Insights into the Synthesis, Secretion and Curing of Barnacle Cyprid Adhesive via Transcriptomic and Proteomic Analyses of the Cement Gland
Abstract
1. Introduction
2. Results
2.1. Transcriptome Sequencing, Assembly and Annotation
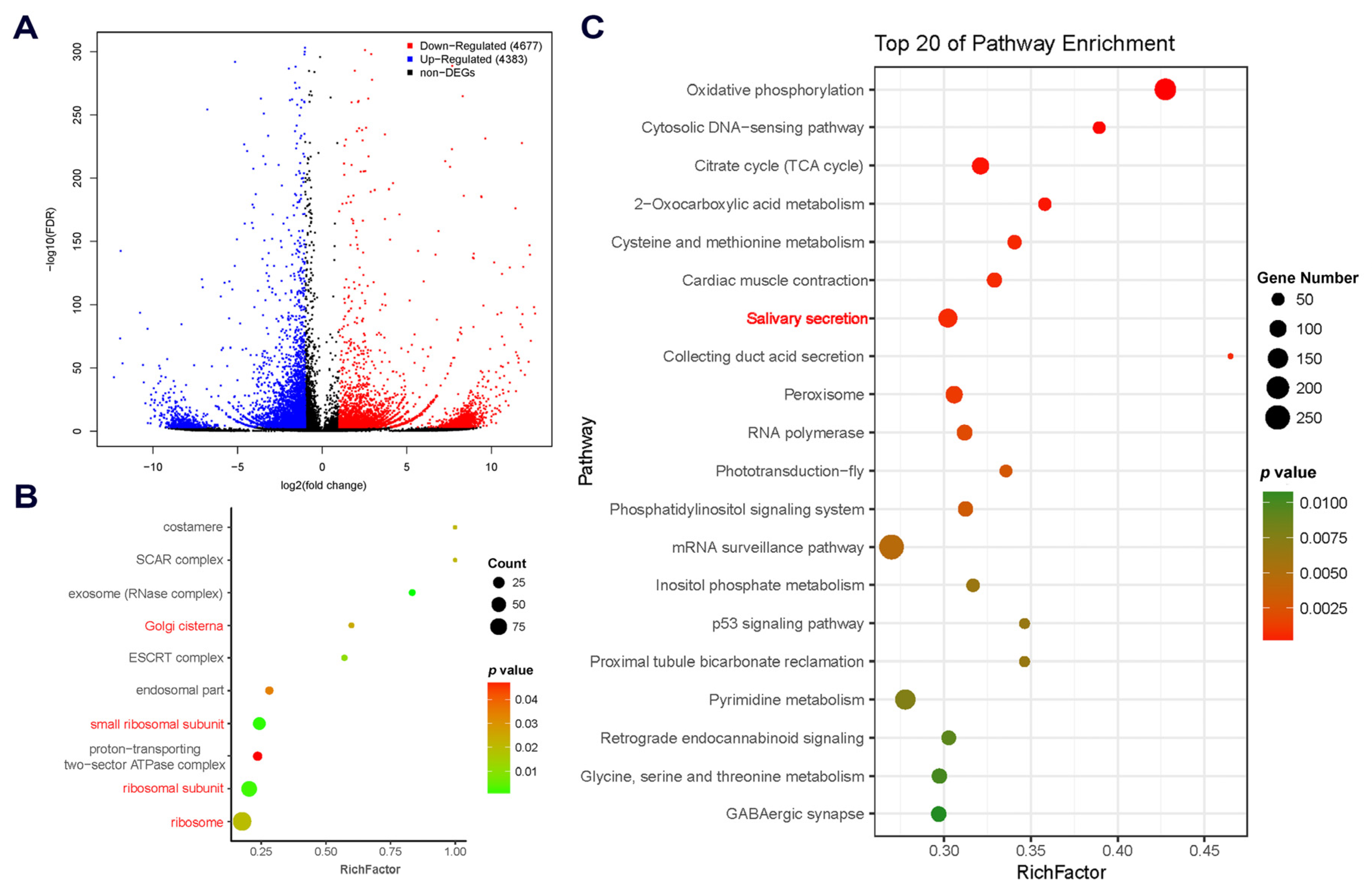
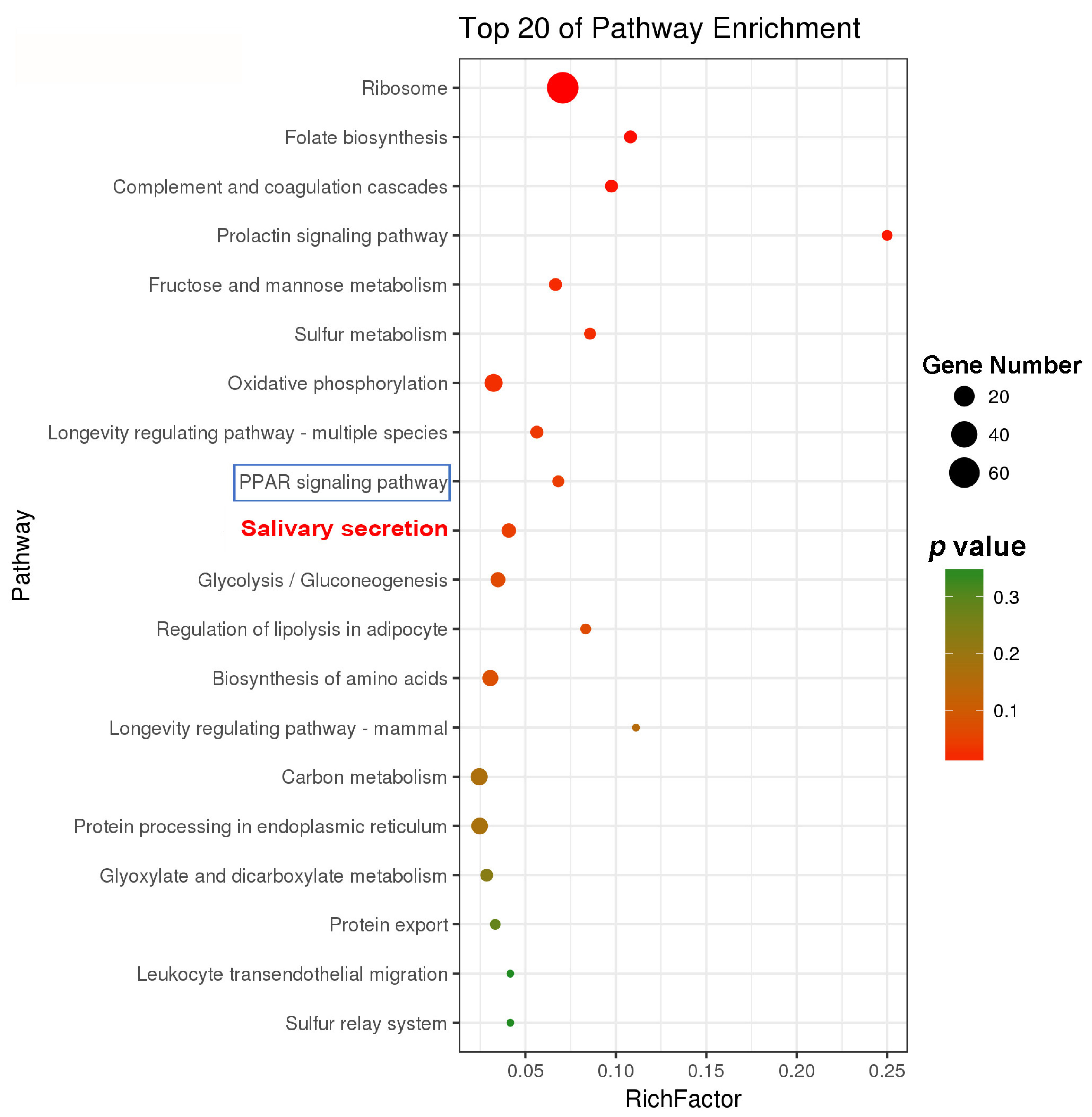
2.2. Comparative Transcriptomic Analysis and Characterization of DEGs
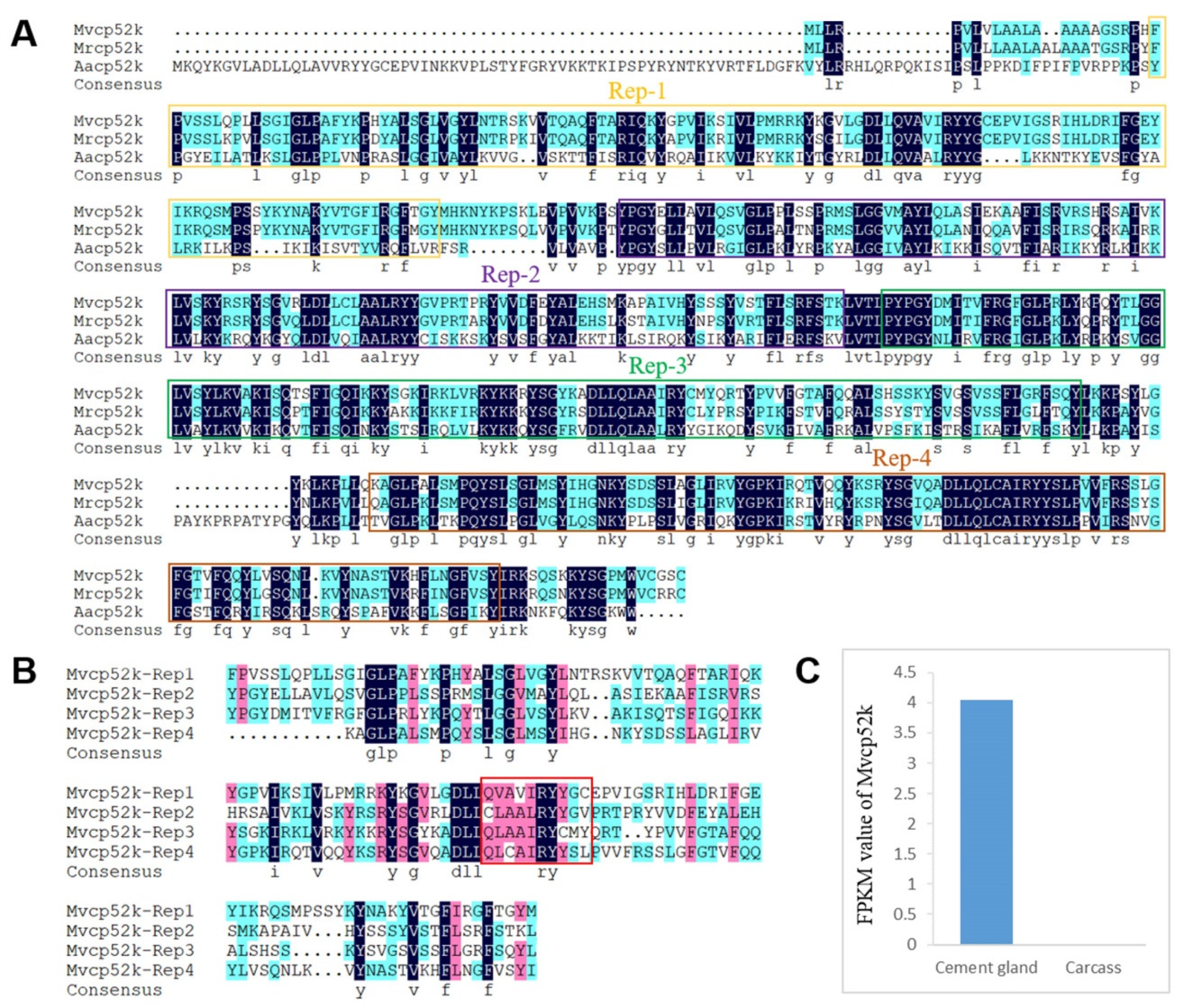
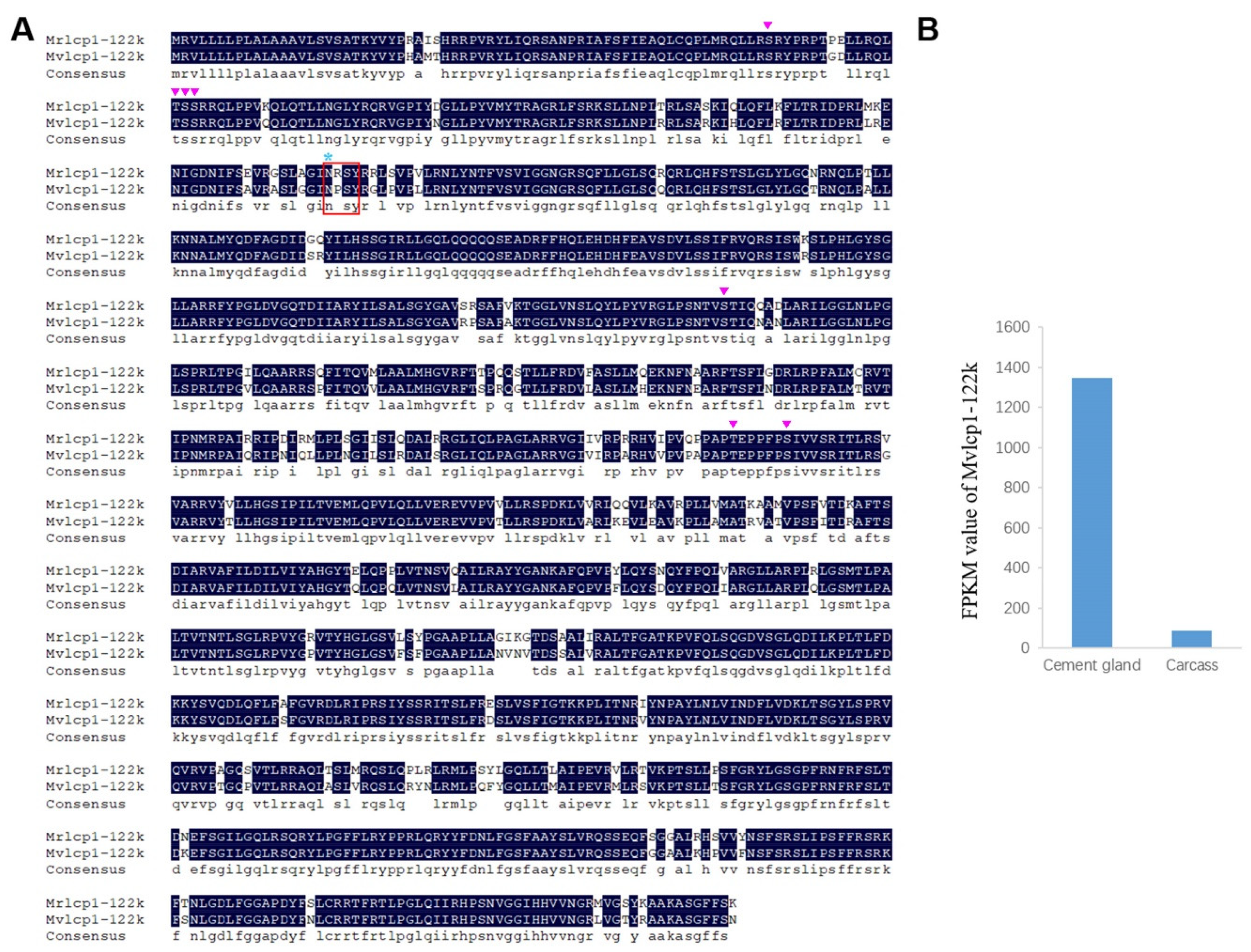
2.3. Characterization of Cement Proteins in the Cement Gland
2.4. Characterization of Enzymes in the Cement Gland
2.5. Proteomic Analysis of the Cement Gland
3. Discussion
4. Materials and Methods
4.1. Larval Culture
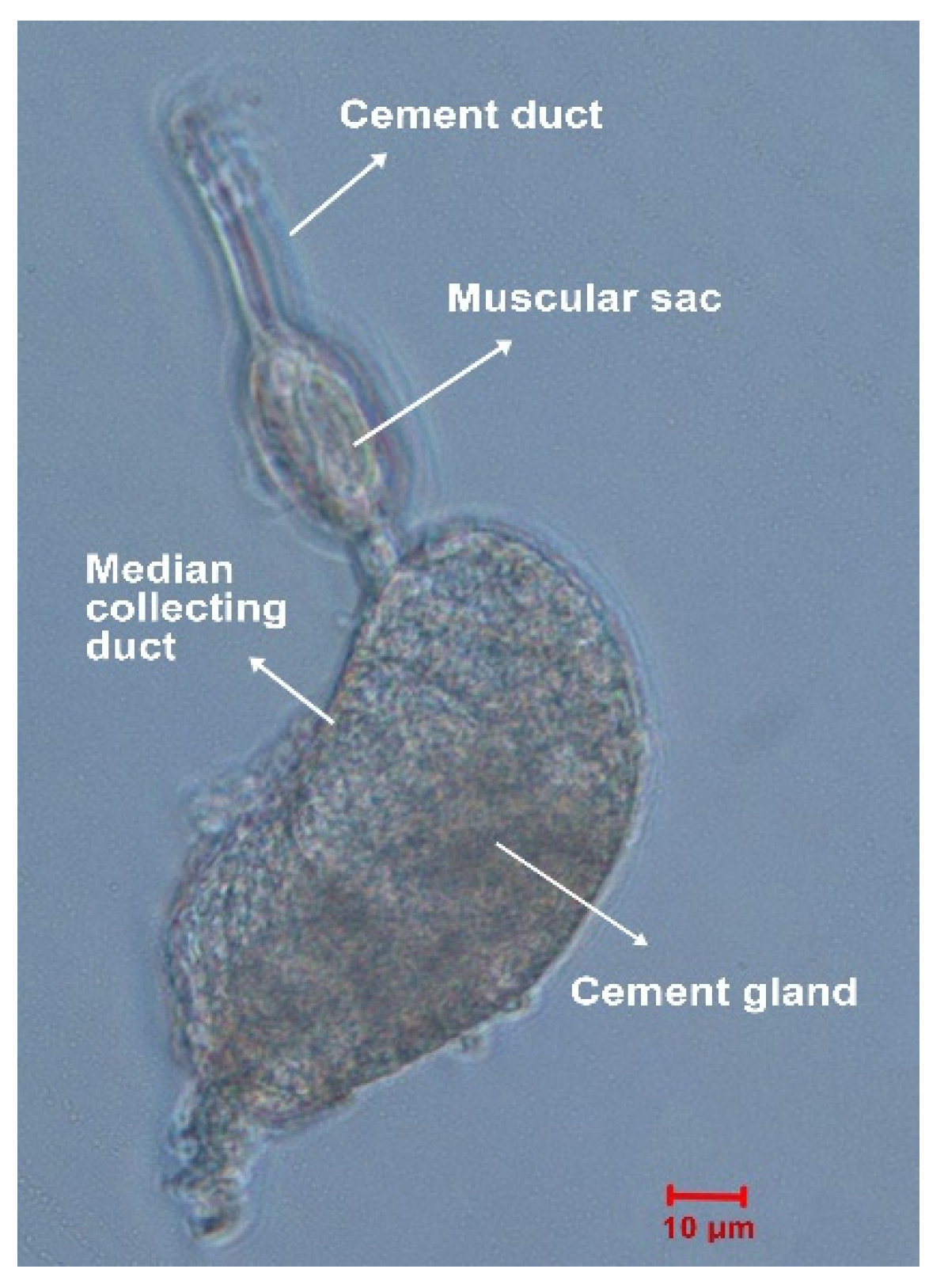
4.2. Cement Gland Dissection
4.3. RNA Extraction, cDNA Library Construction and Sequencing
4.4. De Novo Assembly and Annotation
4.5. Comparative Transcriptomic Analysis
4.6. Protein Extraction and in-Solution Digestion
4.7. LC–MS/MS Analysis
4.8. Sequence Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holm, E.R. Barnacles and biofouling. Integr. Comp. Biol. 2012, 52, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Walker, G. The Biochemical Composition of the Cement of two Barnacle Species, Balanus Hameri and Balanus Crenatus. J. Mar. Biol. Assoc. U. K. 1972, 52, 429–435. [Google Scholar] [CrossRef]
- Kamino, K. Barnacle Underwater Attachment. In Biological Adhesives; Smith, A.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 153–176. [Google Scholar] [CrossRef]
- Kamino, K. Mini-review: Barnacle adhesives and adhesion. Biofouling 2013, 29, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.-Y.; Chen, L.; Xu, Y. Mini-review: Molecular mechanisms of antifouling compounds. Biofouling 2013, 29, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Walker, G. A study of the cement apparatus of the cypris larva of the barnacle Balanus balanoides. Mar. Biol. 1971, 9, 205–212. [Google Scholar] [CrossRef]
- Gohad, N.V.; Aldred, N.; Hartshorn, C.M.; Lee, Y.J.; Cicerone, M.T.; Orihuela, B.; Clare, A.S.; Rittschof, D.; Mount, A.S. Synergistic roles for lipids and proteins in the permanent adhesive of barnacle larvae. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Cheung, P.J.; Nigrelli, R.F. Secretory activity of the cement gland in different developmental stages of the barnacle Balanus eburneus. Mar. Biol. 1975, 32, 99–103. [Google Scholar] [CrossRef]
