Abstract
This review examines the current state of knowledge regarding toxins from anthozoans (sea anemones, coral, zoanthids, corallimorphs, sea pens and tube anemones). We provide an overview of venom from phylum Cnidaria and review the diversity of venom composition between the two major clades (Medusozoa and Anthozoa). We highlight that the functional and ecological context of venom has implications for the temporal and spatial expression of protein and peptide toxins within class Anthozoa. Understanding the nuances in the regulation of venom arsenals has been made possible by recent advances in analytical technologies that allow characterisation of the spatial distributions of toxins. Furthermore, anthozoans are unique in that ecological roles can be assigned using tissue expression data, thereby circumventing some of the challenges related to pharmacological screening.
Keywords:
Cnidaria; Anthozoa; Medusozoa; venom; toxins; transcriptomics; proteomics; spatiotemporal expression; ecology 1. Introduction to Animal Venoms
Within the animal kingdom, biotic interactions such as predation and competition are key driving forces for the evolution of species [1,2]. Traits that enhance the success of these interactions, and thus increase survival, are diverse and widespread. The utilisation of venom during interactions with prey, predators and competitors is one such trait, and has evolved on over a hundred separate occasions across at least eight animal phyla [3]. Venomous animals have received considerable interest throughout history, with Aristotle’s “Historia Animalium” serving as the oldest surviving document that references venomous creatures [4,5]. While initial investigations of animal venoms were prompted by the need to develop antivenom strategies [6], studying these complex chemical cocktails has resulted more recently in toxins being employed as molecular tools and as potential candidates for pharmacological development [7,8,9]. This renewed interested in toxins has also enhanced understanding of venom regulation and production.
By definition, venom is a collection of molecules that, when introduced into another animal via a wound, antagonistically interferes with its physiological processes [10]. Venoms typically contain an assortment of salts, amino acids, neurotransmitters, and bioactive proteins and peptides, collectively referred to as toxins [10,11,12,13,14]. Proteins and peptides typically make up the bulk of toxins, and have evolved from physiological proteins and peptides that have been functionally recruited into venoms. However, the relative importance of the various underlying genetic mechanisms governing the compositional evolution of venoms is still poorly understood [15,16]. Venoms are delivered to their victims through specialised delivery structures, which are extremely diverse across venomous lineages [3]. In the vast majority of venomous lineages, these delivery structures are connected to a single set of venom producing tissue(s), forming what is known as centralised venom systems [3]. Alternatively, cells capable of autonomous venom release are distributed throughout the entire body of some venomous organisms, as observed in phylum Cnidaria [17]. Regardless of their composition or associated structures, animals may employ venom as a chemical weapon in instances of prey capture, defence against predators, intraspecific competition, and a number of other diverse functions [3].
As venom is critical to the livelihood of venomous animals, natural selection has resulted in diverse strategies to regulate toxin production and enhance toxin suitability for specific ecological roles. Most venomous animals employ these chemical weapons primarily in order to immobilise prey and/or facilitate feeding [3]. Conversely, venoms may also serve a defensive role by causing intense, instantaneous and localised pain [11,18,19,20]. The same venom is often used for both types of encounters, with toxins that target neuronal communication capable of eliciting both pain and paralysis [21,22,23]. However, this is not the case in the assassin bug and cone snails, which exhibit regionalisation in venom production, within either the same venom duct or distinct anatomical structures [22,24]. This partitioning allows the production of separate venoms for offensive and defensive interactions, thereby reducing the number of toxins depleted during each encounter [22,24]. The ability to produce multiple venoms is evidently beneficial, although to date this has only been observed in a limited number of organisms with a centralised venom system. In cnidarians, there is no centralised morphological structure responsible for the production of venom, and the mechanisms responsible for producing multiple venoms are likely to be more nuanced [16,25]. Furthermore, given the distribution of venom apparatus across multiple functional anatomical tissues, this phylum provides a unique opportunity to ascertain the ecological roles of venom.
2. Phylum Cnidaria
Phylum Cnidaria is the most ancient known venomous lineage, having emerged at least 600 million years ago, and includes over 10,000 species [26]. Remarkably, however, only 273 toxins from this phylum are recorded in the ToxProt database [27] as of mid-February 2020. Cnidarians (sea anemones, corals, jellyfish, myxozoans and hydra) are unique among other venomous phyla in that venom production often occurs throughout the entire organism rather than in a single or limited number of discrete anatomical structures. Nematocysts are the highly specialised organelles secreted by the Golgi apparatus that are responsible for storing and discharging venom [17]. The presence of these single-use venom delivery structures is the distinguishing feature of this phylum [17,28].
Recent phylogenomic analyses have resolved three major cnidarian lineages—Anthozoa, Endocnidozoa and Medusozoa (Figure 1) [29]. Class Anthozoa is a monophyletic clade that is further divided into subclasses Octocorallia (soft corals, sea pens, sea fans), Ceriantharia (tube anemones) and Hexacorallia (zoanthids, sea anemones, true coral, corallimorphs). Scyphozoans (true jellyfish), staurozoans (stalked jellyfish), cubozoans (box jellyfish) and hydrozoans (hydroids, hydromedusae, siphonophores) collectively form clade Medusozoa. Endocnidozoa is comprised exclusively of the freshwater and marine obligate parasites, Myxozoa and Polypodiozoa; this clade possesses a number of cnidarian-restricted genes and nematocyst-homologous structures (polar capsules) but as yet no toxin families have been detected [30]. Traits such as symbiosis, coloniality and life cycle vary within and among cnidarians classes. Solitary and colonial forms are observed across all three clades. Hosting of photosynthetic endosymbionts occurs in a subset of species from Anthozoa and Medusozoa but not Endocnidozoa [29]. A complex life cycle involving both sessile polyp and mobile medusa stages is restricted to Medusozoa but appears to have been lost in class Staurozoa [29]. This high level of diversity within Cnidaria has evolved over at least 600 million years and has implications for the regulation of venom production.
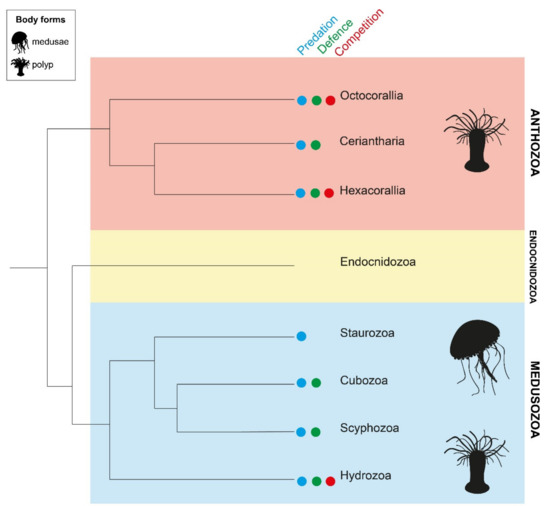
Figure 1.
Relationships among cnidarian lineages, Anthozoa (red), Endocnidozoa (yellow) and Medusozoa (blue), based on phylogenomic reconstruction [29]. Sessile polyp forms are observed in both Anthozoa and Medusozoa, while mobile medusae are confined to the Medusozoa (black symbols on right). The presence of venom components in Endocnidozoa and their functions have not yet been verified [30]. In the other classes, it is recognised that venom is utilised as a tool during both predation (blue circle) and defence (green circle). However, venom is only known to be deployed during intraspecific competition (red circle) by Octocorallia, Hexacorallia and Hydrozoa. The ecological significance of venom in some classes requires further characterisation.
Venom was previously thought to be utilised principally for predation in Cnidaria [11,31]. However, cnidarians are now identified as one of only two phyla known to use venom for all three of the major ecological functions of venom, namely predation, defence and intraspecific competition [3,32]. The extent to which an organism relies upon venom as a defensive strategy is highly variable even within groups [33,34]. In Hexacorallia, for instance, nematocyst discharge in response to simultaneous mechanical and chemical stimuli was observed in all actiniarian species, only 40% of zoanthid species, but no corallimorphians that were tested [33]. These data were substantiated by the field observation that reef fish consistently refused to consume cnidarians with nematocyst defences but not their defenceless counterparts [33]. Likewise, only some hydroid species are dependent upon nematocyst envenomation for protection from predators [34]. Alternate chemical defence via noxious secondary metabolites has also been documented in Anthozoa and Hydrozoa, particularly in species that inhabit coral reefs [33,34]. Within these two classes, the selective utilisation of venom for interspecific or even intraspecific combat has also been observed [35,36,37,38]. Evidence for roles of venom in other classes is limited, with Staurozoa and Ceriantharia remaining the most understudied cnidarian classes to date [39]. Thus, while venom appears to be ubiquitously employed during feeding in anthozoans and medusozoans, further characterisation is required to understand the spectrum of venom usage across the diverse life histories of Cnidaria.
