Cladodionen Is a Potential Quorum Sensing Inhibitor Against Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results
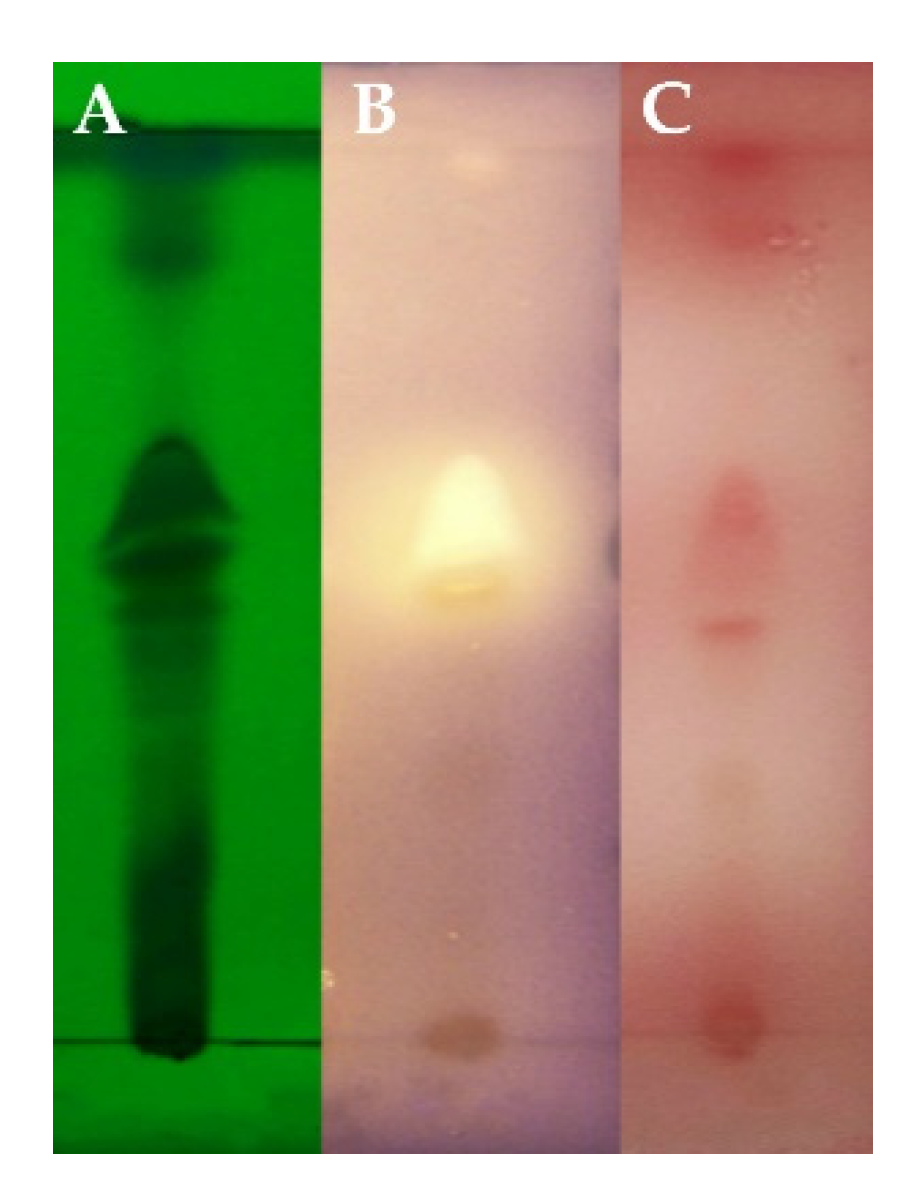
2.1. Screening of Fungi and Identification of Active Compound
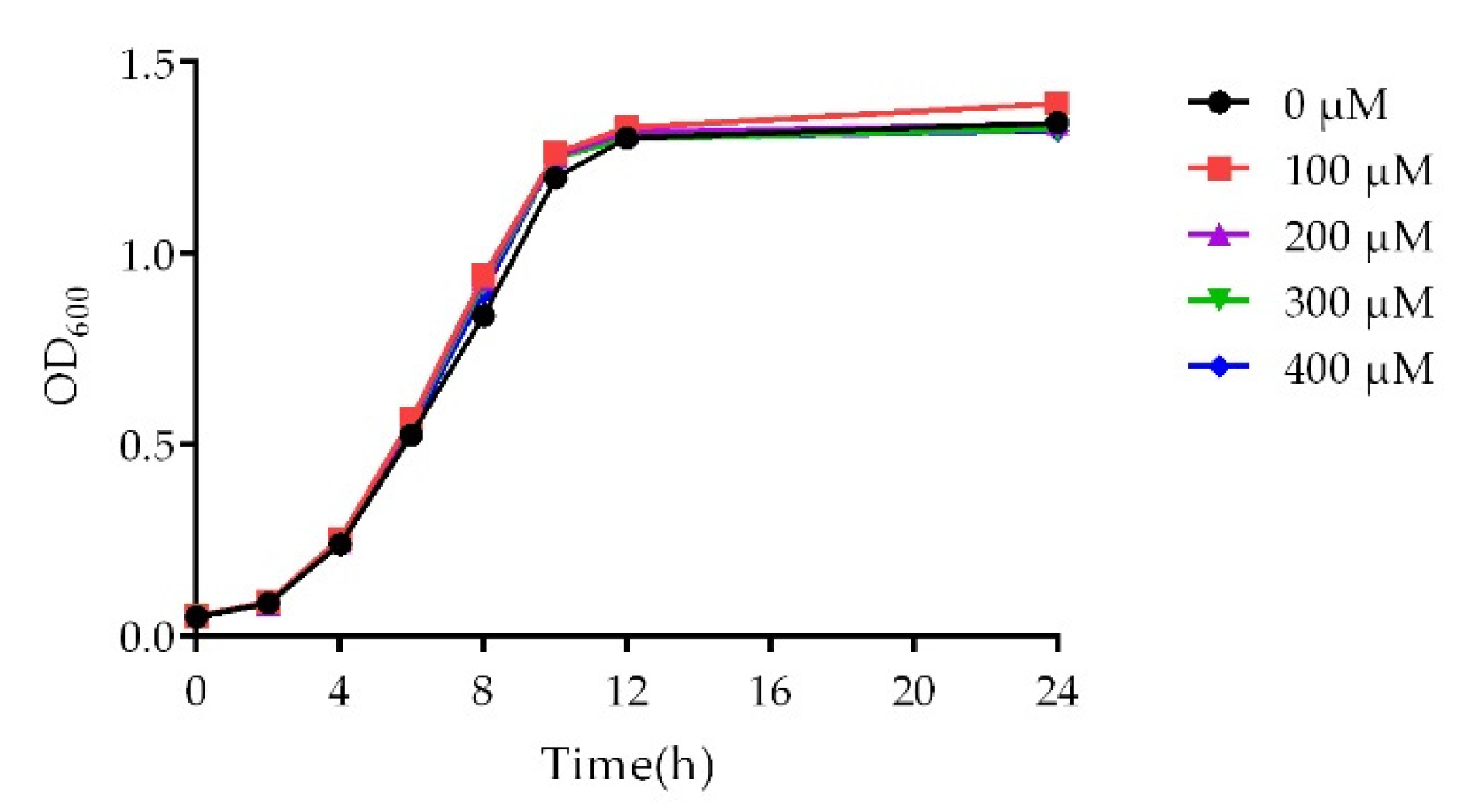
2.2. Growth Curve Analysis
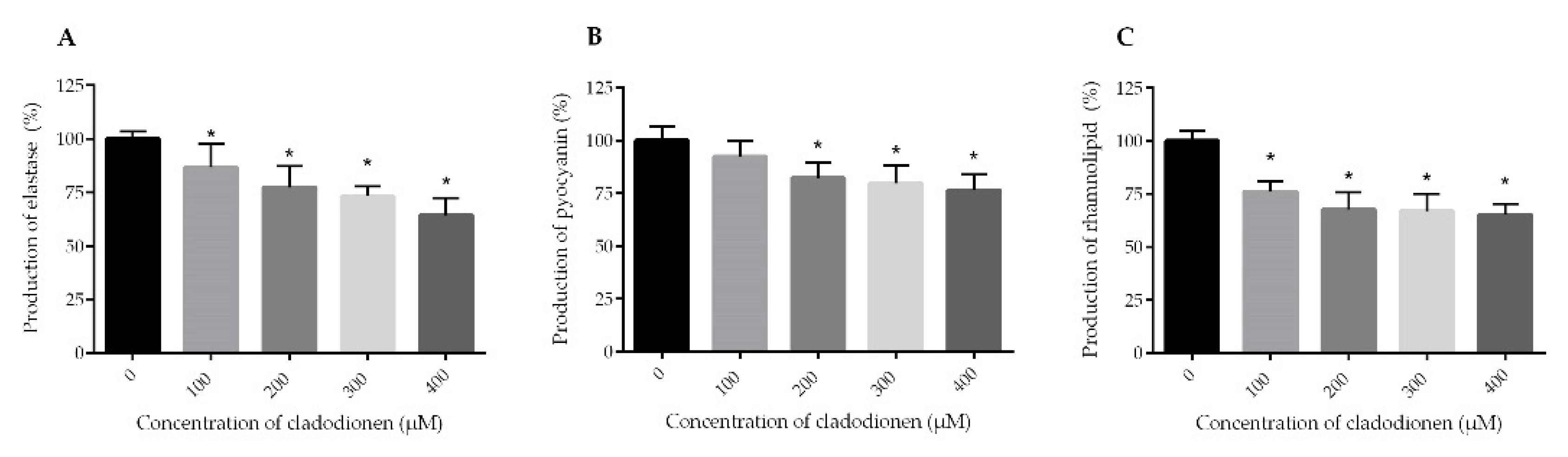
2.3. Effects of Cladodionen on the Production of Virulence Factors
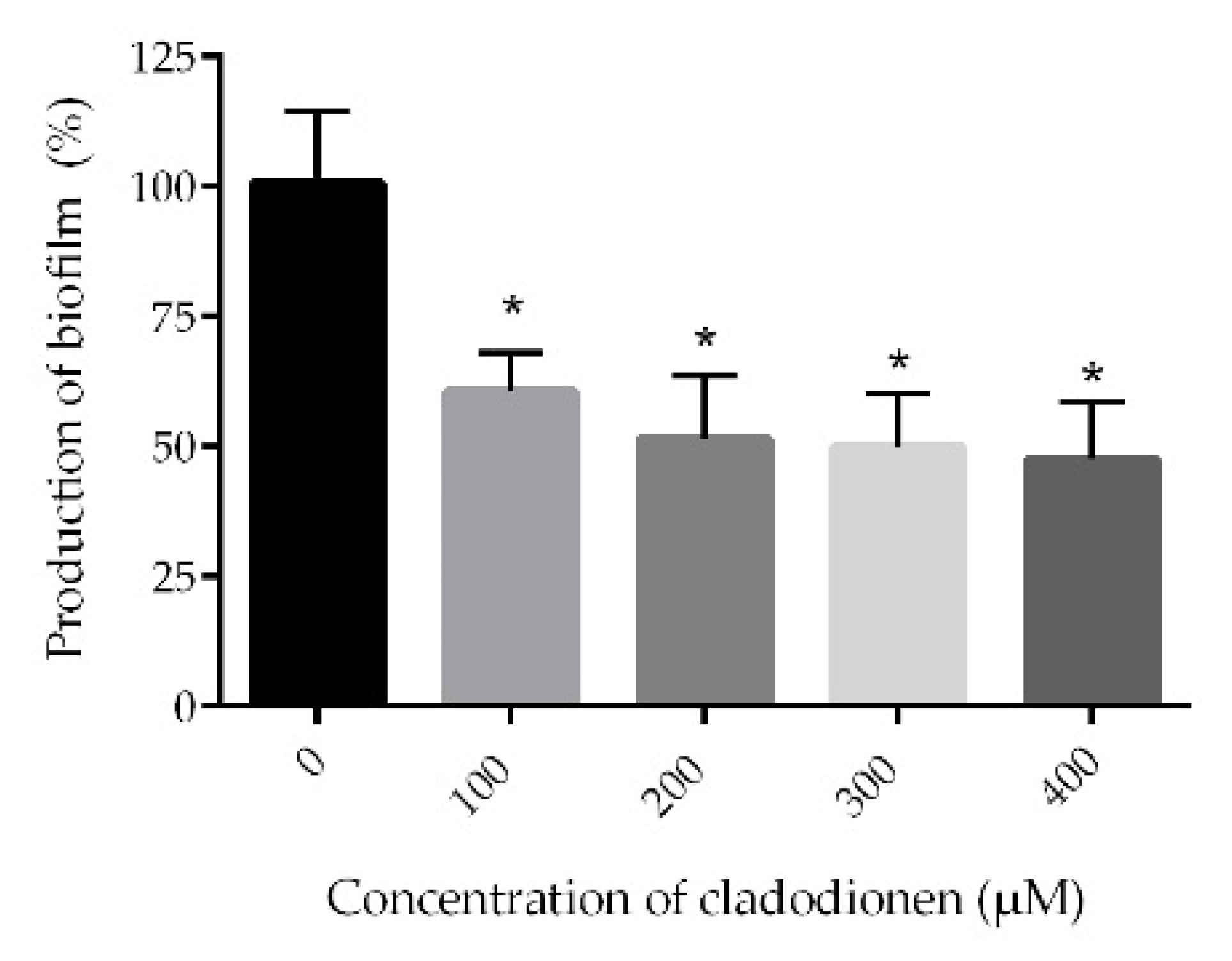
2.4. Biofilm Assay
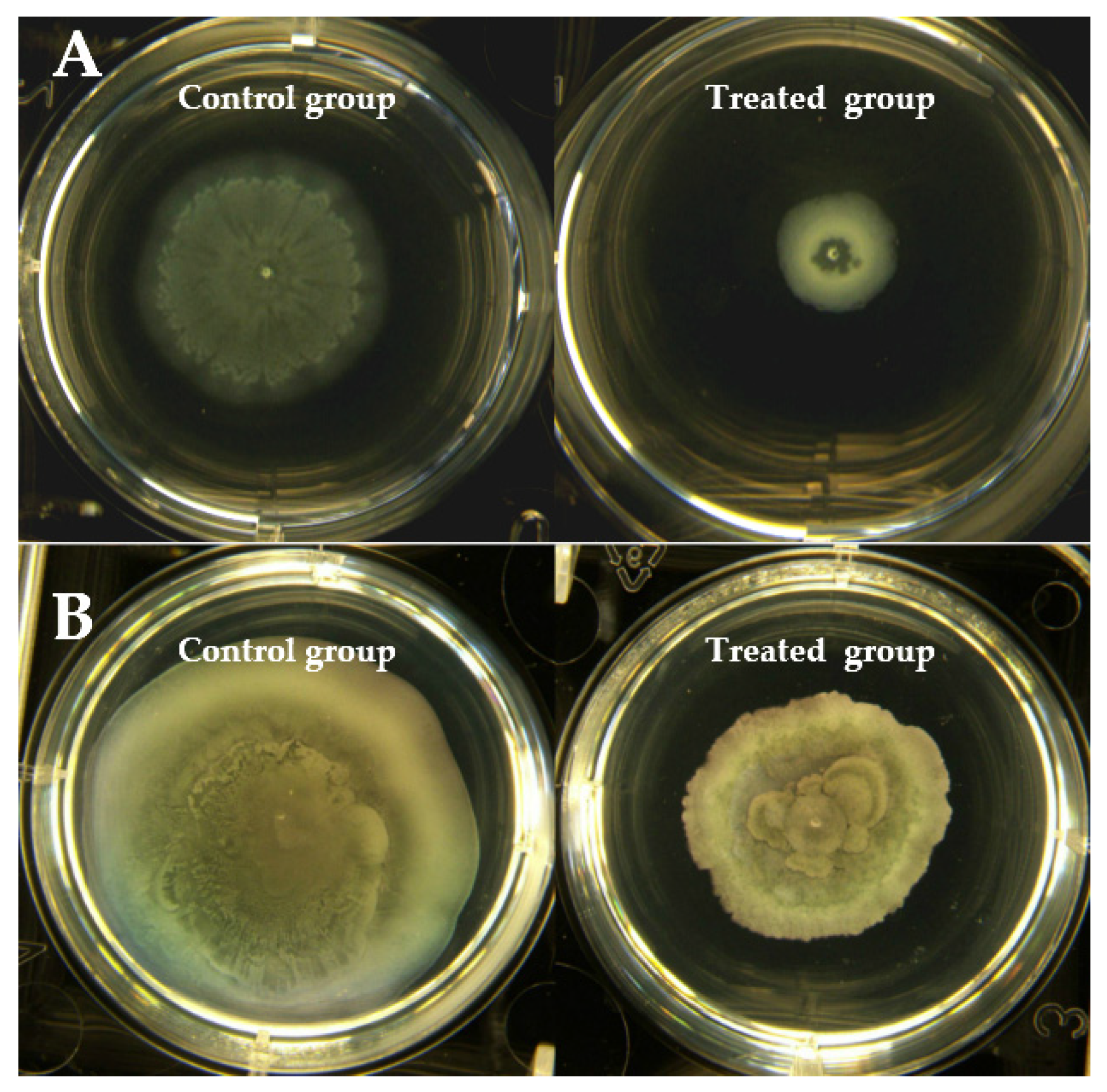
2.5. Swarming and Swimming Motility
2.6. Real-Time RT-PCR
2.7. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Isolation of Marine Fungi and Preparation of the Crude Extracts
4.3. Screening for QSIs
4.4. Species Identification of Selected Fungus
4.5. Purification of QSIs
4.6. Growth Curve Assay
4.7. Effects of Cladodionen on the Production of Virulence Factors
4.7.1. Elastase Activity Assay
4.7.2. Pyocyanin Assay
4.7.3. Rhamnolipid Assay
4.8. Biofilm Assay
4.9. Swimming and Swarming Motility Assay
4.10. Real-Time RT-PCR
4.11. Molecular Docking Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sadikot, R.T.; Blackwell, T.S.; Christman, J.W.; Prince, A.S. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 1209–1223. [Google Scholar] [CrossRef]
