1. Introduction
Conotoxins (CTxs) are highly sophisticated set of neuropharmacological weapons formed during the long-term evolution. Most of the CTxs are composed of 12 to 40 amino acid residues and contain 2 to 3 pairs of disulfide bonds. CTxs are among smallest nucleic acid-encoded animal neurotoxin peptides, and they are also small peptides with the highest disulfide bond density [
1]. CTxs can specifically act on voltage-gated ion channels (Na
+, K
+, Ca
2+), ligand-gated ion channels (nAchR, 5-HT
3R, NMDAR) and G protein-coupled receptors (neurotensin receptors and vasopressin receptors), have received extensive attention in the field of neuroscience and new drug development. For example, α, αA, and ψ-CTxs antagonize nAchR competitively and noncompetitively [
2]. ω-CTxs selectively inhibit various subtypes of voltage-sensitive Ca
2+ channels [
3]. κ-CTxs selection the voltage-sensitive K
+ channel are inhibited [
4], while μ and μO-CTxs selectively act on the voltage-sensitive Na
+ channel [
5,
6,
7]. In addition, some Conotoxins, which do not contain or contain a pair of disulfide bonds, can selectively act on vasopressin receptors (such as conopressin) [
8], NMDA receptors (such as conantokins) [
9], serotonin type 3 (5-HT3) receptors [
10]. CTxs can distinguish and recognize many different subtypes or heterostructures of ion channels and neural receptors and are widely used in life science research; several CTxs have been marketed or entered clinical research as analgesics and antiepileptic drugs. According to their feeding habits, cone snails are either fish-hunting (piscivorous class), mollusk-hunting (molluscivorous class), or worm-hunting (vermivorous class). Fish-hunting cone snails are not the largest category of cone snails, but the activity of CTxs on mammals is obvious. There are many types of cone snails, and each type of cone snails contains different toxins. Therefore, studying new species of cone snails means obtaining a new structure of toxins, which means that it may have new functions.
Conus achatinus is a fish-hunting cone snails. At present, little research has been conducted at home and abroad on the toxins of this cone snail [
11,
12,
13]. We have conducted preliminary research, taking advantage of the South China Sea resources to purify and characterize a class of containing D-amino acid CTxs. We also explored the role of these CTxs in ion channels and analgesic activity.
3. Discussion
Using NCBI website, multimode search was conducted, conotoxin-Ac1 was identified as the mature region of the reported sequence of conotoxin Ac3.1 from the same species
Conus achatinus (Sequence ID: P0CH24.1) [
14]. The full-length cDNA of Ac3.1 was cloned from the venom-duct transcriptome with a combined PCR and RACE approach, and the amino acid sequence of Ac3.1 precursor was deduced and assigned to M-superfamily in the previous research. In this study, we performed peptides purification and sequencing to analyze and identify the mature toxins conotoxin-Ac1 and conotoxin-Ac1-O6P, located the accurate cleavage site of mature peptide formation, but also confirmed the high expression of conotoxin Ac3.1 preliminarily identified in the transcript level. It is reported in the literature that most CTxs contain disulfide bonds [
15], while conotoxin-Ac1 in
Conus achatinus is cysteine-free linear toxin. Linear toxins, as in
Conus achatinus, are presented in high abundance. This situation is still rare and has strong research value. conotoxin-Ac1 contains 15 amino acid residues, including six aromatic amino acid residues, N-terminal (YYLY) and C-terminal (WW) are highly hydrophobic and lipophilic, while the intermediate (ROENS) is hydrophilic. Current research indicates that CTxs do not contain cysteine in cone snails can be roughly divided into six categories (
Table 7). The activity of these CTxs are also different. Mo1659, which acts on the K
+ channel; Conomap-Vt can excite muscle tissue, but its role in receptors has not been determined; contulakin-G acts on the neurotensin receptor [
16]; conantokins can block NMDAR. According to current literatures, conantokins are the only peptide-type NMDAR inhibitors with strong neuroprotective effects, and also have good anti-pain effects [
17]. By electrophysiological experiments, we found that conotoxin-Ac1 can act on NMDAR ion channel. in addition to conantokins, which are reported in the literature, conotoxin-Ac1 is natural peptide that inhibits NMDAR activity.
