The Biotechnological Potential of the Marine Diatom Skeletonema dohrnii to the Elevated Temperature and pCO2
Abstract
1. Introduction
2. Results
2.1. Physiological and Biochemical Response
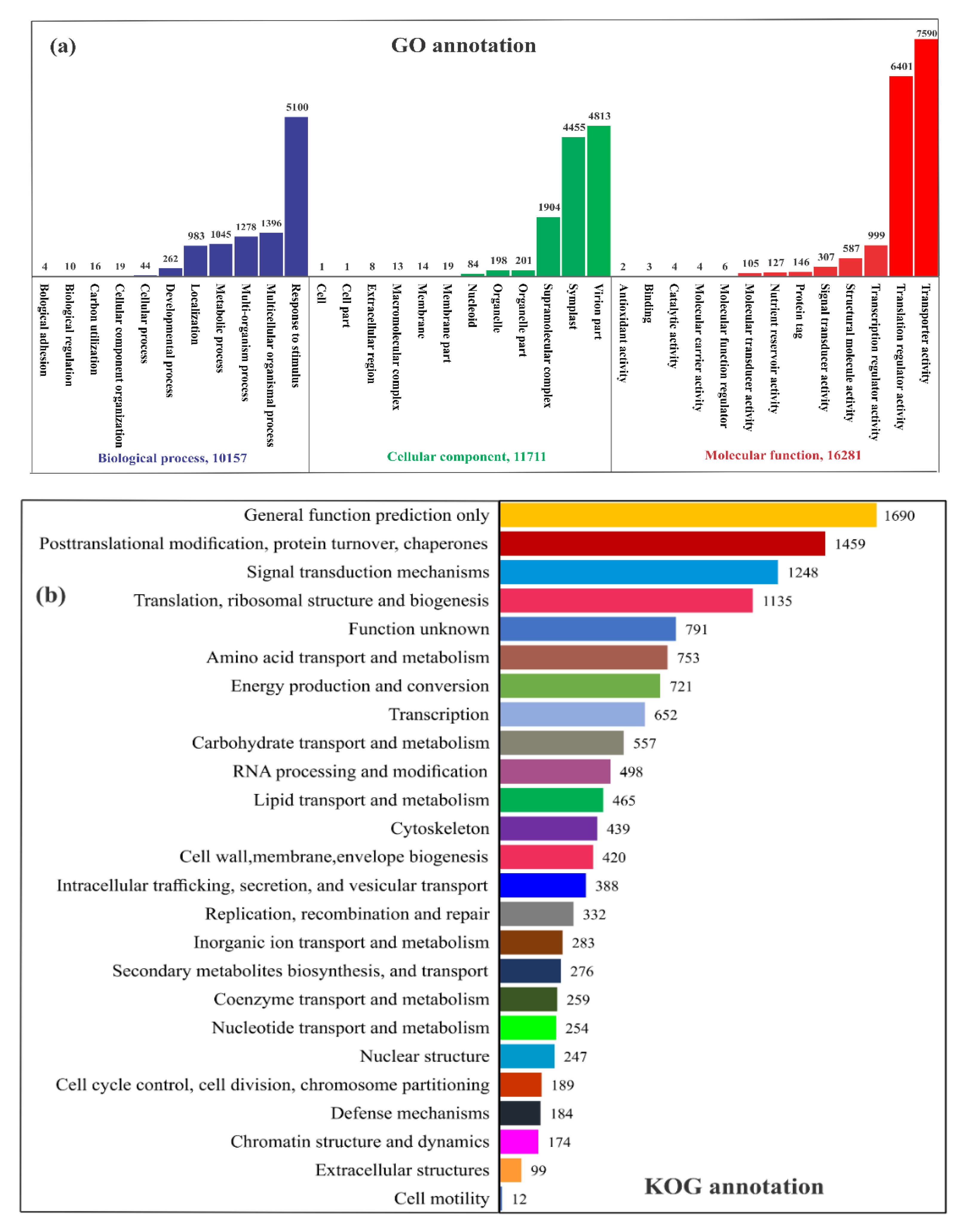
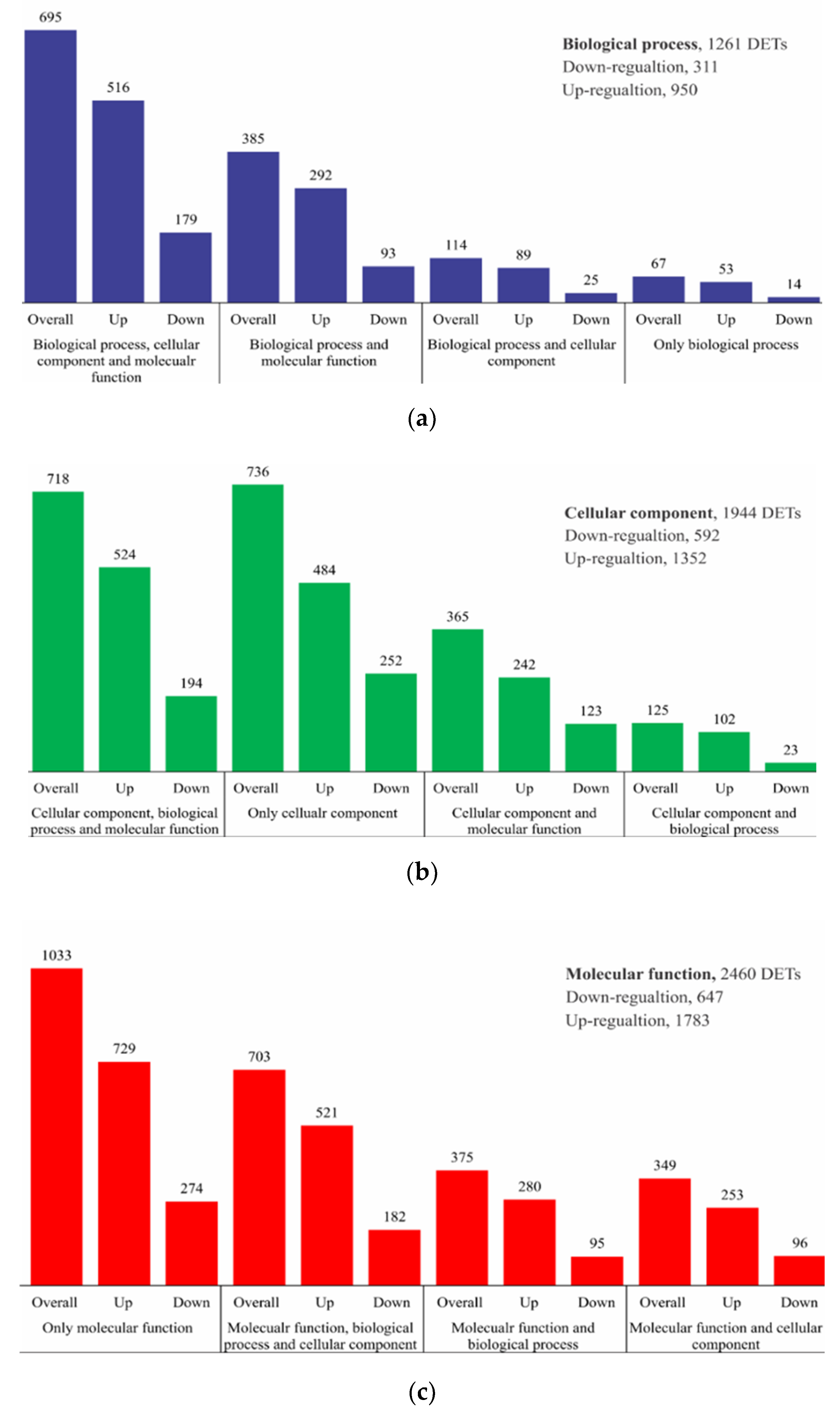
2.2. RNA Sequencing, de Novo Assembly, and Functional Annotation
2.3. Membrane Transporters
2.4. Carotenoid Biosynthesis and Chlorophyll Metabolism
2.5. Phenylalanine, Tyrosine and Tryptophan Biosynthesis
2.6. Phenylpropanoid and Flavonoid Biosynthesis
2.7. Lipid Metabolism and Fatty Acid Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Experimental Setup, Species Collection and Culture Condition
4.2. Seawater Carbonate System and Parameters
4.3. Determination of Growth Rate and Cell Density
4.4. Determination of Pigments
4.5. Determination of Biochemical Analysis
4.6. RNA Isolation
4.7. Library Construction and Sequencing
4.8. Quality Control and de Novo Assembly
4.9. Functional Annotation, DETs Detection and Pathway Enrichment Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Lum, K.K.; Kim, J.; Lei, X.G. Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J. Anim. Sci. Biotechnol. 2013, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wommack, K.E.; Chen, F. Abundance and distribution of Synechococcus spp. and cyanophages in the Chesapeake Bay. Appl. Environ. Microbiol. 2011, 77, 7459–7468. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Carotenuto, Y.; Vitiello, V.; Buttino, I.; Romano, G.; Hwang, J.S.; Ianora, A. Effects of the oxylipin-producing diatom Skeletonema marinoi on gene expression levels of the calanoid copepod Calanus sinicus. Mar. Genom. 2015, 24, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Sabia, A.; Clavero, E.; Pancaldi, S.; Salvado Rovira, J. Effect of different CO2 concentrations on biomass, pigment content, and lipid production of the marine diatom Thalassiosira pseudonana. Appl. Microbiol. Biotechnol. 2018, 102, 1945–1954. [Google Scholar] [CrossRef]
- Ekinci, K.; Erdal, I.; Uysal, Ö.; Uysal, F.Ö.; Tunce, H.; Doğan, A. Anaerobic Digestion of Three Microalgae Biomasses and Assessment of Digestates as Biofertilizer for Plant Growth. Environ. Prog. Sustain. Energy 2018, 38, e13024. [Google Scholar] [CrossRef]
- Rashid, N.; Park, W.K.; Selvaratnam, T. Binary culture of microalgae as an integrated approach for enhanced biomass and metabolites productivity, wastewater treatment, and bioflocculation. Chemosphere 2018, 194, 67–75. [Google Scholar] [CrossRef]