- Walker, G. The histology, histochemistry and ultrastructure of the cement apparatus of three adult sessile barnacles, Elminius modestus, Balanus balanoides and Balanus hameri. Mar. Biol. 1970, 7, 239–248. [Google Scholar] [CrossRef]
- Walker, G. The early development of the cement apparatus in the barnacle, Balanus balanoides (L.) (Crustacea: Cirripedia). J. Exp. Mar. Biol. Ecol. 1973, 12, 305–314. [Google Scholar] [CrossRef]
- Fears, K.P.; Orihuela, B.; Rittschof, D.; Wahl, K.J. Acorn Barnacles Secrete Phase-Separating Fluid to Clear Surfaces Ahead of Cement Deposition. Adv. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Kamino, K.; Inoue, K.; Maruyama, T.; Takamatsu, N.; Harayama, S.; Shizuri, Y. Barnacle cement proteins - Importance of disulfide bonds in their insolubility. J. Biol. Chem. 2000, 275, 27360–27365. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K.; Nakano, M.; Kanai, S. Significance of the conformation of building blocks in curing of barnacle underwater adhesive. Febs J. 2012, 279, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Urushida, Y.; Nakano, M.; Uchiyama, S.; Kamino, K. Calcite-specific coupling protein in barnacle underwater cement. Febs J. 2007, 274, 6436–6446. [Google Scholar] [CrossRef]
- Urushida, Y.; Nakano, M.; Matsuda, S.; Inoue, N.; Kanai, S.; Kitamura, N.; Nishino, T.; Kamino, K. Identification and functional characterization of a novel barnacle cement protein. Febs J. 2007, 274, 4336–4346. [Google Scholar] [CrossRef] [PubMed]
- He, L.S.; Zhang, G.; Wang, Y.; Yan, G.Y.; Qian, P.Y. Toward understanding barnacle cementing by characterization of one cement protein-100kDa in Amphibalanus amphitrite. Biochem. Biophys. Res. Commun. 2018, 495, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, Y.Q.; Liu, Z.M.; Wu, W.J.; Hu, B.R. Protein Aggregation Formed by Recombinant cp19k Homologue of Balanus albicostatus Combined with an 18 kDa N-Terminus Encoded by pET-32a(+) Plasmid Having Adhesion Strength Comparable to Several Commercial Glues. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Lin, H.-C.; Wong, Y.H.; Tsang, L.M.; Chu, K.H.; Qian, P.-Y.; Chan, B.K.K. First study on gene expression of cement proteins and potential adhesion-related genes of a membranous-based barnacle as revealed from Next-Generation Sequencing technology. Biofouling 2014, 30, 169–181. [Google Scholar] [CrossRef]
