The Phylum Bryozoa: From Biology to Biomedical Potential
Abstract
1. Introduction
2. Bryozoans
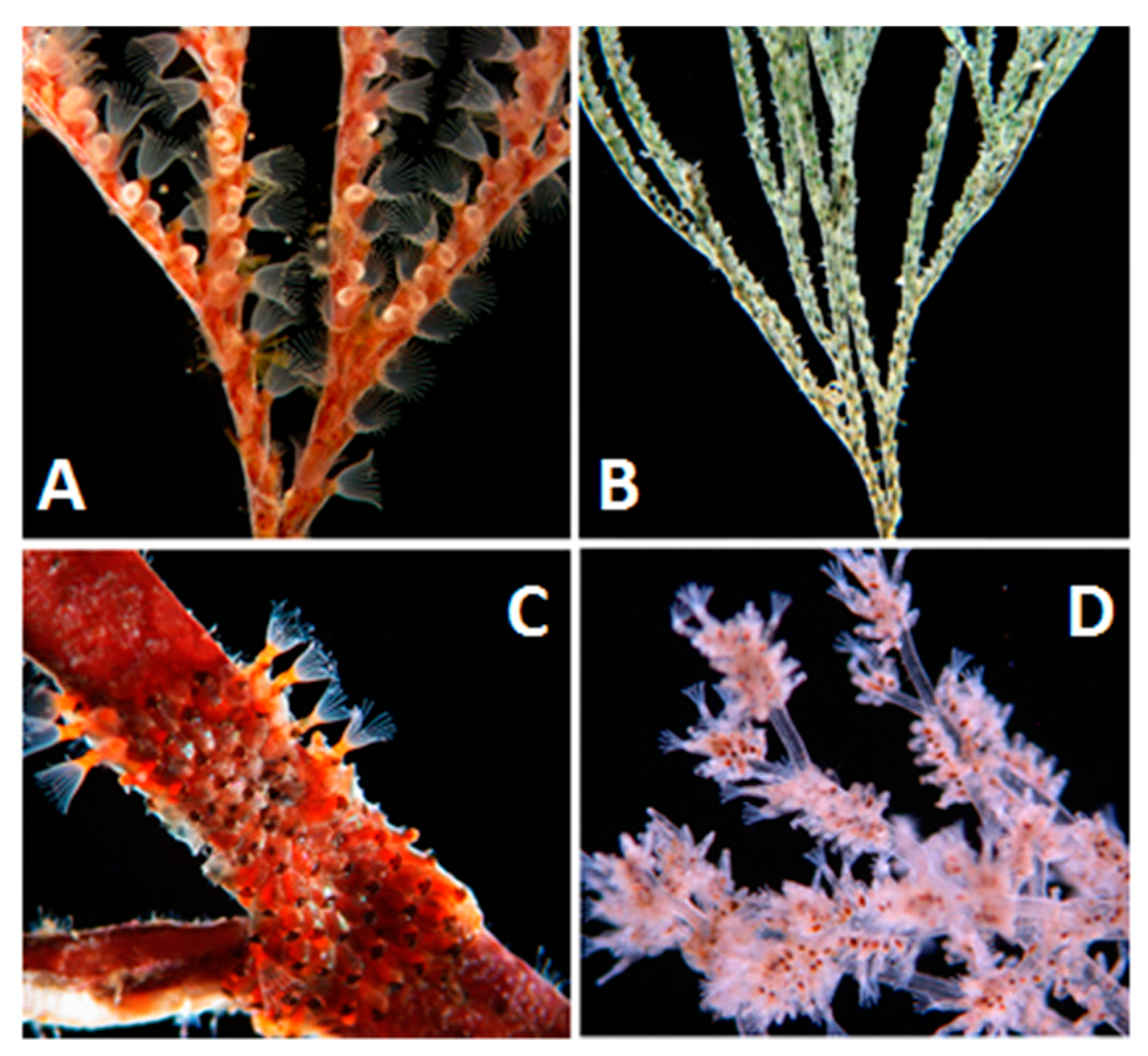
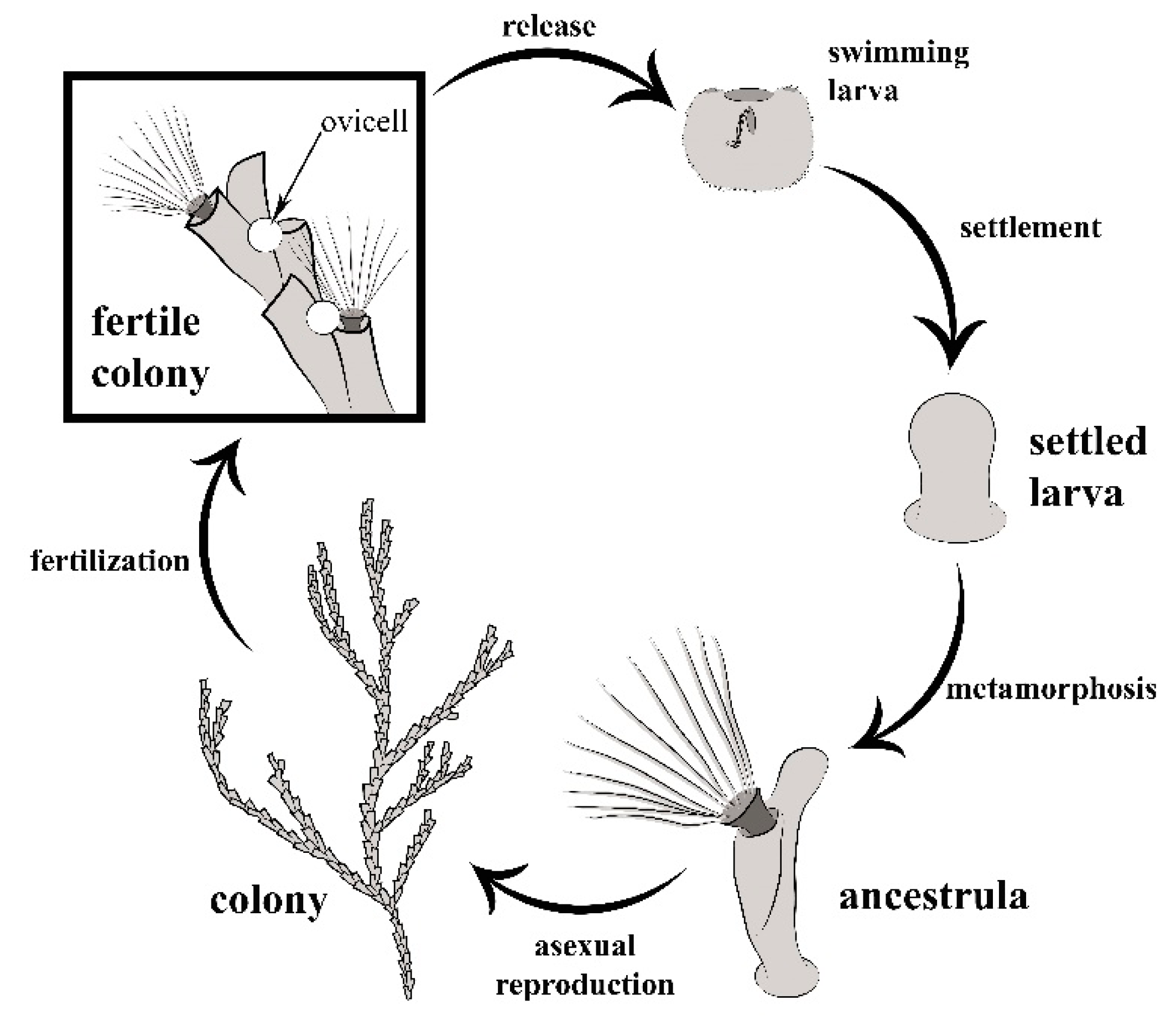
2.1. General Biology
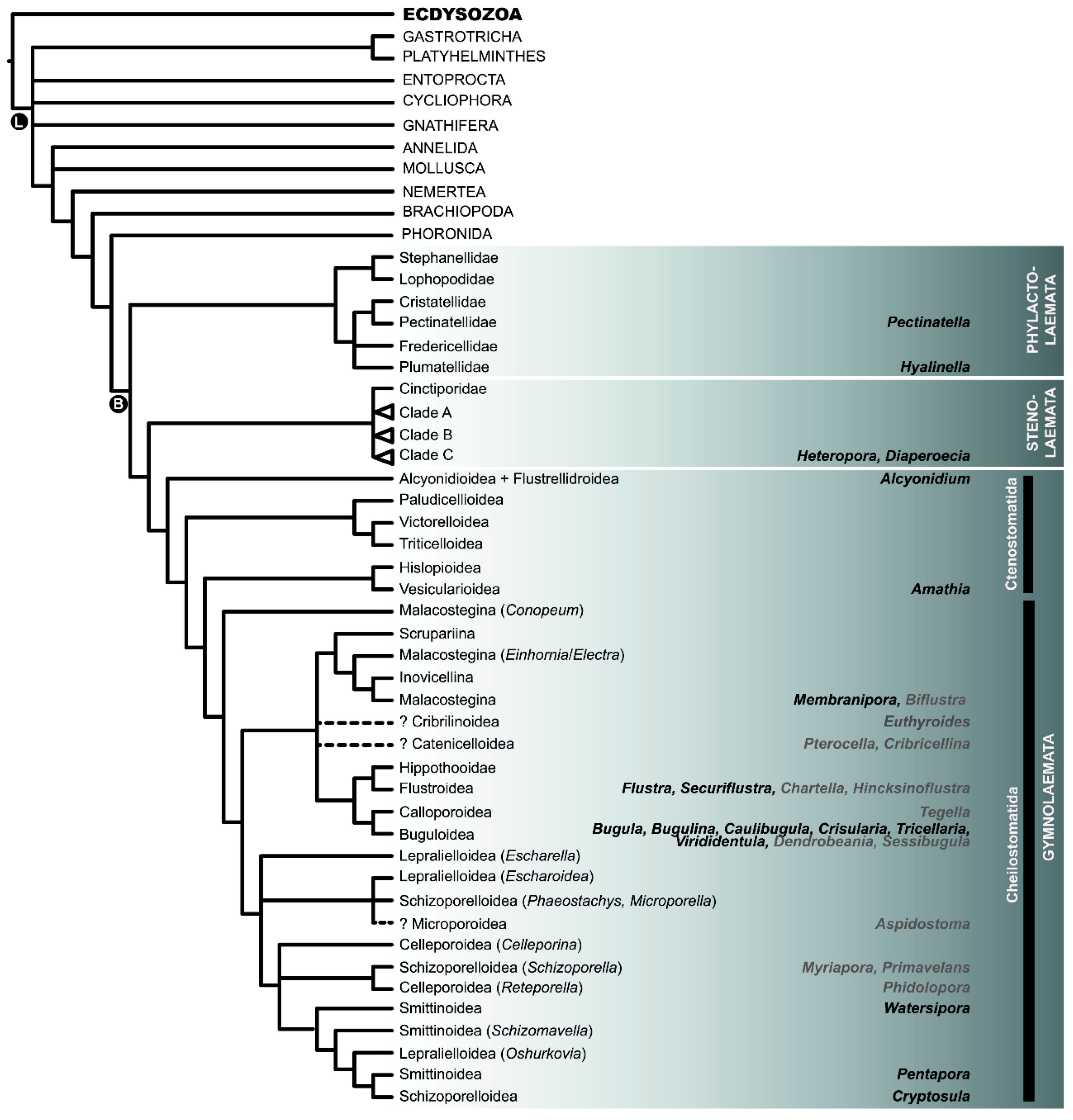
2.2. Phylogeny
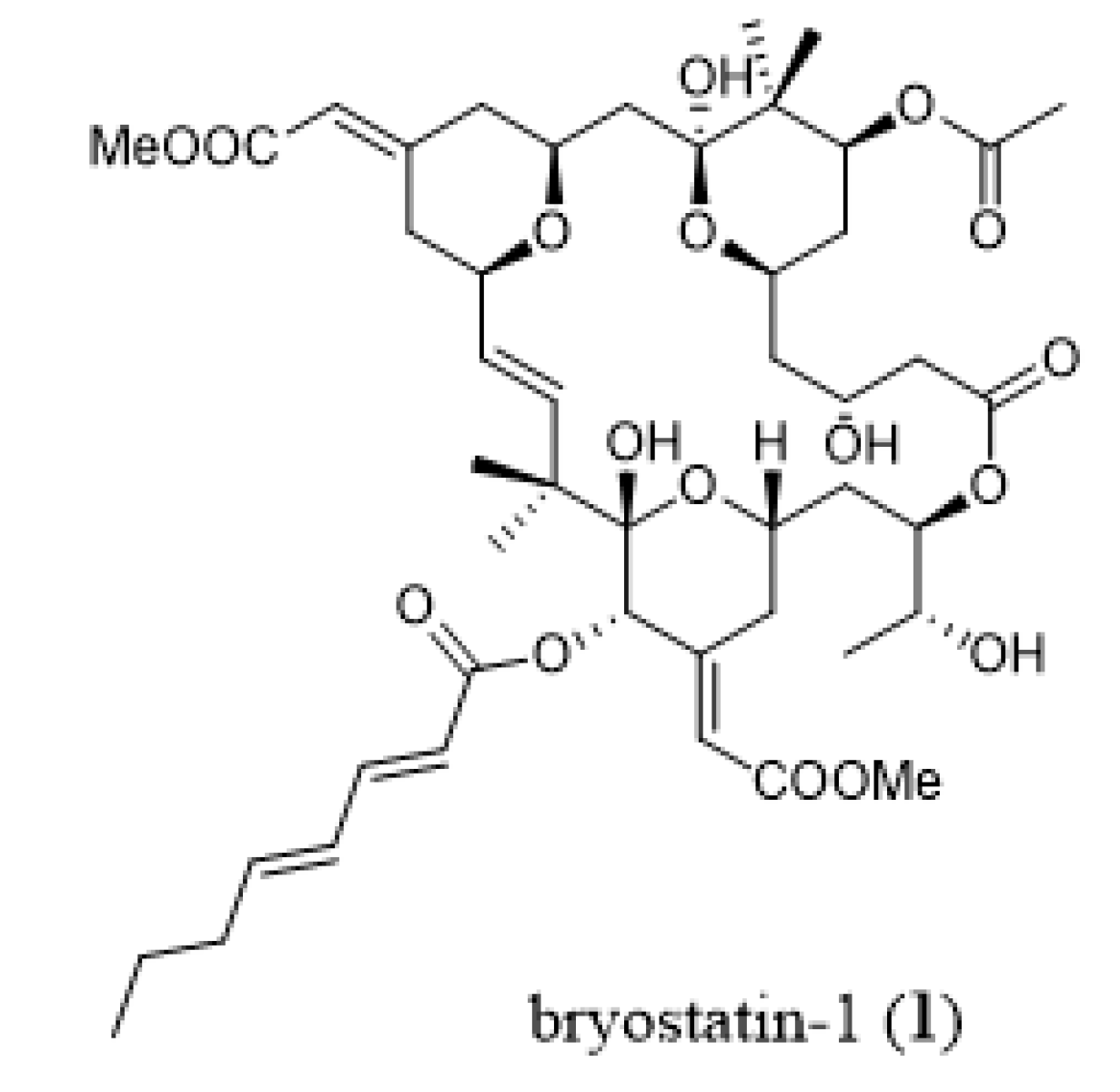
2.3. Chemoecology
3. Bryozoans as Sources of Novel Anticancer Drugs
3.1. Cancer Stem Cells
3.2. Multidrug Resistance (MDR) Phenotype
3.3. Resistance to Pro-Apoptotic Stimuli
4. Bryozoan-Derived Metabolites Active in the Brain
4.1. Alzheimer’s Disease (AD)
4.2. Post-Stroke Activities
4.3. Antiparkinson Activity
5. Antiviral Bryozoan-Derived Metabolites
5.1. Human Immunodeficiency Virus-1 (HIV-1)
5.2. Chikungunya Virus (CHIKV)
5.3. Polio Virus
6. Antiparasitic Bryozoan-Derived Metabolites
6.1. Antitrypanosomal Activity
6.2. Nematocidal Activity
6.3. Anti-Plasmodial Activity
7. Sourcing of Bryozoan-Derived Compounds of Medicinal Interest
7.1. Harvesting Wild Bryozoans
7.2. Culturing Bryozoans
7.3. Heterologous Production
7.4. Compound Extraction
8. Synthesis
8.1. Chemical Synthesis
- a)
- Further research into the development of shorter synthetic routes that produce bioactive compounds for downstream exploitation by the pharmaceutical industry.