- Mondal, M.; Goswami, S.; Ghosh, A.; Oinam, G.; Tiwari, O.; Das, P.; Gayen, K.; Mandal, M.; Halder, G. Production of biodiesel from microalgae through biological carbon capture: A review. 3 Biotech 2017, 7, 99. [Google Scholar] [CrossRef]
- Trobajo, R.; Ibañez, C.; Clavero, E.; Salvadó, J.; Jørgensen, S.E. Modelling the response of microalgae to CO2 addition. Ecol. Model. 2014, 294, 42–50. [Google Scholar] [CrossRef]
- Nascimento, I.A.; Cabanelas, I.T.D.; Santos, J.N.; Nascimento, M.A.; Sousa, L.; Sansone, G. Biodiesel yields and fuel quality as criteria for algal-feedstock selection: Effects of CO2-supplementation and nutrient levels in cultures. Algal Res. 2015, 8, 53–60. [Google Scholar] [CrossRef]
- Hildebrand, M.; Davis, A.K.; Smith, S.R.; Traller, J.C.; Abbriano, R. The place of diatoms in the biofuels industry. Biofuels 2012, 3, 221–240. [Google Scholar] [CrossRef]
- Joseph, M.M.; Renjith, K.; John, G.; Nair, S.M.; Chandramohanakumar, N. Biodiesel prospective of five diatom strains using growth parameters and fatty acid profiles. Biofuels 2017, 8, 81–89. [Google Scholar] [CrossRef]
- Kurpan Nogueira, D.P.; Silva, A.F.; Araújo, O.Q.F.; Chaloub, R.M. Impact of temperature and light intensity on triacylglycerol accumulation in marine microalgae. Biomass Bioenergy 2015, 72, 280–287. [Google Scholar] [CrossRef]
- Smith, S.R.; Dupont, C.L.; McCarthy, J.K.; Broddrick, J.T.; Oborník, M.; Horák, A.; Füssy, Z.; Cihlář, J.; Kleessen, S.; Zheng, H. Evolution and regulation of nitrogen flux through compartmentalized metabolic networks in a marine diatom. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Mus, F.; Toussaint, J.P.; Cooksey, K.E.; Fields, M.W.; Gerlach, R.; Peyton, B.M.; Carlson, R.P. Physiological and molecular analysis of carbon source supplementation and pH stress-induced lipid accumulation in the marine diatom Phaeodactylum tricornutum. Appl. Microbiol. Biotechnol. 2013, 97, 3625–3642. [Google Scholar] [CrossRef]
- Di Dato, V.; Di Costanzo, F.; Barbarinaldi, R.; Perna, A.; Ianora, A.; Romano, G. Unveiling the presence of biosynthetic pathways for bioactive compounds in the Thalassiosira rotula transcriptome. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Muhseen, Z.T.; Xiong, Q.; Chen, Z.; Ge, F. Proteomics studies on stress responses in diatoms. Proteomics 2015, 15, 3943–3953. [Google Scholar] [CrossRef]
