Anti-Inflammatory Activity of Glycolipids and a Polyunsaturated Fatty Acid Methyl Ester Isolated from the Marine Dinoflagellate Karenia mikimotoi
Abstract
1. Introduction
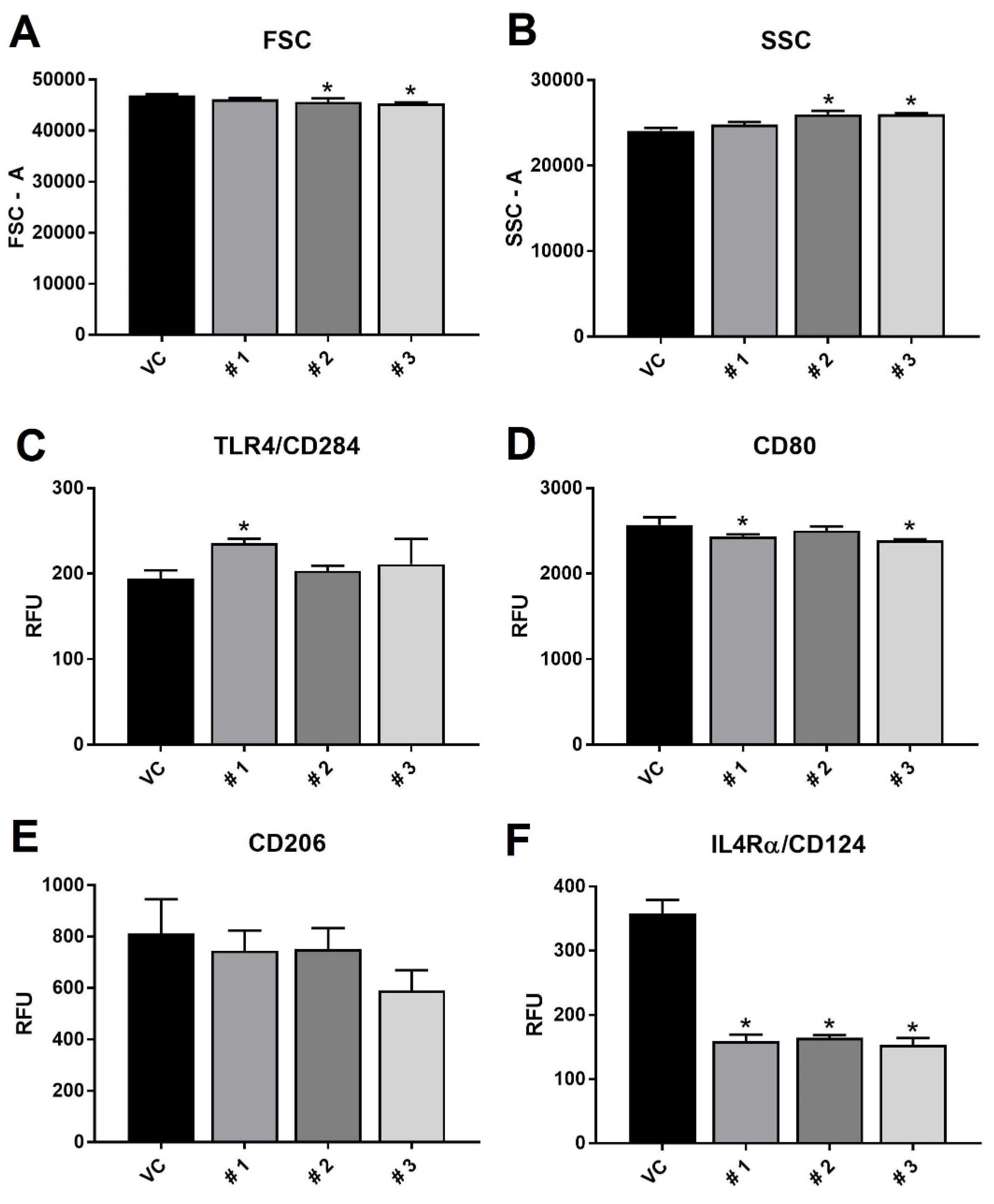
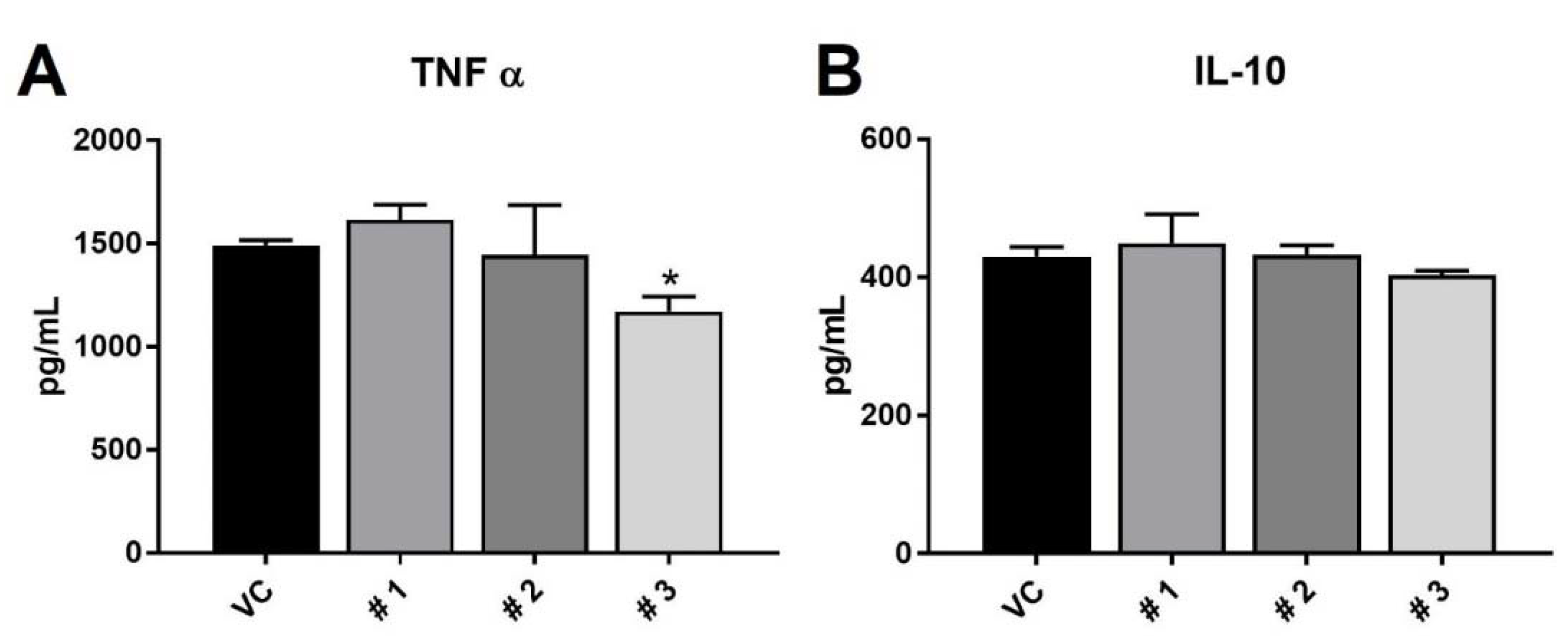
2. Results and Discussion
3. Experimental Section
3.1. Strain and Cultivation
3.2. Extraction and Isolation
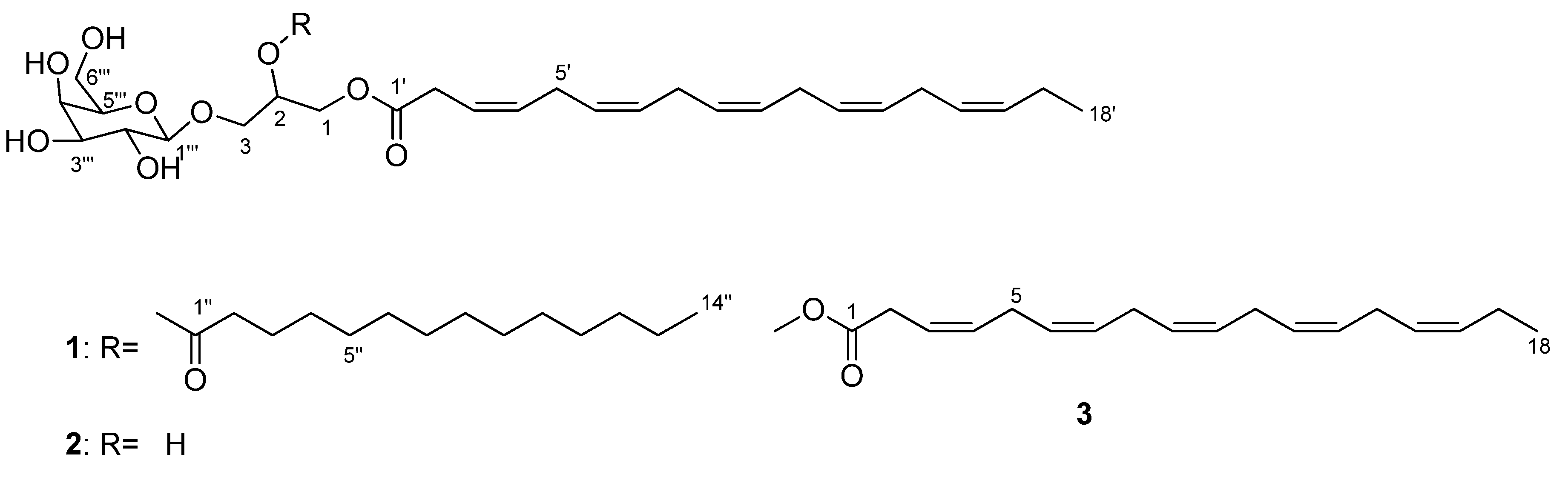
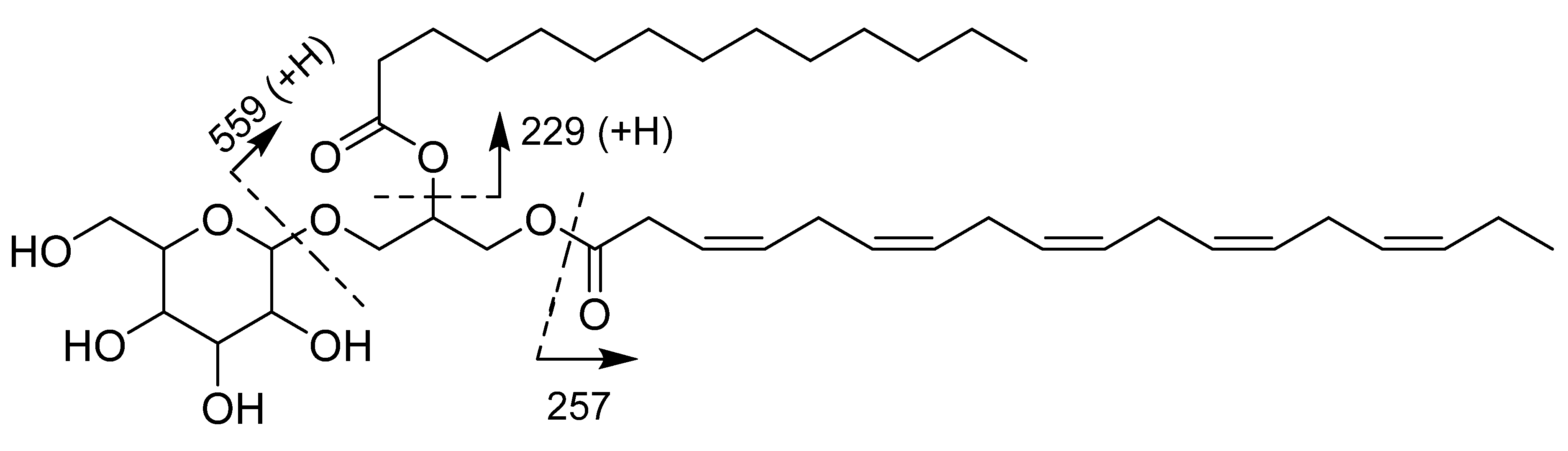
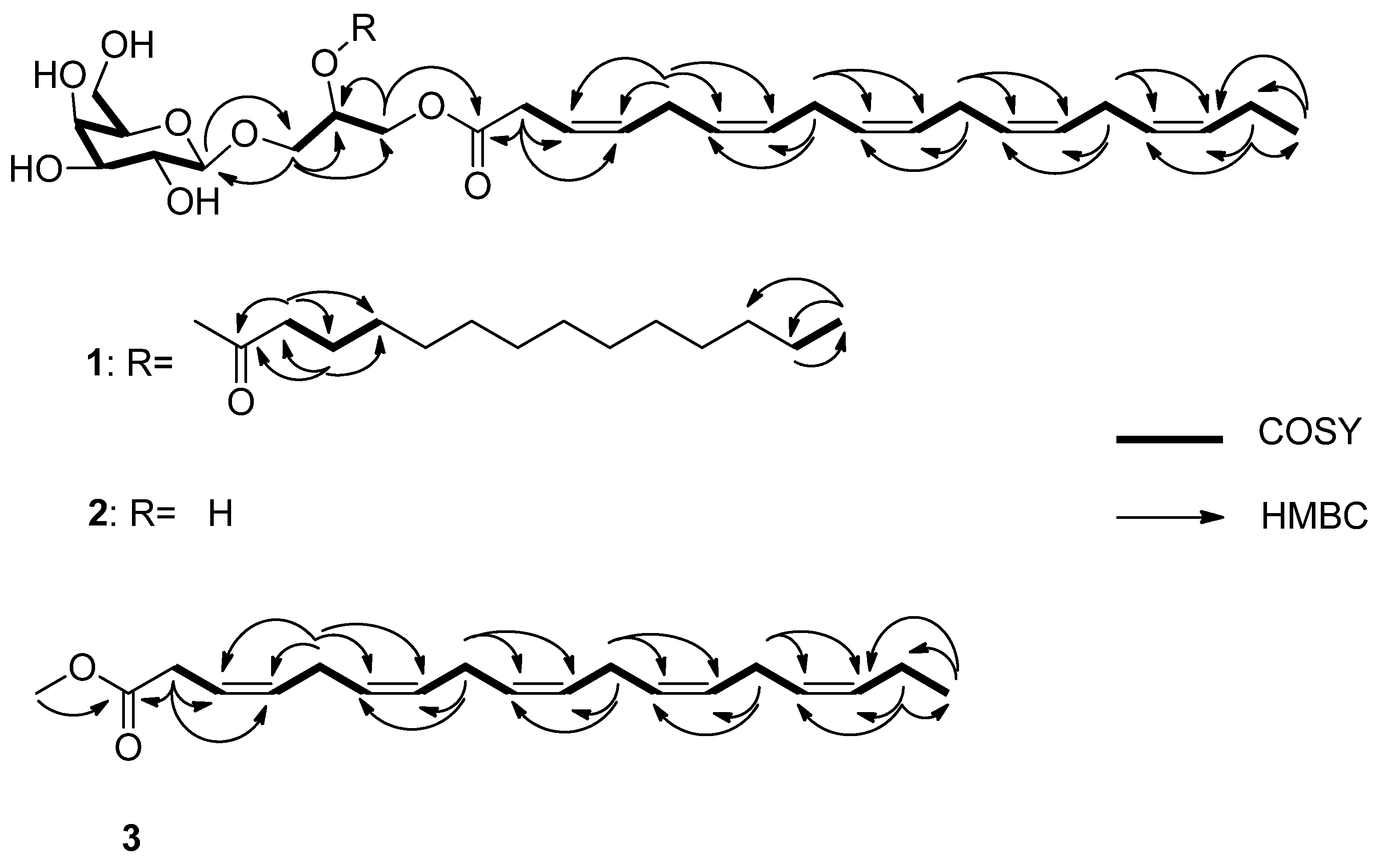
3.3. Structure Elucidation
3.3.1. Monogalactosyldiacylglycerol 1
3.3.2. Monogalactosylmonoacylglycerol 2
3.3.3. Methyl (3Z,6Z,9Z,12Z,15Z)-octadeca-3,6,9,12,15-pentaenoate 3
3.4. Antimicrobial Bioassay Testing
3.5. Flow Cytometry and ELISA Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Imada, C.; Koseki, N.; Kamata, M.; Kobayashi, T.; Hamada-Sato, N. Isolation and characterization of antibacterial substances produced by marine actinomycetes in the presence of seawater. Actinomycetologica 2007, 21, 27–31. [Google Scholar] [CrossRef]
- Fenical, W.; Jensen, P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Dahl, E.; Tangen, K. 25 years experience with Gyrodinium aureolum in Norwegian waters 1993. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 15–21. [Google Scholar]
- Davidson, K.; Miller, P.; Wilding, T.A.; Shutler, J.; Bresnan, E.; Kennington, K.; Swan, S. A large and prolonged bloom of Karenia mikimotoi in Scottish waters in 2006. Harmful Algae 2009, 8, 349–361. [Google Scholar] [CrossRef]
