Azaphilones from the Red Sea Fungus Aspergillus falconensis
Abstract
1. Introduction
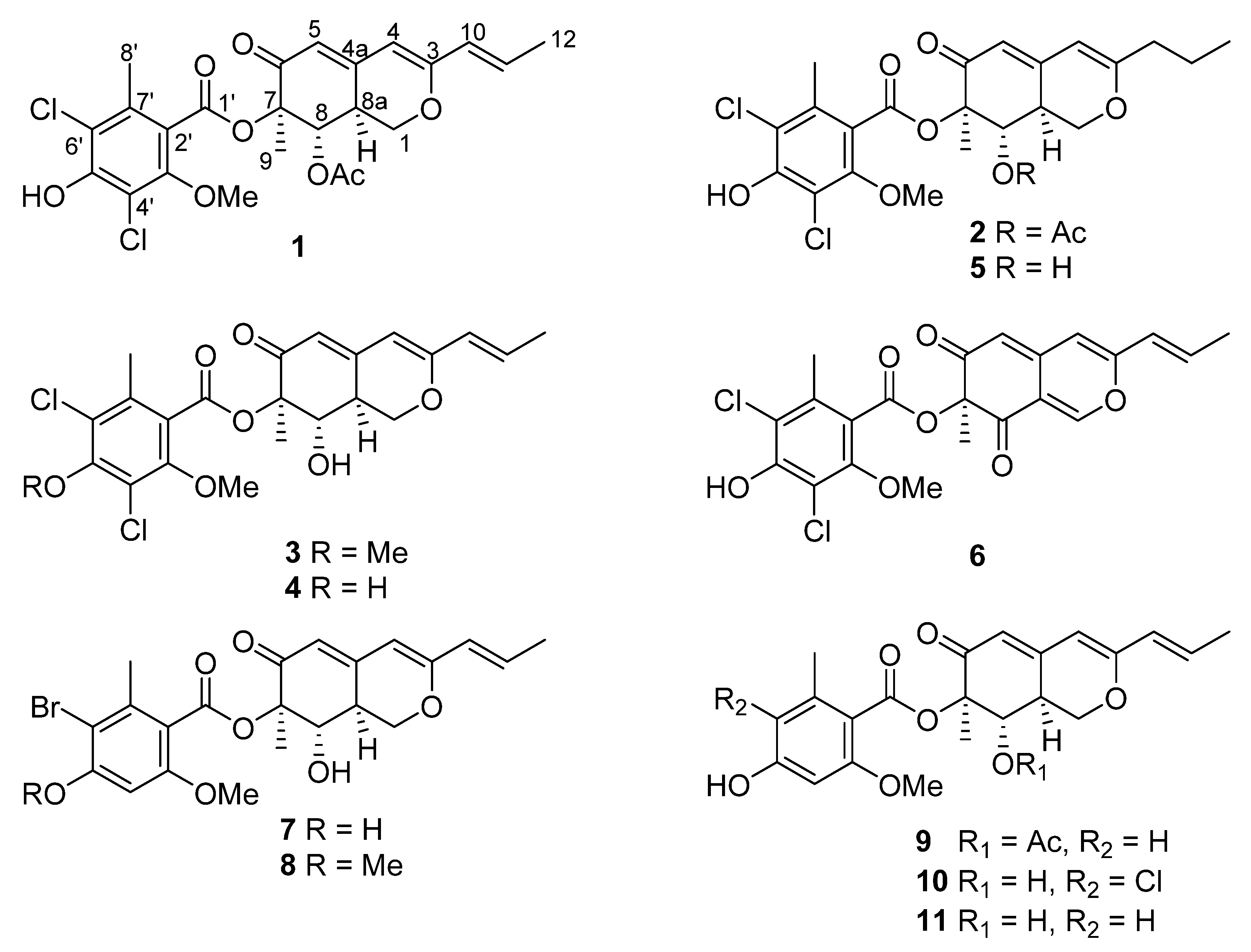
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.4. Crystallographic Analysis of Compound 5
3.5. Triple Negative Breast Cancer Studies
3.5.1. Cell Culture and Chemicals
3.5.2. NF-κB Inhibitory Assay
3.5.3. Cell Viability Assay
3.5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jimenez, C. Marine natural products in medicinal chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Wang, Y.T.; Xue, Y.R.; Liu, C.H. A brief review of bioactive metabolites derived from deep-sea fungi. Mar. Drugs 2015, 13, 4594–4616. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Jensen, P.R.; Cheng, X.C. Halimide, A Cytotoxic Marine Natural Product, and Derivatives Thereof. U.S. Patent No. 6,069,146, 30 May 2000. [Google Scholar]
- Petersen, L.E.; Kellermann, M.Y.; Schupp, P.J. Secondary metabolites of marine microbes: From natural products chemistry to chemical ecology. In YOUMARES 9-The oceans: Our Research, our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 159–180. [Google Scholar]
- Available online: https://www.beyondspringpharma.com/ChannelPage/index.aspx (accessed on 6 November 2019).