- Heydarizadeh, P.; Boureba, W.; Zahedi, M.; Huang, B.; Moreau, B.; Lukomska, E.; Couzinet-Mossion, A.; Wielgosz-Collin, G.; Martin-Jézéquel, V.; Bougaran, G. Response of CO2-starved diatom Phaeodactylum tricornutum to light intensity transition. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160396. [Google Scholar] [CrossRef]
- Thangaraj, S.; Shang, X.; Sun, J.; Liu, H. Quantitative Proteomic Analysis Reveals Novel Insights into Intracellular Silicate Stress-Responsive Mechanisms in the Diatom Skeletonema dohrnii. Int. J. Mol. Sci. 2019, 20, 2540. [Google Scholar] [CrossRef]
- Yao, G.; Peng, C.; Zhu, Y.; Fan, C.; Jiang, H.; Chen, J.; Cao, Y.; Shi, Q. High-Throughput Identification and Analysis of Novel Conotoxins from Three Vermivorous Cone Snails by Transcriptome Sequencing. Mar. Drugs 2019, 17, 193. [Google Scholar] [CrossRef]
- Wang, J.K.; Seibert, M. Prospects for commercial production of diatoms. Biotechnol. Biofuels 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wichuk, K.; Brynjolfsson, S. Developing diatoms for value-added products: Challenges and opportunities. New Biotechnol. 2015, 32, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Campbell, D.A. Photophysiological responses of marine diatoms to elevated CO2 and decreased pH: A review. Funct. Plant Biol. 2014, 41, 449. [Google Scholar] [CrossRef]
- Gao, K.; Beardall, J.; Häder, D.-P.; Hall-Spencer, J.M.; Gao, G.; Hutchins, D.A. Effects of Ocean Acidification on Marine Photosynthetic Organisms Under the Concurrent Influences of Warming, UV Radiation, and Deoxygenation. Front. Mar. Sci. 2019, 6, 322. [Google Scholar] [CrossRef]
- Sun, J.; Hutchins, D.A.; Feng, Y.; Seubert, E.L.; Caron, D.A.; Fu, F.-X. Effects of changingpCO2and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatomPseudo-nitzschia multiseries. Limnol. Oceanogr. 2011, 56, 829–840. [Google Scholar] [CrossRef]
- Li, G.; Campbell, D.A. Rising CO2 interacts with growth light and growth rate to alter photosystem II photoinactivation of the coastal diatom Thalassiosira pseudonana. PLoS ONE 2013, 8, e55562. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, K.; Riebesell, U. CO2-induced seawater acidification affects physiological performance of the marine diatom Phaeodactylum tricornutum. Biogeosciences 2010, 7, 2915–2923. [Google Scholar] [CrossRef]
- Mejía, L.M.; Isensee, K.; Méndez-Vicente, A.; Pisonero, J.; Shimizu, N.; González, C.; Monteleone, B.; Stoll, H. B content and Si/C ratios from cultured diatoms (Thalassiosira pseudonana and Thalassiosira weissflogii): Relationship to seawater pH and diatom carbon acquisition. Geochim. Cosmochim. Acta 2013, 123, 322–337. [Google Scholar] [CrossRef]
