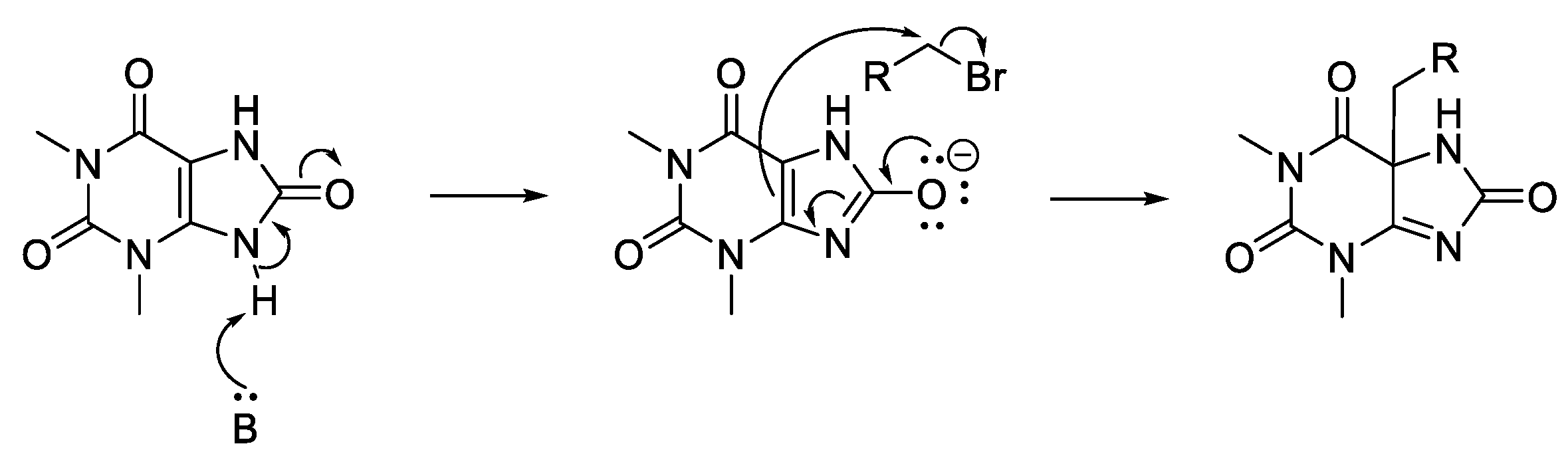
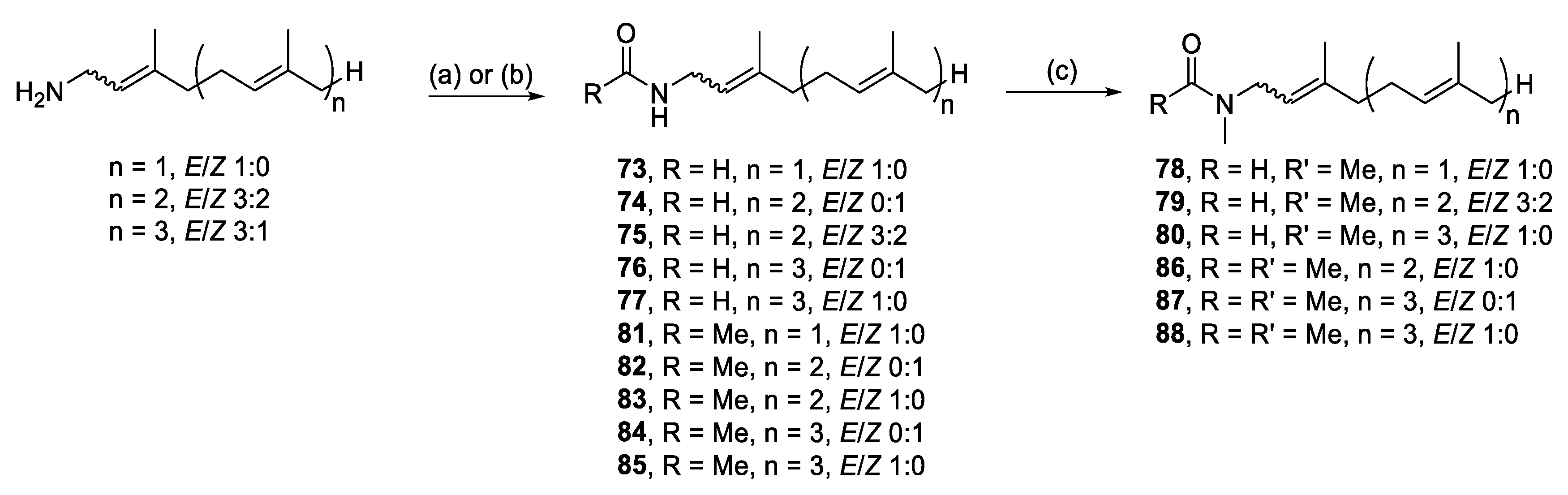
3.2. General Alkylation Procedure for Synthesis of 4–21, 27–50, 54–64, 67–72
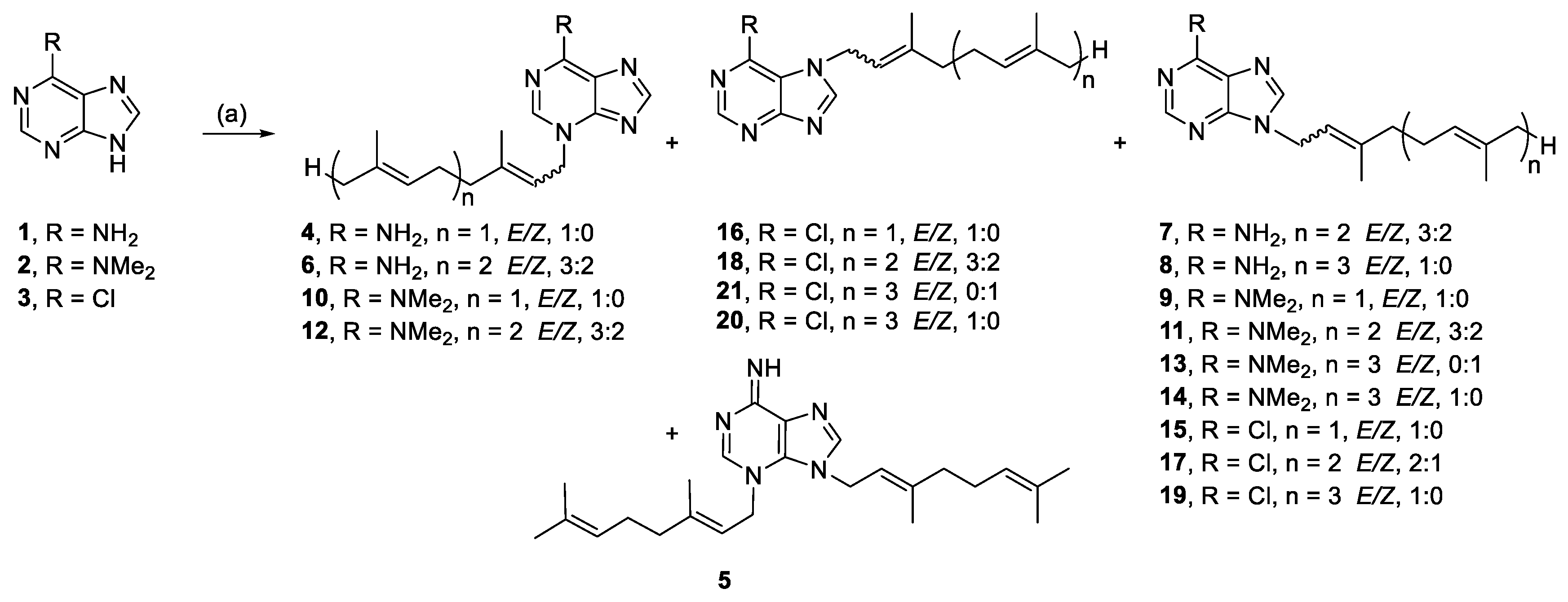
3.2.1. (E)-3-(3,7-Dimethylocta-2,6-dien-1-yl)-3H-purin-6-amine (4) and 3,9-bis((E)-3,7-dimethylocta-2,6-dien-1-yl)-3,9-dihydro-6H-purin-6-imine (5)
Adenine (1.00 mmol, 134.7 mg) and K2CO3 (1.31 mmol, 181.3 mg) were stirred in DMF (2 mL) at RT for 10 min before dropwise addition of geranyl bromide (1.2 mmol, 250.6 mg). The reaction was stirred for 21 h, then poured onto H2O (6 mL) and extracted with EA (3 × 2 mL). The combined extracts were washed with H2O (3 × 2 mL), then brine (1 × 2 mL) and dried over anhydrous MgSO4. The dried residue was purified by silica gel flash chromatography (5% MeOH/EA) to yield 4 and 5.
Compound 4: 51.9 mg (19%), pale-yellow crystals; Rf = 0.80 (5% MeOH/EA); 1H NMR (500 MHz, CDCl3): δ 8.06 (s, 1H, H-8), 8.02 (s, 1H, H-2), 5.49 (t, J = 7.3 Hz, 1H, CH=), 5.07–5.03 (m, 1H, CH=), 5.01 (d, J = 7.3 Hz, 2H, NCH2), 2.12 (br s, 4H, 2 × CH2), 1.83 (s, 3H, CH3), 1.66 (s, 3H, CH3), 1.57 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 154.3 (C, C-6), 153.9 (CH, C-8), 150.9 (C, C-4), 145.0 (C=), 141.7 (CH, C-2), 132.5 (C=), 123.4 (CH=), 120.8 (C, C-5), 116.2 (CH=), 47.4 (NCH2), 39.6 (CH2), 26.2 (CH2), 25.8 (CH3), 17.9 (CH3), 16.8 (CH3); IR (film from CH2Cl2): νmax 3231, 3067, 2966, 2912, 2853 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C15H22N5 272.1870; Found 272.1875 (Δ = 1.8 ppm); HRESIMS/MS (40 eV) m/z (%): 136.0612 (100), 81.0700 (17).