3. Venom Evolution across Cnidaria
Venom composition differs substantially among cnidarian classes, with the classes Scyphozoa, Hydrozoa and Anthozoa sharing only six proteins among their soluble nematocyst proteins, which likely have largely house-keeping functions [40]. The proportion of shared protein content is significantly lower for nematocyst proteins (2%) compared to the total proteome (15%) [40]. While venoms of scyphozoans and hydrozoans exert similar biochemical effects, sea anemone venom is unique in that it consists predominantly of peptide neurotoxins [40,41]. However, whether an abundance of neurotoxins is characteristic of anthozoan venom cannot be determined until the taxonomic bias in available data is addressed and knowledge of coral venoms improves. Furthermore, comparison of soluble nematocyst proteomes from eight cnidarian species indicates that approximately one third of all toxin protein families identified are present in both Anthozoa and Medusozoa, although no representative of Staurozoa was included [42]. Of the remaining toxin families, four were taxonomically restricted to a single class and 15 were absent in at least one class, and there was no correlation between toxin family absence and presence and phylogenetic relatedness [42]. In fact, the reported loss of numerous toxin families in Cubozoa was associated with an erroneous phylogenetic reconstruction that placed Cubozoa external to Anthozoa and Medusozoa [42]. The incorrect placement of Cubozoa, however, is likely to be a consequence of using presence/absence variation of only known toxins from the ToxProt database for their phylogenetic reconstruction.
Within subclass Hexacorallia, actiniarians (Figure 2) show the greatest biological and anatomical diversity [43]. Sea anemones are distributed across almost every marine environment, from the depths of the oceans to coastal intertidal zones, and from tropical waters to Antarctica [43,44,45]. Their success lies in part in their ability to respond to different environmental pressures [46]. Within actiniarians, ecological interactions and environmental conditions are key drivers of the expression of toxin genes, rather than the retention and expansion of gene families [16,47,48]. Comparative analysis supports the minimal impact of environmental factors on the toxin gene complement, revealing that closely related cnidarian species have more similar toxin gene complements than those that share an ecological niche [16]. Moreover, phylogenetic investigations of sequence variation in cnidarian toxin genes consistently report that toxin gene distribution correlates with species relatedness [49,50,51,52]. These results suggest that speciation is an important driver of toxin gene complement and sequence variation. However, the influence of ecological factors on toxin expression results in dynamic spatial and temporal patterns of venom composition [16,47,48].
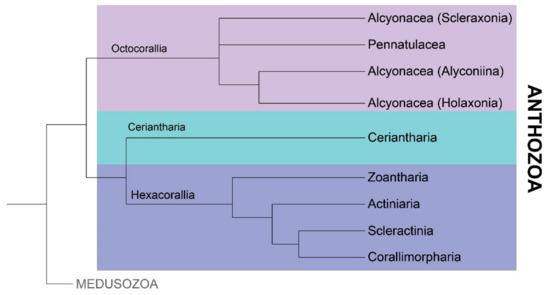
Figure 2.
Proposed phylogeny of class Anthozoa based on recent analysis by Kayal et al. [29]. Subclass Octocorallia contains sea pens (order Pennatulacea) and soft corals (order Alcyonacea), while all tube anemones are found in subclass Ceriantharia. Zooanthids (order Zoantharia), sea anemones (order Actiniaria), stony corals (order Scleractinia) and corallimorphians (order Corallimorpharia) belong to subclass Hexacorallia.
4. Geographic, Ontogenetic and Prey-Associated Venom Variation
Recent investigations of medically important species such as the box jellyfish, Chironex fleckeri, have facilitated exploration of interspecies patterns of venom composition. C. fleckeri (class Cubozoa), is regarded as the most lethal jellyfish in the world and is associated with approximately 70 recorded fatalities since 1884 in Australia [53,54]. Comparison of Queensland and Western Australian populations revealed geographic heterogeneity in the composition and potency of their venom arsenal [55]. Similar variability in venom protein content was observed in the scyphozoan giant jellyfish, Nemopilema nomura, sourced from a number of locations in the Yellow Sea [56]. In contrast, when clonal fragments of the scleractinian coral, Tubastraea coccinea, were reciprocally transplanted between inshore and offshore sites for a six week period, no changes to the abundance and composition of recognised toxins were detected, despite altered expression of non-toxin peptides [57]. Whether this unchanged venom profile is a consequence of similarity in biotic communities between the two locations, the short duration of this study, or is a common attribute of corals and other sessile cnidarians, remains to be determined.
An ontogenetic-driven dietary shift compounds venom complexity in C. fleckeri; as medusae mature, they begin preying upon fish in addition to crustaceans. This transition is accompanied by an increase in mastigophores (large-volume nematocysts) and an increased diversity of toxin peptides, which together produce a potent venom specialised for targeting fish [58,59]. Similarly, the number and volume of heteroneme nematocysts, including mastigophores, were found to increase in siphonophore tentilla with a diet of fish compared to those with a diet of copepods [60]. The venom from cubozoan species that consistently prey upon shrimp is also capable of eliciting death in fish, but the amount of venom required exceeds the surface area of both nematocyst-laden tentacles and their prey, making it impractical for them to subdue fish [58]. Hydrozoans also appear to undergo dramatic shifts in venom composition across life stages, and changes in nematocyst type have even been observed in multiple cubozoan species during the transition from polyp to medusa, independent of dietary shifts [61,62,63].
An altered venom protein profile in the absence of nematocyst variation also occurs in the jellyfish Carukia barnesi [64,65]. Augmentation of neckchieves, nematocyst bands within tentacles that are postulated to function as a prey attractant, is observed in mature C. barnesi, as is an increase in frequency of twitching of this structure to actively lure fish [65,66,67]. These morphological changes provide preliminary evidence that a difference in predatory behaviour accompanies a shift in venom composition during the maturation of some cubozoan species. Thus, a combination of changes to venom and nematocyst profiles, as well as feeding behaviours, which occur during metamorphosis, facilitates a change in diet in cnidarian species.
This intraspecific variability in cnidarian venoms is reminiscent of similar geographical and ontogenetic changes observed in highly-studied terrestrial venomous taxa, particularly snakes, where venom composition is often attributed to changes in diet [68,69,70,71,72,73]. Therefore, it can now be appreciated that within phylum Cnidaria, different patterns of venom composition emerge in response to changes in ecological factors associated with life history transitions, such as diet, and that these patterns are probably driven by changes in gene regulation and expression, as observed in snakes [74].
5. Colonial Regionalisation and Functional Divisions
Cnidarians can exist as solitary polyps or as colonies, with coloniality common among corals, zoanthids, hydroids and also select sea anemones [29]. Colonies are comprised of many physically connected individuals, termed zooids, formed through asexual reproduction but with variable morphological forms and functional responsibilities [75,76]. Perhaps the most well-known toxin associated with colonial cnidarians is palytoxin. First isolated from the Palythoa genus around 1970, and since isolated in Heteractis crispa, this non-proteinaceous toxin poses a serious human health threat, with exposure having the potential to rapidly cause death via heart failure [77,78,79]. In marine environments, colonial zoanthids employ this chemical weapon to deter predators and also during spatial competition. The abundance of this toxin and its potency (LC50) in Artemia salina were found to vary within regions of single colonies and among reef sites [80]. Within a colony, crude organic extract (COE) was found to be most potent in peripheral regions, where encounters with competing organisms were most likely, compared to central regions. Similarly, differences in COE potency was observed among four Caribbean reef sites; this variability was not significantly associated with differences in reef biodiversity and depth. However, there was a positive correlation between COE yield and reef diversity at one site, providing preliminary evidence for interplay between increased competition and increased demand for toxins [80]. As palytoxin is unlikely to fully account for these observed differences in toxicity, with any number of other chemical components also present in the COE, further research will be required to substantiate these findings and explore the role of environmental factors in driving intracolony and intercolony toxin variability.
Within colonial hydrozoans, functions such as prey capture, defence, digestion and reproduction are divided among three polyp groups—gastrozooids, gonozoids and dactylzooids [81]. Through differential gene expression analysis, genes with key roles in generating functional and structural diversity within colonies have been identified. Furthermore, toxin genes were found to be differentially expressed between specialised polyp types [82]. Using RNA-seq analysis, 75% of putative toxin genes identified were found to be significantly differentially expressed between zooid forms in Hydractinia symbiolongicarpus. While toxin families may be present across multiple zooids, the overall venom composition reflected and supported the functions of gastrozooids, gonozoids and dactylzooids in digestion, reproduction and feeding, respectively. Hence, subdivision of labour within colonial hydrozoans is enabled by toxin cocktails that are unique to each zooid type [63,81]. Furthermore, toxin arsenals of functionally distinct structures/tissues are also likely to have diverged in their composition.