- b)
- Increased investigations into simplified analogues of bryozoan origin. The design and synthesis of structurally and stereochemically simplified bioactive compounds inspired by natural products will in itself result in shorter synthetic pathways that should be amenable to scale-up. These simplified analogues could be initially accessed through synthetic derivatizations and manipulations, or through modification of biosynthetic pathways.
- c)
- Increased development of processes aiming at the production of bioactive compounds or advanced biosynthetic intermediates. Multidisciplinary collaborations involving the fields of genetics [223,224], microbiology [225,226], and bioengineering [227] should allow for the identification of the microbial producers of important compounds, the genetic “up-scaling” required for production of the compounds and the expertise needed for large scale production and subsequent isolation of the desired bioactive compounds. These strategies are likely necessary to replace the large-scale cultivation/collection of marine organisms, utilized for some marine-derived compounds which, to date, have often resulted in enough compound for clinical trials (but unfortunately not enough for clinical application) [228]. It should also be noted that some of these biotechnological approaches could be aimed at the production of advanced intermediates which could then be utilized further as semisynthetic strategies to afford the bioactive natural products or their derivatives/analogues. For reviews on this theme regarding accessing the bryostatins, see [228,229]. Wu et al. (2020) recently published a comprehensive review entitled “Unlocking the Drug Potential of the Bryostatin Family: Recent Advances in Product Synthesis and Biomedical Applications”. It could be argued that as more compounds of bryozoan origin are being identified as having valuable medicinal application (essentially on a yearly basis [9,230]), the pressure of combining advanced biotechnology and synthetic organic chemistry to provide significant amounts (gram to kilogram scale for clinical trials and even larger amounts for clinical application) will surely increase. It is thus up to the combined fields of natural products isolation and characterization, biochemical and biological evaluations, microbiology, biotechnology, and synthetic chemistry to come up with solutions for this important challenge.
8.2. Partial Synthesis
8.3. Total Synthesis
8.4. Biosynthesis
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Pettit, G.R. Progress in the discovery of biosynthetic anticancer drugs. J. Nat. Prod. 1996, 59, 812–821. [Google Scholar] [CrossRef]
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.A.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomic era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- David, B.; Wolfender, J.L.; Dias, D.A. The pharmaceutical industry and natural products: Hystorical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef]
- Vinothkumar, S.; Parameswaran, P.S. Recent advances in marine drug research. Biotechnol. Adv. 2013, 31, 1826–1845. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef]
- Tian, X.R.; Tang, H.F.; Tian, X.L.; Hu, J.J.; Huang, L.L.; Gustafson, K.R. Review of bioactive secondary metabolites from marine bryozoans in the progress of new drugs discovery. Future Med. Chem. 2018, 10, 1497–1514. [Google Scholar] [CrossRef]
- Wu, R.; Chen, H.; Chang, N.; Xu, Y.; Jiao, J.; Zhang, H. Unlocking the drug potential of the bryostatin family: Recent advances on product synthesis and biomedical applications. Chem. Eur. J. 2020, 26, 1166–1195. [Google Scholar] [CrossRef]
- Figuerola, B.; Avila, C. The Phylum Bryozoa as promising source of anticancer drugs. Mar. Drugs 2019, 17, 477. [Google Scholar] [CrossRef]
- Bock, P.E.; Gordon, D.P. Phylum Bryozoa Ehrenberg, 1831. Zootaxa 2013, 3703, 67–74. [Google Scholar] [CrossRef]
- McKinney, F.K.; Jackson, J.B.C. Bryozoan Evolution, Special Topics in Paleontology Series; Unwin Hyman: Boston, MA, USA, reprinted by University of Chicago Press, Chicago, IL, USA; 1989. [Google Scholar]
- O’Dea, A. Relation of form to life habit in free-living cupuladriid bryozoans. Aquat. Biol. 2009, 7, 1–18. [Google Scholar] [CrossRef]
- Ostrovsky, A.N. Evolution of Sexual Reproduction in Marine Invertebrates: Example of Gymnolaemate Bryozoans; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Gordon, D.P. The Aging Process in Bryozoans. In Biology of Bryozoans; Woollacott, R.M., Zimmer, R.L., Eds.; Academic Press: New York, NY, USA, 1977; pp. 335–376. [Google Scholar]
- Rønsted, N.; Symonds, M.R.; Birkholm, T.; Christensen, S.B.; Meerow, A.W.; Molander, M.; Mølgaard, P.; Petersen, G.; Rasmussen, N.; van Staden, J.; et al. Can phylogeny predict chemical diversity and potential medicinal activity of plants? A case study of Amaryllidaceae. BMC Evol. Biol. 2012, 12, 182. [Google Scholar] [CrossRef]
- Ernst, M.; Saslis-Lagoudakis, C.H.; Grace, O.M.; Nilsson, N.; Simonsen, H.T.; Horn, J.W.; Rønsted, N. Evolutionary prediction of medicinal properties in the genus Euphorbia, L. Sci. Rep. 2016, 6, 30531. [Google Scholar] [CrossRef]
- Saslis-Lagoudakis, C.H.; Savolainen, V.; Williamson, E.M.; Forest, F.; Wagstaff, S.J.; Baral, S.R.; Watson, M.F.; Pendry, C.A.; Hawkins, J.A. Phylogenies reveal predictive power of traditional medicine in bioprospecting. Proc. Natl. Acad. Sci. USA 2012, 109, 15835–15840. [Google Scholar] [CrossRef]
- Ruggiero, M.A.; Gordon, D.P.; Orrell, T.M.; Bailly, N.; Bourgoin, T.; Brusca, R.C.; Cavalier-Smith, T.; Guiry, M.D.; Kirk, P.M. A higher level classification of all living organisms. PLoS ONE 2015, 10, e0119248. [Google Scholar] [CrossRef]
- Giribet, G.; Edgecombe, G.D. The Invertebrate Tree of Life; Princeton University Press: Princeton, NJ, USA, 2020. [Google Scholar]
- Bock, P. Bryozoa. Accessed through World Register of Marine Species. 2014. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=146142 (accessed on 20 January 2018).