- Zhang, Z.; He, X.; Wu, G.; Liu, C.; Lu, C.; Gu, Q.; Che, Q.; Zhu, T.; Zhang, G.; Li, D. Aniline-tetramic acids from the deep-sea-derived fungus Cladosporium sphaerospermum L3P3 cultured with the HDAC inhibitor SAHA. J. Nat. Prod. 2018, 81, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Hertweck, C. Hidden biosynthetic treasures brought to light. Nat. Chem. Biol. 2009, 5, 450–452. [Google Scholar] [CrossRef]
- Daletos, G.; Ebrahim, W.; Ancheeva, E.; El-Neketi, M.; Lin, W.; Chaidir, C. Microbial co-culture and OSMAC approach as strategies to induce cryptic fungal biogenetic gene clusters. In Chemical Biology of Natural Products; Grothaus, P., Cragg, G.M., Newman, D.J., Eds.; CRC press: Boca Raton, FL, USA, 2017; pp. 233–284. [Google Scholar]
- Hofs, R.; Walker, M.; Zeeck, A. Hexacyclinic acid, a polyketide from Streptomyces with a novel carbon skeleton. Angew. Chem. Int. Ed. 2000, 39, 3258–3261. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.L.; Fang, Y.C.; Zhu, T.J.; Gu, Q.Q.; Zhu, W.M. Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi. J. Nat. Prod. 2008, 71, 985–989. [Google Scholar] [CrossRef]
- Saleem, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef]
- Ge, H.M.; Yu, Z.G.; Zhang, J.; Wu, J.H.; Tan, R.X. Bioactive alkaloids from endophytic Aspergillus fumigatus. J. Nat. Prod. 2009, 72, 753–755. [Google Scholar] [CrossRef]
- Frank, M.; Ozkaya, F.C.; Muller, W.E.G.; Hamacher, A.; Kassack, M.U.; Lin, W.; Liu, Z.; Proksch, P. Cryptic secondary metabolites from the sponge-associated fungus Aspergillus ochraceus. Mar. Drugs 2019, 17, 99. [Google Scholar] [CrossRef]
- El-Kashef, D.H.; Daletos, G.; Plenker, M.; Hartmann, R.; Mandi, A.; Kurtan, T.; Weber, H.; Lin, W.; Ancheeva, E.; Proksch, P. Polyketides and a dihydroquinolone alkaloid from a marine-derived strain of the fungus Metarhizium marquandii. J. Nat. Prod. 2019, 82, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Kuppers, L.; Ebrahim, W.; El-Neketi, M.; Ozkaya, F.C.; Mandi, A.; Kurtan, T.; Orfali, R.S.; Muller, W.E.G.; Hartmann, R.; Lin, W.H.; et al. Lactones from the sponge-derived fungus Talaromyces rugulosus. Mar. Drugs 2017, 15, 359. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.B.; Hubka, V.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef]
- Itabashi, T.; Nozawa, K.; Miyaji, M.; Udagawa, S.; Nakajima, S.; Kawai, K. Falconensins A, B, C, and D, new compounds related to azaphilone from Emericella falconensis. Chem. Pharm. Bull. 1992, 40, 3142–3144. [Google Scholar] [CrossRef]