- Lindahl, O. On the development of a Gyrodinium aureolum occurrence on the Swedish west coast in 1982. Mar. Biol. 1983, 77, 143–150. [Google Scholar] [CrossRef]
- Raine, R.; O’Boyle, S.; O’Higgins, T.; White, M.; Patching, J.; Cahill, B.; McMahon, T. A satellite and field portrait of a Karenia mikimotoi bloom off the south coast of Ireland, August 1998. Hydrobiologia 2001, 465, 187–193. [Google Scholar] [CrossRef]
- Hodgkiss, I.; Yang, Z. New and dominant species from Sam Xing wan, sai Kung during the 1998 massive fish killing red tide in Hong Kong. Harmful Algal Blooms 2000, 62, 65. [Google Scholar]
- Gentien, P.; Lunven, M.; Lazure, P.; Youenou, A.; Crassous, M.P. Motility and autotoxicity in Karenia mikimotoi (Dinophyceae). Philos. Trans. R Soc. B Biol. Sci. 2007, 362, 1937–1946. [Google Scholar] [CrossRef]
- Tatters, A.O.; Muhlstein, H.I.; Tomas, C.R. The hemolytic activity of Karenia selliformis and two clones of Karenia brevis throughout a growth cycle. J. Appl. Phycol. 2010, 22, 435–442. [Google Scholar] [CrossRef]
- Mooney, B.D.; Nichols, P.D.; De Salas, M.F.; Hallegraeff, G.M. Lipid, fatty acid, and sterol composition of eight species of Kareniaceae (Dinophyta): Chemotaxonomy and putative lipid phycotoxins. J. Phycol. 2007, 43, 101–111. [Google Scholar] [CrossRef]
- Yasumoto, T.; Underdal, B.; Aune, T.; Hormazabal, V.; Skulberg, O.M.; Oshima, Y. Screening for hemolytic and ichthyotoxic components of Chryssochromulina polylepis and Gyrodinium aureolum from Norwegian coastal waters. In Toxic Marine Phytoplankton; Graneli, E., Sundstrom, B., Edler, L., Anderson, D.M., Eds.; Elsevier: New York, NY, USA, 1990; pp. 436–440. [Google Scholar]