- Singh, V.K.; Mishra, A.; Jha, B. Anti-quorum Sensing and anti-biofilm activity of Delftia tsuruhatensis extract by attenuating the quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2017, 7, E337. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [PubMed]
- McKnight, S.L.; Iglewski, B.H.; Pesci, E.C. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 2000, 182, 2702–2708. [Google Scholar] [CrossRef]
- Lee, J.; Wu, J.; Deng, Y.; Wang, J.; Wang, C.; Wang, J.; Chang, C.; Dong, Y.; Williams, P.; Zhang, L.H. A cell-cell communication signal integrates quorum sensing and stress response. Nat. Chem. Biol. 2013, 9, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.P.; Pesci, E.C.; Iglewski, B.H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1997, 179, 5756–5767. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Deziel, E.; He, J.; Lepine, F.; Lesic, B.; Castonguay, M.H.; Milot, S.; Tampakaki, A.P.; Stachel, S.E.; Rahme, L.G. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol. Microbiol. 2006, 62, 1689–1699. [Google Scholar] [CrossRef]
- Shih, P.C.; Huang, C.T. Effects of quorum-sensing deficiency on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. J. Antimicrob. Chemother. 2002, 49, 309–314. [Google Scholar] [CrossRef]
- Davey, M.E.; Caiazza, N.C.; O’Toole, G.A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003, 185, 1027–1036. [Google Scholar] [CrossRef]