- He, L.-S.; Zhang, G.; Qian, P.-Y. Characterization of Two 20kDa-Cement Protein (cp20k) Homologues in Amphibalanus amphitrite. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- So, C.R.; Scancella, J.M.; Fears, K.P.; Essock-Burns, T.; Haynes, S.E.; Leary, D.H.; Diana, Z.; Wang, C.Y.; North, S.; Oh, C.S.; et al. Oxidase Activity of the Barnacle Adhesive Interface Involves Peroxide-Dependent Catechol Oxidase and Lysyl Oxidase Enzymes. Acs Appl. Mater. Interfaces 2017, 9, 11493–11505. [Google Scholar] [CrossRef]
- So, C.R.; Fears, K.P.; Leary, D.H.; Scancella, J.M.; Wang, Z.; Liu, J.L.; Orihuela, B.; Rittschof, D.; Spillmann, C.M.; Wahl, K.J. Sequence basis of Barnacle Cement Nanostructure is Defined by Proteins with Silk Homology. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Wang, Z.; Leary, D.H.; Liu, J.; Settlage, R.E.; Fears, K.P.; North, S.H.; Mostaghim, A.; Essock-Burns, T.; Haynes, S.E.; Wahl, K.J.; et al. Molt-dependent transcriptomic analysis of cement proteins in the barnacle Amphibalanus amphitrite. BMC Genomics 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Power, A.M.; Klepal, W.; Zheden, V.; Jonker, J.; McEvilly, P.; von Byern, J. Mechanisms of Adhesion in Adult Barnacles. In Biological Adhesive Systems: From Nature to Technical and Medical Application; von Byern, J., Grunwald, I., Eds.; Springer: Vienna, Austria, 2010; pp. 153–168. [Google Scholar] [CrossRef]
- Dickinson, G.H.; Yang, X.; Wu, F.H.; Orihuela, B.; Rittschof, D.; Beniash, E. Localization of Phosphoproteins within the Barnacle Adhesive Interface. Biol. Bull. 2016, 230, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Crisp, D.J. Mechanisms of adhesion of fouling organisms. In Proceedings of the 3rd International Congress on Marine Corrosion and Fouling, Gaithersburg, MD, USA, 2–6 October 1972; pp. 691–709. [Google Scholar]
- Lindner, E.; Dooley, C.A. Chemical bonding in cirriped adhesive. In Proceedings of the 3rd International Congress on Marine Corrosion and Fouling, Gaithersburg, MD, USA, 2–6 October 1972; pp. 653–673. [Google Scholar]