7. Functional Anatomy and Venom Variation
Previous characterisation of cnidarian venoms has relied largely on tentacle tissue, where the greatest concentration of nematocytes can be observed [17,40,98,99,100]. However, this disregards the widespread nature of nematocytes and provides only a glimpse of the complexity and dynamic nature of the venom landscape. Venom analyses utilising next generation sequencing (NGS) or proteomics offer evidence for the presence of toxins in various morphological structures where nematocytes are found, such as the actinopharynx, mesenterial filaments, column and physa [50,84,101]. Furthermore, Bastos et al. [102] reported that tentacle extracts from Bunodosoma cangicum (Anthozoa; Hexacorallia; Actiniaria) were unable to induce apoptosis in zebrafish hepatocytes in vitro, but column vesicular extracts from the same organism exerted haemolytic and apoptotic effects, consistent with cytolytic toxins. Likewise, some neurotoxins are localised to ectodermal gland cells rather than nematocytes in the sea anemones Nematostella vectensis and Anthopleura elegantissima [103]. Therefore, it appears that the regulation of venom composition across tissue types is considerably more complex than the relatively simple structure of these animals would suggest.
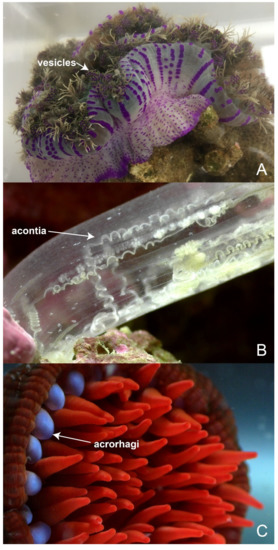
While there can be a shared pool of nematocyst types within a genus, species can be distinguished by variable patterns in the size and localisation of nematocysts [104]. The nematocyst populations of discrete anatomical regions have been detailed in several sea anemone species [90,105,106,107] as well as jellyfish [108,109,110,111], hydromedusae [104], tube anemones [112] and corals [113]. Through these studies, it has become apparent that nematocyst densities vary across the body plan (Figure 4) and that different structures have different proportions of nematocyst types [84,114]. While most tissues contain an assortment of different nematocysts, there are some nematocyst types that are confined to a single tissue, as is the case with very large P-mastigophores and acontial filaments [113]. How these specific nematocysts enhance the predator deterrent power of acontia has yet to be explored, but these observations suggest that the distribution of nematocysts is in part related to the differing functions of tissues.
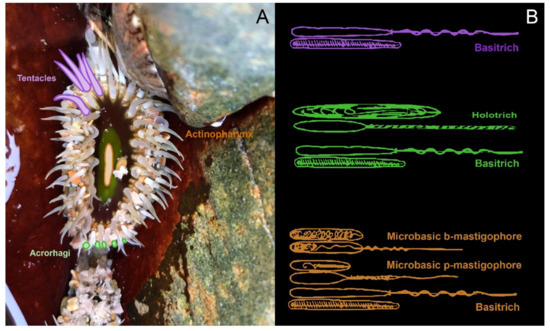
Figure 4.
The nematocyst profiles of various tissues in Oulactis muscosa, based on cnidom data [105]. (A) Tentacles, acrorhagi and actinopharynx of O. muscosa are shaded purple, green and orange, respectively; (B) the corresponding nematocyst types present in each region are shown in the same colour.
The relationship between nematocyst and toxin expression profiles provides additional insight into the functional basis of nematocyst variation. Fast-performance liquid chromatography has been used to verify that a difference in nematocyst type is correlated with a difference in venom profiles in C. fleckeri [109]. Additionally, homologues of a single protein toxin (the actinoporin equinatoxin) were found to be restricted to a specific nematocyst type in Hydra magnipapillata [115]. However, even within a single nematocyst type, subpopulations can be distinguished based on toxin expression profiles [84]. These data, in combination with recent results showing strong differential expression of Cnidarian-specific genes (including those encoding toxins) among different cell types [116], provide evidence for the region-specific production of venoms in a single organism.
The dynamic landscape of sea anemone venoms has been explored in multiple studies. Nematostella vectensis, the starlet anemone, is a leading cnidarian model organism owing to the availability of a genome sequence and its ability to be cultured within a laboratory environment [117,118]. Many of the insights into spatiotemporal expression of toxins across the complex life cycle of cnidarians and the development of cnidocytes are based on this species [84,103,116,119]. Recognition of venom arsenal changes with developmental stage and an alternative mechanism of envenomation via ectodermal gland cells are examples of the discoveries made from the study of this species [84,103].
The tissue-specific nature of venom composition in actiniarians has also been explored using four species from the superfamily Actinioidea: Actinia tenebrosa, Anemonia sulcata, Heteractis crispa and Megalactis griffithsi [16,50]. Comparison of toxin-like genes across the tentacles, mesenteries and column in A. sulcata, H. crispa and M. griffithsi revealed that the expression of toxins differs among tissues [50]. Toxin expression was consistently lowest in the column and highest in the tentacle or mesenterial filaments depending on the species [50]. This highlights that, while many toxins are expressed throughout the body, tissues with a primary role in envenomation are characterised by an upregulated expression of venom components. Interestingly, toxins from tentacles and mesenterial filaments also show convergence to proteins from other venomous clades, including spiders, snakes, wasps, cephalopods and cone snails [50]. Many of these are among the most highly expressed transcripts within a tissue, such as those with high sequence homology to calglandulin (snake) and venomous translationally-controlled tumour protein (TCTP) homologues (spider and snake), which function in secretion of toxins from the venom gland [120] and the inflammatory activity of venom [121,122], respectively. While these studies emphasise the differences in venom profiles across different tissues in sea anemones, metalloproteases and sea anemone type 2 potassium channel Kunitz-type toxins consistently had the greatest number of transcripts [50], supporting a degree of conservation in toxin expression within superfamily Actinioidea.
Building upon this, it has been demonstrated that toxin expression profiles show different degrees of similarity across tissues in Actinia tenebrosa. In this anemone, tentacles and acrorhagi share greater toxin expression similarity with each other than with mesenteries [16]. Furthermore, functional specialisation of venoms in each tissue type is supported by expression of toxin and toxin-like genes and gene ontology (GO) enrichment analysis [16]. Thus, the biological functions of a tissue seem to drive the composition of tissue-specific venom profiles and functionally similar tissues are more likely to have similar toxin expression profiles. However, it cannot be discounted that developmental constraints of the tissue are responsible for, or also contribute to, this expression pattern rather than just the biological function.
8. Characterising Toxin Expression Patterns
The diminishing cost and technological advances in sequencing technologies will result in more ‘omics’ datasets for venomous taxa becoming available in coming years, highlighted by the recent publication of multiple cnidarian genomes [118,123,124,125,126,127]. These genomic data represent a rich resource for comparative studies and the elucidation of venom evolution. Functional characterisation is still required for many of the currently identified toxins as this cannot be ascertained from a genome, transcriptome or proteome alone [128,129]. However, by studying toxins in conjunction with their expression patterns, invaluable inferences can be drawn regarding their potential ecological significance.
Platforms for studying toxins in situ include in situ hybridisation, immunohistochemistry, matrix-assisted laser desorption/ionisation mass spectrometry imaging (MALDI–MSI, henceforth simply MSI) and potentially spatial transcriptomics. Of these, in situ hybridisation (ISH) has been used to detect peptide and protein toxins in venomous species since the 1990s [130,131] and remains a leading technique to visualise toxins. The basis of ISH is that the location of a nucleic acid can be visualised using a complementary labelled probe specific to the gene or protein of interest [132]. Elucidating patterns of toxin gene expression across cell types and ontogenetic stages has been achieved through the application of ISH approaches in the model sea anemone species N. vectensis [84,101,133]. Furthermore, this approach has enabled the identification of novel and recruited genes with a nematocyte-specific expression pattern [119]. Conversely, immunohistochemistry (IHC) detects the location of peptides in an organism by exploiting antigen–antibody interactions, with the success of IHC dependent upon developing an antibody that is specific and fit for purpose, which is not without its challenges [134]. IHC can be used to complement findings of ISH studies—for example, through a combination of ISH and IHC, it was established that glycerotoxin expression is restricted to a subset of cells in the pharyngeal lobes of bloodworm venom apparatus [135]. Additionally, IHC has been used to demonstrate localisation of sticholysins to tentacles and mesenterial filaments in Stichodactyla helianthus [136] and Nv1 (N. vectensis toxin 1) to the ectodermal gland cells of Nematostella vectensis [103]. However, the probes utilised in both methods are developed for a single target nucleic acid or peptide within a single species, and thus the broad application of these technologies beyond model species is somewhat limited.
