the Non-saxitoxin Producing Alexandrium tamutum Reveals New Insights on Harmful Dinoflagellates. Marine Drugs. 2020; 18(8):386. https://doi.org/10.3390/md18080386
Chicago/Turabian StyleVingiani, Giorgio Maria, Dārta Štālberga, Pasquale De Luca, Adrianna Ianora, Daniele De Luca, and Chiara Lauritano. 2020. "De novo Transcriptome of the Non-saxitoxin Producing Alexandrium tamutum Reveals New Insights on Harmful Dinoflagellates" Marine Drugs 18, no. 8: 386. https://doi.org/10.3390/md18080386
APA StyleVingiani, G. M., Štālberga, D., De Luca, P., Ianora, A., De Luca, D., & Lauritano, C. (2020). De novo Transcriptome of the Non-saxitoxin Producing Alexandrium tamutum Reveals New Insights on Harmful Dinoflagellates. Marine Drugs, 18(8), 386. https://doi.org/10.3390/md18080386