Compound 5: 7.8 mg (3%), pale-yellow oil; Rf = 0.04 (10% MeOH/EA); 1H NMR (600 MHz, CDCl3): δ 7.68 (s, 1H, H-8), 7.26 (s, 1H, H-2), 5.47 (t, J = 7.3 Hz, 1H, CH=), 5.35 (t, J = 7.6 Hz, 1H, CH=), 5.15 (d, J = 7.3 Hz, 2H, NCH2), 5.08–5.00 (m, 2H, 2 × CH=), 4.60 (d, J = 7.1 Hz, 2H, NCH2), 2.15–2.03 (m, 8H, 4 × CH2), 1.78 (s, 3H, CH3), 1.77 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.65 (s, 3H, CH3), 1.58 (s, 3H, CH3), 1.57 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 155.2 (C, C-6), 144.9 (CH, C-8), 144.6 (C, C-4), 143.5 (C=), 143.0 (C=), 140.5 (CH, C-2), 132.43 (C=), 132.38 (C=), 123.7 (CH=), 123.4 (CH=), 117.0 (CH=), 116.4 (CH=), 112.6 (C, C-5), 45.7 (NCH2), 46.1 (NCH2), 39.63 (CH2), 39.60 (CH2), 26.23 (CH2), 26.22 (CH2), 25.9 (CH3), 25.8 (CH3), 17.88 (CH3), 17.85 (CH3), 16.79 (CH3), 16.76 (CH3); IR (film from CH2Cl2): νmax 2966, 2916, 2855, 1629 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C25H38N5 408.3122; Found 408.3129 (Δ = 1.7 ppm); HRESIMS/MS (20 eV) m/z (%): 272.1851 (41), 136.0608 (100), 81.0698 (20).
3.2.2. 3-((6E)-3,7,11-Trimethyldodeca-2,6,10-trien-1-yl)-3H-purin-6-amine (6) and 9-((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-9H-purin-6-amine (7)
Adenine (0.51 mmol, 68.3 mg), K2CO3 (0.52 mmol, 71.9 mg) and farnesyl bromide (0.55 mmol, 157.0 mg) in DMF (2 mL) at 50 °C for 27 h yielded 6 and 7, with modified work up-H2O (6 mL) was added to the reaction filtrate and stored in the fridge until precipitate formed. The isolated solid was purified by chromatography.
Compound 6: 22.4 mg (13%), pale-yellow crystals; Rf = 0.17 (5% MeOH/EA); 3:2 E/Z, data for major isomer: 1H NMR (600 MHz, CDCl3): δ 8.05 (s, 1H, H-8), 8.00 (s, 1H, H-2), 5.51–5.45 (m, 1H, CH=), 5.10–5.03 (m, 2H, 2 × CH=), 5.01 (d, J = 7.3 Hz, 2H, NCH2), 2.15–2.09 (m, 4H, 2 × CH2), 2.05–1.98 (m, 2H, CH2), 1.98–1.92 (m, 2H, CH2), 1.83 (s, 3H, CH3), 1.65 (s, 3H, CH3), 1.57 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 154.5 (C, C-6), 154.1 (CH, C-8), 150.9 (C, C-4), 144.9 (C=), 141.6 (CH, C-2), 136.1 (C=), 131.5 (C=), 124.3 (CH=), 123.3 (CH=), 121.1 (C, C-5), 116.2 (CH=), 47.4 (NCH2), 39.8 (CH2), 39.6 (CH2), 26.8 (CH2), 26.2 (CH2), 25.8 (CH3), 17.8 (CH3), 16.9 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3231, 3065, 2965, 2915, 2855, 1704 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C20H30N5 340.2496; Found 340.2505 (Δ = 2.6 ppm); HRESIMS/MS (40 eV) m/z (%): 136.0610 (100), 119.0345 (13).
Compound 7: 3.6 mg (2%), white powder; Rf = 0.30 (EA); 3:2 E/Z, NMR data for major isomer: 1H NMR (500 MHz, CDCl3): δ 8.38 (s, 1H, H-2), 7.78 (s, 1H, H-8), 5.59 (br s, 2H, NH2), 5.45 (t, J = 7.1 Hz, 1H, CH=), 5.13–5.03 (m, 2H, 2 × CH=), 4.78 (d, J = 7.1 Hz, 2H, NCH2), 2.19–2.08 (m, 4H, 2 × CH2), 2.08–1.92 (m, 4H, 2 × CH2), 1.81 (s, 3H, CH3), 1.67 (s, 6H, 2 × CH3), 1.59 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 155.4 (C, C-6), 153.0 (CH, C-2), 150.1 (C, C-4), 143.0 (C=), 140.2 (CH, C-8), 135.9 (C=), 131.6 (C=), 124.3 (CH=), 123.5 (CH=), 119.7 (C, C-5), 117.5 (CH=), 41.4 (NCH2), 39.8 (CH2), 39.6 (CH2), 26.8 (CH2), 26.3 (CH2), 25.9 (CH2), 17.9 (CH3), 16.7 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3307, 3140, 2965, 2924, 2865, 1601 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C20H30N5 340.2496; Found 340.2501 (Δ = 1.5 ppm); HRESIMS/MS (40 eV) m/z (%): 136.0613 (100), 119.0346 (12).
3.2.3. 9-((2E,6E,10E)-3,7,11,15-Tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-9H-purin-6-amine (8)
Adenine (0.69 mmol, 93.8 mg), K2CO3 (0.78 mmol, 108.0 mg) and geranylgeranyl bromide (0.73 mmol, 259 mg) in DMF (1 mL) for 27 h yielded 8, 9.9 mg (4%), pale-yellow crystals. Rf = 0.32 (EA); 1H NMR (500 MHz, CDCl3): δ 8.37 (s, 1H, H-2), 7.77 (s, 1H, H-8), 5.77 (br s, 2H, NH2), 5.44 (t, J = 7.2 Hz, 1H, CH=), 5.11–5.05 (m, 3H, 3 × CH=), 4.77 (d, J = 7.2 Hz, 2H, NCH2), 2.16–2.08 (m, 4H, 2 × CH2), 2.08–2.01 (m, 4H, 2 × CH2), 2.01–1.92 (m, 4H, 2 × CH2), 1.81 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.59 (s, 9H, 3 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 155.5 (C, C-6), 153.0 (CH, C-2), 150.1 (C, C-4), 143.0 (C=), 140.1 (CH, C-8), 135.9 (C=), 135.2 (C=), 131.4 (C=), 124.5 (CH=), 124.2 (CH=), 123.5 (CH=), 119.7 (C, C-5), 117.5 (CH=), 41.3 (NCH2), 39.9 (CH2), 39.8 (CH2), 39.6 (CH2), 26.9 (CH2), 26.7 (CH2), 26.3 (CH2), 25.8 (CH3), 17.8 (CH3), 16.7 (CH3), 16.2 (CH3), 16.1 (CH3); IR (film from CH2Cl2): νmax 3468, 3324, 3153, 3051, 2969 2918 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C25H38N5 408.3122; Found 408.3129 (Δ = 1.7 ppm); HRESIMS/MS (40 eV) m/z (%): 136.0614 (100).
3.2.4. (E)-9-(3,7-Dimethylocta-2,6-dien-1-yl)-N,N-dimethyl-9H-purin-6-amine (9) and (E)-3-(3,7-dimethylocta-2,6-dien-1-yl)-N,N-dimethyl-3H-purin-6-amine (10)
6-(Dimethylamino)purine (0.17 mmol, 28.3 mg), Na2CO3/K2CO3 (1:1, 108 mg) and geranyl bromide (0.42 mmol, 90.8 mg) in DMF (3 mL) for 48 h yielded 9 and 10, with modified work up–concentration under reduced pressure.
Compound 9: 9.2 mg (18%), white powder; Rf = 0.12 (1:2 EA/PE); 1H NMR (600 MHz, CDCl3): δ 8.35 (s, 1H, H-2), 7.69 (s, 1H, H-8), 5.43 (t, J = 7.1 Hz, 1H, CH=), 5.05 (t, J = 6.1 Hz, 1H, CH=), 4.75 (d, J = 7.1 Hz, 2H, NCH2), 3.53 (br s, 6H, 2 × NCH3), 2.15–2.04 (m, 4H, 2 × CH2) 1.79 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.58 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 155.1 (C, C-6), 152.5 (CH, C-2), 150.5 (C, C-4), 142.5 (C=), 137.9 (CH, C-8), 132.2 (C=), 123.7 (CH=), 120.3 (C, C-5), 117.9 (CH=), 41.1 (NCH2), 39.6 (CH2), 38.7 (2 × NCH3), 26.3 (CH2), 25.8 (CH3), 17.9 (CH3), 16.6 (CH3); IR (film from CH2Cl2): νmax 2963, 2919, 1637 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C17H26N5 300.2183; Found 300.2184 (Δ = 0.3 ppm). HRESIMS/MS (40 eV) m/z (%): 164.0914 (100), 149.0683 (11), 121.0503 (16).
Compound 10: 6.3 mg (12%), white powder; Rf = 0.21 (5% MeOH/EA); 1H NMR (600 MHz, CDCl3): δ 8.00 (s, 1H, H-8), 7.95 (s, 1H, H-2), 5.48 (t, J = 6.9 Hz, 1H, CH=), 5.07–5.02 (m, 1H, CH=), 4.95 (d, J = 7.1 Hz, 2H, NCH2), 3.92 (br s, 3H, NCH3), 3.34 (br s, 3H, NCH3), 2.17–2.08 (m, 4H, 2 × CH2), 1.81 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.58 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 153.4 (C, C-6), 152.6 (C, C-8), 150.7 (C, C-4), 144.4 (C=), 140.3 (CH, C-2), 132.4 (C=), 123.6 (CH=), 121.6 (C, C-5), 116.6 (CH=), 46.9 (NCH2), 39.9 (NCH3), 39.6 (CH2), 38.1 (NCH3), 26.3 (CH2), 25.8 (CH3), 17.9 (CH3), 16.7 (CH3); IR (film from CH2Cl2): νmax 3077, 2964, 2922, 1607 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C17H26N5 300.2183; Found 300.2184 (Δ = 0.3 ppm); HRESIMS/MS (20 eV) m/z (%): 164.0914 (100).
3.2.5. N,N-Dimethyl-9-((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-9H-purin-6-amine (11) and N,N-dimethyl-3-((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-3H-purin-6-amine (12)
6-(Dimethylamino)purine (0.088 mmol, 14.4 mg), K2CO3 (0.16 mmol, 22.3 mg) and farnesyl bromide (0.11 mmol, 31.6 mg) in DMF (1 mL) at 50 °C for 21 h yielded 11 and 12.