- Matsubara, K.; Matsumoto, H.; Mizushina, Y.; Mori, M.; Nakajima, N.; Fuchigami, M.; Yoshida, H.; Hada, T. Inhibitory effect of glycolipids from spinach on in vitro and ex vivo angiogenesis. Oncol. Rep. 2005, 14, 157–160. [Google Scholar]
- Wang, R.; Furumoto, T.; Motoyama, K.; Okazaki, K.; Kondo, A.; Fukui, H. Possible antitumor promoters in Spinacia oleracea (Spinach) and comparison of their contents among cultivars. Biosci. Biotechnol. Biochem. 2002, 66, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K. High resistance to oxygen radicals and heat is caused by a galactoglycerolipid in Microbacterium sp. M874. J. Biochem. 2000, 127, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Guo, C.T.; Matsufugi, M.; Yoshimoto, A.; Inagaki, M.; Higuchi, R.; Suzuki, Y. Influenza A virus-binding activity of glycoglycerolipids of aquatic bacteria. J. Biochem. 2000, 127, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Janwitayanuchit, W.; Suwanborirux, K.; Patarapanich, C.; Pummangura, S.; Lipipun, V.; Vilaivan, T. Synthesis and anti-herpes simplex viral activity of monoglycosyl diglycerides. Phytochemistry 2003, 64, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Rossi, C.; Marcolongo, G.; Di Lena, A.; Venzo, A.; Barrie, C.P.; Corda, D. Selective in vivo anti-inflammatory action of the galactolipid monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005, 524, 159–168. [Google Scholar] [CrossRef]