- Waeschenbach, A.; Cox, C.J.; Littlewood, D.T.J.; Porter, J.S.; Taylor, P.D. First molecular estimate of cyclostome bryozoan phylogeny confirms extensive homoplasy among skeletal characters used in traditional taxonomy. Mol. Phylogenet. Evol. 2009, 52, 241–251. [Google Scholar] [CrossRef]
- Laumer, C.E.; Bekkouche, N.; Kerbl, A.; Goetz, F.; Neves, R.C.; Serensen, M.V.; Kristensen, R.M.; Hejnol, A.; Dunn, C.W.; Giribet, G.; et al. Spiralian phylogeny informs the evolution of microscopic lineages. Curr. Biol. 2015, 25, 2000–2006. [Google Scholar] [CrossRef]
- Nesnidal, M.P.; Helmkampf, M.; Meyer, A.; Witek, A.; Bruchhaus, I.; Ebersberger, I.; Hankeln, T.; Lieb, B.; Struck, T.H.; Hausdorf, B. New phylogenomic data support the monophyly of Lophophorata and an Ectoproct-Phoronid clade and indicate that Polyzoa and Kryptrochozoa are caused by systematic bias. BMC Evol. Biol. 2013, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Waeschenbach, A.; Taylor, P.D.; Littlewood, D.T.J. A molecular phylogeny of bryozoans. Mol. Phylogenet. Evol. 2012, 62, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Balounová, Z.; Pechoušková, E.; Rajchard, J.; Joza, V.; Šinko, J. World-wide distribution of the bryozoan Pectinatella magnifica (Leidy 1851). Eur. J. Environ. Sci. 2013, 3, 96–100. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Cui, Y. Pectinatella magnifica (Leidy, 1851) (Bryozoa, Phylactolaemata), a biofouling bryozoan recently introduced to China. Chin. J. Oceanol. Limnol. 2016, 35, 815–820. [Google Scholar] [CrossRef]
- Hartikainen, H.; Waeschenbach, A.; Wöss, E.; Wood, T.; Okamura, B. Divergence and species discrimination in freshwater bryozoans (Bryozoa: Phylactolaemata). Zool. J. Linn. Soc. 2013, 168, 61–80. [Google Scholar] [CrossRef]
- Okuyama, M.; Wada, H.; Ishii, T. Phylogenetic relationships of freshwater bryozoans (Ectoprocta, Phylactolaemata) inferred from mitochondrial ribosomal DNA sequences. Zool. Scr. 2006, 35, 243–249. [Google Scholar] [CrossRef]
- Hirose, M.; Dick, M.H.; Mawatari, S.F. Are plumatellid statoblasts in freshwater bryozoans phylogenetically informative? Zool. Sci. 2011, 28, 318–326. [Google Scholar] [CrossRef]
- Rubini, A.; Pieroni, G.; Elia, A.C.; Zippilli, L.; Paolocci, F.; Taticchi, M.I. Novel morphological and genetic tools to discriminate species among the family Plumatellidae (Phylactolaemata, Bryozoa). Hydrobiologia 2011, 664, 81–93. [Google Scholar] [CrossRef]
- Pejin, B.; Ciric, A.; Karaman, I.; Horvatovic, M.; Glamoclija, J.; Nikolic, M.; Sokovic, M. In vitro antibiofilm activity of the freshwater bryozoan Hyalinella punctata: A case study of Pseudomonas aeruginosa PAO1. J. Nat. Prod. Res. 2016, 30, 1847–1850. [Google Scholar] [CrossRef]
- Pejin, B.; Nakarada, D.; Novakovic, M.; Tesevic, V.; Savic, A.; Radotic, K.; Mojovic, M. Antioxidant volatiles of the freshwater bryozoan Hyalinella punctata. J. Nat. Prod. Res. 2014, 28, 1471–1475. [Google Scholar] [CrossRef]
- Kollár, P.; Šmejkal, K.; Salmonová, H.; Vlková, E.; Lepšová-Skácelová, O.; Balounová, Z.; Rajchard, J.; Cvačka, J.; Jaša, L.; Babica, P.; et al. Assessment of Chemical Impact of Invasive Bryozoan Pectinatella magnifica on the Environment: Cytotoxicity and Antimicrobial Activity of P. magnifica Extracts. Molecules 2016, 21, 1476. [Google Scholar] [CrossRef]
- Pejin, B.; Glamoclija, J.; Ciric, A.; Radotic, K.; Vajs, V.; Tesevic, V.; Hegedis, A.; Karaman, I.; Horvatovic, M.; Sokovic, M. Antimicrobial activity of the freshwater bryozoan Hyalinella punctata (Hancock, 1850). Digest J. Nanomater. Biostruct. 2012, 7, 1021–1026. [Google Scholar]
- Pejin, B.; Savic, A.G.; Hegedis, A.; Karaman, I.; Horvatovic, M.; Mojovic, M. A bryozoan species may offer novel antioxidants with anti-carbon-dioxide anion radical activity. J. Nat. Prod. Res. 2014, 28, 2057–2060. [Google Scholar] [CrossRef]
- Knight, S.; Gordon, D.P.; Lavery, S.D. A multi-locus analysis of phylogenetic relationships within cheilostome bryozoans supports multiple origins of ascophoran frontal shields. Mol. Phylogenet. Evol. 2011, 61, 351–362. [Google Scholar] [CrossRef]
- Taylor, P.D.; Waeschenbach, A.; Florence, W. Phylogenetic position and systematics of the bryozoans Tennysonia: Further evidence for convergence and plasticity in skeletal morphology among cyclostome bryozoans. Zootaxa 2011, 3010, 58–68. [Google Scholar] [CrossRef]
- Taylor, P.D.; Waeschenbach, A.; Smith, A.M.; Gordon, D.P. In search of phylogenetic congruence between molecular and morphological data in bryozoans with extreme adult skeletal heteromorphy. Syst. Biodivers. 2015, 3, 525–544. [Google Scholar] [CrossRef]
- Tischer, M.; Ayer, S.W.; Andersen, R.J. Nitrophenols from Northest Pacific bryozoans. Comp. Biochem. Physiol. 1986, 84, 43–45. [Google Scholar]
- Fehlauer-Ale, K.H.; Vieira, L.M.; Winston, J.E. Molecular and morphological characterization of Amathia distans Busk and Amathia brasiliensis Busk (Bryozoa: Ctenostomata) from the tropical and subtropical Western Atlantic. Zootaxa 2011, 2962, 49–62. [Google Scholar] [CrossRef]
- Waeschenbach, A.; Vieira, L.M.; Reverter-Gil, O.; Souto-Derungs, J.; Nascimento, K.B.; Fehlauer-Ale, K.H. A phylogeny of Vesiculariidae (Bryozoa, Ctenostomata) supports synonymization of three genera and reveals possible cryptic diversity. Zool. Scr. 2015, 44, 667–683. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, H.-J.; Liu, X.-F.; Chen, W.-S.; Tang, H.-F.; Lin, H.-W. Oxygenated steroids from marine bryozoan Biflustra grandicella. Biochem. Syst. Ecol. 2009, 37, 686–689. [Google Scholar] [CrossRef]
- Blackman, A.J.; Ralph, C.E.; Skelton, B.W.; White, A.H. Two sulphur-containing isoquinoline alkaloids from the bryozoan Biflustra perfragilis. Aust. J. Chem. 1993, 46, 213–220. [Google Scholar] [CrossRef]
- Gordon, D.P. Towards a Phylogeny of Cheilostomes—Morphological Models of Frontal Wall/Shield Evolution. In Proceedings of the 11th International Bryozoology Association Conference, Balboa, Republic of Panama, 26–31 January 1998; pp. 17–37. [Google Scholar]
- Morris, B.D.; Prinsep, M.R. Euthyroideones, novel brominated quinone methides from the bryozoan Euthyroides episcopalis. J. Org. Chem. 1998, 63, 9545–9547. [Google Scholar] [CrossRef]
- Dufrasne, F.; Gelbcke, M.; Neve, J.; Kiss, R.; Kraus, J.L. Quinone methides and their prodrugs: A subtle equilibrium between cancer promotion, prevention, and cure. Curr. Med. Chem. 2011, 18, 3995–4011. [Google Scholar] [CrossRef] [PubMed]
- Prinsep, M.R.; Yao, B.; Nicholson, B.K.; Gordon, D.P. The pterocellins, bioactive alkaloids from the marine bryozoan Pterocella vesiculosa. Phytochem. Rev. 2004, 3, 325–331. [Google Scholar] [CrossRef]
- Prinsep, M.R. Further pterocellins from the New Zealand marine bryozoan Pterocella vesiculosa. J. Nat. Prod. 2008, 71, 134–136. [Google Scholar] [CrossRef]
- Till, M.; Prinsep, M.R. 5-Bromo-8-methoxy-1-methyl-β-carboline, an alkaloid from the New Zealand marine bryozoan Pterocella vesiculosa. J. Nat. Prod. 2009, 72, 796–798. [Google Scholar] [CrossRef]
- Prinsep, M.R.; Blunt, J.W.; Munro, M.H.G. New cytotoxic β-carboline alkaloids from the marine bryozoan, Cribricellina cribraria. J. Nat. Prod. 1991, 54, 1068–1076. [Google Scholar] [CrossRef]
- Harwood, D.T.; Urban, S.; Blunt, J.W.; Munro, M.H.G. β-Carboline alkaloids from a New Zealand marine bryozoan, Cribricellina cribraria. Nat. Prod. Res. 2003, 17, 15–19. [Google Scholar] [CrossRef]
- Sharp, J.H.; Winson, M.K.; Porter, J.S. Bryozoan metabolites: An ecological perspective. Nat. Prod. Rep. 2007, 24, 659–673. [Google Scholar] [CrossRef]
- Lindquist, N. Palatability of invertebrate larvae to corals and sea anemones. Mar. Biol. 1996, 126, 745–755. [Google Scholar] [CrossRef]
- Lindquist, N.; Hay, M.E. Palatability and chemical defense of marine invertebrate larvae. Ecol. Monographs 1996, 66, 431–450. [Google Scholar] [CrossRef]
- Lopanik, N.; Lindquist, N.; Targett, N. Potent cytotoxins produced by a microbial symbiont protect host larvae from predation. Oecologia 2004, 139, 131–139. [Google Scholar] [CrossRef]