- Torstensson, A.; Chierici, M.; Wulff, A. The influence of increased temperature and carbon dioxide levels on the benthic/sea ice diatom Navicula directa. Polar Biol. 2011, 35, 205–214. [Google Scholar] [CrossRef]
- Crawfurd, K.J.; Raven, J.A.; Wheeler, G.L.; Baxter, E.J.; Joint, I. The response of Thalassiosira pseudonana to long-term exposure to increased CO2 and decreased pH. PLoS ONE 2011, 6, e26695. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Gao, K. Physiological responses of the marine diatom Thalassiosira pseudonana to increased pCO2 and seawater acidity. Mar. Environ. Res. 2012, 79, 142–151. [Google Scholar] [CrossRef]
- Jasinski, M.; Ducos, E.; Martinoia, E.; Boutry, M. The ATP-binding cassette transporters: Structure, function, and gene family comparison between rice and Arabidopsis. Plant Physiol. 2003, 131, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Kurelec, B. The multixenobiotic resistance mechanism in aquatic organisms. Crit. Rev. Toxicol. 1992, 22, 23–43. [Google Scholar] [CrossRef]
- Scherer, C.; Wiltshire, K.; Bickmeyer, U. Inhibition of multidrug resistance transporters in the diatom Thalassiosira rotula facilitates dye staining. Plant Physiol. Biochem. 2008, 46, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Schafer, L.; Sandmann, M.; Woitsch, S.; Sandmann, G. Coordinate up-regulation of carotenoid biosynthesis as a response to light stress in Synechococcus PCC7942. Plant Cell Environ. 2006, 29, 1349–1356. [Google Scholar] [CrossRef]
- Grünewald, K.; Eckert, M.; Hirschberg, J.; Hagen, C. Phytoene desaturase is localized exclusively in the chloroplast and up-regulated at the mRNA level during accumulation of secondary carotenoids in Haematococcus pluvialis (Volvocales, Chlorophyceae). Plant Physiol. 2000, 122, 1261–1268. [Google Scholar] [CrossRef]
- Jakob, T.; Goss, R.; Wilhelm, C. Unusual pH-dependence of diadinoxanthin de-epoxidase activation causes chlororespiratory induced accumulation of diatoxanthin in the diatom Phaeodactylum tricornutum. J. Plant Physiol. 2001, 158, 383–390. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef]