Firstly, design and chemical synthesis of conotoxin-Ac1 mutants was performed. The alanine replacement method replaces each non-alanine residue site with alanine, removes the active group on the side chain, and replaces it with a small methyl group without other functional groups. This methyl group is small and can be used to identify the positions of key amino acids. We used the alanine substitution method to mutate all non-alanine residues in conotoxin-Ac1 through solid-phase synthesis of peptides, numbered 3–16. Prior results indicate that conantokins are antagonists of NMDAR, in which the C-terminal residues of this class of peptides are amidated, also, the conotoxin-Ac1 C-terminal Thr15 is mutated to amidated Thr15, which is conotoxin-Ac1-15* (17). Conantokins all contain γ-carboxyglutamic acid, which is a site for binding to metal ions. It was found that the γ-carboxyglutamic acid arrangement must meet the requirements of “i, i + 4, i + 7, i + 11”; therefore, Glu10 is mutated to γ-carboxyglutamic acid, or both Glu10 and Trp14 are mutated to γ-carboxyglutamic acid, which are conotoxin-Ac1-E10γ (18), conotoxin-Ac1-E10γW14γ (19), or conotoxin-Ac1-E10γW14γ15 * (20). The accuracy of each synthesized CTx polypeptide was analyzed by HPLC and MS, and then the changes in the secondary structure of conotoxin-Ac1 by each amino acid were analyzed by CD spectroscopy. In the PB buffer solution, the proportion of the α-helix structure in conotoxin-Ac1 was 44.9%. Except for conotoxin-Ac1-Y5A (7), the other mutants also had α-helices as their main secondary structure. Although conotoxin-Ac1-Y2A (4), conotoxin-Ac1-Y3A (5) and conotoxin-Ac1-Y5A (7) all mutate tyrosine to alanine, their effects on the secondary structure are not the same, suggesting that amino acids have a secondary effect. The influence of structure is related not only to the type of amino acid but also to the site where it is located.
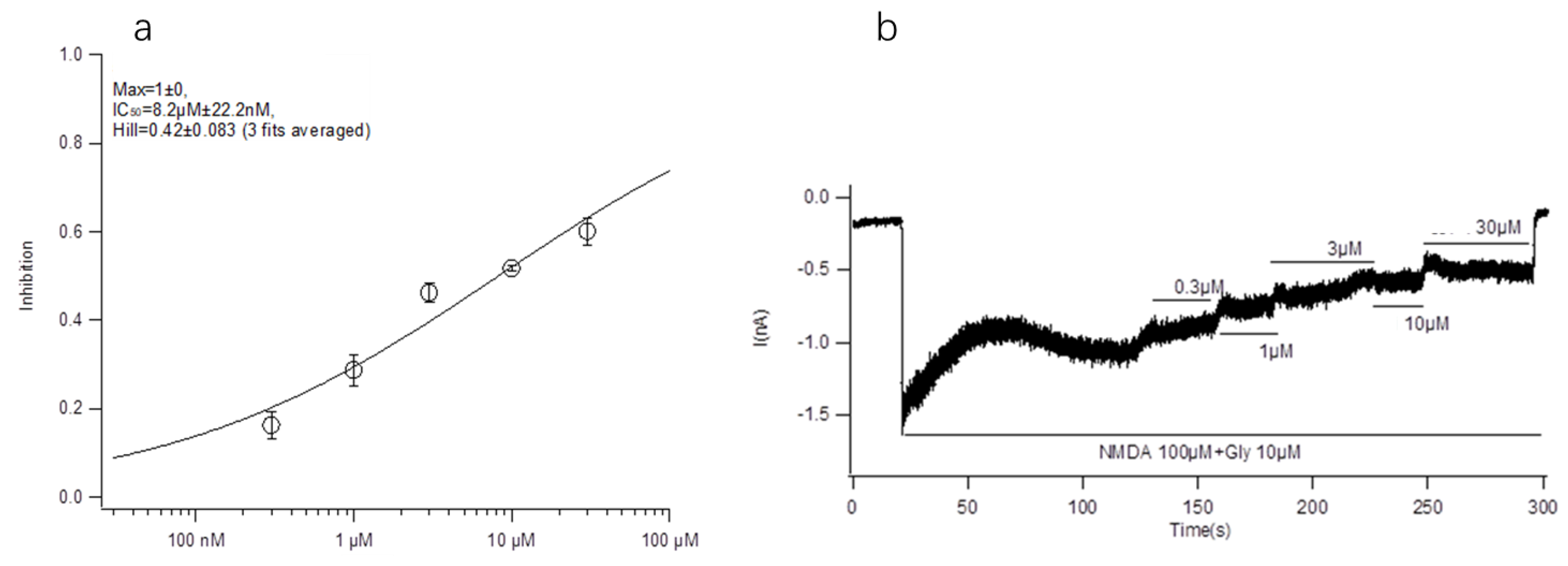
The main purpose of this paper is to explore the structure-activity relationship between conotoxin-Ac1 and its target. We first analyzed the effects of 10 μM conotoxin-Ac1 and conotoxin-Ac1-O6P on the activity of Na+, K+, Ca2+, and NMDAR ion channels using whole-cell patch-clamp technology. The results showed that 10 μM conotoxin-Ac1 and conotoxin-Ac1-O6P had almost no inhibitory effect on Na+ and K+, 10 μM conotoxin-Ac1-O6P had an inhibitory effect on Ca2+ ion