Compound 11: 5.6 mg (17%), white powder; Rf = 0.29 (1:1 EA/PE); 3:2 E/Z, NMR data for major isomer: 1H NMR (500 MHz, CDCl3): δ 8.36 (s, 1H, H-2), 7.70 (s, 1H, H-8), 5.44 (t, J = 7.1 Hz, 1H, CH=), 5.11–5.03 (m, 2H, 2 × CH=), 4.75 (d, J = 7.1 Hz, 2H, NCH2), 3.53 (br s, 6H, 2 × NCH3), 2.16–2.06 (m, 4H, 2 × CH2), 2.06–1.99 (m, 2H, CH2), 1.99–1.92 (m, 2H, CH2), 1.81 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.58 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 155.1 (C, C-6), 152.5 (CH, C-2), 150.5 (C, C-4), 142.5 (C=), 137.9 (CH, C-8), 135.9 (C=), 131.5 (C=), 124.4 (CH=), 120.3 (C, C-5), 117.9 (CH=), 41.2 (NCH2), 39.8 (CH2), 39.6 (CH2), 38.7 (2 × NCH3), 26.8 (CH2), 26.3 (CH2), 25.9 (CH3), 17.8 (CH3), 16.7 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3051, 2961, 2917, 2856, 1589 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C22H34N5 368.2809; Found 368.2817 (Δ = 2.2 ppm); HRESIMS/MS (20 eV) m/z (%): 164.0894 (100).
Compound 12: 9.5 mg (29%), white powder; Rf = 0.25 (10% MeOH/EA); 3:2 E/Z, NMR data for major isomer: 1H NMR (600 MHz, CDCl3): δ 8.01 (s, 1H, H-8), 7.95 (s, 1H, H-2), 5.48 (t, J = 7.3 Hz, 1H, CH=), 5.09–5.03 (m, 2H, 2 × CH=), 4.96 (d, J = 7.3 Hz, 2H, NCH2), 3.92 (br s, 3H, NCH3), 3.33 (br s, 3H, NCH3), 2.17–2.08 (m, 4H, 2 × CH2), 2.05–1.99 (m, 2H, CH2), 1.99–1.94 (m, 2H, CH2), 1.82 (s, 3H, CH3), 1.66 (s, 3H, CH3), 1.58 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 153.4 (C, C-6), 152.4 (CH, C-8), 150.5 (C, C-4), 144.5 (C=), 140.4 (CH, C-2), 136.1 (C=), 131.5 (C=), 124.4 (CH=), 123.3 (CH=), 121.6 (C, C-5), 116.5 (CH=), 46.9 (NCH2), 39.9 (NCH3), 39.8 (CH2), 39.6 (CH2), 38.1 (NCH3), 26.8 (CH2), 26.2 (CH2), 25.8 (CH3), 17.8 (CH3), 16.8 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 2963, 2924, 2856, 1608 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C22H34N5 368.2809; Found 368.2818 (Δ = 2.4 ppm); HRESIMS/MS (40 eV) m/z (%): 164.0932 (100), 81.0704 (14).
3.2.6. N,N-Dimethyl-9-((2Z,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-9H-purin-6-amine (13) and N,N-dimethyl-9-((2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-9H-purin-6-amine (14)
6-(Dimethylamino)purine (0.14 mmol, 23.4 mg), K2CO3 (0.16 mmol, 22.7 mg) and geranylgeranyl bromide (0.15 mmol, 51.5 mg) in DMF (1 mL) for 44 h yielded 13 and 14.
Compound 13: 4.5 mg (28%), white solid; Rf = 0.21 (1:2 EA/PE); 1H NMR (500 MHz, CDCl3): δ 8.35 (s, 1H, H-2), 7.70 (s, 1H, H-8), 5.43 (t, J = 7.1 Hz, 1H, CH=), 5.15–5.04 (m, 3H, 3 × CH=), 4.75 (d, J = 7.1 Hz, 2H, NCH2), 3.53 (br s, 6H, 2 × NCH3), 2.27–2.20 (m, 2H, CH2), 2.18–2.10 (m, 2H, CH2), 2.10–2.01 (m, 4H, 2 × CH2), 2.01–1.91 (m, 4H, 2 × CH2), 1.79 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.61 (s, 3H, CH3), 1.59 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 155.1 (C, C-6), 152.5 (CH, C-2), 150.5 (C, C-4), 142.4 (C=), 137.9 (CH, C-8), 136.3 (C=), 135.2 (C=), 131.4 (C=), 124.5 (CH=), 124.2 (CH=), 123.3 (CH=), 120.7 (C, C-5), 118.7 (CH=), 41.0 (NCH2), 39.9 (CH2), 39.8 (CH2), 38.6 (2 × NCH3), 32.3 (CH2), 26.9 (CH2), 26.7 (CH2), 26.5 (CH2), 25.9 (CH3), 23.6 (CH3), 17.8 (CH3), 16.20 (CH3), 16.16 (CH3); IR (film from CH2Cl2): νmax 3043, 2921, 2854, 1590 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C27H42N5 436.3435; Found 436.3430 (Δ = −1.1 ppm); HRESIMS/MS (40 eV) m/z (%): 164.0912 (100).
Compound 14: 6.7 mg (14%), white solid; Rf = 0.18 (1:2 EA/PE); 1H NMR (500 MHz, CDCl3): δ 8.36 (s, 1H, H-2), 7.70 (s, 1H, H-8), 5.43 (t, J = 7.0 Hz, 1H, CH=), 5.12–5.04 (m, 3H, 3 × CH=), 4.75 (d, J = 7.1 Hz, 2H, NCH2), 3.56 (br s, 6H, 2 × NCH3), 2.15–2.08 (m, 4H, 2 × CH2), 2.08–2.01 (m, 4H, 2 × CH2), 1.99–1.93 (m, 4H, 2 × CH2), 1.80 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.59 (s, 3H, CH3), 1.58 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 155.1 (C, C-6), 152.5 (CH, C-2), 150.5 (C, C-4), 142.5 (C=), 137.9 (CH, C-8), 135.9 (C=), 135.2 (C=), 131.4 (C=), 124.5 (CH=), 124.2 (CH=), 123.5 (CH=), 120.3 (C, C-5), 117.9 (CH=), 41.1 (NCH2), 39.9 (CH2), 39.8 (CH2), 39.6 (CH2), 38.6 (2 × NCH3), 26.9 (CH2), 26.7 (CH2), 26.3 (CH2), 25.8 (CH3), 17.8 (CH3), 16.7 (CH3), 16.2 (CH3), 16.1 (CH3); IR (film from CH2Cl2): νmax 3104, 2962, 2917, 2855, 1590 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C27H42N5 436.3435; Found 436.3466 (Δ = 7.1 ppm); HRESIMS/MS (40 eV) m/z (%): 164.0873 (100).
3.2.7. (E)-6-Chloro-9-(3,7-dimethylocta-2,6-dien-1-yl)-9H-purine (15) and (E)-6-chloro-7-(3,7-dimethylocta-2,6-dien-1-yl)-7H-purine (16)
Following the general alkylation procedure, also previously published using 80 °C [
54], 6-chloropurine (0.30 mmol, 45.9 mg), K
2CO
3 (0.62 mmol, 85.6 mg) and geranyl bromide (0.33 mmol, 71.8 mg) in DMF (1 mL) for 21 h yielded
15 and
16.
Compound
15: 39.4 mg (46%), colourless oil; R
f = 0.48 (2:3 EA/PE); IR data and select
1H NMR data previously reported in CD
3OD [
54];
1H NMR (300 MHz, CDCl
3): δ 8.72 (s, 1H, H-2), 8.07 (s, 1H, H-8), 5.42 (t,
J = 7.2 Hz, 1H, CH=), 5.05–4.97 (m, 1H, CH=), 4.84 (d,
J = 7.3 Hz, 2H, NCH
2), 2.09 (s, 4H, 2 × CH
2), 1.81 (s, 3H, CH
3), 1.64 (s, 3H, CH
3), 1.55 (s, 3H, CH
3);
13C{
1H} NMR (150 MHz, CDCl
3): δ 151.9 (CH, C-2), 151.8 (C, C-6), 150.9 (C, C-4), 144.8 (CH, C-8), 144.1 (C=), 132.4 (C, C-5), 131.8 (C=), 123.4 (CH=), 116.7 (CH=), 41.9 (NCH
2), 39.5 (CH
2), 26.1 (CH
2), 25.8 (CH
3), 17.8 (CH
3), 16.7 (CH
3); HRESIMS
m/
z: [M + H]
+ Calcd. for C
15H
20ClN
4 291.1371; Found 291.1371 (Δ = 0.0 ppm); HRESIMS/MS (40 eV)
m/
z (%): 157.0074 (22), 155.0105 (57), 119.0346 (100).
Compound
16: 17.2 mg (20%), colourless oil; R
f = 0.16 (2:3 EA/PE); IR data and select
1H NMR data previously reported in CD
3OD [
54];
1H NMR (300 MHz, CDCl
3): δ 8.85 (s, 1H, H-2), 8.23 (s, 1H, H-8), 5.43 (t,
J = 6.8 Hz, 1H, CH=), 5.07 (d,
J = 7.0 Hz, 2H, NCH
2), 5.05–5.00 (m, 1H, CH=), 2.13 (s, 4H, 2 × CH
2), 1.80 (s, 3H, CH
3), 1.66 (s, 3H, CH
3), 1.58 (s, 3H, CH
3);
13C{
1H} NMR (150 MHz, CDCl
3): δ 162.2 (C, C-6), 152.5 (CH, C-2), 148.4 (CH, C-8), 144.2 (C=), 143.3 (C, C-4), 132.6 (C=), 123.3 (CH=), 122.7 (C, C-5), 117.1 (CH=), 45.4 (NCH
2), 39.5 (CH
2), 26.1 (CH
2), 25.8 (CH
3), 17.9 (CH
3), 16.8 (CH
3); HRESIMS
m/
z: [M + H]
+ Calcd. for C
15H
20ClN
4 291.1371; Found 291.1365 (Δ = −2.1 ppm); HRESIMS/MS (40 eV)
m/
z (%): 157.0062 (100), 155.0081 (40).
3.2.8. 6-Chloro-9-((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-9H-purine (17) and 6-chloro-7-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-9H-purine (18)
6-Chloropurine (0.31 mmol, 48.5 mg), K2CO3 (0.6 mmol, 83 mg) and farnesyl bromide (0.33 mmol, 94.0 mg) in DMF (2 mL) at 50 °C for 24 h yielded 17 and 18.