- Dickinson, G.H.; Vega, I.E.; Wahl, K.J.; Orihuela, B.; Beyley, V.; Rodriguez, E.N.; Everett, R.K.; Bonaventura, J.; Rittschof, D. Barnacle cement: A polymerization model based on evolutionary concepts. J. Exp. Biol. 2009, 212, 3499–3510. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K. Absence of cross-linking via trans-glutaminase in barnacle cement and redefinition of the cement. Biofouling 2010, 26, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Kamino, K. Amyloid-like conformation and interaction for the self-assembly in barnacle underwater cement. Biochemistry 2015, 54, 826–835. [Google Scholar] [CrossRef]
- Bose, U.; Wang, T.; Zhao, M.; Motti, C.A.; Hall, M.R.; Cummins, S.F. Multiomics analysis of the giant triton snail salivary gland, a crown-of-thorns starfish predator. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Aldred, N.; Chan, V.B.S.; Emami, K.; Okano, K.; Clare, A.S.; Mount, A.S. Chitin is a functional component of the larval adhesive of barnacles. Commun. Biol. 2020, 3, 31. [Google Scholar] [CrossRef]
- He, Y.H.; Sun, C.J.; Jiang, F.H.; Yang, B.; Li, J.X.; Zhong, C.; Zheng, L.; Ding, H.B. Lipids as integral components in mussel adhesion. Soft Matter 2018, 14, 7145–7154. [Google Scholar] [CrossRef]
- Buffet, J.P.; Corre, E.; Duvernois-Berthet, E.; Fournier, J.; Lopez, P.J. Adhesive gland transcriptomics uncovers a diversity of genes involved in glue formation in marine tube-building polychaetes. Acta Biomater. 2018, 72, 316–328. [Google Scholar] [CrossRef]
- Yan, G.Y.; Zhang, G.; Huang, J.M.; Lan, Y.; Sun, J.; Zeng, C.; Wang, Y.; Qian, P.Y.; He, L.S. Comparative Transcriptomic Analysis Reveals Candidate Genes and Pathways Involved in Larval Settlement of the Barnacle Megabalanus volcano. Int. J. Mol. Sci. 2017, 18, 2253. [Google Scholar] [CrossRef]
- Chandramouli, K.H.; Al-Aqeel, S.; Ryu, T.; Zhang, H.; Seridi, L.; Ghosheh, Y.; Qian, P.Y.; Ravasi, T. Transcriptome and proteome dynamics in larvae of the barnacle Balanus Amphitrite from the Red Sea. BMC Genomics 2015, 16, 1063. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Hu, Y.H.; Ma, K.Y.; Jiang, X.S.; Ho, C.H.; Tsang, L.M.; Yi, L.F.; Leung, R.W.T.; Chu, K.H. CrusTF: A comprehensive resource of transcriptomes for evolutionary and functional studies of crustacean transcription factors. BMC Genomics 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Hennebert, E.; Maldonado, B.; Ladurner, P.; Flammang, P.; Santos, R. Experimental