- Itabashi, T.; Nozawa, K.; Nakajima, S.; Kawai, K. A new azaphilone, falconensin H, from Emericella falconensis. Chem. Pharm. Bull. 1993, 41, 2040–2041. [Google Scholar] [CrossRef]
- Itabashi, T.; Ogasawara, N.; Nozawa, K.; Kawai, K. Isolation and structures of new azaphilone derivatives, falconensins E−G, from Emericella falconensis and absolute configurations of falconensins A−G. Chem. Pharm. Bull. 1996, 44, 2213–2217. [Google Scholar] [CrossRef]
- Yasukawa, K.; Itabashi, T.; Kawai, K.; Takido, M. Inhibitory effects of falconensins on 12-O-tetradecanoylphorbol-13-acetate-induced inflammatory ear edema in mice. J. Nat. Med. 2008, 62, 384–386. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Pielka, E.; Lipinski, A.; Jelen, M.; Kielan, W.; Agrawal, S. Clinical validation of nuclear factor kappa B expression in invasive breast cancer. Tumour Biol. 2018, 40. [Google Scholar] [CrossRef]
- Ogasawara, N.; Kawai, K.I. Hydrogenated azaphilones from Emericella falconensis and E. fruticulosa. Phytochemistry 1998, 47, 1131–1135. [Google Scholar] [CrossRef]
- Gao, J.M.; Yang, S.X.; Qin, J.C. Azaphilones: Chemistry and biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef]
- Huang, H.; Wang, F.; Luo, M.; Chen, Y.; Song, Y.; Zhang, W.; Zhang, S.; Ju, J. Halogenated anthraquinones from the marine-derived fungus Aspergillus sp. SCSIO F063. J. Nat. Prod. 2012, 75, 1346–1352. [Google Scholar] [CrossRef]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Crystallogr. Sect. A 1983, 39, 876–881. [Google Scholar] [CrossRef]
- Flack, H.D.; Bernardinelli, G. Absolute structure and absolute configuration. Acta Crystallogr. Sect. A 1999, 55, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Flack, H.D.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 2008, 20, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Flack, H.D.; Sadki, M.; Thompson, A.L.; Watkin, D.J. Practical applications of averages and differences of Friedel opposites. Acta Crystallogr. Sect. A 2011, 67, 21–34. [Google Scholar] [CrossRef]
- Osmanova, N.; Schultze, W.; Ayoub, N. Azaphilones: A class of fungal metabolites with diverse biological activities. Phytochem. Rev. 2010, 9, 315–342. [Google Scholar] [CrossRef]
- Kjer, J.; Debbab, A.; Aly, A.H.; Proksch, P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 2010, 5, 479–490. [Google Scholar] [CrossRef]
- Bruker. Bruker AXS: Apex2, data collection program for the CCD area-detector system; SAINT, data reduction and frame integration program for the CCD area-detector system; Bruker: Billerica, MA, USA, 2014−2015. [Google Scholar]