- Nishino, H.; Murakoshi, M.; Ii, T.; Takemura, M.; Kuchide, M.; Kanazawa, M.; Mou, X.Y.; Wada, S.; Masuda, M.; Ohsaka, Y. Carotenoids in cancer chemoprevention. Cancer Metastasis Rev. 2002, 21, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Dyhrman, S.T.; Jenkins, B.D.; Rynearson, T.A.; Saito, M.A.; Mercier, M.L.; Alexander, H.; Whitney, L.P.; Drzewianowski, A.; Bulygin, V.V.; Bertrand, E.M. The transcriptome and proteome of the diatom Thalassiosira pseudonana reveal a diverse phosphorus stress response. PLoS ONE 2012, 7, e33768. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Li, Y.Y.; Zhang, H.; Liu, J.L.; Xie, Z.X.; Lin, L.; Wang, D.Z. Quantitative Proteomics Reveals Common and Specific Responses of a Marine Diatom Thalassiosira pseudonana to Different Macronutrient Deficiencies. Front. Microbiol. 2018, 9, 2761. [Google Scholar] [CrossRef]
- Alipanah, L.; Rohloff, J.; Winge, P.; Bones, A.M.; Brembu, T. Whole-cell response to nitrogen deprivation in the diatom Phaeodactylum tricornutum. J. Exp. Bot. 2015, 66, 6281–6296. [Google Scholar] [CrossRef] [PubMed]
- Bromke, M.A. Amino Acid biosynthesis pathways in diatoms. Metabolites 2013, 3, 294–311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, F.; Xu, H.-B.; Luo, C.-X.; Wu, H.-Y.; Zhu, M.-M.; Lu, W.; Ji, X.; Zhou, Q.-G.; Zhu, D.-Y. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat. Med. 2010, 16, 1439. [Google Scholar] [CrossRef]
- Yin, W.B.; Amaike, S.; Wohlbach, D.J.; Gasch, A.P.; Chiang, Y.M.; Wang, C.C.; Bok, J.W.; Rohlfs, M.; Keller, N.P. An Aspergillus nidulans bZIP response pathway hardwired for defensive secondary metabolism operates through aflR. Mol. Microbiol. 2012, 83, 1024–1034. [Google Scholar] [CrossRef]
- De la Torre, F.; Canas, R.A.; Pascual, M.B.; Avila, C.; Canovas, F.M. Plastidic aspartate aminotransferases and the biosynthesis of essential amino acids in plants. J. Exp. Bot. 2014, 65, 5527–5534. [Google Scholar] [CrossRef]
- Pyne, M.E.; Narcross, L.; Martin, V.J.J. Engineering Plant Secondary Metabolism in Microbial Systems. Plant Physiol. 2019, 179, 844–861. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Ogawa, M.; Kuwahara, A.; Hanada, A.; Kamiya, Y.; Yamaguchi, S. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell 2004, 16, 367–378. [Google Scholar] [CrossRef]
- Bermudez, R.; Feng, Y.; Roleda, M.Y.; Tatters, A.O.; Hutchins, D.A.; Larsen, T.; Boyd, P.W.; Hurd, C.L.; Riebesell, U.; Winder, M. Long-Term Conditioning to Elevated pCO2 and Warming Influences the Fatty and Amino Acid Composition of the Diatom Cylindrotheca fusiformis. PLoS ONE 2015, 10, e0123945. [Google Scholar] [CrossRef]
- James, C.; Al-Hinty, S.; Salman, A. Growth and ω3 fatty acid and amino acid composition of microalgae under different temperature regimes. Aquaculture 1989, 77, 337–351. [Google Scholar] [CrossRef]