- Lopanik, N.; Gustafson, K.R.; Lindquist, N. Structure of bryostatin 20: A symbiont-produced chemical defense for larvae of the host bryozoan, Bugula neritina. J. Nat. Prod. 2004, 67, 1412–1414. [Google Scholar] [CrossRef]
- Pettit, G.R.; Day, J.F.; Hartwell, J.L.; Wood, H.B. Antineoplastic components of marine animals. Nature 1970, 227, 962–963. [Google Scholar] [CrossRef]
- Woollacott, R.M.; Zimmer, R.L. A simplified placenta-like system for the transport of extraembryonic nutrients during embryogenesis of Bugula neritina (Bryozoa). J. Morphol. 1975, 147, 355–378. [Google Scholar] [CrossRef]
- Woollacott, R.M. Association of bacteria with bryozoan larvae. Mar. Biol. 1981, 65, 155–158. [Google Scholar] [CrossRef]
- Haygood, M.G.; Davidson, S.K. Small-subunit rRNA genes and in situ hybridization with oligonucleotides specific for the bacterial symbionts in the larvae of the bryozoan Bugula neritina and proposal of “Candidatus Endobugula sertula”. Appl. Environ. Microbiol. 1997, 63, 4612–4616. [Google Scholar] [CrossRef]
- Davidson, S.K.; Haygood, M.G. Identification of sibling species of the bryozoan Bugula neritina that produce different anticancer bryostatins and harbor distinct strains of the bacterial symbiont “Candidatus Endobugula sertula”. Biol. Bull. 1999, 196, 273–280. [Google Scholar] [CrossRef]
- Davidson, S.K.; Allen, S.W.; Lim, G.E.; Anderson, C.M.; Haygood, M.G. Evidence for the biosynthesis of bryostatins by the bacterial symbiont “Candidatus Endobugula sertula” of the bryozoan Bugula neritina. Appl. Environ. Microbiol. 2001, 67, 4531–4537. [Google Scholar] [CrossRef]
- Sharp, K.H.; Davidson, S.K.; Haygood, M.G. Localization of ‘Candidatus Endobugula sertula’ and the bryostatins throughout the life cycle of the bryozoan Bugula neritina. ISME J. 2007, 1, 693–702. [Google Scholar] [CrossRef]
- Fehlauer-Ale, K.H.; Mackie, J.A.; Lim-Fong, G.E.; Ale, E.; Pie, M.R.; Waeschenbach, A. Cryptic species in the cosmopolitan Bugula neritina complex (Bryozoa, Cheilostomata). Zool. Scr. 2014, 43, 193–205. [Google Scholar] [CrossRef]
- Mackie, J.A.; Keough, M.J.; Christidis, L. Invasion patterns inferred from cytochrome oxidase I sequences in three bryozoans, Bugula neritina, Watersipora subtorquata, and Watersipora arcuata. Mar. Biol. 2006, 149, 285–295. [Google Scholar] [CrossRef]
- McGovern, T.M.; Hellberg, M.E. Cryptic species, cryptic endosymbionts, and geographical variation in chemical defences in the bryozoan Bugula neritina. Mol. Ecol. 2003, 12, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, G.J. Biogeography and Adaptation: Patterns of Marine Life; Harvard University Press: Cambridge, MA, USA, 1978. [Google Scholar]
- Linneman, J.; Paulus, D.; Lim-Fong, G.; Lopanik, N.B. Latitudinal variation of a defensive symbiosis in the Bugula neritina (Bryozoa) sibling species complex. PLoS ONE 2014, 9, e108783. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Herald, C.L. Antineoplastic agents. 118. Isolation and structure of bryostatin 9. J. Nat. Prod. 1986, 49, 661–664. [Google Scholar] [CrossRef]
- Lim, G.E.; Haygood, M.G. “Candidatus Endobugula glebosa”, a specific bacterial symbiont of the marine bryozoan Bugula simplex. Appl. Environ. Microbiol. 2004, 70, 4921–4929. [Google Scholar] [CrossRef]
- Lopanik, N.B. Chemical defensive symbioses in the marine environment. Funct. Ecol. 2014, 28, 328–340. [Google Scholar] [CrossRef]
- Fehlauer-Ale, K.H.; Winston, J.E.; Tilbrook, K.J.; Nascimento, K.B.; Vieira, L.M. Identifying monophyletic groups within Bugula sensu lato (Bryozoa, Buguloidea). Zool. Scr. 2015, 44, 334–347. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N.; Hashimoto, K. Bioactive marine metabolites. VIII. Isolation of an antimicrobial blue pigment from the bryozoan Bugula dentata. Cell. Mol. Life Sci. 1986, 42, 84. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Marwood, J.F.; Murphy, P.T.; Wells, R.J. A blue pigment from a compound ascidian. Aust. J. Chem. 1982, 35, 215–217. [Google Scholar] [CrossRef]
- Wasserman, H.H.; Friedland, D.J.; Morrison, D.A. A novel dipyrrolyldipyrromethene prodigiosin analog from Serratia marcescens. Tetrahedron Lett. 1968, 9, 641–644. [Google Scholar] [CrossRef]
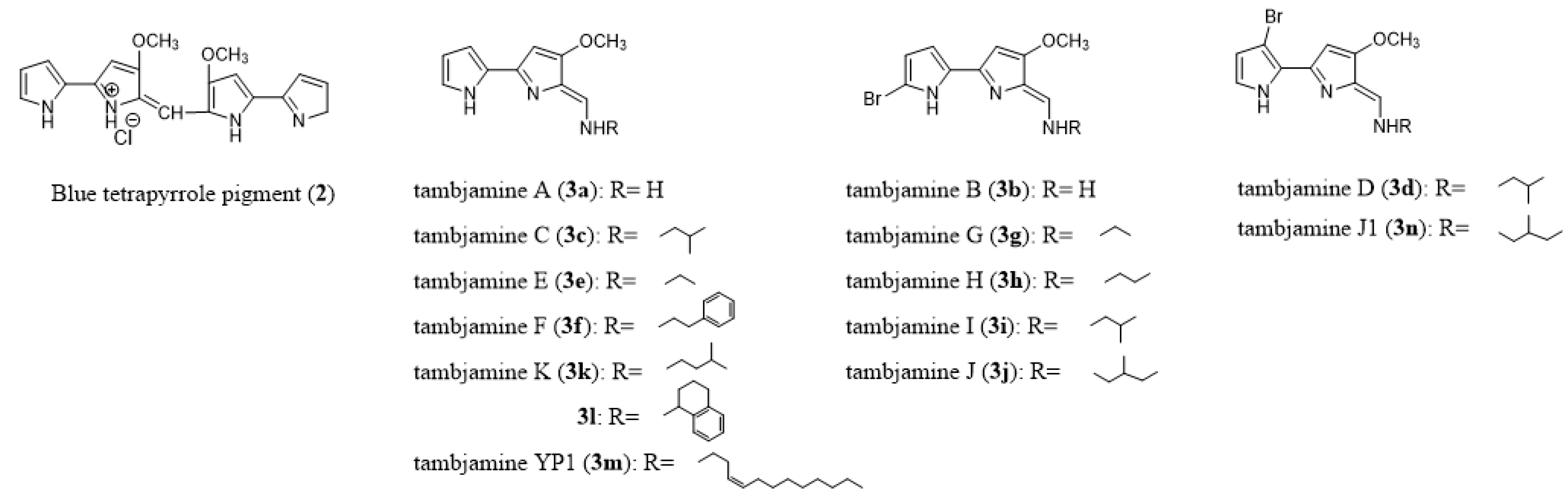
- Blackman, A.J.; Li, C.P. New tambjamine alkaloids from the marine bryozoan Bugula dentata. Aust. J. Chem. 1994, 47, 1625–1629. [Google Scholar] [CrossRef]
- Carté, B.; Faulkner, D.J. Defensive metabolites from three nembrothid nudibranchs. J. Org. Chem. 1983, 48, 2314–2318. [Google Scholar] [CrossRef]
- Carté, B.; Faulkner, D.J. Role of secondary metabolites in feeding associations between a predatory nudibranch, two grazing nudibranchs, and a bryozoan. J. Chem. Ecol. 1986, 12, 795–804. [Google Scholar] [CrossRef]
- Carbone, M.; Irace, C.; Costagliola, F.; Castelluccio, F.; Villani, G.; Calado, G.; Padula, V.; Cimino, G.; Cervera, J.L.; Santamaria, R.; et al. A new cytotoxic tambjamine alkaloid from the Azorean nudibranch Tambja ceutae. Bioorg. Med. Chem. Lett. 2010, 20, 2668–2670. [Google Scholar] [CrossRef]
- Pereira, F.R.; Berlinck, R.G.S.; Rodrigues Filho, E.; Veloso, K.; Ferreira, A.G.; Padula, V. Secondary metabolites from the nudibranch Tambja stegosaurifirmis, Hypselodoris tajenis and Okenia zoobotryon and from bryozoans Zoobotryon verticillatum and Bugula dentate from the Brazilian coastline. Quim. Nova 2012, 35, 2194–2201. [Google Scholar] [CrossRef][Green Version]
- Lindquist, N.; Fenical, W. New tambjamine class alkaloids from the marine ascidian Atapozoa sp. and its nudibranch predators. Origin of the tambjamines in Atapozoa. Experientia 1991, 47, 504–506. [Google Scholar] [CrossRef]
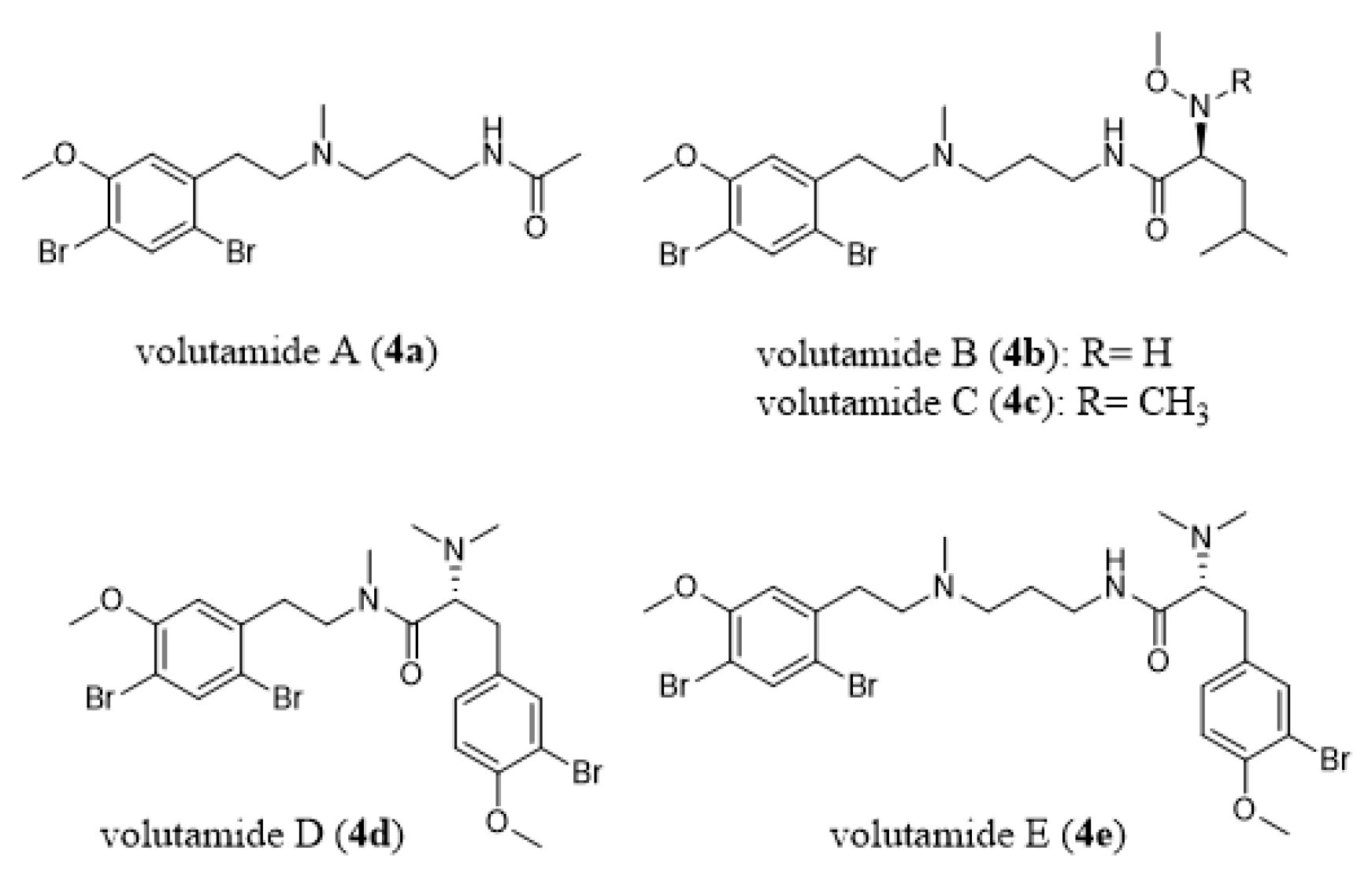
- Montanari, A.M.; Fenical, W.; Lindquist, N.; Lee, A.Y.; Clardy, J. Volutamides A-E, halogenated alkaloids with antifeedant properties from the Atlantic bryozoan Amathia convoluta. Tetrahedron 1996, 52, 5371–5380. [Google Scholar] [CrossRef]
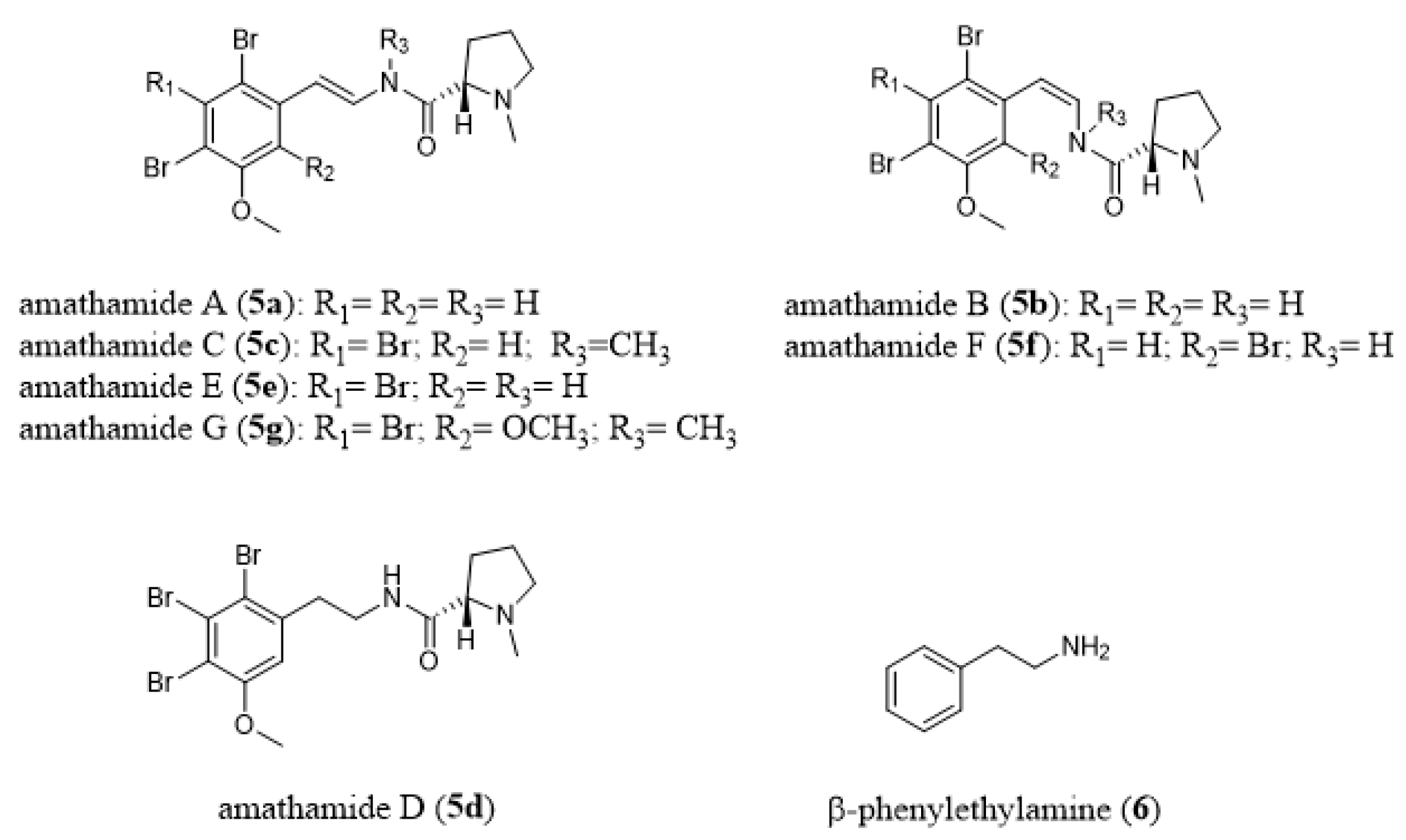
- Walls, J.T.; Blackman, A.J.; Ritz, D.A. Distribution of amathamide alkaloids within single colonies of the bryozoan Amathia wilsoni. J. Chem. Ecol. 1991, 17, 1871–1881. [Google Scholar] [CrossRef]
- Blackman, A.J.; Green, R.D. Further amathamide alkaloids from the bryozoan Amathia wilsoni. Aust. J. Chem. 1987, 40, 1655–1662. [Google Scholar] [CrossRef]
- Blackman, A.J.; Matthews, D. Amathamide alkaloids from the marine bryozoan Amathia wilsoni Kirkpatrick. Heterocycles 1985, 23, 2829–2833. [Google Scholar] [CrossRef]
- Blackman, A.J.; Eldershaw, T.P.D.; Garland, S.M. Alkaloids from two further Amathia bryozoan species. Aust. J. Chem. 1993, 46, 401–405. [Google Scholar] [CrossRef]
- Blackman, A.J.; Fu, S.L. A β-phenylethylamine-derived possible biosynthetic precursor to the amathamides, alkaloids from the bryozoan Amathia wilsoni. J. Nat. Prod. 1989, 52, 436–438. [Google Scholar] [CrossRef]
- Ramírez-Osuna, M.; Aguirre, G.; Somanathan, R.; Molins, E. Asymmetric synthesis of amathamides A and B: Novel alkaloids isolated from Amathia wilsoni. Tetrahedron Asymmetry 2002, 13, 2261–2266. [Google Scholar] [CrossRef]
- Khan, F.A.; Ahmad, S. Synthesis of reported and revised structures of amathamide D and synthesis of convolutamine F, H and lutamide A, C. J. Org. Chem. 2012, 77, 2389–2397. [Google Scholar] [CrossRef]
- Ahmad, S.; Choudhurya, S.; Khan, F.A. Synthesis of marine brominated alkaloid amathamide F: A palladium-catalyzed enamide synthesis. Tetrahedron 2015, 71, 4192–4202. [Google Scholar] [CrossRef][Green Version]
- Walls, J.T.; Blackman, A.J.; Ritz, D.A. Localisation of the amathamide alkaloids in surface bacteria of Amathia wilsoni Kirkpatrick, 1888 (Bryozoa: Ctenostomata). Hydrobiologia 1995, 297, 163–172. [Google Scholar] [CrossRef]
- Sherwood, J.; Walls, J.T.; Ritz, D.A. Amathamide Alkaloids in the Pycnogonid, Stylopallene Longicauda, Epizoic on the Chemically Defended Bryozoan, Amathia wilsoni. Papers Proc. R. Soc. Tasman. 1998, 132, 65–70. [Google Scholar] [CrossRef]