- Tanaka, R.; Sakano, Y.; Nagatsu, A.; Shibuya, M.; Ebizuka, Y.; Goda, Y. Synthesis of digalactosyl diacylglycerols and their structure–inhibitory activity on human lanosterol synthase. Bioorg. Med. Chem. Lett. 2005, 15, 159–162. [Google Scholar] [CrossRef]
- Nagatsu, A.; Watanabe, M.; Ikemoto, K.; Hashimoto, M.; Murakami, N.; Sakakibara, J.; Tokuda, H.; Nishino, H.; Iwashima, A.; Yazawa, K. Synthesis and structure—anti-tumor-promoting activity relationship of monogalactosyl diacylglycerols. Bioorg. Med. Chem. Lett. 1994, 4, 1619–1622. [Google Scholar] [CrossRef]
- Colombo, D.; Compostella, F.; Ronchetti, F.; Scala, A.; Toma, L.; Mukainaka, T.; Nagatsu, A.; Konoshima, T.; Tokuda, H.; Nishino, H. Inhibitory effects of monoacylated 2-O-β-galactosylglycerols on Epstein–Barr virus activation: The significant role of the hexanoyl chain. Cancer Lett. 1999, 143, 1–4. [Google Scholar] [CrossRef]
- Cateni, F.; Bonivento, P.; Procida, G.; Zacchigna, M.; Scialino, G.; Banfi, E. Chemoenzymatic synthesis and in vitro studies on the hydrolysis of antimicrobial monoglycosyl diglycerides by pancreatic lipase. Bioorg. Med. Chem. Lett. 2007, 17, 1971–1978. [Google Scholar] [CrossRef]
- Leblond, J.D.; Dodson, J.; dahmen, J.I. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. VII. Evidence against galactolipid production and plastid presence in the heterotrophic, basal dinoflagellate, Oxyrrhis marina. Eur. J. Phycol. 2013, 48, 309–317. [Google Scholar] [CrossRef]