In contrast to targeted approaches, high-throughput omics technologies aim to capture the entire DNA, RNA or protein complement within a cell, tissue or organism [137,138]. In addition, MSI offers the opportunity to map the distribution of hundreds to thousands of peptides within a histological tissue section simultaneously, without the need for peptide isolation [139]. Therefore, MSI offers the opportunity to analyse peptide mixtures and evaluate peptide localisation for any species. This approach has been applied to the imaging of venom toxins from sea anemones, snakes and centipedes to date [16,25,140,141,142,143,144]. Identification of venom components directly from MSI spectra, however, remains non-trivial. MSI of toxins is therefore most informative when analysed in light of a venom peptidome obtained by more “traditional” venomic approaches, such as combined transcriptomic and venom proteomic analyses [25]. It has also contributed to understanding the variable tissue expression patterns of toxins within order Actiniaria, having been employed to visualise both widely distributed and highly localised toxins in A. tenebrosa [16,25,140]. Taking into account the presence of enzymes within sea anemone venom, the use of MSI as a novel assay to investigate the regulation of enzyme activity also confers considerable utility [144].
9. Conclusions
Phylum Cnidaria represents the oldest extant venomous lineage and includes many medically important species, such as the box jellyfish Chironex fleckeri. However, the venom of this group remains relatively understudied compared to several of their terrestrial venomous counterparts. It has been established that, in response to environmental stimuli, a single venomous animal can produce multiple venoms with distinct compositions. In cnidarians, these multiple venom profiles are driven in part by pressures related to geographical location as well as ontogenetic stages and associated dietary shifts. However, the colonial organisation of some taxa and the distribution of ‘stinging cells’ throughout the entire body plan augment the complexity of venom production and its regulation.
While venom is used to perform biological functions across every anatomical region in sea anemones (order Actiniaria, subclass Hexacorallia, class Anthozoa), the distinct requirements of each tissue necessitate the expression of unique tissue-specific venom cocktails. In particular, the highly specialised structures exclusively utilised for intraspecific aggression or defence in actiniarians are likely to be accompanied by equally specialised venom profiles. Through recent technological advances which have given rise to methods such as MSI, it is now possible to visualise these spatiotemporal patterns of venom constituents on a large scale. Therefore, by studying toxin distribution in conjunction with knowledge of the functions performed by specific tissues, it is possible to formulate hypotheses on the ecological significance of these peptides without additional functional data, which are often obtained through ecologically non-relevant pharmacological assays. Thus, actiniarians represent a unique opportunity to study toxin pharmacology, structure, and evolution in light of their endogenous functions, due to the discrete ecological roles of the different sea anemone tissues.
Author Contributions
Conceptualization, L.M.A. and P.J.P.; original draft preparation, L.M.A. and P.J.P.; draft review and editing, L.M.A., R.S.N., E.A.B.U., D.A.H. and P.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Norwegian Research Council (FRIPRO-YRT Fellowship no. 287462 to E.A.B.U.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paterson, S.; Vogwill, T.; Buckling, A.; Benmayor, R.; Spiers, A.; Thomson, N.; Quail, M.; Smith, F.; Walker, D.; Libberton, B.; et al. Antagonistic coevolution accelerates molecular evolution. Nature 2010, 464, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Langerhans, R.B. Evolutionary consequences of predation: avoidance, escape, reproduction, and diversification. In Predation in Organisms; Elewa, A.M.T., Ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Schendel, V.; Rash, D.L.; Jenner, A.R.; Undheim, A.B.E. The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution. Toxins 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N. Animal venom studies: Current benefits and future developments. World J. Biol. Chem. 2015, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.W.; Czerwinski, A.; Norton, R.S. Peptide therapeutics from venom: Current status and potential. Bioorg. Med. Chem. 2018, 26, 2738–2758. [Google Scholar] [CrossRef]
- Zhang, Y. Why do we study animal toxins? Zool. Res. 2015, 36, 183–222. [Google Scholar] [CrossRef]
- King, G.F. Venoms as a platform for human drugs: translating toxins into therapeutics. Expert Opin. Biol. Th. 2011, 11, 1469–1484. [Google Scholar] [CrossRef]
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discovery 2003, 2, 790–802. [Google Scholar] [CrossRef]
- Norton, R.S. Enhancing the therapeutic potential of peptide toxins. Expert Opin. Drug Discovery 2017, 12, 611–623. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.A.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu. Rev. Genomics Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: the evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Beress, L. Biologically active compounds from coelenterates. Pure Appl. Chem. 1982, 54, 1981–1994. [Google Scholar] [CrossRef]
- Honma, T.; Shiomi, K. Peptide toxins in sea anemones: structural and functional aspects. Mar. Biotechnol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Norton, R.S. Structures of sea anemone toxins. Toxicon 2009, 54, 1075–1088. [Google Scholar] [CrossRef]
- Jenner, R.A.; von Reumont, B.M.; Campbell, L.I.; Undheim, E.A.B. Parallel Evolution of Complex Centipede Venoms Revealed by Comparative Proteotranscriptomic Analyses. Mol. Biol. Evol. 2019, 36, 2748–2763. [Google Scholar] [CrossRef]
- Surm, J.M.; Smith, H.L.; Madio, B.; Undheim, E.A.; King, G.F.; Hamilton, B.R.; Van der Burg, C.A.; Pavasovic, A.; Prentis, P.J. A process of convergent amplification and tissue-specific expression dominates the evolution of toxin and toxin-like genes in sea anemones. Mol. Ecol. 2019, 28, 2272–2289. [Google Scholar] [CrossRef]
- Fautin, D.G. Structural diversity, systematics, and evolution of cnidae. Toxicon 2009, 54, 1054–1064. [Google Scholar] [CrossRef]
- Church, J.E.; Hodgson, W.C. The pharmacological activity of fish venoms. Toxicon 2002, 40, 1083–1093. [Google Scholar] [CrossRef]
- Peiren, N.; Vanrobaeys, F.; de Graaf, D.C.; Devreese, B.; Van Beeumen, J.; Jacobs, F.J. The protein composition of honeybee venom reconsidered by a proteomic approach. Biochim. Biophys. Acta Proteins Proteom. 2005, 1752, 1–5. [Google Scholar] [CrossRef]