- Mukherjee, S.; Moustafa, D.A.; Stergioula, V.; Smith, C.D.; Goldberg, J.B.; Bassler, B.L. The PqsE and RhlR proteins are an autoinducer synthase-receptor pair that control virulence and biofilm development in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2018, 115, 9411–9418. [Google Scholar] [CrossRef]
- Bottomley, M.J.; Muraglia, E.; Bazzo, R.; Carfi, A. Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer. J. Biol. Chem. 2007, 282, 13592–13600. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Dai, X.; Sun, J.; Bu, X.; Weng, C.; Li, H.; Zhu, H. A diketopiperazine factor from Rheinheimera aquimaris QSI02 exhibits anti-quorum sensing activity. Sci. Rep. 2016, 6, E39637. [Google Scholar] [CrossRef] [PubMed]
- Ilangovan, A.; Fletcher, M.; Rampioni, G.; Pustelny, C.; Rumbaugh, K.; Heeb, S.; Camara, M.; Truman, A.; Chhabra, S.R.; Emsley, J.; et al. Structural basis for native agonist and synthetic inhibitor recognition by the Pseudomonas aeruginosa quorum sensing regulator PqsR (MvfR). PLoS Pathog. 2013, 9, E1003508. [Google Scholar] [CrossRef]
- Kitao, T.; Lepine, F.; Babloudi, S.; Walte, F.; Steinbacher, S.; Maskos, K.; Blaesse, M.; Negri, M.; Pucci, M.; Zahler, B.; et al. Molecular insights into function and competitive inhibition of Pseudomonas aeruginosa multiple virulence factor regulator. mBio 2018, 9, e02158-17. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ding, T.; Li, J. Medicinal purposes: Bioactive metabolites from marine-derived organisms. Mini-Rev. Med. Chem. 2019, 19, 138–164. [Google Scholar] [CrossRef]
- Imhoff, J.F. Natural products from marine fungi—Still an underrepresented resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef]
- Zhu, G.; Kong, F.; Wang, Y.; Fu, P.; Zhu, W. Cladodionen, a cytotoxic hybrid polyketide from the marine-derived Cladosporium sp. OUCMDZ-1635. Mar. Drugs 2018, 16, 71. [Google Scholar] [CrossRef]
- Liang, X.; Huang, Z.H.; Ma, X.; Qi, S.H. Unstable tetramic acid derivatives from the deep-sea-derived fungus Cladosporium sphaerospermum EIODSF 008. Mar. Drugs 2018, 16, 448. [Google Scholar] [CrossRef]
- Rischer, M.; Lee, S.; Eom, H.; Park, H.; Vollmers, J.; Kaster, A.K.; Shin, Y.-H.; Oh, D.-C.; Kim, K.H.; Beemelmanns, C. Spirocyclic cladosporicin A and cladosporiumins I and J from a Hydractinia-associated Cladosporium sphaerospermum SW67. Org. Chem. Front. 2018, 6, 1084–1093. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Villeret, B.; Bastaert, F.; Kheir, S.; Hatton, A.; Cazes, A.; Xing, Z.; Sermet-Gaudelus, I.; Garcia-Verdugo, I.; Edelman, A.; et al. Pseudomonas aeruginosa LasB protease impairs innate immunity in mice and humans by targeting a lung epithelial cystic fibrosis transmembrane regulator-IL-6-antimicrobial-repair pathway. Thorax 2018, 73, 49–61. [Google Scholar] [CrossRef]