- Taucher, J.; Jones, J.; James, A.; Brzezinski, M.; Carlson, C.; Riebesell, U.; Passow, U. Combined effects of CO2 and temperature on carbon uptake and partitioning by the marine diatoms T halassiosira weissflogii and D actyliosolen fragilissimus. Limnol. Oceanogr. 2015, 60, 901–919. [Google Scholar] [CrossRef]
- Mouradov, A.; Spangenberg, G. Flavonoids: A metabolic network mediating plants adaptation to their real estate. Front. Plant Sci. 2014, 5, 620. [Google Scholar] [CrossRef]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.-P.; Matros, A.; Peterek, S.; Schijlen, E.G.; Hall, R.D.; Bovy, A.G.; Luo, J. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, Y.; Wang, B.; Wu, T. Bivalent RNA interference to increase isoflavone biosynthesis in soybean (Glycine max). Braz. Arch. Biol. Technol. 2014, 57, 163–170. [Google Scholar] [CrossRef]
- Wang, X.W.; Liang, J.R.; Luo, C.S.; Chen, C.P.; Gao, Y.H. Biomass, total lipid production, and fatty acid composition of the marine diatom Chaetoceros muelleri in response to different CO2 levels. Bioresour. Technol. 2014, 161, 124–130. [Google Scholar] [CrossRef]
- Abida, H.; Dolch, L.J.; Mei, C.; Villanova, V.; Conte, M.; Block, M.A.; Finazzi, G.; Bastien, O.; Tirichine, L.; Bowler, C.; et al. Membrane glycerolipid remodeling triggered by nitrogen and phosphorus starvation in Phaeodactylum tricornutum. Plant Physiol. 2015, 167, 118–136. [Google Scholar] [CrossRef]
- Kroth, P.G.; Chiovitti, A.; Gruber, A.; Martin-Jezequel, V.; Mock, T.; Parker, M.S.; Stanley, M.S.; Kaplan, A.; Caron, L.; Weber, T.; et al. A model for carbohydrate metabolism in the diatom Phaeodactylum tricornutum deduced from comparative whole genome analysis. PLoS ONE 2008, 3, e1426. [Google Scholar] [CrossRef]
- Zulu, N.N.; Zienkiewicz, K.; Vollheyde, K.; Feussner, I. Current trends to comprehend lipid metabolism in diatoms. Prog. Lipid Res. 2018, 70, 1–16. [Google Scholar] [CrossRef]
- Wu, Y.; Campbell, D.A.; Irwin, A.J.; Suggett, D.J.; Finkel, Z.V. Ocean acidification enhances the growth rate of larger diatoms. Limnol. Oceanogr. 2014, 59, 1027–1034. [Google Scholar] [CrossRef]
- Tsuzuki, M.; Ohnuma, E.; Sato, N.; Takaku, T.; Kawaguchi, A. Effects of CO2 concentration during growth on fatty acid composition in microalgae. Plant Physiol. 1990, 93, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Sato, N. Modulation of lipid and fatty acid content by carbon dioxide in Chlamydomonas reinhardtii. Plant Sci. 1989, 61, 17–21. [Google Scholar] [CrossRef]