- de Graaf, D.C.; Aerts, M.; Danneels, E.; Devreese, B. Bee, wasp and ant venomics pave the way for a component-resolved diagnosis of sting allergy. J. Proteomics 2009, 72, 145–154. [Google Scholar] [CrossRef]
- Brodie, E.D. Toxins and venoms. Curr. Biol. 2009, 19, R931–R935. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Dutertre, V.; Fry, B.G.; Antunes, A.; Venter, D.J. Evolution of separate predation-and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef]
- Cecilia, A.P.; Kellee, M.M.; Joseph, R.S. Venom variation during prey capture by the cone snail, Conus textile. PLoS ONE 2014, 9, e98991. [Google Scholar] [CrossRef]
- Walker, A.A.; Mayhew, M.L.; Jin, J.; Herzig, V.; Undheim, E.A.B.; Sombke, A.; Fry, B.G.; Meritt, D.J.; King, G.F. The assassin bug Pristhesancus plagipennis produces two distinct venoms in separate gland lumens. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Madio, B.; Peigneur, S.; Chin, Y.K.; Hamilton, B.R.; Henriques, S.T.; Smith, J.J.; Cristofori-Armstrong, B.; Dekan, Z.; Boughton, B.A.; Alewood, P.F. PHAB toxins: a unique family of predatory sea anemone toxins evolving via intra-gene concerted evolution defines a new peptide fold. Cell Mol. Life Sci. 2018, 75, 4511–4524. [Google Scholar] [CrossRef]
- Jouiaei, M.; Yanagihara, A.A.; Madio, B.; Nevalainen, T.J.; Alewood, P.F.; Fry, B.G. Ancient venom systems: a review on cnidaria toxins. Toxins 2015, 7, 2251–2271. [Google Scholar] [CrossRef]
- Jungo, F.; Bairoch, A. Tox-Prot, the toxin protein annotation program of the Swiss-Prot protein knowledgebase. Toxicon 2005, 45, 293–301. [Google Scholar] [CrossRef]
- Watson, G.M.; Hessinger, D.A. Cnidocyte mechanoreceptors are tuned to the movements of swimming prey by chemoreceptors. Science 1989, 243, 1589–1591. [Google Scholar] [CrossRef]
- Kayal, E.; Bentlage, B.; Sabrina Pankey, M.; Ohdera, A.H.; Medina, M.; Plachetzki, D.C.; Collins, A.G.; Ryan, J.F. Phylogenomics provides a robust topology of the major cnidarian lineages and insights on the origins of key organismal traits. BMC Evol. Biol. 2018, 18, 68. [Google Scholar] [CrossRef]
- Shpirer, E.; Diamant, A.; Cartwright, P.; Huchon, D. A genome wide survey reveals multiple nematocyst-specific genes in Myxozoa. BMC Evol. Biol. 2018, 18, 138. [Google Scholar] [CrossRef]
- Sunagar, K.; Moran, Y. The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals. PLoS Genet 2015, 11, e1005596. [Google Scholar] [CrossRef]
- Daly, M. Functional and Genetic Diversity of Toxins in Sea Anemones. In Evolution of Venomous Animals and Their Toxins; Malhotra, A., Ed.; Springer: Dordrecht, The Netherlands, 2017; pp. 87–104. [Google Scholar] [CrossRef]
- Hines, D.E.; Pawlik, J.R. Assessing the antipredatory defensive strategies of Caribbean non-scleractinian zoantharians (Cnidaria): is the sting the only thing? Mar. Biol. 2012, 159, 389–398. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Lindquist, N. Hydroid defenses against predators: the importance of secondary metabolites versus nematocysts. Oecologia 2000, 124, 280–288. [Google Scholar] [CrossRef]
- Francis, L. Intraspecific aggression and its effect on the distribution of Anthopleura elegantissima and some related sea anemones. Biol. Bull. 1973, 144, 73–92. [Google Scholar] [CrossRef]
- Bigger, C.H. Interspecific and Intraspecific Acrorhagial Aggressive-Behavior among Sea-Anemones—A Recognition of Self and Not-Self. Biol. Bull. 1980, 159, 117–134. [Google Scholar] [CrossRef]
- Ayre, D. Inter-genotype aggression in the solitary sea anemone Actinia tenebrosa. Mar. Biol. 1982, 68, 199–205. [Google Scholar] [CrossRef]
- Lane, S.M.; Briffa, M. Immune function and the decision to deploy weapons during fights in the beadlet anemone. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef]
- Miranda, L.S.; Mills, C.E.; Hirano, Y.M.; Collins, A.G.; Marques, A.C. A review of the global diversity and natural history of stalked jellyfishes (Cnidaria, Staurozoa). Mar. Biodivers. 2018, 48, 1695–1714. [Google Scholar] [CrossRef]
- Rachamim, T.; Morgenstern, D.; Aharonovich, D.; Brekhman, V.; Lotan, T.; Sher, D. The dynamically evolving nematocyst content of an anthozoan, a scyphozoan, and a hydrozoan. Mol. Biol. Evol. 2015, 32, 740–753. [Google Scholar] [CrossRef]
- Frazão, B.; Vasconcelos, V.; Antunes, A. Sea anemone (Cnidaria, Anthozoa, Actiniaria) toxins: an overview. Mar. Drugs 2012, 10, 1812–1851. [Google Scholar] [CrossRef]
- Jaimes-Becerra, A.; Chung, R.; Morandini, A.C.; Weston, A.J.; Padilla, G.; Gacesa, R.; Ward, M.; Long, P.F.; Marques, A.C. Comparative proteomics reveals recruitment patterns of some protein families in the venoms of Cnidaria. Toxicon 2017, 137, 19–26. [Google Scholar] [CrossRef]
- Daly, M.; Chaudhuri, A.; Gusmão, L.; Rodríguez, E. Phylogenetic relationships among sea anemones (Cnidaria: Anthozoa: Actiniaria). Mol. Phylogenet. Evol. 2008, 48, 292–301. [Google Scholar] [CrossRef]
- Fautin, D.G.; Malarky, L.; Soberon, J. Latitudinal diversity of sea anemones (Cnidaria: Actiniaria). Biol. Bull. 2013, 224, 89–98. [Google Scholar] [CrossRef]
- Fautin, D.G. Hexacorallians of the World. Available online: http://geoportal.kgs.ku.edu/hexacoral/anemone2/index.cfm (accessed on 7 February 2020).
- Sunagar, K.; Morgenstern, D.; Reitzel, A.M.; Moran, Y. Ecological venomics: How genomics, transcriptomics and proteomics can shed new light on the ecology and evolution of venom. J. Proteomics 2016, 135, 62–72. [Google Scholar] [CrossRef]
- O’Hara, E.P.; Caldwell, G.S.; Bythell, J. Equistatin and equinatoxin gene expression is influenced by environmental temperature in the sea anemone Actinia equina. Toxicon 2018, 153, 12–16. [Google Scholar] [CrossRef]
- Sachkova, M.Y.; Macrander, J.; Surm, J.M.; Aharoni, R.; Menard-Harvey, S.S.; Klock, A.; Leach, W.B.; Reitzel, A.M.; Moran, Y. Population Specific Adaptations in Venom Production to Abiotic Stressors in a Widely Distributed Cnidarian. bioRxiv 2020. [Google Scholar] [CrossRef]
- Jouiaei, M.; Sunagar, K.; Federman Gross, A.; Scheib, H.; Alewood, P.F.; Moran, Y.; Fry, B.G. Evolution of an Ancient Venom: Recognition of a Novel Family of Cnidarian Toxins and the Common Evolutionary Origin of Sodium and Potassium Neurotoxins in Sea Anemone. Mol. Biol. Evol. 2015, 32, 1598–1610. [Google Scholar] [CrossRef]
- Macrander, J.; Broe, M.; Daly, M. Tissue-specific venom composition and differential gene expression in sea anemones. Genome Biol. Evol. 2016, 8, 2358–2375. [Google Scholar] [CrossRef]
- Macrander, J.; Brugler, M.R.; Daly, M. A RNA-seq approach to identify putative toxins from acrorhagi in aggressive and non-aggressive Anthopleura elegantissima polyps. BMC Genomics 2015, 16. [Google Scholar] [CrossRef]
- Macrander, J.; Daly, M. Evolution of the Cytolytic Pore-Forming Proteins (Actinoporins) in Sea Anemones. Toxins 2016, 8, 368. [Google Scholar] [CrossRef]