- Sheldrick, G.M. SADABS: Area-Detector Absorption Correction; University of Goettingen: Goettingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond (Version 4), Crystal and Molecular Structure Visualization; Crystal Impact−K, Brandenburg & H. Putz Gbr: Bonn, Germany, 2009. [Google Scholar]

| No. | 1 a,c | 2 a,c | 7 b | 8 a,c | 9 b |
|---|---|---|---|---|---|
| 1 | 67.8, CH2 | 68.2, CH2 | 68.5, CH2 | 68.4, CH2 | 68.0, CH2 |
| 3 | 160.2, C | 168.2, C | 160.5, C | 160.2, C | 160.2, C |
| 4 | 102.4, CH | 100.9, CH | 102.8, CH | 102.6 CH | 102.8, CH |
| 4a | 149.4, C | 149.2, C | 150.3, C | 149.9, C | 148.9, C |
| 5 | 116.6, CH | 115.7, CH | 116.6, CH | 116.6, CH | 117.1, CH |
| 6 | 192.3, C | 192.7, C | 193.8, C | 193.4, C | 193.3, C |
| 7 | 83.4, C | 83.4, C | 85.6, C | 85.4, C | 82.5, C |
| 8 | 70.1, CH | 70.4, CH | 69.8, CH | 69.7, CH | 70.7, CH |
| 8a | 38.1, CH | 38.0, CH | 37.8, CH | 37.5, CH | 38.2, CH |
| 9 | 17.9, CH3 | 18.2, CH3 | 16.8, CH3 | 16.6, CH3 | 18.2, CH3 |
| 10 | 124.9, CH | 36.4, CH2 | 125.4, CH | 125.3, CH | 125.2, CH |
| 11 | 134.1, CH | 20.0, CH2 | 133.9, CH | 133.6, CH | 133.9, CH |
| 12 | 18.2, CH3 | 13.6, CH3 | 18.4, CH3 | 18.2, CH3 | 18.4, CH3 |
| 1′ | 164.3, C | 164.4, C | 165.3, C | 164.9, C | 166.2, C |
| 2′ | 122.2, C | 122.3, C | 117.4, C | 117.2, C | 115.5, C |
| 3′ | 152.8, C | 152.9, C | 156.2, C | 157.3, C | 159.0, C |
| 4′ | 112.9, C | 112.8, C | 97.1, CH | 93.8, CH | 96.9, CH |
| 5′ | 149.2, C | 149.8, C | 154.0, C | 155.8, C | 157.6, C |
| 6′ | 117.0, C | 117.1, C | 105.3, C | 106.6, C | 109.2, CH |
| 7′ | 134.3, C | 134.5, C | 137.4, C | 138.1, C | 140.0, C |
| 8′ | 16.9, CH3 | 17.1, CH3 | 20.2, CH3 | 20.1, CH3 | 19.6, CH3 |
| 8-OAc | 170.0, C | 170.1, C | 169.7, C | ||
| 20.5, CH3 | 20.8, CH3 | 20.8, CH3 | |||
| 3′-OMe | 62.4, CH3 | 62.6, CH3 | 56.5, CH3 | 56.3, d CH3 | 56.0, CH3 |
| 5′-OMe | 56.4, d CH3 |
| No. | 1 a | 2 a | 7 b | 8 a | 9 b |
|---|---|---|---|---|---|
| 1 | 4.34, dd (11.0, 5.0); 3.96, dd (13.0, 11.0) | 4.29, dd (11.0, 5.1); 3.93, dd (13.2, 11.0) | 4.77, dd (10.5, 5.1); 3.84, dd (13.0, 10.5) | 4.77, dd (10.6, 5.0); 3.84, dd (13.0, 10.6) | 4.32, dd (10.8, 5.0); 3.93, dd (13.0, 10.8) |
| 4 | 5.59, s | 5.53, s | 5.56, s | 5.57, s | 5.57, s |
| 5 | 5.86, d (1.7) | 5.79, d (1.9) | 5.80, d (1.6) | 5.80, d (1.8) | 5.85, d (1.9) |
| 8 | 6.11, d (10.7) | 6.09, d (10.7) | 4.74, d (10.7) | 4.75, d (10.7) | 6.11, d (10.7) |