strategies for the identification and characterization of adhesive proteins in animals: A review. Interface Focus 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Cartharius, K.; Frech, K.; Grote, K.; Klocke, B.; Haltmeier, M.; Klingenhoff, A.; Frisch, M.; Bayerlein, M.; Werner, T. Matlnspector and beyond: Promoter analysis based on transcription factor binding sites. Bioinformatics 2005, 21, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Walker, G.; Holland, D.; Crisp, D. An energy budget for the free-swimming and metamorphosing larvae of Balanus balanoides (Crustacea: Cirripedia). Mar. Biol. 1979, 55, 221–229. [Google Scholar] [CrossRef]
- Thiyagarajan, V.; Harder, T.; Qian, P.Y. Relationship between cyprid energy reserves and metamorphosis in the barnacle Balanus amphitrite Darwin (Cirripedia; Thoracica). J. Exp. Mar. Biol. Ecol. 2002, 280, 79–93. [Google Scholar] [CrossRef]
- Wang, S.; You, Z.; Feng, M.; Che, J.; Zhang, Y.; Qian, Q.; Komatsu, S.; Zhong, B. Analyses of the Molecular Mechanisms Associated with Silk Production in Silkworm by iTRAQ-Based Proteomics and RNA-Sequencing-Based Transcriptomics. J. Proteome Res. 2016, 15, 15–28. [Google Scholar] [CrossRef]
- Huang, H.J.; Lu, J.B.; Li, Q.; Bao, Y.Y.; Zhang, C.X. Combined transcriptomic/proteomic analysis of salivary gland and secreted saliva in three planthopper species. J. Proteomics 2018, 172, 25–35. [Google Scholar] [CrossRef]
- Alvarenga, É.R.; Mendes, T.M.; Magalhães, B.F.; Siqueira, F.F.; Dantas, A.E.; Barroca, T.M.; Horta, C.C.; Kalapothakis, E. Transcriptome analysis of the Tityus serrulatus scorpion venom gland. Open J. Genet. 2012, 2, 210. [Google Scholar] [CrossRef]
- Qian, P.Y.; Lau, S.C.K.; Dahms, H.U.; Dobretsov, S.; Harder, T. Marine biofilms as mediators of colonization by marine macroorganisms: Implications for antifouling and aquaculture. Mar. Biotechnol. 2007, 9, 399–410. [Google Scholar] [CrossRef]
- Yanai, H.; Savitsky, D.; Tamura, T.; Taniguchi, T. Regulation of the cytosolic DNA-sensing system in innate immunity: A current view. Curr. Opin. Immunol. 2009, 21, 17–22. [Google Scholar] [CrossRef]
- Okano, K.; Shimizu, K.; Satuito, C.; Fusetani, N. Visualization of cement exocytosis in the cypris cement gland of the barnacle Megabalanus rosa. J. Exp. Biol. 1996, 199, 2131–2137. [Google Scholar] [PubMed]