- Fenner, P.J.; Williamson, J.A. Worldwide deaths and severe envenomation from jellyfish stings. Med. J. Aust. 1996, 165, 658. [Google Scholar] [CrossRef]
- Currie, B.J.; Jacups, S.P. Prospective study of Chironex fleckeri and other box jellyfish stings in the “Top End” of Australia’s Northern Territory. Med. J. Aust. 2005, 183, 631–636. [Google Scholar] [CrossRef]
- Winter, K.L.; Isbister, G.K.; McGowan, S.; Konstantakopoulos, N.; Seymour, J.E.; Hodgson, W.C. A pharmacological and biochemical examination of the geographical variation of Chironex fleckeri venom. Toxicol. Lett. 2010, 192, 419–424. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, H.; Li, R.; Liu, S.; Xing, R.; Li, P. Insights into individual variations in nematocyst venoms from the giant jellyfish Nemopilema nomurai in the Yellow Sea. Sci. Rep. 2019, 9, 3361. [Google Scholar] [CrossRef]
- Kitahara, M.V.; Jaimes-Becerra, A.; Gamero-Mora, E.; Padilla, G.; Doonan, L.B.; Ward, M.; Marques, A.C.; Morandini, A.C.; Long, P.F. Reciprocal transplantation of the heterotrophic coral Tubastraea coccinea (Scleractinia: Dendrophylliidae) between distinct habitats did not alter its venom toxin composition. Ecol. Evol. 2020, 00, 1–10. [Google Scholar] [CrossRef]
- Kintner, A.H.; Seymour, J.E.; Edwards, S.L. Variation in lethality and effects of two Australian chirodropid jellyfish venoms in fish. Toxicon 2005, 46, 699–708. [Google Scholar] [CrossRef]
- Carrette, T.; Alderslade, P.; Seymour, J. Nematocyst ratio and prey in two Australian cubomedusans, Chironex fleckeri and Chiropsalmus sp. Toxicon 2002, 40, 1547–1551. [Google Scholar] [CrossRef]
- Damian-Serrano, A.; Haddock, S.H.D.; Dunn, C.W. The Evolution of Siphonophore Tentilla as Specialized Tools for Prey Capture. bioRxiv. 2019. [CrossRef]
- Carrette, T.; Straehler-Pohl, I.; Seymour, J. Early Life History of Alatina cfmoseri Populations from Australia and Hawaii with Implications for Taxonomy (Cubozoa: Carybdeida, Alatinidae). PLoS ONE 2014, 9, e84377. [Google Scholar] [CrossRef]
- Straehler-Pohl, I.; Jarms, G. Morphology and life cycle of Carybdea morandinii, sp. nov. (Cnidaria), a cubozoan with zooxanthellae and peculiar polyp anatomy. Zootaxa 2011, 2755, 36–56. [Google Scholar] [CrossRef]
- Klompen, A.; Sanders, S.; Cartwright, P. Influences of functional variation on venom expression in hydractiniid hydrozoans. Presented at the Gordon Research Conference: Venom Evolution, Function and Biomedical Applications, Mount Snow, VT, USA, 5–10 August 2018. [Google Scholar]
- Courtney, R.; Browning, S.; Seymour, J. Early Life History of the ‘Irukandji’ Jellyfish Carukia barnesi. PLoS ONE 2016, 11, e0151197. [Google Scholar] [CrossRef]
- Underwood, A.H.; Seymour, J.E. Venom ontogeny, diet and morphology in Carukia barnesi, a species of Australian box jellyfish that causes Irukandji syndrome. Toxicon 2007, 49, 1073–1082. [Google Scholar] [CrossRef]
- Courtney, R.; Sachlikidis, N.; Jones, R.; Seymour, J. Prey Capture Ecology of the Cubozoan Carukia barnesi. PLoS One 2015, 10, e0124256. [Google Scholar] [CrossRef]
- Gershwin, L.-A. Two new species of jellyfishes (Cnidaria: Cubozoa: Carybdeida) from tropical Western Australia, presumed to cause Irukandji Syndrome. Zootaxa 2005, 1084, 1–30. [Google Scholar] [CrossRef]
- Augusto-de-Oliveira, C.; Stuginski, D.R.; Kitano, E.S.; Andrade-Silva, D.; Liberato, T.; Fukushima, I.; Serrano, S.M.; Zelanis, A. Dynamic Rearrangement in Snake Venom Gland Proteome: Insights into Bothrops jararaca Intraspecific Venom Variation. J. Proteome Res. 2016, 15, 3752–3762. [Google Scholar] [CrossRef]
- Goncalves-Machado, L.; Pla, D.; Sanz, L.; Jorge, R.J.B.; Leitao-De-Araujo, M.; Alves, M.L.M.; Alvares, D.J.; De Miranda, J.; Nowatzki, J.; de Morais-Zani, K.; et al. Combined venomics, venom gland transcriptomics, bioactivities, and antivenomics of two Bothrops jararaca populations from geographic isolated regions within the Brazilian Atlantic rainforest. J. Proteomics 2016, 135, 73–89. [Google Scholar] [CrossRef]
- Huang, H.W.; Liu, B.S.; Chien, K.Y.; Chiang, L.C.; Huang, S.Y.; Sung, W.C.; Wu, W.G. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteomics 2015, 128, 92–104. [Google Scholar] [CrossRef]
- Massey, D.J.; Calvete, J.J.; Sanchez, E.E.; Sanz, L.; Richards, K.; Curtis, R.; Boesen, K. Venom variability and envenoming severity outcomes of the Crotalus scutulatus scutulatus (Mojave rattlesnake) from Southern Arizona. J. Proteomics 2012, 75, 2576–2587. [Google Scholar] [CrossRef]
- Dowell, N.L.; Giorgianni, M.W.; Kassner, V.A.; Selegue, J.E.; Sanchez, E.E.; Carroll, S.B. The Deep Origin and Recent Loss of Venom Toxin Genes in Rattlesnakes. Curr. Biol. 2016, 26, 2434–2445. [Google Scholar] [CrossRef]
- Lyons, K.; Dugon, M.M.; Healy, K. Diet Breadth Mediates the Prey Specificity of Venom Potency in Snakes. Toxins 2020, 12, 74. [Google Scholar] [CrossRef]
- Margres, M.J.; Wray, K.P.; Hassinger, A.T.; Ward, M.J.; McGivern, J.J.; Moriarty Lemmon, E.; Lemmon, A.R.; Rokyta, D.R. Quantity, not quality: rapid adaptation in a polygenic trait proceeded exclusively through expression differentiation. Mol. Biol. Evol. 2017, 34, 3099–3110. [Google Scholar] [CrossRef]
- Dunn, C. Siphonophores. Curr. Biol. 2009, 19, R233–R234. [Google Scholar] [CrossRef]
- Dunn, C.W.; Wagner, G.P. The evolution of colony-level development in the Siphonophora (Cnidaria:Hydrozoa). Dev. Genes Evol. 2006, 216, 743–754. [Google Scholar] [CrossRef]
- Ramos, V.; Vasconcelos, V. Palytoxin and analogs: biological and ecological effects. Mar. Drugs 2010, 8, 2021–2037. [Google Scholar] [CrossRef]
- Wu, C.H. Palytoxin: Membrane mechanisms of action. Toxicon 2009, 54, 1183–1189. [Google Scholar] [CrossRef]
- Mahnir, V.M.; Kozlovskaya, E.P.; Kalinovsky, A.I. Sea anemone Radianthus macrodactylus—A new source of palytoxin. Toxicon 1992, 30, 1449–1456. [Google Scholar] [CrossRef]
- Guppy, R.; Ackbarali, C.; Ibrahim, D. Toxicity of crude organic extracts from the zoanthid Palythoa caribaeorum: A biogeography approach. Toxicon 2019, 167, 117–122. [Google Scholar] [CrossRef]
- Klompen, A.; Sanders, S.; Cartwright, P. Differentially expressed venoms in functionally specialized polyps of the colonial hydrozoan Hydractinia symbiolongicarpus. Presented at the 7th European Evolution and Development Conference, Galway, Ireland, 265–29 June 2018. [Google Scholar]
- Sanders, S.M.; Shcheglovitova, M.; Cartwright, P. Differential gene expression between functionally specialized polyps of the colonial hydrozoan Hydractinia symbiolongicarpus (Phylum Cnidaria). BMC Genom. 2014, 15, 406. [Google Scholar] [CrossRef]
- McCloskey, B. Illustrated Glossary of Sea Anemone Anatomy. Available online: http://archive.li/L7PMk#selection-187.0–186.1 (accessed on 7 February 2020).