- Zhang, H.P.; Kamano, Y.; Kizu, H.; Itokawa, H.; Pettit, G.R.; Herald, C.L. Convolutamines A-E, Novel β-phenylethylamine alkaloids from marine bryozoan Amathia convoluta. Chem. Lett. 1994, 23, 2271–2274. [Google Scholar] [CrossRef]
- Kamano, Y.; Kotake, A.; Hashima, H.; Hayakawa, I.; Hiraide, H.; Zhang, H.P.; Kizu, H.; Komiyama, K.; Hayashi, M.; Pettit, G.R. Three new alkaloids, convolutamines F and G, and Convolutamydine E, from the Floridian marine bryozoan Amathia convoluta. Collect. Czech. Chem. Commun. 1999, 64, 1147–1153. [Google Scholar] [CrossRef]
- Hashima, H.; Hayashi, M.; Kamano, Y.; Sato, N. Synthesis and biological activities of the marine bryozoan alkaloids convolutamines A, C and F, and lutamides A and C. Bioorg. Med. Chem. 2000, 8, 1757–1766. [Google Scholar] [CrossRef]
- Narkowicz, C.Z.; Blackman, A.J.; Lacey, E.; Gill, J.H.; Heiland, K. Convolutindole A and convolutamine H, new nematocidal brominated alkaloids from the marine bryozoan Amathia convoluta. J. Nat. Prod. 2002, 65, 938–941. [Google Scholar] [CrossRef]
- Davis, R.A.; Sykes, M.; Avery, V.M.; Camp, D.; Quinn, R.J. Convolutamines I and J, antitrypanosomal alkaloids from the bryozoan Amathia tortusa. Bioorg. Med. Chem. 2011, 19, 6615–6619. [Google Scholar] [CrossRef]
- Figuerola, B.; Nunez-Pons, L.; Moles, J.; Avila, C. Feeding repellence in Antarctic bryozoans. Naturwissenschaften 2013, 100, 1069–1081. [Google Scholar] [CrossRef]
- Figuerola, B.; Nunez-Pons, L.; Monleon-Getino, T.; Avila, C. Chemo-ecological interactions in Antarctic bryozoans. Polar. Biol. 2014, 37, 1017–1030. [Google Scholar] [CrossRef]
- Figuerola, B.; Sala-Comorera, L.; Angulo-Preckler, C.; Vazquez, J.; Montes, M.J.; Garcia-Aljaro, C.; Mercade, E.; Blanch, A.R.; Avila, C. Antimicrobial activity of Antarctic bryozoans: An ecological perspective with potential for clinical applications. Mar. Environ. Res. 2014, 101, 52–59. [Google Scholar] [CrossRef]
- Taboada, S.; Nunez-Pons, L.; Avila, C. Feeding repellence of Antarctic and sub-Antarctic benthic invertebrates against the omnivorous sea star Odontaster validus. Polar. Biol. 2013, 36, 13–25. [Google Scholar] [CrossRef]
- Mathew, M.; Bean, K.I.; Temate-Tiagueu, Y.; Caciula, A.; Mandoiu, I.I.; Zelikovsky, A.; Lopanik, N.B. Influence of symbiont-produced bioactive natural products on holobiont fitness in the marine bryozoan, Bugula neritina via protein kinase C (PKC). Mar. Biol. 2016, 163, 44. [Google Scholar] [CrossRef]
- Mathew, M.; Schwaha, T.; Ostrovsky, A.N.; Lopanik, N.B. Symbiont-dependent sexual reproduction in marine colonial invertebrate: Morphological and molecular evidence. Mar. Biol. 2018, 165, 14. [Google Scholar] [CrossRef]
- Dube, F.; Golsteyn, R.; Dufresne, L. Protein Kinase C and Meiotic Maturation of Surf Clam Oocytes. Biochem. Biophys. Res. Commun. 1987, 142, 1072–1076. [Google Scholar] [CrossRef]
- Eckberg, W.R.; Carroll, A.G. Protein kinase C and phospholipase C are involved in germinal vesicle breakdown in Chaetopterus oocytes. Dev. Growth Diff. 1986, 29, 491–498. [Google Scholar]
- Eckberg, W.R.; Szuts, E.Z.; Carroll, A.G. Protein kinase C activity, protein phosphorylation and germinal vesicle breakdown in Spisula oocytes. Dev. Biol. 1987, 124, 57–64. [Google Scholar] [CrossRef]
- Eckberg, W.R.; Johnson, M.R.; Palazzo, R.E. Regulation of maturation-promoting factor by protein kinase C in Chaetopterus oocytes. Invert. Reprod. Dev. 1996, 30, 71–79. [Google Scholar] [CrossRef]
- Pejin, B.; Mojovic, M.; Savic, A.G. Novel antitumour natural products from the phylum Bryozoa. Biol. Serbica 2013, 35, 3–14. [Google Scholar]
- Skropeta, D.; Wei, L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014, 31, 999–1025. [Google Scholar] [CrossRef]
- Hegazy, M.E.; Mohamed, T.A.; Alhammady, M.A.; Shaheen, A.M.; Reda, E.H.; Elshamy, A.I.; Aziz, M.; Paré, P.W. Molecular architecture and biomedical leads of terpenes from red sea marine invertebrates. Mar. Drugs 2015, 13, 3154–3181. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Zhou, B.B.; Zhang, H.; Damelin, M.; Geles, K.G.; Grindley, J.C.; Dirks, P.B. Tumour-initiating cells: Challenges and opportunities for anticancer drug discovery. Nat. Rev. Drug Discov. 2009, 8, 806–823. [Google Scholar] [CrossRef]
- Zhao, J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol. Ther. 2016, 160, 145–158. [Google Scholar] [CrossRef]
- Valent, P.; Bonnet, D.; De Maria, R.; Lapidot, T.; Copland, M.; Melo, J.V.; Chomienne, C.; Ishikawa, F.; Schuringa, J.J.; Stassi, G.; et al. Cancer stem cell definitions and terminology: The devil is in the details. Nat. Rev. Cancer 2012, 12, 767–775. [Google Scholar] [CrossRef]
- Jørgensen, H.G.; Allan, E.K.; Mountford, J.C.; Richmond, L.; Harrison, S.; Elliott, M.A.; Holyoake, T.L. Enhanced CML stem cell elimination in vitro by bryostatin priming with imatinib mesylate. Exp. Hematol. 2005, 33, 1140–1146. [Google Scholar] [CrossRef]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, A.J.; Chen, M.; Liu, J.J. ABC transporter inhibitors in reversing multidrug resistance to chemotherapy. Curr. Drug Targets 2015, 16, 1356–1371. [Google Scholar] [CrossRef]
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef]
- Abraham, I.; El Sayed, K.; Chen, Z.S.; Guo, H. Current status on marine products with reversal effect on cancer multidrug resistance. Mar. Drugs 2012, 10, 2312–2321. [Google Scholar] [CrossRef]
- Long, S.; Sousa, E.; Kijjoa, A.; Pinto, M.M. Marine natural products as models to circumvent multidrug resistance. Molecules 2016, 21, 892. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Koprowska, K.; Majchrzak, K.; Hartman, M.; Czyz, M. Natural compounds’ activity against cancer stem-like or fast-cycling melanoma cells. PLoS ONE 2014, 9, e90783. [Google Scholar] [CrossRef]
- Al-Katib, A.M.; Smith, M.R.; Kamanda, W.S.; Pettit, G.R.; Hamdan, M.; Mohamed, A.N.; Chelladurai, B.; Mohammad, R.M. Bryostatin 1 down-regulates mdr1 and potentiates vincristine cytotoxicity in diffuse large cell lymphoma xenografts. Clin. Cancer Res. 1998, 4, 1305–1314. [Google Scholar]
- Clamp, A.; Jayson, G.C. The clinical development of the bryostatins. Anticancer Drugs 2002, 13, 673–683. [Google Scholar] [CrossRef]
- De Lorenzo, M.S.; Yamaguchi, K.; Subbaramaiah, K.; Dannenberg, A.J. Bryostatin-1 stimulates the transcription of cyclooxygenase-2: Evidence for an activator protein-1-dependent mechanism. Clin. Cancer Res. 2003, 9, 5036–5043. [Google Scholar]
- Mohammad, R.M.; Diwakaran, H.; Maki, A.; Emara, M.A.; Pettit, G.R.; Redman, B.; Al-Khatib, A. Bryostatin 1 induces apoptosis and augments inhibitory effects of vincristine in human diffuse large cell lymphoma. Leuk. Res. 1995, 19, 667–673. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Limvarapuss, C.; Hamdy, N.; Dutcher, B.S.; Beck, F.W.; Wall, N.R.; Al-Katib, A.M. Treatment of a de novo fludarabine resistant-CLL xenograft model with bryostatin 1 followed by fludarabine. Int. J. Oncol. 1999, 14, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.M.; Wall, N.R.; Dutcher, J.A.; Al-Katib, A.M. The addition of bryostatin 1 to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy improves response in a CHOP-resistant human diffuse large cell lymphoma xenograft model. Clin. Cancer Res. 2000, 6, 4950–4956. [Google Scholar]
- Basu, A.; Lazo, J.S. Sensitization of human cervical carcinoma cells to cis-diamminedichloroplatinum(II) by bryostatin 1. Cancer Res. 1992, 52, 3119–3124. [Google Scholar]
- Wall, N.R.; Mohammad, R.M.; Al-Katib, A.M. Bax: Bcl-2 ratio modulation by bryostatin 1 and novel antitubulin agents is important for susceptibility to drug induced apoptosis in the human early pre-B acute lymphoblastic leukemia cell line, Reh. Leuk. Res. 1999, 23, 881–888. [Google Scholar] [CrossRef]
- Wender, P.A.; Schrier, A.J. Total synthesis of bryostatin 9. J. Am. Chem. Soc. 2011, 133, 9228–9231. [Google Scholar] [CrossRef]