Compound 17: 47.4 mg (43%), colourless oil; Rf = 0.24 (1:2 EA/PE); 2:1 E/Z, NMR data for major isomer: 1H NMR (600 MHz, CDCl3): δ 8.72 (s, 1H, H-2), 8.08 (s, 1H, H-8), 5.42 (t, J = 7.2 Hz, 1H, CH=), 5.06–5.00 (m, 2H, 2 × CH=), 4.85 (d, J = 7.3 Hz, 2H, NCH2), 2.13–2.07 (m, 4H, 2 × CH2), 2.02–1.96 (m, 2H, CH2), 1.95–1.90 (m, 2H, CH2), 1.82 (s, 3H, CH3), 1.63 (s, 3H, CH3), 1.55 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 152.0 (C, C-2), 151.8 (C, C-6), 151.0 (C, C-4), 144.9 (C, C-8), 144.1 (C=), 136.0 (C=), 132.3 (C, C-5), 131.5 (C=), 124.3 (CH=), 123.3 (CH=), 116.6 (CH=), 41.9 (NCH2), 39.8 (CH2), 39.5 (CH2), 26.8 (CH2), 26.2 (CH2), 25.8 (CH3), 17.8 (CH3), 16.8 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3115, 2969, 2930, 1335, 939, 637 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C20H28ClN4 359.1997; Found 359.1993 (Δ = −1.1 ppm); HRESIMS/MS (40 eV) m/z (%): 157.0073 (40), 155.0103 (100), 119.0343 (47), 81.0697 (47).
Compound 18: 21.3 mg (19%), colourless oil; Rf = 0.20 (1:1 EA/PE); E/Z 3:2, NMR data for major isomer: 1H NMR (600 MHz, CDCl3): δ 8.86 (s, 1H, H-2), 8.23 (s, 1H, H-8), 5.44 (t, J = 7.0 Hz, 1H, CH=), 5.08 (d, J = 7.1 Hz, 2H, NCH2), 5.07–5.04 (m, 2H, 2 × CH=), 2.18–2.09 (m, 4H, 2 × CH2), 2.05–1.99 (m, 2H, CH2), 1.98–1.93 (m, 2H, CH2), 1.82 (s, 3H, CH3), 1.65 (s, 3H, CH3), 1.58 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 162.0 (C, C-6), 152.4 (CH, C-2), 148.5 (CH, C-8), 144.2 (C=), 143.3 (C, C-4), 136.2 (C=), 131.5 (C=), 124.2 (CH=), 123.2 (CH=), 122.6 (C, C-5), 117.0 (CH=), 45.4 (NCH2), 39.8 (CH2), 39.5 (CH2), 26.8 (CH2), 26.2 (CH2), 25.8 (CH3), 17.8 (CH3), 16.9 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3053, 2981, 1264, 732 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C20H28ClN4 359.1997; Found 359.2002 (Δ = 1.4 ppm); HRESIMS/MS (40 eV) m/z (%): 157.0001 (27), 155.0031 (66), 119.0287 (31), 95.0806 (23), 93.0651 (13), 81.0660 (100), 79.0539 (15).
3.2.9. 6-Chloro-9-((2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-9H-purine (19), 6-chloro-7-((2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-7H-purine (20), and 6-chloro-7-((2Z,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-7H-purine (21)
Following the general alkylation procedure, also previously published [
55], 6-chloropurine (0.30 mmol, 45.6 mg), K
2CO
3 (0.35 mmol, 48.2 mg) and geranylgeranyl bromide (0.32 mmol, 111 mg) in DMF (1 mL) for 27 h yielded
19,
20, and
21.Compound
19: 19.4 mg (19%), colourless oil; R
f = 0.51 (2:3 EA/PE);
1H and
13C NMR data previously reported [
55]; IR (film from CH
2Cl
2): ν
max 3070, 2966, 2922, 2855, 1592, 1560, 1335 cm
−1; HRESIMS
m/
z: [M + H]
+ Calcd. for C
25H
36ClN
4 427.2623; Found 427.2614 (Δ = −2.1 ppm); HRESIMS/MS (40 eV)
m/
z (%): 157.0068 (39), 155.0098 (100), 119.0334 (21).
Compound
20: 15.2 mg (15%), colourless oil; R
f = 0.21 (2:3 EA/PE);
1H and
13C NMR data previously reported [
55]; IR (film from CH
2Cl
2): ν
max 3053, 2971, 2931, 733 cm
−1; HRESIMS
m/
z: [M + H]
+ Calcd. for C
25H
36ClN
4 427.2623; Found 427.2642 (Δ = 4.4 ppm).
Compound 21: 16.8 mg (49%), colourless oil; Rf = 0.3 (2:3 EA/PE); 1H NMR (500 MHz, CDCl3): δ 8.87 (s, 1H, H-2), 8.23 (s, 1H, H-8), 5.45 (t, J = 7.1 Hz, 1H, CH=), 5.15–5.08 (m, 3H, 3 × CH=), 5.07 (d, J = 7.1 Hz, 2H, NCH2), 2.28–2.21 (m, 2H, CH2), 2.21–2.13 (m, 2H, CH2), 2.10–2.02 (m, 4H, 2 × CH2), 2.02–1.93 (m, 4H, 2 × CH2), 1.84 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.62 (s, 3H, CH3), 1.59 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 162.1 (C, C-6), 152.5 (CH, C-2), 148.4 (CH, C-8), 144.1 (C=), 143.2 (C, C-4), 136.9 (C=), 135.4 (C=), 131.3 (C=), 124.4 (CH=), 124.1 (CH=), 122.9 (CH=), 122.5 (C, C-5), 117.9 (CH=), 45.2 (NCH2), 39.9 (CH2), 39.8 (CH2), 32.5 (CH2), 26.9 (CH2), 26.7 (CH2), 26.3 (CH2), 25.8 (CH3), 23.6 (CH3), 17.8 (CH3), 16.18 (CH3), 16.16 (CH3); IR (film from CH2Cl2): νmax 3055, 2973, 2932, 733 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C25H36ClN4 427.2623; Found 427.2634 (Δ = 2.6 ppm); HRESIMS/MS (40 eV) m/z (%): 157.0086 (32), 155.0118 (100), 121.10185 (10), 119.03516 (16), 109.1013 (11), 107.0858 (15).
3.2.10. 3,7-Bis((E)-3,7-dimethylocta-2,6-dien-1-yl)-3,7-dihydro-1H-purine-2,6-dione (27)
Xanthine (2.1 mmol, 311.5 mg), K2CO3 (2.0 mmol, 272.2 mg) and geranyl bromide (2.4 mmol, 521 mg) in DMF (3 mL) for 48 h yielded 27, with modified work up—concentrating under reduced pressure, 44.6 mg (9%), colourless oil. Rf = 0.15 (1:2 EA/PE); 1H NMR (600 MHz, CDCl3): δ 8.10 (br s, 1H, NH), 7.55 (s, J = 2.8 Hz, 1H, H-8), 5.43 (t, J = 6.7 Hz, 1H, CH=), 5.34 (t, J = 6.4 Hz, 1H, CH=), 5.07–5.01 (m, 2H, 2 × CH=), 4.89 (d, J = 7.3 Hz, 2H, NCH2), 4.66 (d, J = 6.9 Hz, 2H, NCH2), 2.15–2.09 (m, 4H, 2 × CH2), 2.09–2.03 (m, 2H, CH2), 2.02–1.96 (m, 2H, CH2), 1.84 (s, 3H, CH3), 1.77 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.63 (s, 3H, CH3), 1.59 (s, 3H, CH3), 1.56 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 154.7 (C, C-6), 150.62 (C, C-2 or C-4), 150.58 (C, C-2 or C-4), 143.8 (C=), 140.8 (CH=), 140.7 (CH, C-8), 132.4 (C=), 131.8 (C=), 124.0 (CH=), 123.6 (CH=), 118.0 (CH=), 117.2 (CH=), 107.5 (C, C-5), 44.7 (NCH2), 40.9 (NCH2), 39.7 (CH2), 39.6 (CH2), 26.5 (CH2), 26.3 (CH2), 25.84 (CH3), 25.80 (CH3), 17.9 (CH3), 17.8 (CH3), 16.7 (CH3), 16.6 (CH3); IR (film from CH2Cl2): νmax 3423, 3176, 3115, 3052, 2967, 2926, 1679 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C25H37N4O2 425.2911; Found 425.2910 (Δ = −0.2 ppm); HRESIMS/MS (10 eV) m/z (%): 289.1638 (70), 153.0395 (100).
3.2.11. 3,7-Bis((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-3,7-dihydro-1H-purine-2,6-dione (28)
Xanthine (2.0 mmol, 306.3 mg), K2CO3 (3.0 mmol, 420.3 mg) and farnesyl bromide (2.4 mmol, 683.4 mg) in DMF (3 mL) for 25 h yielded 28, 19.3 mg (3%), colourless oil. Rf = 0.25 (1:2 EA/PE); 3:2 E/Z, NMR data for major isomer: 1H NMR (600 MHz, CDCl3): δ 8.57 (br s, 1H, NH), 7.54 (s, 1H, H-8), 5.43 (t, J = 6.6 Hz, 1H, CH=), 5.34 (t, J = 6.8 Hz, 1H, CH=), 5.12–5.02 (m, 4H, 4 × CH=), 4.89 (d, J = 7.2 Hz, 2H, NCH2), 4.66 (d, J = 6.9 Hz, 2H, NCH2), 2.16–1.89 (m, 16H, 8 × CH2), 1.85 (s, 3H, CH3), 1.78 (s, 3H, CH3), 1.66 (s, 6H, 2 × CH3), 1.58 (s, 9H, 3 × CH3), 1.55 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 154.9 (C, C-6), 150.7 (C, C-2 or C-4), 150.6 (C, C-2 or C-4), 143.8 (C=), 141.1 (C=), 140.8 (CH, C-8), 136.0 (C=), 135.4 (C=), 131.5 (C=), 131.4 (C=), 124.4 (CH=), 124.3 (CH=), 123.9 (CH=), 123.4 (CH=), 118.0 (CH=), 117.2 (CH=), 107.5 (C, C-5), 44.7 (NCH2), 40.8 (NCH2), 39.79 (CH2), 39.78 (CH2), 39.7 (CH2), 39.6 (CH2), 26.81 (CH2), 26.78 (CH2), 26.4 (CH2), 26.3 (CH2), 25.84 (CH3), 25.83 (CH3), 17.82 (CH3), 17.81 (CH3), 16.74 (CH3), 16.67 (CH3), 16.2 (CH3), 16.1 (CH3); IR (film from CH2Cl2): νmax 3166, 3065, 2964, 2927, 2856, 1686 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C35H53N4O2 561.4163; Found 561.4169 (Δ = 1.1 ppm); HRESIMS/MS (20 eV) m/z (%): 561.1128 (39), 357.2262 (48), 153.0391 (100).