| 8a | 2.98, dddd (13.0, 10.7, 5.0, 1.7) | 2.94, dddd (13.2, 10.7, 5.1, 1.9) | 2.88, dddd (13.0, 10.7, 5.1, 1.6) | 2.88, dddd (13.0, 10.7, 5.0, 1.8) | 2.96, dddd (13.0, 10.7, 5.0, 1.9) |
| 9 | 1.56, s | 1.56, s | 1.47, s | 1.47, s | 1.54, s |
| 10 | 5.91, dq (15.4, 1.3) | 2.20, m | 5.90, dq (15.4, 1.4) | 5.90, dd (15.4, 1.5) | 5.90, dd (15.3, 1.6) |
| 11 | 6.45, dq (15.4, 7.0) | 1.58, m | 6.47, dq (15.4, 7.0) | 6.46, dd (15.4, 7.0) | 6.42, dd (15.3, 7.0) |
| 12 | 1.88, dd (7.0, 1.3) | 0.94, t (7.4) | 1.87, dd (7.0, 1.4) | 1.88, dd (7.0, 1.5) | 1.87, dd (7.0, 1.6) |
| 4′ | 6.52, s | 6.38, s | 6.23, s | ||
| 6′ | 6.22, s | ||||
| 8′ | 2.50, s | 2.50, s | 2.48, s | 2.51, s | 2.39, s |
| 8-OAc | 2.17, s | 2.16, s | 2.15, s | ||
| 3′-OMe | 3.83, s | 3.83, s | 3.83, s | 3.92, c s | 3.74, s |
| 5′-OH | 6.04, s | 6.04, s | |||
| 5′-OMe | 3.89, c s |
| Compound | NF-κB inhibition | Cell viability inhibition |
|---|---|---|
| Falconensin O (1) | 15.7 ± 0.7 | > 200 |
| Falconensin A (3) | 53.2 ± 21.4 | 89.7 ± 9.1 |
| Falconensin M (4) | 56.5 ± 8.3 | > 200 |
| Falconensin N (5) | 71.0 ± 7.3 | > 200 |
| Falconensin H (6) | 72.0 ± 28.1 | > 400 |
| Falconensin Q (7) | 11.9 ± 2.1 | > 200 |
| Falconensin R (8) | 14.6 ± 1.7 | 126.8 ± 5.4 |
| Falconensin S (9) | 20.1 ± 5.6 | > 200 |
| Falconensin I (11) | 19.5 ± 2.5 | > 400 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Kashef, D.H.; Youssef, F.S.; Hartmann, R.; Knedel, T.-O.; Janiak, C.; Lin, W.; Reimche, I.; Teusch, N.; Liu, Z.; Proksch, P. Azaphilones from the Red Sea Fungus Aspergillus falconensis. Mar. Drugs 2020, 18, 204. https://doi.org/10.3390/md18040204
El-Kashef DH, Youssef FS, Hartmann R, Knedel T-O, Janiak C, Lin W, Reimche I, Teusch N, Liu Z, Proksch P. Azaphilones from the Red Sea Fungus Aspergillus falconensis. Marine Drugs. 2020; 18(4):204. https://doi.org/10.3390/md18040204
Chicago/Turabian StyleEl-Kashef, Dina H., Fadia S. Youssef, Rudolf Hartmann, Tim-Oliver Knedel, Christoph Janiak, Wenhan Lin, Irene Reimche, Nicole Teusch, Zhen Liu, and Peter Proksch. 2020. "Azaphilones from the Red Sea Fungus Aspergillus falconensis" Marine Drugs 18, no. 4: 204. https://doi.org/10.3390/md18040204
APA StyleEl-Kashef, D. H., Youssef, F. S., Hartmann, R., Knedel, T.-O., Janiak, C., Lin, W., Reimche, I., Teusch, N., Liu, Z., & Proksch, P. (2020). Azaphilones from the Red Sea Fungus Aspergillus falconensis. Marine Drugs, 18(4), 204. https://doi.org/10.3390/md18040204