channels, and 10 μM conotoxin-Ac1 had a current inhibition percentage of 56.90% on NMDAR ion channels. Therefore, the NMDAR ion channel is the active channel of conotoxin-Ac1. Subsequently, the effects of 10 μM conotoxin-Ac1 and conotoxin-Ac1-O6P on NMDAR ion channel subtypes NR2A and NR2B were investigated. The results showed that the activity inhibition rate of the NR2B ion channel was increased by 10 μM conotoxin-Ac1 to 51.69%, while that of the NR2A ion channel was only 7.22%, the activity inhibition rate of the NR2B ion channel was increased by 10 μM conotoxin-Ac1-O6P to 10.63%, while that of the NR2A ion channel was only 1.85%, The result showed that the absence of the hydroxyl group on O6 of conotoxin-Ac1 causes a five-fold difference at the NMDAR subtypes NR2A and NR2B. The IC50 value of conotoxin-Ac1 on the activity of the NR2B ion channel was 8.22 ± 0.022 μM. It is suggested that conotoxin-Ac1 acts on the NR2B subtype. Finally, we investigated the effect of conotoxin-Ac1 mutants on NR2B and discussed the structure-activity relationship between conotoxin-Ac1 and NR2B. Among the alanine substitution mutants numbered 3–16, conotoxin-Ac1-N1A (3), conotoxin-Ac1-O9A (10), conotoxin-Ac1-E10A (11) and conotoxin-Ac1-S12A (13) all inhibited NR2B. Compared with the inhibitory rate of conotoxin-Ac1 on NR2B, the ratio is reduced by 10 times, and the numbers 3, 10, 11, and 13 are all mutated from polar amino acids to nonpolar amino acids, suggesting that polar amino acids have an important effect on the activity of conotoxin-Ac1. Although all mutations were o to A, the inhibition rate of conotoxin-Ac1-O6A (8) on NR2B was 18.98% greater than that of conotoxin-Ac1-O9A (10) on NR2B, which was approximately 4.27%—approximately four times greater. The abovementioned experimental results show that N1, O9, E10, and S12 are the key active amino acids of conotoxin-Ac1, which play an important role in inhibiting the activity of NR2B. In addition, our mutants, numbered 17–20 based on the structural rule of the NMDAR antagonist conantokins did not show strong anti-NR2B activity. The experimental results suggest that conotoxin-Ac1 and conantokins are not the same NMDAR antagonist.
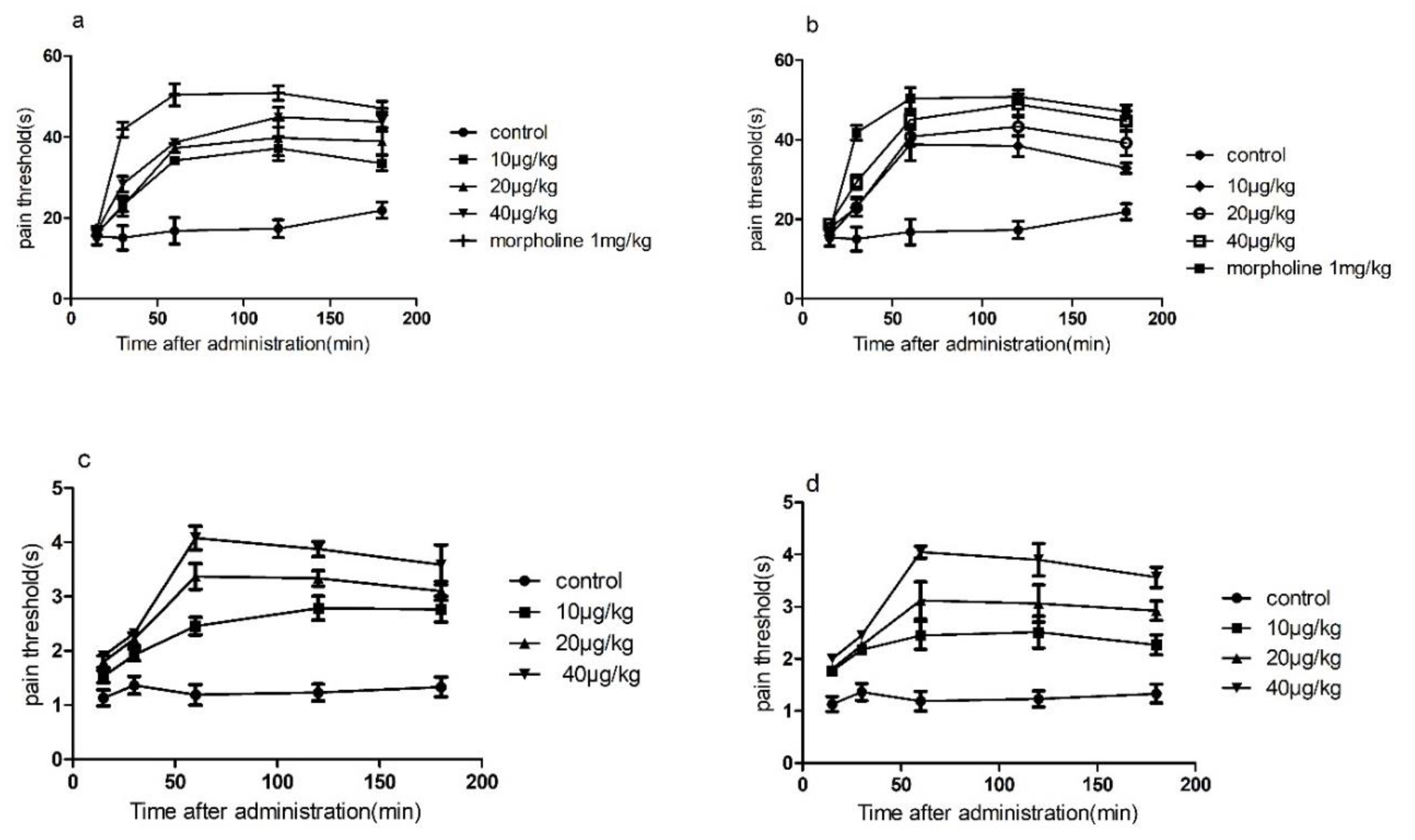