- Odling, K.; Albertsson, C.; Russell, J.T.; Martensson, L.G.E. An in vivo study of exocytosis of cement proteins from barnacle Balanus improvisus (D.) cyprid larva. J. Exp. Biol. 2006, 209, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Sugiya, H. Understanding salivary fluid and protein secretion. Oral Dis. 2002, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Catalan, M.A.; Nakamoto, T.; Melvin, J.E. The salivary gland fluid secretion mechanism. J. Med. Investig. JMI 2009, 56, 192–196. [Google Scholar] [CrossRef]
- Walley, L.J.; Rees, E.I.S. Studies on the Larval Structure and Metamorphosis of Balanus balanoides (L.). Philos. Trans. R. Soc. Lond. B Biol. Sci. 1969, 256, 237–280. [Google Scholar]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef]
- Rocha, M.; Antas, P.; Castro, L.F.C.; Campos, A.; Vasconcelos, V.; Pereira, F.; Cunha, I. Comparative Analysis of the Adhesive Proteins of the Adult Stalked Goose Barnacle Pollicipes pollicipes (Cirripedia: Pedunculata). Mar. Biotechnol. (N. Y.) 2018. [Google Scholar] [CrossRef]
- Clarke, T.H.; Garb, J.E.; Hayashi, C.Y.; Arensburger, P.; Ayoub, N.A. Spider Transcriptomes Identify Ancient Large-Scale Gene Duplication Event Potentially Important in Silk Gland Evolution. Genome Biol. Evol. 2015, 7, 1856–1870. [Google Scholar] [CrossRef]
- Waite, J.H.; Jensen, R.A.; Morse, D.E. Cement precursor proteins of the reef-building polychaete Phragmatopoma californica (Fewkes). Biochemistry 1992, 31, 5733–5738. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [PubMed]
- Morgado, I.; Garvey, M. Lipids in Amyloid-beta Processing, Aggregation, and Toxicity. Adv. Exp. Med. Biol. 2015, 855, 67–94. [Google Scholar] [CrossRef] [PubMed]
- Barlow, D.E.; Dickinson, G.H.; Orihuela, B.; Kulp, J.L., 3rd; Rittschof, D.; Wahl, K.J. Characterization of the adhesive plaque of the barnacle Balanus amphitrite: Amyloid-like nanofibrils are a major component. Langmuir 2010, 26, 6549–6556. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Liang, C.; Zhang, X.K.; Li, J.Y.; Huang, J.Y.; Zeng, L.; Ye, Z.H.; Hu, B.R.; Wu, W.J. Amyloid fibril aggregation: An insight into the underwater adhesion of barnacle cement. Biochem. Biophys. Res. Commun. 