- Gray, C.G.; Lasiter, A.D.; Li, C.; Leblond, J.D. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. I. Peridinin-containing taxa. Eur. J. Phycol. 2009, 44, 191–197. [Google Scholar] [CrossRef]
- Leblond, J.D.; Lasiter, A.D. Mono- and digalactosyldiacylglycerol composition of dinoflagellates. II. Lepidodinium chlorophorum, Karenia brevis, and Kryptoperidinium foliaceum, three dinoflagellates with aberrant plastids. Eur. J. Phycol. 2009, 44, 199–205. [Google Scholar] [CrossRef][Green Version]
- Hiraga, Y.; Shikano, T.; Widianti, T.; Ohkata, K. Three new glycolipids with cytolytic activity from cultured marine dinoflagellate Heterocapsa circularisquama. Nat. Prod. Res. 2008, 22, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Bond, T.J.; Herald, D.L.; Penny, M.; Doubek, D.L.; Williams, M.D.; Pettit, R.K.; Hooper, J.N.A. Isolation and structure of spongilipid from the Republic of Singapore marine porifera Spongia cf. hispida. Can. J. Chem. 1997, 75, 920–925. [Google Scholar] [CrossRef]
- Ishii, T.; Okino, T.; Mino, Y. A ceramide and cerebroside from the starfish Asterias amurensis Lu1tken and their plant-growth promotion activities. J. Nat. Prod. 2006, 69, 1080–1082. [Google Scholar] [CrossRef]
- Scribe, P.; Guezennec, J.; Dagaut, J.; Pepe, C.; Saliot, A. Identification of the position and the stereochemistry of the double bond in monounsaturated fatty acid methyl esters by gas chromatography/mass spectrometry of dimethyl disulfide derivatives. Anal. Chem. 1988, 60, 928–931. [Google Scholar] [CrossRef]