- Suffrian, K.; Schulz, K.G.; Gutowska, M.; Riebesell, U.; Bleich, M. Cellular pH measurements in Emiliania huxleyi reveal pronounced membrane proton permeability. New Phytol. 2011, 190, 595–608. [Google Scholar] [CrossRef]
- Rossoll, D.; Bermúdez, R.; Hauss, H.; Schulz, K.G.; Riebesell, U.; Sommer, U.; Winder, M. Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS ONE 2012, 7, 4. [Google Scholar] [CrossRef]
- Rousch, J.M.; Bingham, S.E.; Sommerfeld, M.R. Changes in fatty acid profiles of thermo-intolerant and thermo-tolerant marine diatoms during temperature stress. J. Exp. Mar. Biol. Ecol. 2003, 295, 145–156. [Google Scholar] [CrossRef]
- Van Wagenen, J.; Miller, T.W.; Hobbs, S.; Hook, P.; Crowe, B.; Huesemann, M. Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 2012, 5, 731–740. [Google Scholar] [CrossRef]
- Sayanova, O.; Mimouni, V.; Ulmann, L.; Morant-Manceau, A.; Pasquet, V.; Schoefs, B.; Napier, J.A. Modulation of lipid biosynthesis by stress in diatoms. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160407. [Google Scholar] [CrossRef]
- Peng, K.T.; Zheng, C.N.; Xue, J.; Chen, X.Y.; Yang, W.D.; Liu, J.S.; Bai, W.; Li, H.Y. Delta 5 fatty acid desaturase upregulates the synthesis of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum. J. Agric. Food Chem. 2014, 62, 8773–8776. [Google Scholar] [CrossRef]
- Cook, O.; Hildebrand, M. Enhancing LC-PUFA production in Thalassiosira pseudonana by overexpressing the endogenous fatty acid elongase genes. J. Appl. Phycol. 2015, 28, 897–905. [Google Scholar] [CrossRef]
- Ma, Y.-H.; Wang, X.; Niu, Y.-F.; Yang, Z.-K.; Zhang, M.-H.; Wang, Z.-M.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Antisense knockdown of pyruvate dehydrogenase kinase promotes the neutral lipid accumulation in the diatom Phaeodactylum tricornutum. Microb. Cell Factories 2014, 13, 100. [Google Scholar] [CrossRef]
- Trentacoste, E.M.; Shrestha, R.P.; Smith, S.R.; Glé, C.; Hartmann, A.C.; Hildebrand, M.; Gerwick, W.H. Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19748–19753. [Google Scholar] [CrossRef]
- Sunda, W.G.; Price, N.M.; Morel, F.M. Trace metal ion buffers and their use in culture studies. Algal Cult. Tech. 2005, 4, 35–63. [Google Scholar]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Pierrot, D.; Lewis, E.; Wallace, D. CO2SYS DOS Program Developed for CO2 System Calculations; ORNL/CDIAC-105; Carbon Dioxide Information Analysis Center; Oak Ridge National Laboratory; US Department of Energy: Oak Ridge, TN, USA, 2006.