- Jensen, P.O.; Bjarnsholt, T.; Phipps, R.; Rasmussen, T.B.; Calum, H.; Christoffersen, L.; Moser, C.; Williams, P.; Pressler, T.; Givskov, M.; et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology (Reading, England) 2007, 153, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Lewenza, S.; Marr, A.K.; Hancock, R.E. Identification of genes involved in swarming motility using a Pseudomonas aeruginosa PAO1 mini-Tn5-lux mutant library. J. Bacteriol. 2007, 189, 2164–2169. [Google Scholar] [CrossRef] [PubMed]
- Kohler, T.; Curty, L.K.; Barja, F.; van Delden, C.; Pechere, J.C. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 2000, 182, 5990–5996. [Google Scholar] [CrossRef] [PubMed]
- Steindler, L.; Bertani, I.; De Sordi, L.; Schwager, S.; Eberl, L.; Venturi, V. LasI/R and RhlI/R quorum sensing in a strain of Pseudomonas aeruginosa beneficial to plants. Appl. Environ. Microbiol. 2009, 75, 5131–5140. [Google Scholar] [CrossRef] [PubMed]
- Schobert, R.; Schlenk, A. Tetramic and tetronic acids: An update on new derivatives and biological aspects. Bioorg. Med. Chem. 2008, 16, 4203–4221. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Bayer, M.; Gunasekera, S.; Proksch, P.; Paul, V.J. Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling 2011, 27, 893–905. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, M.; Zhu, X.; Yu, W.; Gong, Q. Equisetin as potential quorum sensing inhibitor of Pseudomonas aeruginosa. Biotechnol. Lett. 2018, 40, 865–870. [Google Scholar] [CrossRef]
- Wang, L.; Zou, S.; Yin, S.; Liu, H.; Yu, W.; Gong, Q. Construction of an effective screening system for detection of Pseudomonas aeruginosa quorum sensing inhibitors and its application in bioautographic thin-layer chromatography. Biotechnol. Lett. 2011, 33, 1381–1387. [Google Scholar] [CrossRef]
- Hossain, M.A.; Lee, S.J.; Park, N.H.; Mechesso, A.F.; Birhanu, B.T.; Kang, J.; Reza, M.A.; Suh, J.W.; Park, S.C. Impact of phenolic compounds in the acyl homoserine lactone-mediated quorum sensing regulatory pathways. Sci. Rep. 2017, 7, E10618. [Google Scholar] [CrossRef]
- Fu, T.K.; Ng, S.K.; Chen, Y.E.; Lee, Y.C.; Demeter, F.; Herczeg, M.; Borbás, A.; Chiu, C.H.; Lan, C.Y.; Chen, C.L.; et al. Rhamnose binding protein as an anti-bacterial agent—Targeting biofilm of Pseudomonas aeruginosa. Mar. Drugs 2019, 17, 355. [Google Scholar] [CrossRef]