3.2.12. (E)-7-(3,7-Dimethylocta-2,6-dien-1-yl)-3-methyl-3,7-dihydro-1H-purine-2,6-dione (29)
3-Methylxanthine (0.17 mmol, 28.0 mg), K2CO3/Na2CO3 (1:1, 48.6 mg) and geranyl bromide (0.36 mmol, 77 mg) in DMF (2 mL) at 50 °C for 48 h yielded 29, with modified work up—the concentrated reaction was filtered from DCM, and additionally recrystallised from PE after chromatography, 16.9 mg (5%), white powder. Rf = 0.2 (1:2 EA/PE); 1H NMR (600 MHz, CDCl3): δ 8.10 (s, 1H, NH), 7.55 (s, 1H, H-8), 5.43 (t, J = 7.3 Hz, 1H, CH=), 5.07–5.03 (m, 1H, CH=), 4.90 (d, J = 7.3 Hz, 2H, NCH2), 3.55 (s, 3H, NCH3), 2.16–2.08 (m, 4H, 2 × CH2), 1.78 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.59 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 154.5 (C, C-6), 150.99 (C, C-2), 150.96 (C, C-4), 143.9 (C=), 140.8 (CH, C-8), 132.4 (C=), 123.5 (CH=), 117.1 (CH=), 107.4 (C, C-5), 44.8 (NCH2), 39.6 (CH2), 29.2 (NCH3), 26.2 (CH2), 25.9 (CH3), 17.9 (CH3), 16.6 (CH3); IR (film from CH2Cl2): νmax 3121, 3021, 2964, 2916, 2826, 1678 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C16H23N4O2 303.1816; Found 303.1812 (Δ = −1.3 ppm); HRESIMS/MS (40 eV) m/z (%): 167.0538 (100), 149.0431 (8), 124.0487 (17).
3.2.13. 3-Methyl-7-((2Z,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-3,7-dihydro-1H-purine-2,6-dione (30) and 3-methyl-7-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-3,7-dihydro-1H-purine-2,6-dione (31)
3-Methylxanthine (0.20 mmol, 32.9 mg), K2CO3 (0.37 mmol, 51.2 mg) and farnesyl bromide (0.34 mmol, 98 mg) in DMF (2 mL) for 21 h yielded 30 and 31.
Compound 30: 4.6 mg (8%), white solid; Rf = 0.20 (1:1 EA/PE); 1H NMR (600 MHz, CDCl3): δ 8.10 (s, 1H, NH), 7.55 (s, 1H, H-8), 5.44 (t, J = 6.9 Hz, 1H, CH=), 5.11–5.05 (m, 2H, 2 × CH=), 4.88 (d, J = 7.4 Hz, 2H, NCH2), 3.54 (s, 3H, NCH3), 2.23–2.17 (m, 2H, CH2), 2.15–2.10 (m, 2H, CH2), 2.07–2.01 (m, 2H, CH2), 1.99–1.93 (m, 2H, CH2), 1.81 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.60 (s, 3H, CH3), 1.59 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 154.5 (C, C-6), 151.0 (C, C-2), 150.9 (C, C-4), 143.7 (C=), 140.8 (CH, C-8), 136.5 (C=), 131.6 (C=), 124.3 (CH=), 123.1 (CH=), 118.0 (CH=), 107.4 (C, C-5), 44.6 (NCH2), 39.8 (CH2), 32.2 (CH2), 29.2 (NCH3), 26.7 (CH2), 26.4 (CH2), 25.9 (CH3), 23.6 (CH3), 17.8 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3400, 3162, 3035, 2969, 2930, 1683 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C21H31N4O2 371.2442; Found 371.2452 (Δ = 2.7 ppm).
Compound 31: 33.1 mg (45%), white solid; Rf = 0.18 (1:1, EA/PE); 2:1 E/Z, NMR data for major isomer: 1H NMR (600 MHz, CDCl3): δ 8.89 (s, 1H, NH), 7.56 (s, 1H, H-8), 5.42 (t, J = 7.3 Hz, 1H, CH=), 5.08–5.04 (m, 2H, 2 × CH=), 4.90 (d, J = 7.3 Hz, 2H, NCH2), 3.54 (s, 3H, NCH3), 2.16–2.07 (m, 4H, 2 × CH2), 2.06–1.99 (m, 2H, CH2), 1.98–1.93 (m, 2H, CH2), 1.78 (s, 3H, CH3), 1.66 (s, 3H, CH3), 1.58 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 154.9 (C, C-6), 151.3 (C, C-2), 150.9 (C, C-4), 143.9 (C=), 140.8 (CH, C-8), 136.0 (C=), 131.5 (C=), 124.3 (CH=), 123.4 (CH=), 117.1 (CH=), 107.5 (C, C-5), 44.7 (NCH2), 39.8 (CH2), 39.6 (CH2), 29.2 (NCH3), 26.8 (CH2), 26.2 (CH2), 25.8 (CH3), 17.8 (CH3), 16.7 (CH3), 16.2 (CH3); HRESIMS m/z: [M + H]+ Calcd. for C21H31N4O2 371.2442; Found 371.2445 (Δ = 0.8 ppm); HRESIMS/MS (40 eV) m/z (%): 167.0552 (100), 124.0506 (87).
3.2.14. 3-Methyl-7-((2Z,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-3,7-dihydro-1H-purine-2,6-dione (32) and 3-methyl-7-((2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-3,7-dihydro-1H-purine-2,6-dione (33)
3-Methylxanthine (0.35 mmol, 40.9 mg), K2CO3 (0.38 mmol, 52.5 mg) and geranylgeranyl bromide (0.37 mmol, 130 mg) in DMF (1 mL) for 44 h yielded 32 and 33.
Compound 32: 10.5 mg (26%), white, waxy solid; Rf = 0.20 (1:1, EA/PE); 1H NMR (600 MHz, CDCl3): δ 8.45 (s, 1H, NH), 7.55 (s, 1H, H-8), 5.44 (t, J = 7.1 Hz, 1H, CH=), 5.12–5.05 (m, 3H, 3 × CH=), 4.88 (d, J = 7.2 Hz, 2H, NCH2), 3.54 (s, 3H, NCH3), 2.23–2.18 (m, 2H, CH2), 2.15–2.09 (m, 2H, CH2), 2.08–2.01 (m, 4H, 2 × CH2), 2.00–1.93 (m, 4H, 2 × CH2), 1.80 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.60 (s, 3H, CH3), 1.59 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 154.6 (C, C-6), 151.1 (C, C-2), 150.9 (C, C-4), 143.7 (C=), 140.8 (CH, C-8), 136.5 (C=), 135.3 (C=), 131.4 (C=), 124.5 (CH=), 124.1 (CH=), 123.1 (CH=), 118.0 (CH=), 107.4 (C, C-5), 44.6 (NCH2), 39.9 (CH2), 39.8 (CH2), 32.3 (CH2), 29.2 (NCH3), 26.9 (CH2), 26.7 (CH2), 26.5 (CH2), 25.8 (CH3), 23.6 (CH3), 17.8 (CH3), 16.20 (CH3), 16.15 (CH3); IR (film from CH2Cl2): νmax 3458, 3159, 2968, 2924, 2852 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C26H39N4O2 439.3068; Found 439.3036 (Δ = −7.2 ppm); HRESIMS/MS (40 eV) m/z (%): 168.0565 (9), 167.0533 (100), 124.0494 (43).
Compound 33: 22.1 mg (18%), white waxy solid; Rf = 0.12 (1:1 EA/PE); 1H NMR (500 MHz, CDCl3): δ 8.94 (s, 1H, NH), 7.56 (s, 1H, H-8), 5.43 (t, J = 7.3 Hz, 1H, CH=), 5.12–5.04 (m, 3H, 3 × CH=), 4.90 (d, J = 7.3 Hz, 2H, NCH2), 3.54 (s, 3H, NCH3), 2.16–2.08 (m, 4H, 2 × CH2), 2.07–2.00 (m, 4H, 2 × CH2), 2.00–1.93 (m, 4H, 2 × CH2), 1.78 (s, 3H, CH3), 1.66 (s, 3H, CH3), 1.59 (s, 9H, 3 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 154.9 (C, C-6), 151.3 (C-2), 150.9 (C-4), 143.9 (C=), 140.8 (CH, C-8), 136.0 (C=), 135.2 (C=), 131.41 (C=), 124.5 (CH=), 124.2 (CH=), 123.4 (CH=), 117.1 (CH=), 107.5 (C, C-5), 44.8 (NCH2), 39.84 (CH2), 39.79 (CH2), 39.6 (CH2), 29.2 (NCH3), 26.9 (CH2), 26.7 (CH2), 26.3 (CH2), 25.8 (CH3), 17.8 (CH3), 16.7 (CH3), 16.2 (CH3), 16.1 (CH3); IR (film from CH2Cl2): νmax 3158, 3121, 3029, 2965, 2924, 2834, 1713, 1679 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C26H39N4O2 439.3068; Found 439.3035 (Δ = −7.5 ppm); HRESIMS/MS (40 eV) m/z (%): 168.0459 (9), 167.0437 (100), 124.0412 (39).
3.2.15. (E)-7-(3,7-Dimethylocta-2,6-dien-1-yl)-1,3-dimethyl-3,7-dihydro-1H-purine-2,6-dione (34)
Theophylline hydrate (2.0 mmol, 391.3 mg), K2CO3/Na2CO3 (1:1, 244 mg) and geranyl bromide (4.0 mmol, 869 mg) in DMF (3 mL) for 3 h yielded 34, with modified work up and modified purification—H2O (9 mL) was added to the reaction and the resulting precipitate was isolated and recrystallised from PE, 277.5 mg (45%), white crystals. 1H NMR (600 MHz, CDCl3): δ 7.53 (s, 1H, H-8), 5.43 (t, J = 7.6 Hz, 1H, CH=), 5.05 (t, J = 6.2 Hz, 1H, CH=), 4.93 (d, J = 7.3 Hz, 2H, NCH2), 3.59 (s, 3H, N(3)CH3), 3.42 (s, 3H, N(1)CH3), 2.15–2.07 (m, 4H, 2 × CH2), 1.78 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.59 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 155.5 (C, C-6), 151.9 (C, C-2), 149.0 (C, C-4), 143.5 (C=), 140.3 (CH, C-8), 132.4 (C=), 123.6 (CH=), 117.5 (CH=), 107.2 (C, C-5), 44.7 (NCH2), 39.6 (CH2), 29.9 (N(3)CH3), 28.1 (N(1)CH3), 26.3 (CH2), 25.9 (CH3), 17.9 (CH3), 16.6 (CH3); IR (neat): νmax 3098, 2964, 2926, 2855, 1695, 1645 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C17H25N4O2 317.1972; Found 317.1978 (Δ = 1.9 ppm); HRESIMS/MS (40 eV) m/z (%): 181.0714 (52), 124.0511 (100).