- Gao, G.; Gao, K.; Giordano, M. Responses to solar radiation of the diatom Skeletonema coastatum (Bacillariphyceae) grown at different Zn(2+) concentrations (1). J. Phycol. 2009, 45, 119–129. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Deriaz, R. Routine analysis of carbohydrates and lignin in herbage. J. Sci. Food Agric. 1961, 12, 152–160. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Waterhouse, R.M.; Seppey, M.; Simao, F.A.; Manni, M.; Ioannidis, P.; Klioutchnikov, G.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO Applications from Quality Assessments to Gene Prediction and Phylogenomics. Mol. Biol. Evol. 2018, 35, 543–548. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef]
- Pruitt, K.D.; Tatusova, T.; Maglott, D.R. NCBI reference sequences (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007, 35, D61–D65. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Leng, N.; Dawson, J.A.; Thomson, J.A.; Ruotti, V.; Rissman, A.I.; Smits, B.M.; Haag, J.D.; Gould, M.N.; Stewart, R.M.; Kendziorski, C. EBSeq: An empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 2013, 29, 1035–1043. [Google Scholar] [CrossRef]
- Tarazona, S.; Garcia-Alcalde, F.; Dopazo, J.; Ferrer, A.; Conesa, A. Differential expression in RNA-seq: A matter of depth. Genome Res. 2011, 21, 2213–2223. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Audic, S.; Claverie, J.-M. The significance of digital gene expression profiles. Genome Res. 1997, 7, 986–995. [Google Scholar] [CrossRef]
| LC (21 °C 400 ppm) | HC (25 °C 1000 ppm) | |
|---|---|---|
| Growth rate (day−1) | 0.76 ± 0.05 * | 1.23 ± 0.15 |
| Cell density (104 cells mL−1) | 233.3 ± 9.07 * | 297.3 ± 9.71 |
| Chlorophyll a (pg cell−1) | 0.20 ± 0.01 * | 0.28 ± 0.02 |
| Carotenoid (pg cell−1) | 0.05 ± 0.07 * | 0.08 ± 0.09 |
| Protein content (pg cell−1) | 3.1 ± 0.2 * | 3.7 ± 0.1 |
| Carbohydrate (pg cell−1) | 0.07 ± 0.5 | 1.4 ± 0.9 |
| Lipid content (pg cell−1) | 13.33 ± 1.5 * | 19.27 ± 1.5 |
| Lipid productivity (mg L−1) | 12.51 ± 0.5 * | 17.35 ± 0.6 |
| pHNBS | DIC (µmol Kg−1) | HCO3− (µmol Kg−1) | CO3−2 (µmol Kg−1) | CO2 (µmol Kg−1) | TA (µmol Kg−1) | |
|---|---|---|---|---|---|---|
| LC (21 °C 400 ppm) | 8.12 ± 0.02 | 2103 ± 11 | 1894 ± 14 | 219 ± 6.0 | 13.6 ± 0.2 | 2355 ± 16 |
| HC (25 °C 1000 ppm) | 7.82 ± 0.01 | 2248 ± 17 | 2216 ± 22 | 126 ± 3.1 | 31.4 ± 1.0 | 2347 ± 14 |
| Functional Annotation | NR Homology | |||
|---|---|---|---|---|
| Number | Percentage | Species | Percentage | |
| Total | 32,884 | 100 | Thalassiosira pseudonana CCMP 1335 | 36.91 |
| NR | 22,261 | 67.70 | Thalassiosira oceanica | 24.90 |
| NT | 2960 | 9.00 | Fragilariopsis cylindrus CCMP 1102 | 2.90 |
| Swiss-Prot | 11,573 | 35.19 | Fistulifera solaris | 2.80 |
| KEGG | 13,654 | 41.52 | Ricinus communis | 1.87 |
| KOG | 13,525 | 41.13 | Others | 30.62 |
| Pfam | 21,179 | 64.41 | ||
| GO | 13,937 | 42.38 | ||
| Intersection | 1487 | 4.52 | ||
| Overall | 25,332 | 77.03 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangaraj, S.; Sun, J. The Biotechnological Potential of the Marine Diatom Skeletonema dohrnii to the Elevated Temperature and pCO2. Mar. Drugs 2020, 18, 259. https://doi.org/10.3390/md18050259
Thangaraj S, Sun J. The Biotechnological Potential of the Marine Diatom Skeletonema dohrnii to the Elevated Temperature and pCO2. Marine Drugs. 2020; 18(5):259. https://doi.org/10.3390/md18050259
Chicago/Turabian StyleThangaraj, Satheeswaran, and Jun Sun. 2020. "The Biotechnological Potential of the Marine Diatom Skeletonema dohrnii to the Elevated Temperature and pCO2" Marine Drugs 18, no. 5: 259. https://doi.org/10.3390/md18050259
APA StyleThangaraj, S., & Sun, J. (2020). The Biotechnological Potential of the Marine Diatom Skeletonema dohrnii to the Elevated Temperature and pCO2. Marine Drugs, 18(5), 259. https://doi.org/10.3390/md18050259