2017, 493, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Ye, Z.; Xue, B.; Zeng, L.; Wu, W.; Zhong, C.; Cao, Y.; Hu, B.; Messersmith, P.B. Self-Assembled Nanofibers for Strong Underwater Adhesion: The Trick of Barnacles. ACS Appl. Mater. Interfaces 2018, 10, 25017–25025. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, J.H.; Stuart, A.E. Currents in the presynaptic terminal arbors of barnacle photoreceptors. Visual Neurosci. 1993, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Chen, Y.S.; Shi, C.M.; Huang, Z.B.; Zhang, Y.; Li, S.K.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2017, 7. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Pertea, G.; Huang, X.; Liang, F.; Antonescu, V.; Sultana, R.; Karamycheva, S.; Lee, Y.; White, J.; Cheung, F.; Parvizi, B.; et al. TIGR Gene Indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics 2003, 19, 651–652. [Google Scholar] [CrossRef]
- Simao, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Iseli, C.; Jongeneel, C.V.; Bucher, P. ESTScan: A program for detecting, evaluating, and reconstructing potential coding regions in EST sequences. In Proceedings of the International Conference on Intelligent Systems for Molecular Biology, Heidelberg, Germany, 6–10 August 1999; pp. 138–148. [Google Scholar]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Liu, H.; Chen, C.H.; Gao, Z.X.; Min, J.M.; Gu, Y.M.; Jian, J.B.; Jiang, X.W.; Cai, H.M.; Ebersberger, I.; Xu, M.; et al. The draft genome of blunt snout bream (Megalobrama amblycephala) reveals the development of intermuscular bone and adaptation to herbivorous diet. Gigascience 2017, 6. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.M.; Qiu, Z.Y.; Huang, Y. De Novo Assembly and Characterization of the Xenocatantops brachycerus Transcriptome. Int. J. Mol. Sci. 2018, 19, 520. [Google Scholar] [CrossRef]
- Audic, S.; Claverie, J.M. The significance of digital gene expression profiles. Genome Res. 1997, 7, 986–995. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Mu, H.; Sun, J.; Heras, H.; Chu, K.H.; Qiu, J.W. An integrated proteomic and transcriptomic analysis of perivitelline fluid proteins in a freshwater gastropod laying aerial eggs. J. Proteomics 2017, 155, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Liu, T.; Liu, C.J.; Song, S.; Zhang, X.; Liu, W.; Jia, H.; Xue, Y.; Guo, A.Y. AnimalTFDB 2.0: A resource for expression, prediction and functional study of animal transcription factors. Nucleic Acids Res. 2015, 43, D76–D81. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H. Predicting Secretory Proteins with SignalP. Methods Mol. Biol. 2017, 1611, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef]