- Barr, P.M.; Lazarus, H.M.; Cooper, B.W.; Schluchter, M.D.; Panneerselvam, A.; Jacobberger, J.W.; Hsu, J.W.; Janakiraman, N.; Simic, A.; Dowlati, A.; et al. Phase II study of bryostatin 1 and vincristine for aggressive non-Hodgkin lymphoma relapsing after an autologous stem cell transplant. Am. J. Hematol. 2009, 84, 484–487. [Google Scholar] [CrossRef]
- Lam, A.P.; Sparano, J.A.; Vinciguerra, V.; Ocean, A.J.; Christos, P.; Hochster, H.; Camacho, F.; Goel, S.; Mani, S.; Kaubisch, A. Phase II study of paclitaxel plus the protein kinase C inhibitor bryostatin-1 in advanced pancreatic carcinoma. Am. J. Clin. Oncol. 2010, 33, 121–124. [Google Scholar] [CrossRef]
- Morgan, R.J., Jr.; Leong, L.; Chow, W.; Gandara, D.; Frankel, P.; Garcia, A.; Lenz, H.J.; Doroshow, J.H. Phase II trial of bryostatin-1 in combination with cisplatin in patients with recurrent or persistent epithelial ovarian cancer: A California cancer consortium study. Investig. New Drugs 2012, 30, 723–728. [Google Scholar] [CrossRef]
- Kollár, P.; Rajchard, J.; Balounová, Z.; Pazourek, J. Marine natural products: Bryostatins in preclinical and clinical studies. Pharm. Biol. 2014, 52, 237–242. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, M.; Joshi, P.; Rawat, D.S. Clinical status of anti-cancer agents derived from marine sources. Anticancer Agents Med. Chem. 2008, 8, 603–617. [Google Scholar] [CrossRef]
- Irie, K.; Yanagita, R.C.; Nakagawa, Y. Challenges to the development of bryostatin-type anticancer drugs based on the activation mechanism of protein kinase Cδ. Med. Res. Rev. 2012, 32, 518–535. [Google Scholar] [CrossRef]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef]
- Nguyen, D.X.; Bos, P.D.; Massagué, J. Metastasis: From dissemination to organ-specific colonization. Nat. Rev. Cancer 2009, 9, 274–285. [Google Scholar] [CrossRef]
- Liu, W.; Vivian, C.J.; Brinker, A.E.; Hampton, K.R.; Lianidou, E.; Welch, D.R. Microenvironmental influences on metastasis suppressor expression and function during a metastatic cell’s journey. Cancer Microenviron. 2014, 7, 117–131. [Google Scholar] [CrossRef]
- Ribelles, N.; Santonja, A.; Pajares, B.; Llacer, C.; Alba, E. The seed and soil hypothesis revisited: Current state of knowledge of inherited genes on prognosis in breast cancer. Cancer Treat. Rev. 2014, 40, 293–299. [Google Scholar] [CrossRef]
- Simpson, C.D.; Anyiwe, K.; Schimmer, A.D. Anoikis resistance and tumor metastasis. Cancer Lett. 2008, 272, 177–185. [Google Scholar] [CrossRef]
- Speirs, C.K.; Hwang, M.; Kim, S.; Li, W.; Chang, S.; Varki, V.; Mitchell, L.; Schleicher, S.; Lu, B. Harnessing the cell death pathway for targeted cancer treatment. Am. J. Cancer Res. 2011, 1, 43–61. [Google Scholar]
- Kornienko, A.; Mathieu, V.; Rastogi, S.K.; Lefranc, F.; Kiss, R. Therapeutic agents triggering nonapoptotic cancer cell death. J. Med. Chem. 2013, 56, 4823–4839. [Google Scholar] [CrossRef]
- Lefranc, F.; Brotchi, J.; Kiss, R. Possible future issues in the treatment of glioblastomas: Special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J. Clin. Oncol. 2005, 23, 2411–2422. [Google Scholar] [CrossRef]
- Melvin, M.S.; Ferguson, D.C.; Lindquist, N.; Manderville, R.A. DNA binding by 4-methoxypyrrolic natural products. Preference for intercalation at AT sites by tambjamine E and prodigiosin. J. Org. Chem. 1999, 64, 6861–6869. [Google Scholar] [CrossRef]
- Cavalcanti, B.C.; Junior, H.V.N.; Seleghim, M.H.R.; Berlinck, R.G.S.; Cunha, G.M.A.; Moraes, M.O.; Pessoa, C. Cytotoxic and genotoxic effects of tambjamine D, an alkaloid isolated from the nudibranch Tambja eliora, on Chinese hamster lung fibroblasts. Chem. Biol. Interact. 2008, 174, 155–162. [Google Scholar] [CrossRef]
- Pinkerton, D.M.; Banwell, M.G.; Garson, M.J.; Kumar, N.; De Moraes, M.O.; Cavalcanti, B.C.; Barros, F.W.A.; Pessoa, C. Antimicrobial and cytotoxic activities of synthetically derived tambjamines C and E-J, BE-18591, and a related alkaloid from the marine bacterium Pseudoalteromonas tunicata. Chem. Biodiv. 2010, 7, 1311–13324. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Aldrich, L.N.; Stoops, S.L.; Crews, B.C.; Marnett, L.J.; Lindsley, C.W. Total synthesis and biological evaluation of tambjamine K and a library of unnatural analogs. Bioorg. Med. Chem. Lett. 2010, 20, 5207–5211. [Google Scholar] [CrossRef]
- Schmitz, F.J.; DeGuzman, F.S.; Choi, Y.H.; Hossain, M.B.; Rizvi, S.K.; van der Helm, D. Biologically active compounds from marine organisms. Pure Appl. Chem. 1990, 62, 1393–1396. [Google Scholar] [CrossRef]
- Choi, Y.H.; Park, A.; Schmitz, A.; Van Altena, I. Perfragilins A and B, cytotoxic isoquinolinequinones from the bryozoan Membranipora perfragilis. J. Nat. Prod. 1993, 56, 1431–1433. [Google Scholar] [CrossRef]
- Rizvi, S.K.; Hossain, M.B.; Van der Helm, D. Structures of perfragilins A and B. Acta Cryst. 1993, 49, 151–154. [Google Scholar] [CrossRef]
- Witte, R.S.; Hsieh, P.; Elson, P.; Oken, M.M.; Trump, D.L. A phase II trial of amonafide, caracemide, and homoharringtonine in the treatment of patients with advanced renal cell cancer. Investig. New Drugs 1996, 14, 409–413. [Google Scholar] [CrossRef]
- Witte, R.S.; Lipsitz, S.; Goodman, T.L.; Asbury, R.F.; Wilding, G.; Strnad, C.M.; Smith, T.J.; Haller, D.G. A phase II trial of homoharringtonine and caracemide in the treatment of patients with advanced large bowel cancer. Investig. New Drugs 1999, 17, 173–177. [Google Scholar] [CrossRef]
- Madaan, K.; Kaushik, D.; Verma, T. Hydroxyurea: A key player in cancer chemotherapy. Expert Rev. Anticancer Ther. 2012, 12, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Pardanani, A. Myeloproliferative neoplasms: A contemporary review. JAMA Oncol. 2015, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.F.; Park, A.; Schmitz, F.J.; Valeriote, F.A. Synthesis of perfragilin A, B and some analogues. Tetrahedron 2003, 59, 1349–1357. [Google Scholar] [CrossRef]
- Arai, T.; Yazawa, K.; Mikami, Y. Isolation and characterization of satellite antibiotics, mimosamycin and chlorocarcins from Streptomyces lavendulae, streptothricin source. J. Antibiot. 1976, 29, 398–407. [Google Scholar] [CrossRef]
- Fukumi, H.; Kurihara, H.; Hata, T.; Tamura, C.; Mishima, H.; Kubo, A.; Arai, T. Mimosamycin, a novel antibiotic produced by Streptomyces lavendulae no. 314: Structure and synthesis. Tetrahedron Lett. 1977, 18, 3825–3828. [Google Scholar] [CrossRef]
- Frincke, J.M.; Faulkner, D.J. Antimicrobial metabolites of the sponge Reniera sp. J. Am. Chem. Soc. 1982, 104, 265–269. [Google Scholar] [CrossRef]
- Plubrukarn, A.; Yuenyongsawad, S.; Thammasaroj, T.; Jitsue, A. Cytotoxic isoquinoline quinones from the Thai sponge Cribrochalina sp. Pharm. Biol. 2003, 41, 439–442. [Google Scholar] [CrossRef]
- Russo, P.; Kisialiou, A.; Lamonaca, P.; Moroni, R.; Prinzi, G.; Fini, M. New drugs from marine organisms in Alzheimer’s disease. Mar. Drugs 2016, 14, 5. [Google Scholar] [CrossRef]
- Sun, M.K.; Alkon, D.L. Bryostatin-1: Pharmacology and therapeutic potential as a CNS drug. CNS Drug Rev. 2006, 12, 1–8. [Google Scholar] [CrossRef]
- Etcheberrigaray, R.; Tan, M.; Dewachter, I.; Kuipéri, C.; Van der Auwera, I.; Wera, S.; Qiao, L.; Bank, B.; Nelson, T.J.; Kozikowski, A.P.; et al. Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc. Natl. Acad. Sci. USA 2004, 101, 11141–11146. [Google Scholar] [CrossRef]
- Schrott, L.M.; Jackson, K.; Yi, P.; Dietz, F.; Johnson, G.S.; Basting, T.F.; Purdum, G.; Tyler, T.; Rios, J.D.; Castor, T.P.; et al. Acute oral Bryostatin-1 administration improves learning deficits in the APP/PS1 transgenic mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 22–31. [Google Scholar] [CrossRef]
- Nelson, T.J.; Sun, M.K.; Lim, C.; Sen, A.; Khan, T.; Chirila, F.V.; Alkon, D.L. Bryostatin Effects on Cognitive Function and PKCɛ in Alzheimer’s Disease Phase IIa and Expanded Access Trials. J. Alzheimers Dis. 2017, 58, 521–535. [Google Scholar] [CrossRef]