- Columbus-Shenkar, Y.Y.; Sachkova, M.Y.; Macrander, J.; Fridrich, A.; Modepalli, V.; Reitzel, A.M.; Sunagar, K.; Moran, Y. Dynamics of venom composition across a complex life cycle. eLife 2018, 7, e35014. [Google Scholar] [CrossRef]
- Bocharova, E.; Kozevich, I. Modes of reproduction in sea anemones (Cnidaria, Anthozoa). Biol. Bull. 2011, 38, 849–860. [Google Scholar] [CrossRef]
- Wallace, C.C. Hexacorals 1: Sea Anemones and Anemone-like Animals (Actiniaria, Zoanthidea, Corallimorpharia, Ceriantharia and Antipatharia). In The Great Barrier Reef: Biology, Environment and Management; Hutchings, P., Kingsford, M., Hoegh-Guldberg, O., Eds.; CSIRO: Collingwood, VIC, Australia, 2008; pp. 198–207. [Google Scholar]
- Schlesinger, A.; Zlotkin, E.; Kramarsky-Winter, E.; Loya, Y. Cnidarian internal stinging mechanism. Proc. R. Soc. B 2009, 276, 1063–1067. [Google Scholar] [CrossRef]
- Han, J.; Zhang, X.; Komiya, T. Integrated Evolution of Cnidarians and Oceanic Geochemistry Before and During the Cambrian Explosion. In The Cnidaria, Past, Present and Future: The World of Medusa and Her Sisters; Goffredo, S., Dubinsky, Z., Eds.; Springer International Publishing: Cham, Switherland, 2016; 2p. [Google Scholar]
- Crowther, A. Character Evolution in Light of Phylogenetic Analysis And Taxonomic Revision of the Zooxanthellate Sea Anemone Families Thalassianthidae and Aliciidae. Ph.D. dissertation, University of Kansas, Lawrence, KS, USA,, 2013. [Google Scholar]
- Östman, C.; Kultima, J.R.; Roat, C.; Rundblom, K. Acontia and mesentery nematocysts of the sea anemone Metridium senile (Linnaeus, 1761) (Cnidaria: Anthozoa). Sci. Mar. 2010, 74, 483–497. [Google Scholar] [CrossRef]
- Lam, J.; Cheng, Y.W.; Chen, W.U.; Li, H.H.; Chen, C.S.; Peng, S.E. A detailed observation of the ejection and retraction of defense tissue acontia in sea anemone (Exaiptasia pallida). PeerJ 2017, 5, 1–11. [Google Scholar] [CrossRef]
- Purcell, J.E. Aggresive function and induced development of catch tentacles in the sea anemone Metridium senile (Coelenterata, Actiniaria). Biol. Bull. 1977, 153, 355–368. [Google Scholar] [CrossRef]
- Watson, G.M.; Mariscal, R.N. Comparative ultrastructure of catch tentacles and feeding tentacles in the sea anemone Haliplanella. Tissue Cell 1983, 15, 939–953. [Google Scholar] [CrossRef]
- Williams, R.B. Acrorhagi, catch tentacles and sweeper tentacles: a synopsis of aggression of actiniarian and scleractinian cnidaria. Hydrobiologia 1991, 216, 539–545. [Google Scholar] [CrossRef]
- Einat, D.L.; Nanette, E.C. Long-term effects of competition on coral growth and sweeper tentacle development. Mar. Ecol.: Prog. Ser. 2006, 313, 115–123. [Google Scholar] [CrossRef][Green Version]
- Sebens, K.P.; Miles, J.S. Sweeper Tentacles in a Gorgonian Octocoral: Morphological Modifications for Interference Competition. Biol. Bull. 1988, 175, 378–387. [Google Scholar] [CrossRef]
- Daly, M. The anatomy, terminology, and homology of acrorhagi and pseudoacrorhagi in sea anemones. Zool. Verh. 2003, 345, 89–102. [Google Scholar]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. Pharmacologically distinct cardiovascular effects of box jellyfish (Chironex fleckeri) venom and a tentacle-only extract in rats. Toxicol. Lett. 2005, 155, 219–226. [Google Scholar] [CrossRef]
- Ponce, D.; Brinkman, D.L.; Potriquet, J.; Mulvenna, J. Tentacle Transcriptome and Venom Proteome of the Pacific Sea Nettle, Chrysaora fuscescens (Cnidaria: Scyphozoa). Toxins 2016, 8, 102. [Google Scholar] [CrossRef]
- Ponce, D.; López-Vera, E.; Aguilar, M.B.; Sánchez-Rodríguez, J. Preliminary Results of the in Vivo and in Vitro Characterization of a Tentacle Venom Fraction from the Jellyfish Aurelia aurita. Toxins 2013, 5, 2420–2433. [Google Scholar] [CrossRef]
- Moran, Y.; Praher, D.; Schlesinger, A.; Ayalon, A.; Tal, Y.; Technau, U. Analysis of soluble protein contents from the nematocysts of a model sea anemone sheds light on venom evolution. Mar. Biotechnol. 2013, 15, 329–339. [Google Scholar] [CrossRef]
- Bastos, C.L., Jr.; Varela, A.S., Jr.; Ferreira, S.P.; Nornberg, B.F.; Boyle, R.T. Who knows not where an anemone does wear his sting? Could polypeptides released from the columnar vesicles of Bunodosoma cangicum induce apoptosis in the ZF-L cell line? Toxicon 2016, 124, 73–82. [Google Scholar] [CrossRef]
- Moran, Y.; Genikhovich, G.; Gordon, D.; Wienkoop, S.; Zenkert, C.; Özbek, S.; Technau, U.; Gurevitz, M. Neurotoxin localization to ectodermal gland cells uncovers an alternative mechanism of venom delivery in sea anemones. Proc. R. Soc. B 2012, 279, 1351–1358. [Google Scholar] [CrossRef]
- Roveta, C.; Pica, D.; Puce, S. The cnidome of Olindias muelleri (Cnidaria: Hydrozoa: Limnomedusae) from South Adriatic Sea. Zoomorphology 2019, 138, 437–442. [Google Scholar] [CrossRef]
- Acuña, F.H.; Excoffon, A.C.; Ricci, L. Composition, biometry and statistical relationships between the cnidom and body size in the sea anemone Oulactis muscosa (Cnidaria: Actiniaria). J. Mar. Biol. Assoc. UK 2007, 87, 415–419. [Google Scholar] [CrossRef]
- Ardelean, A.; Fautin, D. Variability in nematocysts from a single individual of the sea anemone Actinodendron arboreum (Cnidaria: Anthozoa: Actiniaria). Hydrobiologia 2004, 530, 189–197. [Google Scholar] [CrossRef]
- Reft, A.J.; Daly, M. Morphology, distribution, and evolution of apical structure of nematocysts in hexacorallia. J. Morphol. 2012, 273, 121–136. [Google Scholar] [CrossRef]
- Avian, M.; Malej, A. Aurelia polyps and medusae (Scyphozoa; Semaeostomeae;Ulmaridae) in the Northern Adriatic: their cnidome and ecology. In PERSEUS International Workshop “Coming to Grips with the Jellyfish Phenomenon in the Southern European and other Seas: Research to The Rescue of Coastal Managers”; Prieto, L., Deidun, A., Malej, A., Shiganova, T., Tirelli, V., Eds.; Perseus: Cadiz, Spain, 2015; p. 17. [Google Scholar]
- McClounan, S.; Seymour, J. Venom and cnidome ontogeny of the cubomedusae Chironex fleckeri. Toxicon 2012, 60, 1335–1341. [Google Scholar] [CrossRef]
- Di Camillo, C.; Bo, M.; Puce, S.; Tazioli, S.; Bavestrello, G. The cnidome of Carybdea marsupialis (Cnidaria: Cubomedusae) from the Adriatic Sea. J. Mar. Biol. Assoc. UK 2006, 86, 705–709. [Google Scholar] [CrossRef]
- Peach, M.B.; Pitt, K.A. Morphology of the nematocysts of the medusae of two scyphozoans, Catostylus mosaicus and Phyllorhiza punctata (Rhizostomeae): implications for capture of prey. Invertebr. Biol. 2005, 124, 98–108. [Google Scholar] [CrossRef]
- Spier, D.; Stampar, S.N.; Prantoni, A.L. New record of the endangered cerianthid Ceriantheomorphe brasiliensis (Cnidaria: Hexacorallia) in Paranaguá Bay, southern Brazil. Mar. Biodivers. Rec. 2012, 5. [Google Scholar] [CrossRef]
- Strömberg, S.M.; Östman, C. The cnidome and internal morphology of Lophelia pertusa (Linnaeus, 1758) (Cnidaria, Anthozoa). Acta Zool. 2017, 98, 191–213. [Google Scholar] [CrossRef]
- Acuña, F.; Excoffon, A.; McKinstry, S.; Martínez, D. Characterization of Aulactinia (Actiniaria: Actiniidae) species from Mar del Plata (Argentina) using morphological and molecular data. Hydrobiologia 2007, 592, 249–256. [Google Scholar] [CrossRef]
- Hwang, J.S.; Ohyanagi, H.; Hayakawa, S.; Osato, N.; Nishimiya-Fujisawa, C.; Ikeo, K.; David, C.N.; Fujisawa, T.; Gojobori, T. The evolutionary emergence of cell type-specific genes inferred from the gene expression analysis of Hydra. Proc. Natl. Acad. Sci. USA 2007, 104, 14735. [Google Scholar] [CrossRef]
- Sebé-Pedrós, A.; Saudemont, B.; Chomsky, E.; Plessier, F.; Mailhé, M.-P.; Renno, J.; Loe-Mie, Y.; Lifshitz, A.; Mukamel, Z.; Schmutz, S.; et al. Cnidarian Cell Type Diversity and Regulation Revealed by Whole-Organism Single-Cell RNA-Seq. Cell 2018, 173, 1520–1534. [Google Scholar] [CrossRef]