- Ghioni, C.; Porter, A.E.A.; Sadler, I.H.; Tocher, D.R.; Sargent, J.R. Cultured fish cells metabolize octadecapentaenoic acid (all-cis Δ3,6,9,12,15-18:5) to octadecatetraenoic acid (all-cis Δ6,9,12,15-18:4) via its 2-trans intermediate (trans Δ2, all-cis Δ6,9,12,15-18:5). Lipids 2001, 36, 145–153. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hayashi, K.; Kawazoe, K.; Kitagawa, I. Marine natural products. XXIX. Heterosigma-glycolipids I, II, III, and IV, four diacylglyceroglycolipids possessing ω3-polyunsaturated fatty acid residues, from the raphidopycean dinoflagellate Heterosigma akashiwo. Chem. Pharm. Bull. 1992, 40, 1404–1410. [Google Scholar] [CrossRef]
- Iwagawa, T.; Masuda, T.; Nakatani, M. Polyunsaturated fatty acid methyl esters from a soft Coral Xenia sp. Rep. Fac. Sci. Kagoshima Univ. 1993, 26, 63–67. [Google Scholar]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.; Mardani, M.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Keeler, D.M.; Grandal, M.K.; McCall, J.R. Brevenal, a marine natural product, is anti-inflammatory and an immunomodulator of macrophage and lung epithelial cells. Mar. Drugs 2019, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginet, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef]
- Adolf, J.E.; Place, A.R.; Stoecker, D.K.; Harding, L.W., Jr. Modulation of polyunsaturated fatty acids in mixotrophic Karlodinium veneficum (Dinophyceae) and its prey, Storeatula major (Cryptophyceae). J. Phycol. 2007, 43, 1259–1270. [Google Scholar] [CrossRef]