3.2.16. 1,3-Dimethyl-7-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-3,7-dihydro-1H-purine-2,6-dione (35)
Theophylline hydrate (1.85 mmol, 370 mg), K2CO3/Na2CO3 (1:1, 170 mg) and farnesyl bromide (2.0 mmol, 570.5 mg). in DMF (2 mL) at 80 °C for 5 h yielded 35, after modified work up and modified purification as per 34, 333.1 mg (72%), white crystals. 1H NMR (600 MHz, CDCl3): δ 7.53 (s, 1H, H-8), 5.43 (t, J = 7.3 Hz, 1H, CH=), 5.09–5.05 (m, 2H, 2 × CH=), 4.93 (d, J = 7.2 Hz, 2H, NCH2), 3.58 (s, 3H, N(3)CH3), 3.41 (s, 3H, N(1)CH3), 2.16–2.07 (m, 4H, 2 × CH2), 2.03 (m, 2H, CH2), 1.99–1.93 (m, 2H, CH2), 1.79 (s, 3H, CH3), 1.67 (s, 1H, CH3), 1.60 (s, 3H, CH3), 1.59 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 155.5 (C, C-6), 151.9 (C, C-2), 149.0 (C, C-4), 143.5 (C=), 140.3 (CH, C-8), 136.0 (C=), 131.6 (C=), 124.3 (CH=), 123.4 (CH=), 117.5 (CH=), 107.2 (C, C-5), 44.7 (NCH2), 39.8 (CH2), 39.6 (CH2), 29.9 (N(3)CH3), 28.1 (N(1)CH3), 26.8 (CH2), 26.3 (CH2), 25.9 (CH3), 17.8 (CH3), 16.7 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3097, 2695, 2922, 1695, 1646 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C22H33N4O2 385.2598; Found 385.2604 (Δ = 1.6 ppm); HRESIMS/MS (40 eV) m/z (%):181.0718 (100), 124.0506 (73).
3.2.17. 1,3-Dimethyl-7-((2Z,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-3,7-dihydro-1H-purine-2,6-dione (36) and 1,3-dimethyl-7-((2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-3,7-dihydro-1H-purine-2,6-dione (37)
Theophylline hydrate (0.30 mmol, 59.1 mg), K2CO3 (0.34 mmol, 46.5 mg) and geranylgeranyl bromide (0.32 mmol, 111 mg) in DMF (1 mL) for 25 h yielded 36 and 37.
Compound 36: 13.1 mg (36%), white solid; Rf = 0.16 (1:2 EA/PE); 1H NMR (500 MHz, CDCl3): δ 7.52 (s, 1H, H-8), 5.44 (t, J = 7.1 Hz, 1H, CH=), 5.15–5.01 (m, 3H, 3 × CH=), 4.91 (d, J = 7.2 Hz, 2H, NCH2), 3.58 (s, 3H, N(3)CH3), 3.41 (s, 3H, N(1)CH3), 2.24–2.19 (m, 2H, CH2), 2.15–2.10 (m, 2H, CH2), 2.08–2.02 (m, 4H, 2 × CH2), 2.00–1.94 (m, 4H, 2 × CH2), 1.80 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.60 (s, 3H, CH3), 1.59 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 155.4 (C, C-6), 151.9 (C, C-2), 148.9 (C, C-4), 143.3 (C=), 140.3 (CH, C-8), 136.4 (C=), 135.3 (C=), 131.4 (C=), 124.5 (CH=), 124.1 (CH=), 123.1 (CH=), 118.4 (CH=), 107.2 (C, C-5), 44.5 (NCH2), 39.9 (CH2), 39.8 (CH2), 32.3 (CH2), 29.9 (N(3)CH3), 28.1 (N(1)CH3), 26.9 (CH2), 26.7 (CH2), 26.5 (CH2), 25.9 (CH3), 23.6 (CH3), 17.8 (CH3), 16.21 (CH3), 16.15 (CH3); IR (film from CH2Cl2): νmax 3111, 2917, 2853, 1704, 1658 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C27H41N4O2 453.3224; Found 453.3215 (Δ = −2.0 ppm); HRESIMS/MS (40 eV) m/z (%): 181.0700 (100), 124.0496 (36).
Compound 37: 21.1 mg (19%), white solid; Rf = 0.20 (2:3 EA/PE); 1H NMR (500 MHz, CDCl3): δ 7.52 (s, 1H, H-8), 5.43 (t, J = 7.1 Hz, 1H, CH=), 5.12–5.04 (m, 3H, 3 × CH=), 4.92 (d, J = 7.2 Hz, 2H, NCH2), 3.58 (s, 3H, N(3)CH3), 3.40 (s, 3H, N(1)CH3), 2.15–2.07 (m, 4H, 2 × CH2), 2.07–2.01 (m, 4H, 2 × CH2), 1.99–1.93 (m, 4H, 2 × CH2), 1.78 (s, 3H, CH3), 1.66 (s, 3H, CH3), 1.58 (s, 9H, 3 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 155.4 (C, C-6), 151.8 (C, C-2), 148.9 (C, C-4), 143.5 (C=), 140.3 (CH, C-8), 136.0 (C=), 135.2 (C=), 131.4 (C=), 124.5 (CH=), 124.2 (CH=), 123.4 (CH=), 117.5 (CH=), 107.2 (C, C-5), 44.6 (NCH2), 39.8 (CH2), 39.8 (CH2), 39.6 (CH2), 29.9 (N(3)CH3), 28.1 (N(1)CH3), 26.9 (CH2), 26.7 (CH2), 26.3 (CH2), 25.8 (CH3), 17.8 (CH3), 16.7 (CH3), 16.2 (CH3), 16.1 (CH3); IR (film from CH2Cl2): νmax 3110, 2916, 2854, 1704, 1658 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C27H41N4O2 453.3224; Found 453.3205 (Δ = −4.2 ppm); HRESIMS/MS (40 eV) m/z (%): 181.0693 (100), 124.0489 (31).
3.2.18. (E)-7-(3,7-Dimethylocta-2,6-dien-1-yl)-3-methyl-2-thioxo-1,2,3,7-tetrahydro-6H-purin-6-one (38)
2-Mercapto-3-methylhypoxanthine (0.30 mmol, 54.7 mg), K2CO3 (0.61 mmol, 84.3 mg) and geranyl bromide (0.39 mmol, 84.9 mg) in DMF (2 mL) at 70 °C for 30 h yielded 30, after modified work up and modified purification-H2O (7 mL) was added to the reaction and the resulting isolated precipitate was dissolved in in MeOH and DCM (1:1, 5 mL). Partial evaporation yielded a precipitate isolated by filtration and washed with MeOH, 5.4 mg (6%), off-white powder. 1H NMR (600 MHz, CDCl3): δ 9.24 (s, 1H, NH), 7.62 (s, 1H, H-8), 5.43 (t, J = 7.3 Hz, 1H, CH=), 5.07–5.03 (m, 1H, CH=), 4.92 (d, J = 7.3 Hz, 2H, NCH2), 3.93 (s, 3H, NCH3), 2.16–2.08 (m, 4H, 2 × CH2), 1.78 (s, 3H, CH3), 1.69 (s, 3H, CH3), 1.60 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 174.3 (C, C-2), 152.4 (C, C-6), 150.9 (C, C-4), 144.5 (C=), 141.2 (CH, C-8), 132.5 (C=), 123.5 (CH=), 116.8 (CH=), 110.9 (C, C-5), 45.0 (NCH2), 39.6 (CH2), 35.5 (NCH3), 26.2 (CH2), 25.9 (CH3), 17.9 (CH3), 16.7 (CH3); IR (neat): νmax 3122, 2964, 2912, 2853, 1708 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C16H23N4OS 319.1587; Found 319.1591 (Δ = 1.3 ppm); HRESIMS/MS (40 eV) m/z (%): 183.033 (48), 149.0452 (19), 126.99579 (11), 124.0503 (100), 96.0557 (21), 81.0702 (46).
3.2.19. 3-Methyl-2-thioxo-7-((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-1,2,3,7-tetrahydro-6H-purin-6-one (39)
2-Mercapto-3-methylhypoxanthine (0.32 mmol, 57.8 mg), K2CO3 (0.61 mmol, 84.7 mg) and farnesyl bromide (0.39 mmol, 111 mg) in DMF (2 mL) at 50 °C for 41 h yielded 39, 3.3 mg (3%), off-white powder. Rf = 0.19 (1:2 EA/PE); 2:1 E/Z, NMR data for major isomer: 1H NMR (600 MHz, CDCl3): δ 9.31 (s, 1H, NH), 7.62 (s, 1H, H-8), 5.43 (t, J = 7.3 Hz, 1H, CH=), 5.09–5.05 (m, 2H, 2 × CH=), 4.92 (d, J = 7.3 Hz, 2H, NCH2), 3.92 (s, 3H, NCH3), 2.16–2.09 (m, 4H, 2 × CH2), 2.06–2.01 (m, 4H, 2 × CH2), 1.78 (s, 3H, CH3), 1.66 (s, 3H, CH3), 1.59 (s, 3H, CH3), 1.58 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 174.3 (C, C-2), 152.4 (C, C-6), 150.9 (C, C-4), 144.5 (C=), 141.1 (CH, C-8), 136.1 (C=), 131.6 (C=), 124.3 (CH=), 123.3 (CH=), 116.8 (CH=), 110.9 (C, C-5), 45.0 (NCH2), 39.8 (CH2), 39.6 (CH2), 35.5 (NCH3), 26.8 (CH2), 26.2 (CH2), 25.9 (CH3), 17.8 (CH3), 16.7 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3116, 2922, 2854, 1691 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C21H31N4OS 387.2213; Found 387.2216 (Δ = 0.8 ppm); HRESIMS/MS (40 eV) m/z (%): 183.0316 (100), 149.0436 (13), 124.0493 (42).
3.2.20. 3-Methyl-7-((2Z,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-2-thioxo-1,2,3,7-tetrahydro-6H-purin-6-one (40) and 3-methyl-7-((2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-2-thioxo-1,2,3,7-tetrahydro-6H-purin-6-one (41)
2-Mercapto-3-methylhypoxanthine (0.30 mmol, 54.7 mg), K2CO3 (1.1 mmol, 150.8 mg) and geranylgeranyl bromide (0.32 mmol, 111.3 mg) in DMF (1 mL) for 24 h yielded 40 and 41.