- Gutierrez, M.; Choi, M.H.; Tian, B.; Xu, J.; Rho, J.K.; Kim, M.O.; Cho, Y.H.; Yoon, S.C. Simultaneous inhibition of rhamnolipid and polyhydroxyalkanoic acid synthesis and biofilm formation in Pseudomonas aeruginosa by 2-bromoalkanoic acids: Effect of inhibitor alkyl-chain-length. PLoS ONE 2013, 8, e73986. [Google Scholar] [CrossRef] [PubMed]
- Golic, A.; Vaneechoutte, M.; Nemec, A.; Viale, A.M.; Actis, L.A.; Mussi, M.A. Staring at the cold sun: Blue light regulation is distributed within the genus Acinetobacter. PLoS ONE 2013, 8, e55059. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Sun, S.; Li, L.; Dai, X.; Li, H.; He, Q.; Zhu, H. Tyrosol from marine Fungi, a novel quorum sensing inhibitor against Chromobacterium violaceum and Pseudomonas aeruginosa. Bioorg. Chem. 2019, 91, E103140. [Google Scholar] [CrossRef] [PubMed]

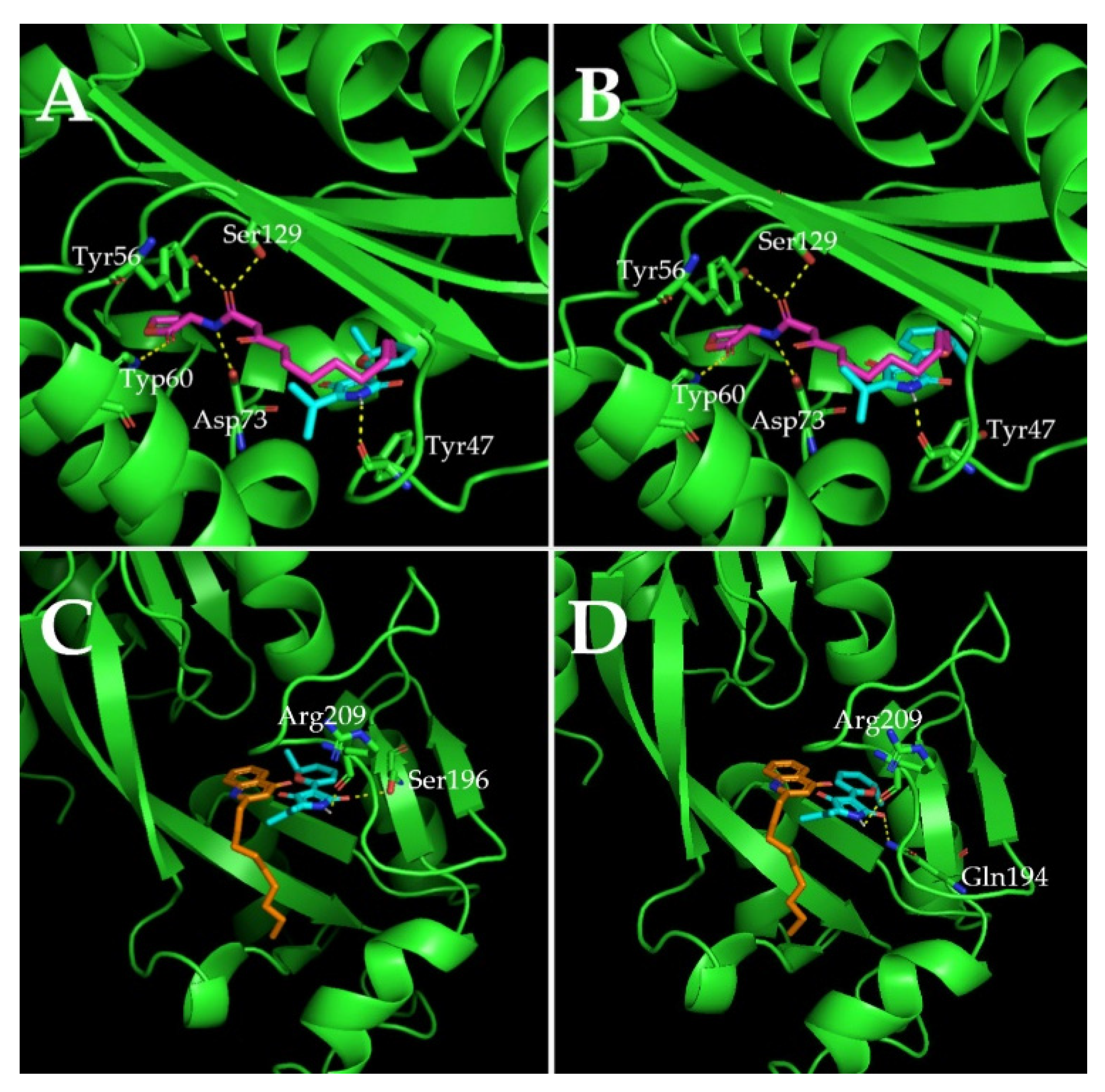
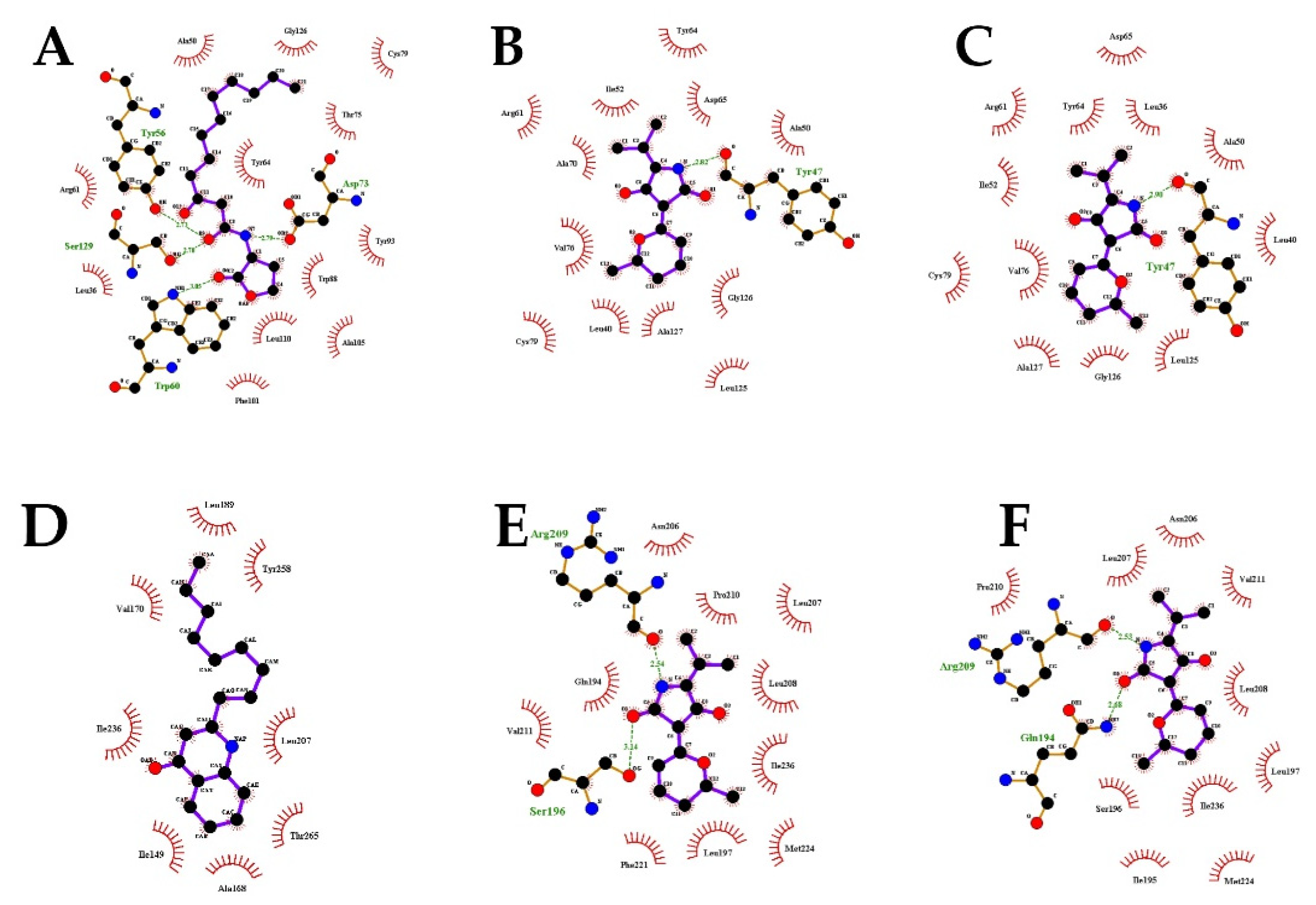
| Molecule | Docking Energy (kcal/mol) | Hydrogen Bonding Interactions | Key Hydrophobic Interactions |
|---|---|---|---|
| 3-oxo-C12-HSL | −5.47 | Ser129, Tyr56, Asp73, Trp60 | Ala50, Gly126, Cys79, Thr75, Tyr64, Tyr93, Trp88, Leu110, Ala105, Phe101, Leu36, Arg61 |
| Cladodionen(a) | −7.38 | Tyr47 | Ala70, Val76, Cys79, Leu40, Ala127, Leu125, Gly126, Ala50, Asp65, Tyr64, Ile52, Arg61 |
| Cladodionen(b) | −7.04 | Tyr47 | Leu36, Val76, Cys79, Leu40, Ala127, Leu125, Gly126, Ala50, Asp65, Tyr64, Ile52, Arg61 |