- Okuyama, H.; Morita, N.; Kogame, K. Occurrence of octadecapentaenoic acid in lipids of a cold stenothermic alga, prymnesiophyte strain B. J. Phycol. 1992, 28, 465–472. [Google Scholar] [CrossRef]
| 1 | ||||
| No. | δC, mult. b | δH (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1, Gly | 64.5, CH2 | 4.25, dd (12.1, 6.3) | 3, 2 | 2, 1’ |
| 4.43, dd (12.1, 3.0) | ||||
| 2, Gly | 71.8, CH | 5.27, m | 1, 3 | |
| 3, Gly | 68.8, CH2 | 3.74, m | 2, 1 | 2, 1, 1‴ |
| 4.00, dd (10.7, 4.0) | ||||
| 1’ | 173.1, C | |||
| 2’ | 33.2, CH2 | 3.14, d (4.9) | 3’ | 1’, 3’, 4’ |
| 3’ | 132.6, CH | 5.55, t (4.9) | 2’ | |
| 4’ | 122.4, CH | 5.55, t (4.9) | 5’ | |
| 6’-7’ | 128.3/129.7, CH | 5.36, m | 5’/8’ | |
| 9’-10’ | 128.3/129.7, CH | 5.36, m | 8’/11’ | |
| 12’-13’ | 128.3/129.7, CH | 5.36, m | 11’/14’ | |
| 15’ | 129.3, CH | 5.36, m | 14’ | |
| 16’ | 132.9, CH | 5.36, m | 17’ | |
| 17’ | 21.6, CH2 | 2.09, quint (7.6) | 16’, 18’ | 15’, 16’, 18’ |
| 18’ | 14.7, CH3 | 0.98, t (7.6) | 17’ | 16’, 17’ |
| 5’ | 26.8c, CH2 | 2.84, m | 4’, 6’ | 3’, 4’, 6’, 7’ |
| 8’ | 26.7c, CH2 | 2.84, m | 7’, 9’ | 6’, 7’, 9’, 10’ |
| 11’ | 26.6c, CH2 | 2.84, m | 10’, 12’ | 9’, 10’, 12’, 13’ |
| 14’ | 26.6c, CH2 | 2.84, m | 13’, 15’ | 12’, 13’, 15’, 16’ |
| 1″ | 174.6, C | |||
| 2″ | 35.2, CH2 | 2.33, t (7.4) | 3″ | 1″, 3″, 4″ |
| 3″ | 26.1, CH2 | 1.61, m | 2″, 4″ | 1″, 2″, 4″ |
| 4″ | 30.6, CH2 | 1.33, m | 3″ | |
| 5″-11″ | 30.2-30.8, CH2 | 1.33, m | ||
| 12″ | 33.1, CH2 | 1.33, m | ||
| 13″ | 23.8, CH2 | 1.33, m | 14″ | 14″ |
| 14″ | 14.5, CH3 | 0.90, t (7.3) | 13″ | 12″, 13″ |