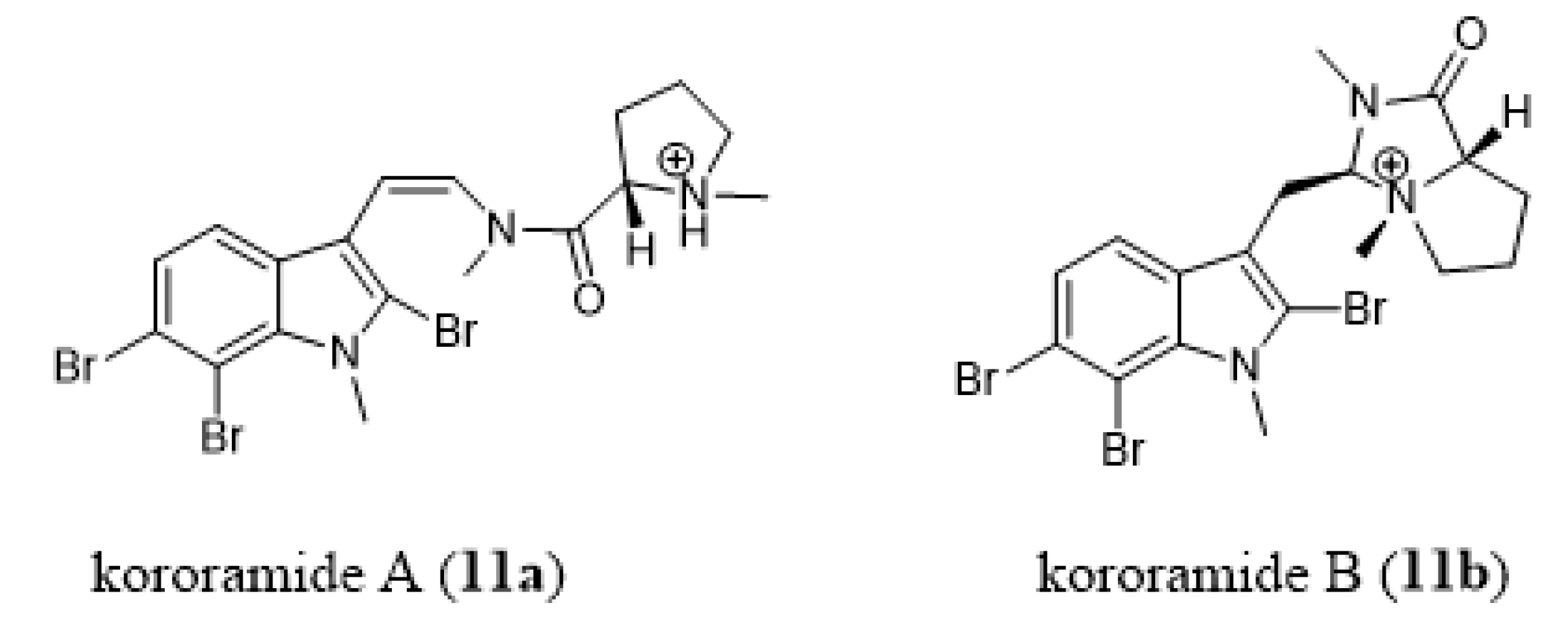
- Carroll, A.R.; Wild, S.J.; Duffy, S.; Avery, V.M. Kororamide A, a new tribrominated indole alkaloid from the Australian bryozoan Amathia tortuosa. Tetrahedron Lett. 2012, 53, 2873–2875. [Google Scholar] [CrossRef]
- Dashti, Y.; Vial, M.L.; Wood, S.A.; Mellick, G.D.; Roullier, C.; Quinn, R.J. Kororamide B, a brominated alkaloid from the bryozoan Amathia tortuosa and its effects on Parkinson’s disease cells. Tetrahedron 2015, 71, 7879–7884. [Google Scholar] [CrossRef]
- Llorach-Pares, L.; Nonell-Canals, A.; Avila, C.; Sanchez-Martinez, M. Kororamides, convolutamines and indole derivatives as possible tau and dual-specificity kinase inhibitors for Alzheimer’s disesase: A computational study. Mar. Drugs 2018, 16, 386. [Google Scholar] [CrossRef]
- Sun, M.K.; Hongpaisan, J.; Nelson, T.J.; Alkon, D.L. Poststroke neuronal rescue and synaptogenesis mediated in vivo by protein kinase C in adult brains. Proc. Natl. Acad. Sci. USA 2008, 105, 13620–13625. [Google Scholar] [CrossRef]
- Tan, Z.; Turner, R.C.; Leon, R.L.; Li, X.; Hongpaisan, J.; Zheng, W.; Logsdon, A.F.; Naser, Z.J.; Alkon, D.L.; Rosen, C.L.; et al. Bryostatin improves survival and reduces ischemic brain injury in aged rats after acute ischemic stroke. Stroke 2013, 44, 3490–3497. [Google Scholar] [CrossRef]
- Mizutani, K.; Sonoda, S.; Wakita, H.; Okazaki, H.; Katoh, Y.; Chihara, T.; Shimpo, K. Effects of exercise and bryostatin-1 on serotonin dynamics after cerebral infarction. Neuroreport 2016, 27, 659–664. [Google Scholar] [CrossRef]
- Pérez, M.; de Vinuesa, A.G.; Sanchez-Duffhues, G.; Marquez, N.; Bellido, M.L.; Muñoz-Fernandez, M.A.; Moreno, S.; Castor, T.P.; Calzado, M.A.; Muñoz, E. Bryostatin-1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency. Curr. HIV Res. 2010, 8, 418–429. [Google Scholar] [CrossRef]
- Diaz, L.; Martinez-Bonet, M.; Sanchez, J.; Fernandez-Pineda, A.; Jimenez, J.L.; Munoz, E.; Moreno, S.; Alvarez, S.; Munoz-Fernandez, A. Bryostatin activates HIV-1 latent expression in human astrocytes through a PKC and NF-ĸB-dependent mechanism. Sci. Rep. 2015, 5, 12442. [Google Scholar] [CrossRef]
- Darcis, G.; Kula, A.; Bouchat, S.; Fujinaga, K.; Corazza, F.; Ait-Ammar, A.; Delacourt, N.; Melard, N.; Kabeya, K.; Vanhulle, C.; et al. An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression. PLoS Pathog. 2015, 11, e1005063. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Bonet, M.; Clemente, M.I.; Serramía, M.J.; Muñoz, E.; Moreno, S.; Muñoz-Fernández, M.A. Synergistic Activation of Latent HIV-1 Expression by Novel Histone Deacetylase Inhibitors and Bryostatin-1. Sci. Rep. 2015, 5, 16445. [Google Scholar] [CrossRef] [PubMed]
- Staveness, D.; Abdelnabi, R.; Near, K.E.; Nakagawa, Y.; Neyts, J.; Delang, L.; Leyssen, P.; Wender, P.A. Inhibition of Chikungunya Virus-Induced Cell Death by Salicylate-Derived Bryostatin Analogues Provides Additional Evidence for a PKC-Independent Pathway. J. Nat. Prod. 2016, 79, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Staveness, D.; Near, K.E.; Wender, P.A.; Delang, L.; Neyts, J.; Leyssen, P. Comparative analysis of the anti-chikungunya virus activity of novel bryostatin analogs confirms the existence of a PKC-independent mechanism. Biochem. Pharmacol. 2016, 20, 15–21. [Google Scholar] [CrossRef]
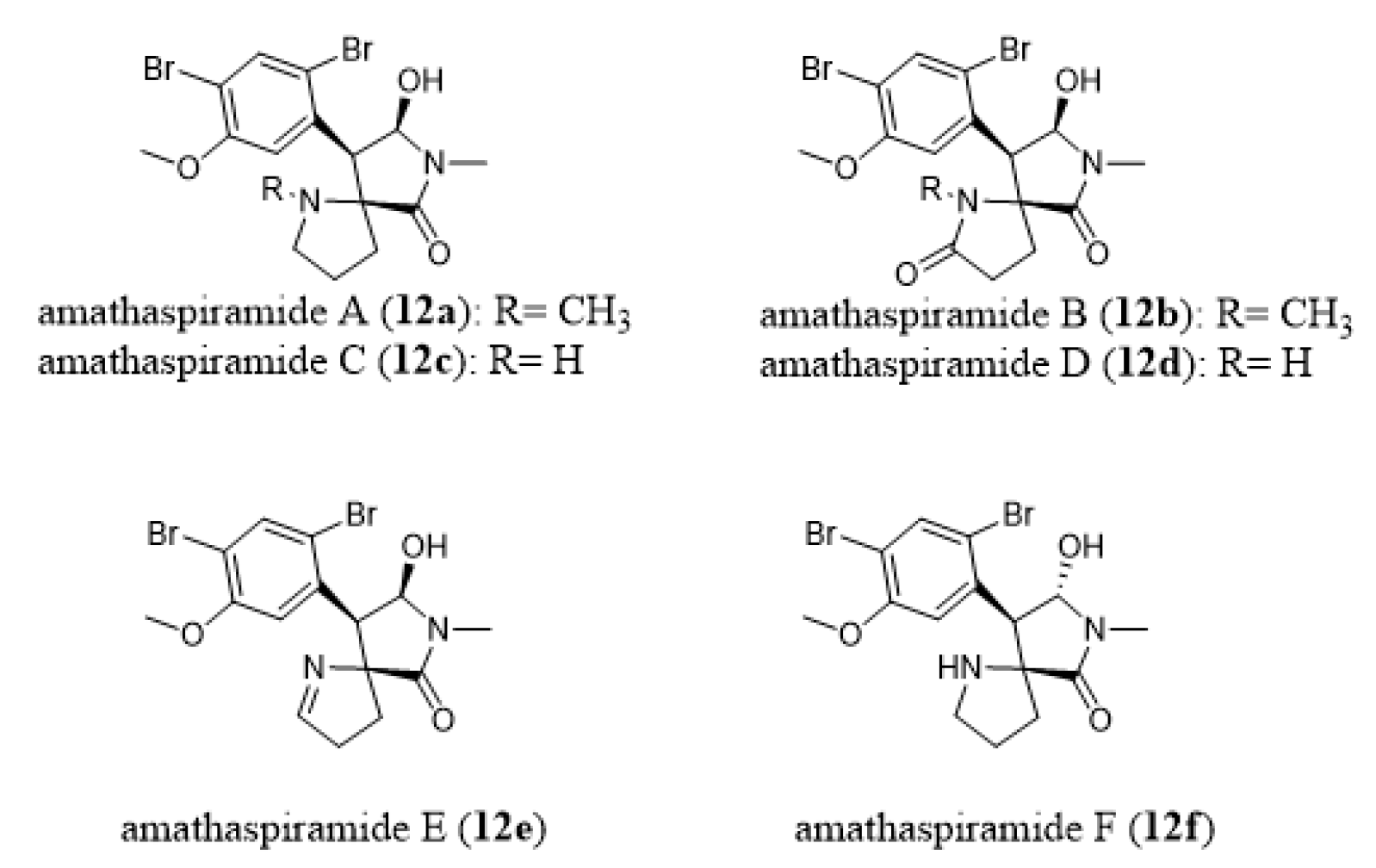
- Morris, B.D.; Prinsep, M.R. Amathaspiramides A-F, novel brominated alkaloids from the marine bryozoan Amathia wilsoni. J. Nat. Prod. 1999, 62, 688–693. [Google Scholar] [CrossRef]
- Shimokawa, J.; Chiyoda, K.; Umihara, H.; Fukuyama, T. Antiproliferative activity of amathaspiramide alkaloids and analogs. Chem. Pharm. Bull. 2016, 64, 1239–1241. [Google Scholar] [CrossRef]
- Chiyoda, K.; Shimokawa, J.; Fukuyama, T. Total Syntheses of All the Amathaspiramides (A-F). Angew Chem. Int. Ed. 2012, 51, 2505–2508. [Google Scholar] [CrossRef]
- Cai, S.L.; Song, R.; Dong, H.Q.; Lin, G.Q.; Sun, X.W. Practical asymmetric synthesis of amathaspiramides B., D, and F. Org. Lett. 2016, 18, 1996–1999. [Google Scholar] [CrossRef]
- Hughes, C.C.; Trauner, D. The Total Synthesis of (−)-Amathaspiramide, F. Angew Chem. Int. Ed. 2002, 41, 4556–4559. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Ayabe, M.; Watanabe, Y.; Okada, T.; Kawamura, K.; Shiada, T.; Ohfune, Y. Total synthesis of (-)-amathaspiramide F. Org. Lett. 2008, 10, 5449–5452. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Ayabe, M.; Watanabe, Y.; Okada, T.; Kawamura, K.; Shiada, T.; Ohfune, Y. Total synthesis of (-)-amathaspiramide F. Tetrahedron 2009, 65, 10355–10364. [Google Scholar] [CrossRef]
- Babokhov, P.; Sanyaolu, A.O.; Oyibo, W.A.; Fagbenro-Beyioku, A.F.; Iriemenam, N.C. A current analysis of chemotherapy strategy for the treatment of human African trypanosomiasis. Pathog. Glob. Health 2013, 107, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.B.; Deydier, S.; Labaied, M.; Monnerat, S.; Stuart, K.; Quinn, R.J. N1,N1-dimethyl-N3-(3-(trifluoromethyl)phenetyl)propane-1,3-diamine, a new lead for the treatment of human African trypanosomiasis. Eur. J. Med. Chem. 2014, 74, 541–551. [Google Scholar] [CrossRef] [PubMed]
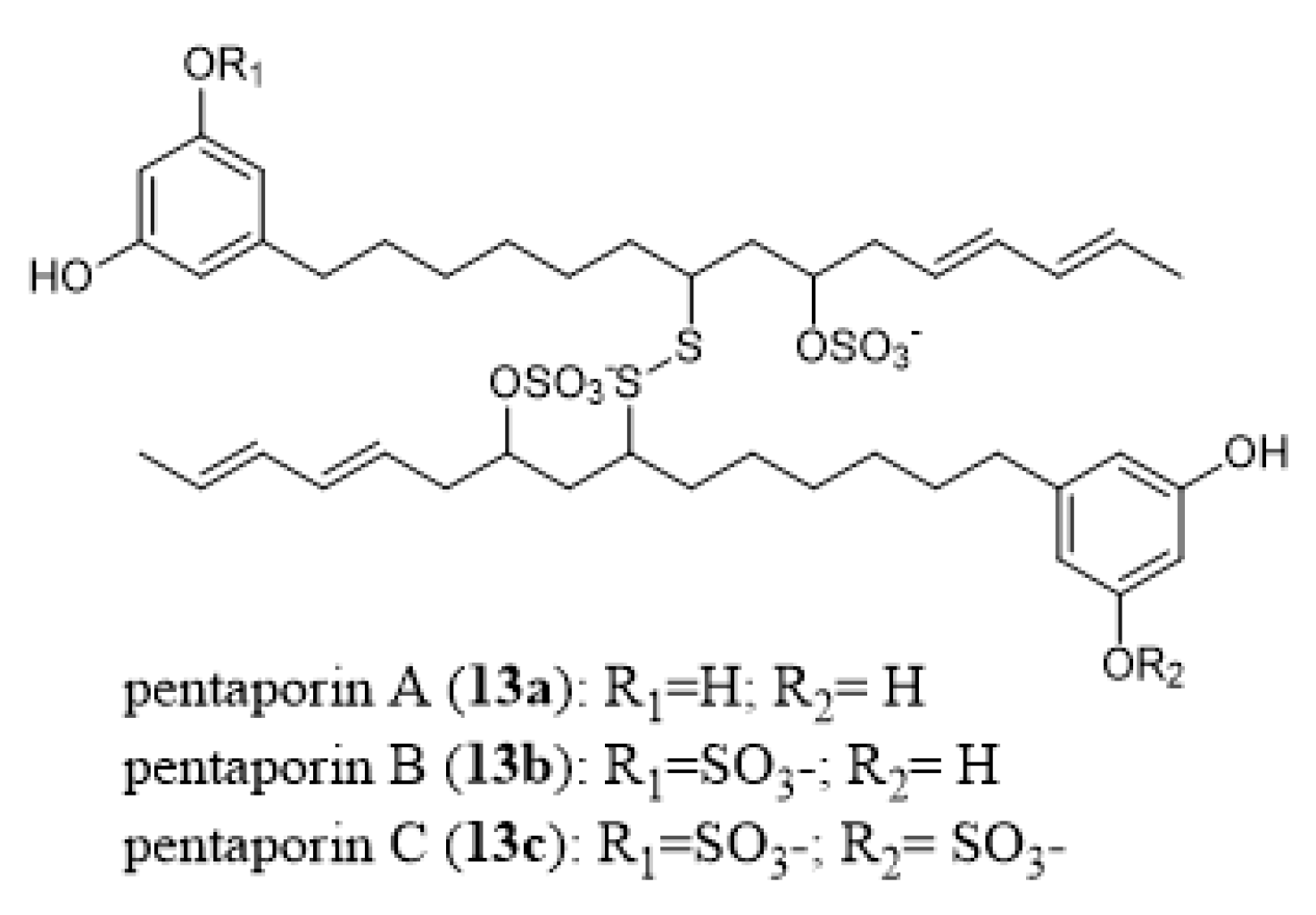
- Eisenbarth, S.; Gehling, M.; Harder, A.; Steffan, B. Pentaporins A, B and C: Disulfides from the marine bryozoan Pentapora fasciata. Tetrahedron 2002, 58, 8461–8464. [Google Scholar] [CrossRef]
- Ramirez-Osuna, M.; Chavez, D.; Hernandez, L.; Molins, E.; Somanathan, R.; Aguirre, G. Synthesis of analogs of amathamide A and their preliminary antimicrobial activity. Molecules 2005, 10, 295–301. [Google Scholar] [CrossRef]
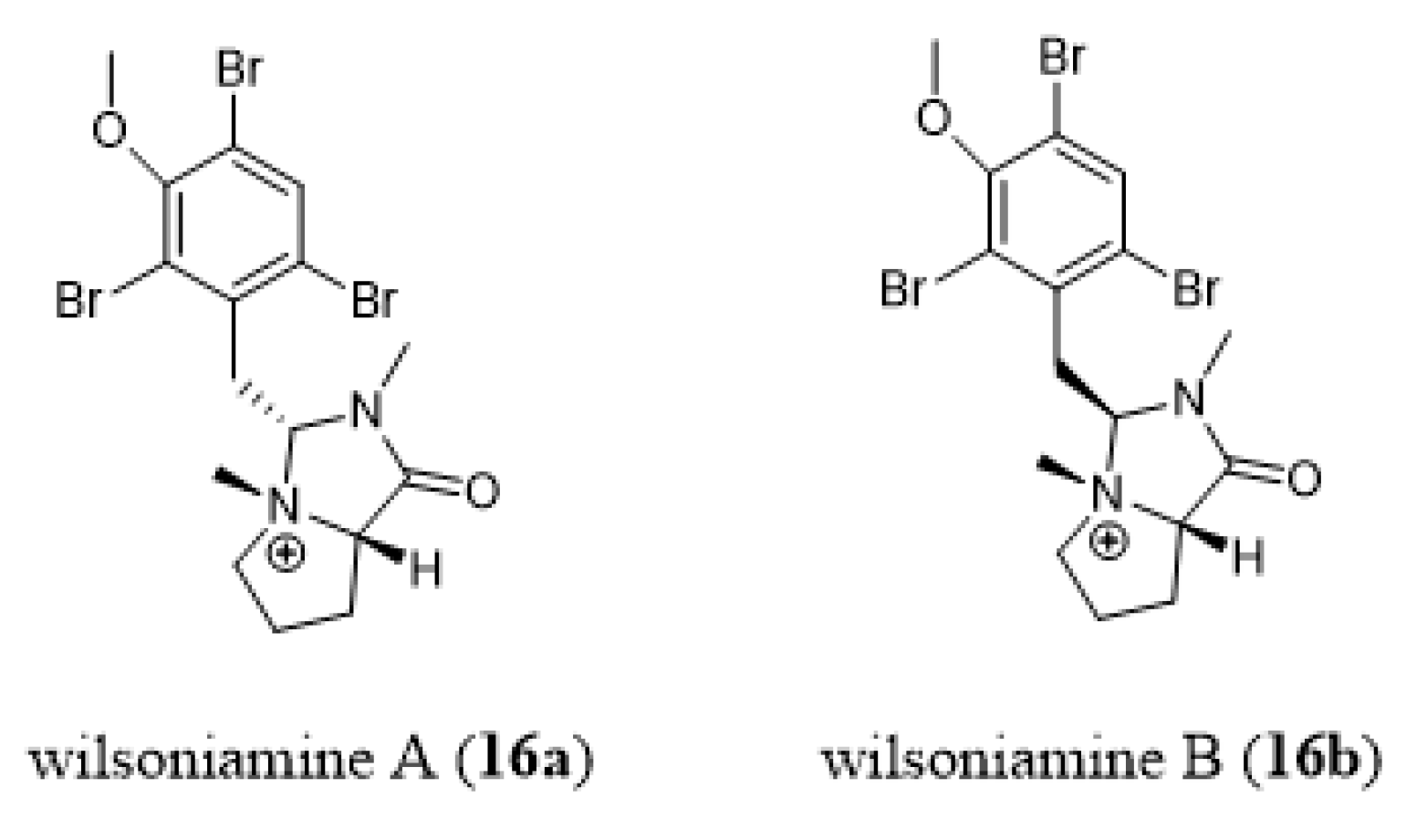
- Carroll, A.R.; Duffy, S.; Sykes, M.; Avery, V.M. Wilsoniamines A and B: Novel alkaloids from the temperate Australian bryozoan, Amathia wilsoni. Org. Biomol. Chem. 2011, 9, 604–609. [Google Scholar] [CrossRef]
- Khan, F.A.; Ahmad, S. Synthesis of wilsoniamines A and B. Tetrahedron Lett. 2013, 54, 2996–2998. [Google Scholar] [CrossRef]
- Milanowski, D.J.; Oku, N.; Cartner, L.K.; Bokesch, H.R.; Williamson, R.T.; Saur´ı, J.; Liu, Y.; Blinov, K.A.; Ding, Y.; Li, X.-C.; et al. Unequivocal determination of caulamidines A and B: Application and validation of new tools in the structure elucidation tool box. Chem. Sci. 2018, 9, 307–314. [Google Scholar] [CrossRef]
- Duffy, S.; Avery, V.M. Development and optimization of a novel 384-well anti-malarial imaging assay validated for high-throughput screening. Am. J. Trop. Med. Hyg. 2012, 86, 84–92. [Google Scholar] [CrossRef]
- Vieira, L.M.; Farrapeira, C.M.R.; Amaral, F.D.; Lira Simone, M.A. Bryozoan biodiversity in Saint Peter and Saint Paul Archipelago, Brazil. Cahiers Biol. Mar. 2012, 53, 159–167. [Google Scholar]
- Miranda, A.A.; Almeida, A.C.S.; Vieira, L.M. Non-native marine bryozoans (Bryozoa: Gymnolaemata) in Brazilian waters: Assessment, dispersal and impacts. Mar. Pollution Bull. 2018, 130, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.; Spencer Jones, M.; Taylor, P.D. The identity of the invasive fouling bryozoan Watersipora subtorquata (d’Orbigny) and some other congeneric species. Zootaxa 2014, 3857, 151–182. [Google Scholar] [CrossRef]
- Schaufelberger, D.E.; Koleck, M.P.; Beutler, J.A.; Vatakis, A.M.; Alvarado, A.B.; Andrews, P.; Marzo, L.V.; Muschnik, G.M.; Roach, J.; Ross, J.T.; et al. The large-scale isolation of bryostatin-1 from Bugula neritina following current good manufacturing practices. J. Nat. Prod. 1991, 54, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Dahms, H.U.; Gao, Q.F.; Hwang, J.S. Optimized maintenance and larval production of the bryozoan Bugula neritina (Bugulidae: Gymnolaemata) in the laboratory. Aquaculture 2007, 265, 169–175. [Google Scholar] [CrossRef]
- Lei, H.; Zhou, X.; Yang, Y.; Xu, T.; Yang, X.; Sun, J.; Yang, B.; Hu, J.; Lin, X.; Long, L.; et al. Bryostatins from South China Sea bryozoan Bugula neritina L. Biochem. Syst. Ecol. 2010, 38, 1231–1233. [Google Scholar] [CrossRef]