Compound 40: 3.2 mg (9%), white powder; Rf = 0.14 (1:3 EA/PE); 1H NMR (500 MHz, CDCl3): δ 9.32 (s, 1H, NH), 7.64 (s, 1H, H-8), 5.45 (t, J = 7.2 Hz, 1H, CH=), 5.13–5.07 (m, 3H, 3 × CH=), 4.92 (d, J = 7.3 Hz, 2H, NCH2), 3.94 (s, 3H, NCH3), 2.26–2.19 (m, 2H, CH2), 2.18–2.11 (m, 2H, CH2), 2.10–2.04 (m, 4H, 2 × CH2), 2.02–1.96 (m, 4H, 2 × CH2), 1.83 (s, 3H, CH3), 1.69 (s, 3H, CH3), 1.62 (s, 3H, CH3), 1.61 (s, 3H, CH3), 1.60 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 174.3 (C, C-2), 152.4 (C, C-6), 150.9 (C, C-4), 144.2 (C=), 141.2 (CH, C-8), 136.6 (C=), 135.3 (C=), 131.5 (C=), 124.5 (CH=), 124.1 (CH=), 123.0 (CH=), 117.7 (CH=), 110.9 (C, C-5), 44.8 (NCH2), 39.9 (CH2), 39.8 (CH2), 35.5 (NCH3), 32.3 (CH2), 26.9 (CH2), 26.7 (CH2), 26.4 (CH2), 25.9 (CH3), 23.6 (CH3), 17.8 (CH3), 16.22 (CH3), 16.16 (CH3); IR (film from CH2Cl2): νmax 3205, 3118, 2965, 2917, 2855, 1696 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C26H39N4OS 455.2839; Found 455.2851 (Δ = 2.6 ppm); HRESIMS/MS (40 eV) m/z (%): 183.0304 (100), 124.0487 (19).
Compound 41: 3.4 mg (3%), white powder; Rf = 0.10 (1:3 EA/PE); 1H NMR (500 MHz, CDCl3): δ 9.29 (s, 1H, NH), 7.62 (s, 1H, H-8), 5.43 (t, J = 7.0 Hz, 1H, CH=), 5.13–5.05 (m, 3H, 3 × CH=), 4.92 (d, J = 7.3 Hz, 2H, NCH2), 3.92 (s, 3H, NCH3), 2.17–2.10 (m, 4H, 2 × CH2), 2.09–2.02 (m, 4H, 2 × CH2), 2.01–1.93 (m, 4H, 2 × CH2), 1.79 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.60–1.58 (m, 9H, 3 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 174.3 (C, C-2), 152.4 (C, C-6), 150.9 (C, C-4), 144.5 (C=), 141.1 (CH, C-8), 136.1 (C=), 135.2 (C=), 131.5 (C=), 124.5 (CH=), 124.2 (CH=), 123.3 (CH=), 116.8 (CH=), 110.9 (C, C-5), 45.0 (NCH2), 39.9 (CH2), 39.8 (CH2), 39.6 (CH2), 35.5 (NCH3), 26.9 (CH2), 26.7 (CH2), 26.2 (CH2), 25.9 (CH3), 17.8 (CH3), 16.7 (CH3), 16.23 (CH3), 16.16 (CH3); IR (film from CH2Cl2): νmax 3212, 3117, 2964, 2917, 2853, 1691 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C26H39N4OS 455.2839; Found 455.2857 (Δ = 4.0 ppm); HRESIMS/MS (40 eV) m/z (%): 183.0306 (100), 124.0487 (13).
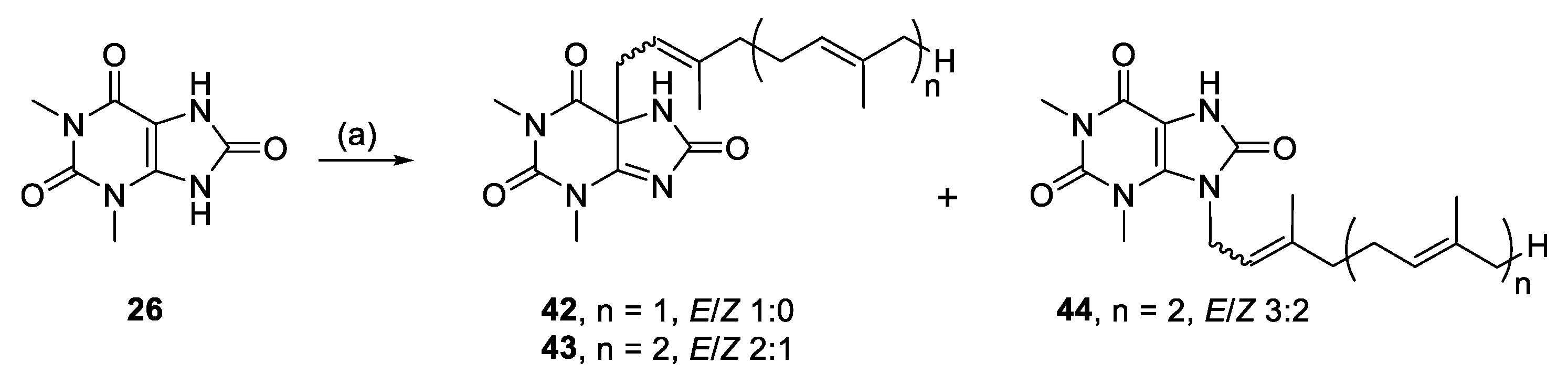
3.2.21. (E)-5-(3,7-Dimethylocta-2,6-dien-1-yl)-1,3-dimethyl-5,7-dihydro-1H-purine-2,6,8(3H)-trione (42)
1,3-Dimethyluric acid (0.12 mmol, 22.6 mg), K2CO3 (0.119 mmol, 16.5 mg) and geranyl bromide (0.13 mmol, 27.6 mg) in DMF (2 mL) for 24 h yielded 42, 3.5 mg (9%), white solid. Rf = 0.23 (1:1 EA/PE); 1H NMR (600 MHz, CDCl3): δ 6.07 (s, 1H, NH), 5.06–5.02 (m, 1H, CH=), 4.95 (t, J = 8.1 Hz, 1H, CH=), 3.49 (s, 3H, N(1)CH3), 3.28 (s, 3H, N(3)CH3), 2.67 (d, J = 8.0 Hz, 2H, NCH2), 2.12–1.99 (m, 4H, 2 × CH2), 1.70 (s, 3H, CH3), 1.61 (s, 3H, CH3), 1.58 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 176.9 (C, C-6), 166.4 (C, C-4), 165.4 (C, C-8), 150.8 (C, C-2), 145.6 (C=), 132.6 (C=), 123.6 (CH=), 112.8 (CH=), 68.1 (C, C-5), 40.6 (NCH2), 39.9 (CH2), 32.0 (N(1)CH3), 29.2 (N(3)CH3), 26.3 (CH2), 25.8 (CH3), 17.9 (CH3), 16.4 (CH3); IR (film from CH2Cl2): νmax 3278, 3106, 2966, 2921, 2857, 1750, 1697, 1609 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C17H25N4O3 333.1910; Found 333.1921 (Δ = 3.3 ppm); HRESIMS/MS (40 eV) m/z (%): 197.0664 (95), 169.0712 (100), 140.0463 (32), 112.0505 (41).
3.2.22. 1,3-Dimethyl-5-((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-5,7-dihydro-1H-purine-2,6,8(3H)-trione (43) and 1,3-dimethyl-9-((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-7,9-dihydro-1H-purine-2,6,8(3H)-trione (44)
1,3-Dimethyluric acid (0.12 mmol, 23.3 mg), K2CO3 (0.12 mmol,16.6 mg) and farnesyl bromide (0.11 mmol, 32.0 mg) in DMF (2 mL) for 19 h yielded 43 and 44.
Compound 43: 19.2 mg (40%), white solid; Rf = 0.38 (1:1 EA/PE); 2:1 E/Z NMR data for major isomer: 1H NMR (600 MHz, CDCl3): δ 6.39 (s, 1H, NH), 5.09–5.02 (m, 2H, 2 × CH=), 4.95 (t, J = 7.9 Hz, 1H, CH=), 3.48 (s, 3H, N(1)CH3), 3.27 (s, 3H, N(3)CH3), 2.68 (d, J = 8.1 Hz, 2H, NCH2), 2.09–2.00 (m, 6H, 3 × CH2), 2.00–1.93 (m, 2H, CH2), 1.66 (s, 3H, CH3), 1.59 (s, 6H, 2 × CH3), 1.58 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 176.8 (C, C-6), 166.4 (C, C-4), 165.6 (C, C-8), 150.9 (C, C-2), 145.7 (C=), 136.0 (C=), 131.5 (C=), 124.4 (CH=), 123.4 (CH=), 112.7 (CH=), 68.2 (C, C-5), 40.5 (NCH2), 40.0 (CH2), 39.8 (CH2), 32.0 (N(1)CH3), 29.2 (N(3)CH3), 26.8 (CH2), 26.3 (CH2), 25.8 (CH3), 17.8 (CH3), 16.4 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3307, 3098, 2964, 2924, 2855, 1695, 1645, 1612 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C22H33N4O3 401.2547; Found 401.2538 (Δ = −2.2 ppm); HRESIMS/MS (40 eV) m/z (%): 197.0639 (100), 169.0699 (40).
Compound 44: 3.3 mg (7%), white solid; Rf = 0.13 (1:1 EA/PE); 3:2 E/Z, NMR data for major isomer: 1H NMR (600 MHz, CDCl3): δ 8.95 (br s, 1H, NH), 5.16–5.11 (m, 1H, CH=), 5.11–5.00 (m, 2H, 2 × CH=), 4.66 (d, J = 5.8 Hz, 2H, NCH2), 3.67 (s, 3H, N(3)CH3), 3.40 (d, J = 2.3 Hz, 3H, N(1)CH3), 2.13–2.07 (m, 2H, CH2), 2.07–1.98 (m, 4H, 2 × CH2), 1.97–1.91 (m, 2H, CH2), 1.75 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.58 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 153.2 (C, C-6), 151.8 (C, C-8), 151.0 (C, C-2), 140.9 (C=), 136.2 (C=), 136.0 (C, C-4), 131.6 (C=), 124.3 (CH=), 123.3 (CH=), 119.5 (CH=), 98.4 (C, C-5), 41.6 (NCH2), 39.8 (CH2), 39.4 (CH2), 31.3 (N(3)CH3), 28.6 (N(1)CH3), 26.8 (CH2), 26.3 (CH2), 25.9 (CH3), 17.8 (CH3), 17.0 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3487, 3174, 3078, 2918, 2854, 1687, 1651 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C22H33N4O3 401.2547; Found 401.2551 (Δ = 1.0 ppm); HRESIMS/MS (40 eV) m/z (%): 197.0658 (100), 169.0707 (57).