- Darling, J.A.; Reitzel, A.R.; Burton, P.M.; Mazza, M.E.; Ryan, J.F.; Sullivan, J.C.; Finnerty, J.R. Rising starlet: the starlet sea anemone, Nematostella vectensis. BioEssays 2005, 27, 211–221. [Google Scholar] [CrossRef]
- Putnam, N.H.; Srivastava, M.; Hellsten, U.; Dirks, B.; Chapman, J.; Salamov, A.; Terry, A.; Shapiro, H.; Lindquist, E.; Kapitonov, V.V.; et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 2007, 317, 86–94. [Google Scholar] [CrossRef]
- Sunagar, K.; Columbus-Shenkar, Y.Y.; Fridrich, A.; Gutkovich, N.; Aharoni, R.; Moran, Y. Cell type-specific expression profiling unravels the development and evolution of stinging cells in sea anemone. BMC Biol. 2018, 16, 108. [Google Scholar] [CrossRef]
- St Pierre, L.; Woods, R.; Earl, S.; Masci, P.P.; Lavin, M.F. Identification and analysis of venom gland-specific genes from the coastal taipan (Oxyuranus scutellatus) and related species. Cell Mol. Life Sci. 2005, 62, 2679–2693. [Google Scholar] [CrossRef]
- Rattmann, Y.D.; Pereira, C.R.; Cury, Y.; Gremski, W.; Marques, M.C.; da Silva-Santos, J.E. Vascular permeability and vasodilation induced by the Loxosceles intermedia venom in rats: involvement of mast cell degranulation, histamine and 5-HT receptors. Toxicon 2008, 51, 363–372. [Google Scholar] [CrossRef]
- Sade, Y.B.; Boia-Ferreira, M.; Gremski, L.H.; da Silveira, R.B.; Gremski, W.; Senff-Ribeiro, A.; Chaim, O.M.; Veiga, S.S. Molecular cloning, heterologous expression and functional characterization of a novel translationally-controlled tumor protein (TCTP) family member from Loxosceles intermedia (brown spider) venom. Int J. Biochem Cell Biol. 2012, 44, 170–177. [Google Scholar] [CrossRef]
- Surm, J.M.; Stewart, Z.K.; Papanicolaou, A.; Pavasovic, A.; Prentis, P.J. The draft genome of Actinia tenebrosa reveals insights into toxin evolution. Ecol. Evol. 2019, 9, 11314–11328. [Google Scholar] [CrossRef]
- Ohdera, A.; Ames, C.L.; Dikow, R.B.; Kayal, E.; Chiodin, M.; Busby, B.; La, S.; Pirro, S.; Collins, A.G.; Medina, M.; et al. Box, stalked, and upside-down? Draft genomes from diverse jellyfish (Cnidaria, Acraspeda) lineages: Alatina alata (Cubozoa), Calvadosia cruxmelitensis (Staurozoa), and Cassiopea xamachana (Scyphozoa). GigaScience 2019, 8. [Google Scholar] [CrossRef]
- Helmkampf, M.; Bellinger, M.R.; Geib, S.M.; Sim, S.B.; Takabayashi, M. Draft Genome of the Rice Coral Montipora capitata Obtained from Linked-Read Sequencing. Genome Biol. Evol. 2019, 11, 2045–2054. [Google Scholar] [CrossRef]
- Ying, H.; Hayward, D.C.; Cooke, I.; Wang, W.; Moya, A.; Siemering, K.R.; Sprungala, S.; Ball, E.E.; Foret, S.; Miller, D.J. The Whole-Genome Sequence of the Coral Acropora millepora. Genome Biol. Evol. 2019, 11, 1374–1379. [Google Scholar] [CrossRef]
- Wilding, C.S.; Fletcher, N.; Smith, E.K.; Prentis, P.; Weedall, G.D.; Stewart, Z. The genome of the sea anemone Actinia equina (L.): Meiotic toolkit genes and the question of sexual reproduction. Mar. Genomics 2020, 100753. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; MacRaild, C.A.; Sunanda, P.; Morales, R.A.; Peigneur, S.; Macrander, J.; Heidi, H.Y.; Daly, M.; Raghothama, S.; Dhawan, V.; et al. Structure, folding and stability of a minimal homologue from Anemonia sulcata of the sea anemone potassium channel blocker ShK. Peptides 2018, 99, 169–178. [Google Scholar] [CrossRef]
- Krishnarjuna, B.; Villegas-Moreno, J.; Mitchell, M.L.; Csoti, A.; Peigneur, S.; Amero, C.; Pennington, M.W.; Tytgat, J.; Panyi, G.; Norton, R.S. Synthesis, folding, structure and activity of a predicted peptide from the sea anemone Oulactis sp. with an ShKT fold. Toxicon 2018, 150, 50–59. [Google Scholar] [CrossRef]
- Lachumanan, R.; Armugam, A.; Durairaj, P.; Gopalakrishnakone, P.; Tan, C.H.; Jeyaseelan, K. In situ hybridization and immunohistochemical analysis of the expression of cardiotoxin and neurotoxin genes in Naja naja sputatrix. J. Histochem. Cytochem. 1999, 47, 551–560. [Google Scholar] [CrossRef]
- Ogawa, T.; Onoue, H.; Nakagawa, K.; Nomura, S.; Sueishi, K.; Hattori, S.; Kihara, H.; Ohno, M. Localization and expression of phospholipases A2 in Trimeresurus flavoviridis (habu snake) venom gland. Toxicon 1995, 33, 1645–1652. [Google Scholar] [CrossRef]
- Wolenski, F.S.; Layden, M.J.; Martindale, M.Q.; Gilmore, T.D.; Finnerty, J.R. Characterizing the spatiotemporal expression of RNAs and proteins in the starlet sea anemone, Nematostella vectensis. Nat. Protoc. 2013, 8, 900–915. [Google Scholar] [CrossRef]
- Sachkova, M.Y.; Singer, S.A.; Macrander, J.; Reitzel, A.M.; Peigneur, S.; Tytgat, J.; Moran, Y. The birth and death of toxins with distinct functions: a case study in the sea anemone Nematostella. Mol. Biol. Evol. 2019, 36. [Google Scholar] [CrossRef]
- Wikramanayake, A.H.; Hong, M.; Lee, P.N.; Pang, K.; Byrum, C.A.; Bince, J.M.; Xu, R.; Martindale, M.Q. An ancient role for nuclear β-catenin in the evolution of axial polarity and germ layer segregation. Nature 2003, 426, 446–450. [Google Scholar] [CrossRef]
- Richter, S.; Helm, C.; Meunier, F.A.; Hering, L.; Campbell, L.I.; Drukewitz, S.H.; Undheim, E.A.; Jenner, R.A.; Schiavo, G.; Bleidorn, C. Comparative analyses of glycerotoxin expression unveil a novel structural organization of the bloodworm venom system. BMC Evol. Biol. 2017, 17, 64. [Google Scholar] [CrossRef]
- Basulto, A.; Pérez, V.M.; Noa, Y.; Varela, C.; Otero, A.J.; Pico, M. Immunohistochemical targeting of sea anemone cytolysins on tentacles, mesenteric filaments and isolated nematocysts of Stichodactyla helianthus. J. Exp. Zool., Part. A 2006, 305, 253–258. [Google Scholar] [CrossRef]
- Horgan, R.P.; Kenny, L.C. ‘Omic’ technologies: genomics, transcriptomics, proteomics and metabolomics. Obstet. Gynaecol. 2011, 13, 189–195. [Google Scholar] [CrossRef]
- Chappell, L.; Russell, A.J.C.; Voet, T. Single-Cell (Multi)omics Technologies. Annu. Rev. Genomics Hum. Genet. 2018, 19, 15–41. [Google Scholar] [CrossRef]
- Angel, P.M.; Caprioli, R.M. Matrix-assisted laser desorption ionization imaging mass spectrometry: in situ molecular mapping. Biochemistry 2013, 52, 3818–3828. [Google Scholar] [CrossRef]
- Chen, X.; Leahy, D.; Van Haeften, J.; Hartfield, P.; Prentis, P.J.; van der Burg, C.A.; Surm, J.M.; Pavasovic, A.; Madio, B.; Hamilton, B.R. A versatile and robust serine protease inhibitor scaffold from Actinia tenebrosa. Mar. Drugs 2019, 17, 701. [Google Scholar] [CrossRef]
- Mitchell, M.L.; Hamilton, B.R.; Madio, B.; Morales, R.A.V.; Tonkin-Hill, G.Q.; Papenfuss, A.T.; Purcell, A.W.; King, G.F.; Undheim, E.A.B.; Norton, R.S. The use of imaging mass spectrometry to study peptide toxin distribution in Australian sea anemones. Aust. J. Chem. 2017, 70, 1235–1237. [Google Scholar] [CrossRef]
- Undheim, E.A.; Sunagar, K.; Hamilton, B.R.; Jones, A.; Venter, D.J.; Fry, B.G.; King, G.F. Multifunctional warheads: Diversification of the toxin arsenal of centipedes via novel multidomain transcripts. J. Proteomics 2014, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Undheim, E.A.; Hamilton, B.R.; Kurniawan, N.D.; Bowlay, G.; Cribb, B.W.; Merritt, D.J.; Fry, B.G.; King, G.F.; Venter, D.J. Production and packaging of a biological arsenal: Evolution of centipede venoms under morphological constraint. Proc. Natl. Acad. Sci. USA 2015, 112, 4026–4031. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.R.; Marshall, D.L.; Casewell, N.R.; Harrison, R.A.; Blanksby, S.J.; Undheim, E.A.B. Mapping enzyme activity on tissue by functional-mass spectrometry imaging. Angew. Chem. Int. Edit. 2019. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).