- Kamano, Y.; Zhang, H.P.; Morita, H.; Itokawa, H.; Shirota, O.; Pettit, G.R.; Herald, D.L.; Herald, C.L. Conformational analysis of a marine antineoplastic macrolide, bryostatin 10. Tetrahedron 1996, 52, 2369–2376. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Herald, C.L.; Tozawa, M. Isolation and structure of bryostatins 5-7. Can. J. Chem. 1985, 63, 1204–1208. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Herald, C.L.; Tozawa, M. Structure of bryostatin 4. An important antineoplastic constituent of geographically diverse Bugula neritina (Bryozoa). J. Am. Chem. Soc. 1984, 106, 6768–6771. [Google Scholar] [CrossRef]
- Ueno, S.; Yanagita, R.C.; Murakami, K.; Murakami, A.; Tokuda, H.; Suzuki, N.; Fujiwara, T.; Irie, K. Identification and biological activities of bryostatins from Japanese bryozoan. Biosci. Biotechnol. Biochem. 2012, 76, 1041–1043. [Google Scholar] [CrossRef][Green Version]
- Piel, J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 2009, 26, 338–362. [Google Scholar] [CrossRef]
- Miller, I.J.; Vanee, N.; Fong, S.S.; Lim-Fong, G.E.; Kwan, J.C. Lack of overt genome reduction in the bryostatin-producing bryozoan symbiont “Candidatus Endobugula sertula”. Appl. Environ. Microbiol. 2016, 82, 6573–6583. [Google Scholar] [CrossRef] [PubMed]
- Julien, B.; Shah, S. Heterologous expression of epothilone biosynthetic genes in Myxococcus xanthus. Antimicrob. Agents Chemother. 2002, 46, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Mutka, S.C.; Carney, J.R.; Liu, Y.Q.; Kennedy, J. Heterologous production of epothilone C and D in Escherichia coli. Biochemistry 2006, 45, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.M.; Katz, L.; Khosla, C. Engineered biosynthesis of a complete macrolactone in a heterologous host. Science 1994, 265, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Sudek, S.; Lopanik, N.B.; Waggoner, L.E.; Hildebrand, M.; Anderson, C.; Liu, H.; Patel, A.; Sherman, D.H.; Haygood, M.G. Identification of the putative bryostatin polyketide synthase gene cluster from “Candidatus Endobugula sertula”, the uncultivated microbial symbiont of the marine bryozoan Bugula neritina. J. Nat. Prod. 2007, 70, 67–74. [Google Scholar] [CrossRef]
- Moran, N.A.; McCutcheon, J.P.; Nakabachi, A. Genomics and Evolution of Heritable Bacterial Symbionts. Ann. Rev. Genet. 2008, 42, 165–190. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Pfeifer, B.A. Bacterial hosts for natural product production. Mol. Pharm. 2008, 5, 212–225. [Google Scholar] [CrossRef]
- Fujii, I. Heterologous expression systems for polyketide synthases. Nat. Prod. Rep. 2009, 26, 155–169. [Google Scholar] [CrossRef]
- Gao, X.; Wang, P.; Tang, Y. Engineered polyketide biosynthesis and biocatalysis in Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 88, 1233–1242. [Google Scholar] [CrossRef]
- Weissman, K.J. Genetic engineering of modular PKSs: From combinatorial biosynthesis to synthetic biology. Nat. Prod. Rep. 2016, 33, 203–230. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef]
- Tyler, A.J.; Morgan, M.V.C.; Aratow, N.A.; Estee, S.A.; Sashidhara, K.V.; Steven, T.; Loveridge, S.T.; Segraves, N.L.; Crews, P. Assessing pressurized liquid extraction for the high-throughput extraction of marine-sponge-derived natural products. J. Nat. Prod. 2010, 73, 359–364. [Google Scholar] [CrossRef]
- Hale, K.J.; Manaviazar, S. New Approaches to the Total Synthesis of the Bryostatin Antitumor Macrolides. Chem. Asian J. 2010, 5, 704–754. [Google Scholar] [CrossRef] [PubMed]
- Manaviazar, S.; Hale, K.J. Total Synthesis of Bryostatin 1: A Short Route. Angew. Chem. Int. Ed. 2011, 50, 8786–8789. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.G.M.; Dasari, R.; Chandra, S.; Kiss, R.; Kornienko, A. Marine Invertebrate Metabolites with Anticancer Activities: Solutions to the “Supply Problem”. Mar. Drugs 2016, 14, 98. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef]
- Radjasa, O.K.; Vaske, Y.M.; Navarro, G.; Vervoort, H.C.; Tenney, K.; Linington, R.G.; Crews, P. Highlights of marine invertebrate-derived biosynthetic products: Their biomedical potential and possible production by microbial associants. Bioorg. Med. Chem. 2011, 19, 6658–6674. [Google Scholar] [CrossRef]
- Liebezeit, G. Aquaculture of “Non-Food Organisms” for Natural Substance Production. In Marine Biotechnology II; Gal, Y.L., Ulber, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–28. [Google Scholar]
- Lang, S.; Huners, M.; Lurtz, V. Bioprocess Engineering Data on the Cultivation of Marine Prokaryotes and Fungi. In Marine Biotechnology II; Gal, Y.L., Ulber, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 29–62. [Google Scholar]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Trindade-Silva, A.E.; Lim-Fong, G.E.; Sharp, K.H.; Haygood, M.G. Bryostatins: Biological context and biotechnological prospects. Curr. Opin. Biotechnol. 2010, 21, 834–842. [Google Scholar] [CrossRef]
- Wender, P.A.; Hinkle, K.W.; Koehler, M.F.; Lippa, B. The rational design of potential chemotherapeutic agents: Synthesis of bryostatin analogues. Med. Res. Rev. 1999, 19, 388–407. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Drugs and drug candidates from marine sources: An assessment of the current “State of Play”. Planta Med. 2016, 82, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Loy, B.A.; Schrier, A.J. Translating Nature’s library: The bryostatins and function-oriented synthesis. Isr. J. Chem. 2011, 51, 453–472. [Google Scholar] [CrossRef]
- Ruan, B.F.; Zhu, H.L. The chemistry and biology of the bryostatins: Potential PKC inhibitors in clinical development. Curr. Med. Chem. 2012, 19, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Irie, K.; Yanagita, R.C. Synthesis and biological activities of simplified analogs of the natural PKC ligands, bryostatin-1 and aplysiatoxin. Chem. Rec. 2014, 14, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Sengupta, D.; Herald, C.L.; Sharkey, N.A.; Blumberg, P.M. Synthetic conversion of bryostatin 2 to bryostatin 1 and related bryopyrans. Canad. J. Chem. 1991, 69, 856–860. [Google Scholar] [CrossRef]
- Pettit, G.R.; Sengupta, D.; Blumberg, P.M.; Lewin, N.E.; Schmidt, J.M.; Kraft, A.S. Structural modifications of bryostatin 2. Anticancer Drug Des. 1992, 7, 101–114. [Google Scholar]
- Lopanik, N.B.; Shields, J.A.; Buchholz, T.J.; Rath, C.M.; Hothersall, J.; Haygood, M.G.; Hakansson, K.; Thomas, C.M.; Sherman, D.H. In vivo and in vitro trans-acylation by BryP, the putative bryostatin pathway acyltransferase derived from an uncultured marine symbiont. Chem. Biol. 2008, 15, 1175–1186. [Google Scholar] [CrossRef]
- Buchholz, T.J.; Rath, C.M.; Lopanik, N.B.; Gardner, N.P.; Hakansson, K.; Sherman, D.H. Polyketide β -Branching in Bryostatin Biosynthesis: Identification of Surrogate Acetyl-ACP Donors for BryR, an HMG-ACP Synthase. Chem. Biol. 2010, 17, 1092–1100. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciavatta, M.L.; Lefranc, F.; Vieira, L.M.; Kiss, R.; Carbone, M.; van Otterlo, W.A.L.; Lopanik, N.B.; Waeschenbach, A. The Phylum Bryozoa: From Biology to Biomedical Potential. Mar. Drugs 2020, 18, 200. https://doi.org/10.3390/md18040200
Ciavatta ML, Lefranc F, Vieira LM, Kiss R, Carbone M, van Otterlo WAL, Lopanik NB, Waeschenbach A. The Phylum Bryozoa: From Biology to Biomedical Potential. Marine Drugs. 2020; 18(4):200. https://doi.org/10.3390/md18040200
Chicago/Turabian StyleCiavatta, Maria Letizia, Florence Lefranc, Leandro M. Vieira, Robert Kiss, Marianna Carbone, Willem A. L. van Otterlo, Nicole B. Lopanik, and Andrea Waeschenbach. 2020. "The Phylum Bryozoa: From Biology to Biomedical Potential" Marine Drugs 18, no. 4: 200. https://doi.org/10.3390/md18040200
APA StyleCiavatta, M. L., Lefranc, F., Vieira, L. M., Kiss, R., Carbone, M., van Otterlo, W. A. L., Lopanik, N. B., & Waeschenbach, A. (2020). The Phylum Bryozoa: From Biology to Biomedical Potential. Marine Drugs, 18(4), 200. https://doi.org/10.3390/md18040200