The results of conotoxin-Ac1 and conotoxin-Ac1-O6P animal overall activity evaluation showed that conotoxin-Ac1 and conotoxin-Ac1-O6P can significantly shorten sleep latency and prolong sleep time. It is shown that conotoxin-Ac1 and conotoxin-Ac1-O6P have stabilization effects. The analgesic activity of conotoxin-Ac1 and conotoxin-Ac1-O6P was examined by the hot-plate method and tail-burn method. The results showed that 10, 20, and 40 μg/kg doses of conotoxin-Ac1 and conotoxin-Ac1-O6P all had analgesic activity, and there were significant differences compared with the control group. Electrophysiological results showed that conotoxin-Ac1-O6P, and the absence of the hydroxyl group on O6 of conotoxin-Ac1, caused a five-fold difference at the NMDAR, but the analgesic activity of these two conotoxins are very similar, Therefore, we believe that the relationship between conotoxin-Ac1 analgesic activity and conotoxin-Ac1 inhibitory activity of NMDAR still needs to be studied in subsequent experiments. Subsequently, the analgesic activity of 10 μg/kg doses of the conotoxin-Ac1 mutants was analyzed by the hot-plate method. The conotoxin-Ac1 mutants numbered 3–16 were less than conotoxin-Ac1. conotoxin-Ac1-N1A (3), conotoxin-Ac1-Y2A (4), conotoxin-Ac1-Y3A (5), conotoxin-Ac1-N11A (12), and conotoxin-Ac1-S12A (13) not only reduce analgesic activity but also reach the maximum analgesic effect after 60 min of administration, and conotoxin-Ac1 reaches the maximum analgesia after 120 min of administration. The effects are notably different. In addition, the pain threshold of conotoxin-Ac1-E10A (11) and conotoxin-Ac1-T15A (16) was lower than that of conotoxin-Ac1 after 60 min of administration, and there were significant differences (p < 0.05). The above results show that N1, Y2, Y3, E10, N11, S12, and T15 play important roles in the analgesic activity of conotoxin-Ac1. Combined with electrophysiological experiments, N1 and S12 have significant effects on conotoxin-Ac1 in inhibiting NR2B and analgesic activity, specifically conotoxin-Ac1-15* (17), conotoxin-Ac1-E10γ (18), conotoxin-Ac1-E10γW14γ (19), and conotoxin-Ac1-E10γW14γ15* (20) Although these compounds have strong NR2B-inhibitory activity, they have analgesic activity, but it was higher than that of conotoxin-Ac1; furthermore, the pain thresholds of conotoxin-Ac1-E10γW14γ(19), conotoxin-Ac1-E10γW14γ15* (20) after administration for 30, 60, 120, and 180 min were significantly higher than the pain threshold of 5P1 (p < 0.05). It is suggested that c-terminal amidation and γ amino acid have a strong effect on the analgesic activity of conotoxin-Ac1.