| 1‴ | 105.4, CH | 4.22, d (7.5) | 2‴ | 3, 2‴ |
| 2‴ | 72.5, CH | 3.52, m | 1‴ | 3‴ |
| 3‴ | 75.0, CH | 3.45, dd (9.7, 3.2) | 4‴ | 2‴ |
| 4‴ | 70.3, CH | 3.82, brd (3.2) | 3‴, 5‴ | 2‴ |
| 5‴ | 76.9, CH | 3.51, m | 4‴, 6‴ | 1‴, 4‴, 6‴ |
| 6‴ | 62.5, CH2 | 3.75, m | 5‴ | 4‴, 5‴ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leutou, A.S.; McCall, J.R.; York, R.; Govindapur, R.R.; Bourdelais, A.J. Anti-Inflammatory Activity of Glycolipids and a Polyunsaturated Fatty Acid Methyl Ester Isolated from the Marine Dinoflagellate Karenia mikimotoi. Mar. Drugs 2020, 18, 138. https://doi.org/10.3390/md18030138
Leutou AS, McCall JR, York R, Govindapur RR, Bourdelais AJ. Anti-Inflammatory Activity of Glycolipids and a Polyunsaturated Fatty Acid Methyl Ester Isolated from the Marine Dinoflagellate Karenia mikimotoi. Marine Drugs. 2020; 18(3):138. https://doi.org/10.3390/md18030138
Chicago/Turabian StyleLeutou, Alain S., Jennifer R. McCall, Robert York, Rajeshwar R. Govindapur, and Andrea J. Bourdelais. 2020. "Anti-Inflammatory Activity of Glycolipids and a Polyunsaturated Fatty Acid Methyl Ester Isolated from the Marine Dinoflagellate Karenia mikimotoi" Marine Drugs 18, no. 3: 138. https://doi.org/10.3390/md18030138
APA StyleLeutou, A. S., McCall, J. R., York, R., Govindapur, R. R., & Bourdelais, A. J. (2020). Anti-Inflammatory Activity of Glycolipids and a Polyunsaturated Fatty Acid Methyl Ester Isolated from the Marine Dinoflagellate Karenia mikimotoi. Marine Drugs, 18(3), 138. https://doi.org/10.3390/md18030138