| Molecule | Docking Energy (kcal/mol) | Hydrogen Bonding Interactions | Key Hydrophobic Interactions |
|---|---|---|---|
| NHQ | −5.22 | / | Val170, Ile236, Ile149, Ala168, Thr265, Leu207, Tyr258, Leu189 |
| Cladodionen(a) | −6.85 | Arg209, Ser196 | Gln194, Phe221, Val211, Leu197, Met224, Ile236, Leu208, Leu207, Pro210, Asn206 |
| Cladodionen(b) | −6.81 | Arg209, Gln194 | Ser196, Ile195, Val211, Leu197, Met224, Ile236, Leu208, Leu207, Pro210, Asn206 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhao, L.; Wu, H.; Zhao, C.; Gong, Q.; Yu, W. Cladodionen Is a Potential Quorum Sensing Inhibitor Against Pseudomonas aeruginosa. Mar. Drugs 2020, 18, 205. https://doi.org/10.3390/md18040205
Wang M, Zhao L, Wu H, Zhao C, Gong Q, Yu W. Cladodionen Is a Potential Quorum Sensing Inhibitor Against Pseudomonas aeruginosa. Marine Drugs. 2020; 18(4):205. https://doi.org/10.3390/md18040205
Chicago/Turabian StyleWang, Mengjia, Lu Zhao, Hao Wu, Chaoyue Zhao, Qianhong Gong, and Wengong Yu. 2020. "Cladodionen Is a Potential Quorum Sensing Inhibitor Against Pseudomonas aeruginosa" Marine Drugs 18, no. 4: 205. https://doi.org/10.3390/md18040205
APA StyleWang, M., Zhao, L., Wu, H., Zhao, C., Gong, Q., & Yu, W. (2020). Cladodionen Is a Potential Quorum Sensing Inhibitor Against Pseudomonas aeruginosa. Marine Drugs, 18(4), 205. https://doi.org/10.3390/md18040205