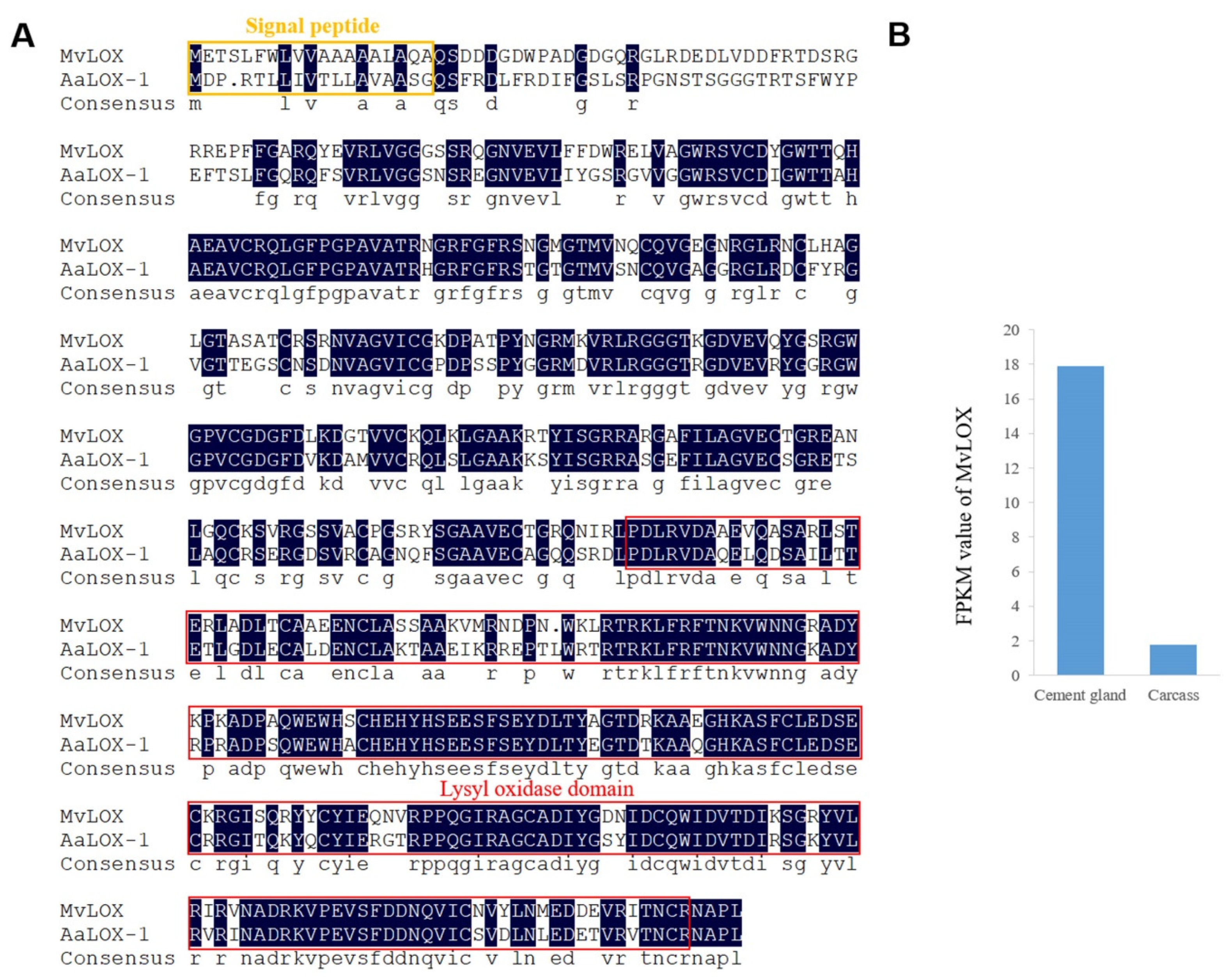
| Result | Cement Gland | Carcass | All |
|---|---|---|---|
| Output result | |||
| Raw reads (M) | 65.60 | 65.60 | |
| Clean reads (M) | 65.36 (99.76%) | 65.44 (99.63%) | |
| Clean read Q20 | 97.43% | 96.80% | |
| Clean read Q30 | 94.11% | 93.34% | |
| Assembly result | |||
| Number of unigenes | 38,538 | 55,573 | 67,299 |
| Unigene mean length (nt) | 679 | 565 | 613 |
| Unigene N50 (nt) | 1092 | 779 | 928 |
| GC (%) | 55.97 | 55.04 | 55.15 |
| Annotation result | |||
| Nr | 19,234 | 21,389 | 27,793 |
| Nt | 11,216 | 12,307 | 15,978 |
| Swiss-Prot | 15,963 | 16,802 | 21,906 |
| KEGG | 15,416 | 16,610 | 21,600 |
| COG | 9078 | 8828 | 12,584 |
| GO | 9258 | 9546 | 6830 |
| Coding sequence prediction | |||
| CDS predicted from BLAST result | 19,624 | 21,842 | 28,522 |
| CDS predicted by ESTScan | 3401 | 4208 | 5360 |
| Total CDSs predicted | 23,025 | 26,050 | 33,882 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, G.; Sun, J.; Wang, Z.; Qian, P.-Y.; He, L. Insights into the Synthesis, Secretion and Curing of Barnacle Cyprid Adhesive via Transcriptomic and Proteomic Analyses of the Cement Gland. Mar. Drugs 2020, 18, 186. https://doi.org/10.3390/md18040186
Yan G, Sun J, Wang Z, Qian P-Y, He L. Insights into the Synthesis, Secretion and Curing of Barnacle Cyprid Adhesive via Transcriptomic and Proteomic Analyses of the Cement Gland. Marine Drugs. 2020; 18(4):186. https://doi.org/10.3390/md18040186
Chicago/Turabian StyleYan, Guoyong, Jin Sun, Zishuai Wang, Pei-Yuan Qian, and Lisheng He. 2020. "Insights into the Synthesis, Secretion and Curing of Barnacle Cyprid Adhesive via Transcriptomic and Proteomic Analyses of the Cement Gland" Marine Drugs 18, no. 4: 186. https://doi.org/10.3390/md18040186
APA StyleYan, G., Sun, J., Wang, Z., Qian, P.-Y., & He, L. (2020). Insights into the Synthesis, Secretion and Curing of Barnacle Cyprid Adhesive via Transcriptomic and Proteomic Analyses of the Cement Gland. Marine Drugs, 18(4), 186. https://doi.org/10.3390/md18040186