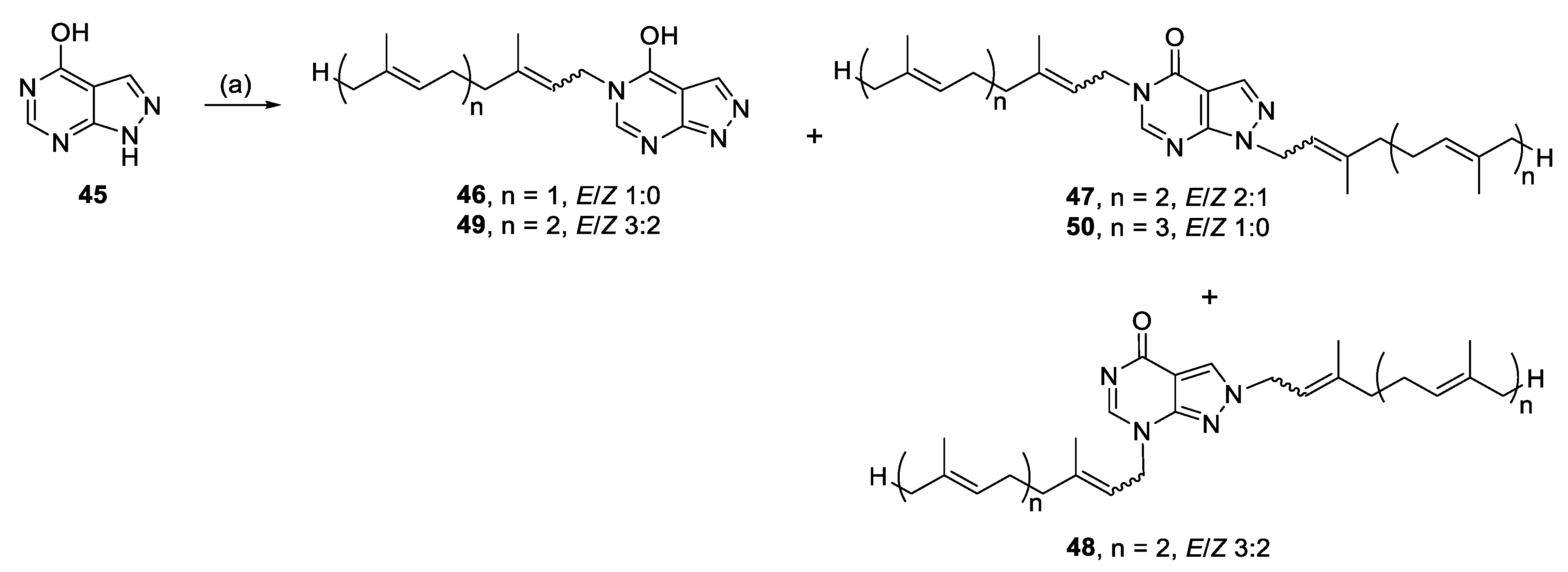
3.2.23. (E)-5-(3,7-Dimethylocta-2,6-dien-1-yl)-5H-pyrazolo[3,4-d]pyrimidin-4-ol (46)
Allopurinol (0.80 mmol, 109.2 mg), K2CO3/Na2CO3 (1:1, 150.8 mg) and geranyl bromide (0.50 mmol, 108 mg) in DMF (10 mL) for 24 h yielded 46-additionally recrystallised from MeOH after chromatography, 4.5 mg (2%), white powder. Rf = 0.06 (1:4 EA/PE); 1H NMR (600 MHz, CDCl3): δ 11.32 (br s, 1H, OH), 8.18 (s, 1H, H-3), 8.02 (s, 1H, H-6), 5.34–5.26 (m, 1H, CH=), 5.07–5.02 (m, 1H, CH=), 4.64 (d, J = 7.2 Hz, 2H, NCH2), 2.16–2.03 (m, 4H, 2 × CH2), 1.82 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.59 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 157.4 (C, C-4), 153.4 (C, C-7a), 149.4 (CH, C-6), 143.0 (C=), 136.4 (CH, C-3), 132.3 (C=), 123.6 (CH=), 118.1 (CH=), 105.9 (C, C-3a), 43.5 (NCH2), 39.6 (CH2), 26.3 (CH2), 25.8 (CH3), 17.9 (CH3), 16.7 (CH3); IR (film from CH2Cl2): νmax 3188, 3080, 2967, 2905, 2791, 1678, 1568 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C15H21N4O 273.1710; Found 273.1715 (Δ = 1.8 ppm); HRESIMS/MS (40 eV) m/z (%): 137.0454 (100), 110.0349 (39).
3.2.24. 1,5-Bis((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (47), 2,7-bis((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-2,7-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (48), and 5-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-5H-pyrazolo[3,4-d]pyrimidin-4-ol (49)
Allopurinol (0.48 mmol, 65.2 mg), K2CO3 (0.51 mmol, 70.5 mg) and farnesyl bromide (0.55 mmol, 157.0 mg) in DMF (2 mL) at 70 °C for 27 h yielded 47, 48, and 49.
Compound 47: 17.2 mg (12%), colourless oil; Rf = 0.24 (1:5 EA/PE); 2:1 E/Z, NMR data for major isomer: 1H NMR (500 MHz, CDCl3): δ 8.09–8.08 (m, 1H, H-6), 7.95–7.94 (m, 1H, H-3), 5.48–5.41 (m, 1H, CH=), 5.34–5.28 (m, 1H, CH=), 5.13–5.04 (m, 4H, 4 × CH=), 4.94 (d, J = 6.9 Hz, 2H, NCH2), 4.61 (d, J = 7.2 Hz, 2H, NCH2), 2.18–1.99 (m, 14H, 7 × CH2), 1.99–1.92 (m, 2H, CH2), 1.85 (s, 3H, CH3), 1.83 (s, 3H, CH3), 1.68 (s, 9H, 3 × CH3), 1.60 (s, 9H, 3 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 157.4 (C, C-4), 151.0 (C, C-7a), 148.4 (CH, C-6), 142.7 (C=), 141.0 (C=), 135.9 (C=), 135.6 (C=), 135.1 (CH, C-3), 131.49 (C=), 131.46 (C=), 124.42 (CH=), 124.37 (CH=), 123.7 (CH=), 123.5 (CH=), 118.4 (CH=), 118.3 (CH=), 105.9 (C, C-3a), 45.4 (NCH2), 43.2 (NCH2), 39.80 (CH2), 39.78 (CH2), 39.64 (CH2), 39.59 (CH2), 26.83 (CH2), 26.81 (CH2), 26.34 (CH2), 26.30 (CH2), 25.9 (CH3), 25.8 (CH3), 17.8 (2 × CH3), 16.74 (CH3), 16.69 (CH3), 16.19 (CH3), 16.15 (CH3); IR (film from CH2Cl2): νmax 3368, 2964, 2925, 2856, 1696, 1582 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C35H53N4O 545.4214; Found 545.4223 (Δ = 1.7 ppm); HRESIMS/MS (40 eV) m/z (%): 137.0458 (100), 81.702 (12).
Compound 48: 6.4 mg (4%), colourless oil; Rf = 0.48 (1:1 EA/PE); 3:2 E/Z, NMR data for major isomer: 1H NMR (500 MHz, CDCl3): δ 8.06 (s, 1H, H-3), 7.95 (s, 1H, H-6), 5.51 (t, J = 6.5 Hz, 1H, CH=), 5.32–5.26 (m, 1H, CH=), 5.13–5.05 (m, 4H, 4 × CH=), 4.89 (d, J = 7.4 Hz, 2H, NCH2), 4.57 (d, J = 7.1 Hz, 2H, NCH2), 2.20–1.93 (m, 16H, 8 × CH2), 1.81 (s, 3H, CH3), 1.79 (s, 3H, CH3), 1.68 (s, 6H, 2 × CH3), 1.62–1.57 (m, 12H, 4 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 158.9 (C, C-4), 158.6 (C, 7a), 148.5 (CH, C-6), 144.5 (C=), 142.1 (C=), 136.0 (C=), 135.8 (C=), 131.7 (C=), 131.4 (C=), 127.0 (CH, C-3), 124.4 (CH=), 124.3 (CH=), 123.5 (CH=), 123.4 (CH=), 118.5 (CH=), 116.7 (CH=), 107.1 (C, C-3a), 51.2 (NCH2), 43.0 (NCH2), 39.77 (CH2), 39.76 (CH2), 39.64 (CH2), 39.62 (CH2), 26.8 (CH2), 26.7 (CH2), 26.4 (CH2), 26.3 (CH2), 25.85 (CH3), 25.84 (CH3), 17.82 (CH3), 17.81 (CH3), 16.8 (CH3), 16.7 (CH3), 16.19 (CH3), 16.15 (CH3); IR (film from CH2Cl2): νmax 3404, 2973, 2934, 1687 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C35H53N4O 545.4214; Found 545.4225 (Δ = 2.0 ppm); HRESIMS/MS (40 eV) m/z (%): 137.0451 (100), 81.0699 (30).
Compound 49: 33.6 mg (21%), white solid; Rf = 0.16 (1:1 EA/PE); 3:2 E/Z, NMR data for major isomer: 1H NMR (600 MHz, CDCl3): δ 12.64 (br s, 1H, OH), 8.20 (s, 1H, H-3), 8.08 (s, 1H, H-6), 5.34–5.28 (m, 1H, CH=), 5.09–5.01 (m, 2H, 2 × CH=), 4.64 (d, J = 7.3 Hz, 2H, NCH2), 2.14–2.04 (m, 4H, 2 × CH2), 2.04–1.96 (m, 2H, CH2), 1.96–1.92 (m, 2H, CH2), 1.82 (s, 3H, CH3), 1.64 (s, 3H, CH3), 1.57 (s, 3H, CH3), 1.56 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 157.4 (C, C-4), 153.3 (C, C-7a), 149.4 (CH, C-6), 143.0 (C=), 136.1 (CH, C-3) 135.9 (C=), 131.5 (C=), 124.3 (CH=), 123.4 (CH=), 118.0 (CH=), 105.9 (C, C-3a), 43.5 (NCH2), 39.7 (CH2), 39.6 (CH2), 26.8 (CH2), 26.3 (CH2), 25.8 (CH3), 17.8 (CH3), 16.7 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3188, 3108, 2967, 2917, 1676 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C20H29N4O 341.2336; Found 341.2340 (Δ = 1.2 ppm); HRESIMS/MS (40 eV) m/z (%): 137.0451 (100), 110.0344 (9).
3.2.25. 1,5-Bis((2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (50)
Allopurinol (0.71 mmol, 96.6 mg), K2CO3 (0.81 mmol, 111.9 mg) and geranylgeranyl bromide (0.73 mmol, 259.7 mg) in DMF (1 mL) for 27 h yielded 50, 6.8 mg (2%), colourless oil. Rf = 0.26 (1:5 EA/PE); 1H NMR (600 MHz, CDCl3): δ 8.07 (s, 1H, H-3), 7.93 (s, 1H, H-6), 5.43 (t, J = 6.8 Hz, 1H, CH=), 5.30 (t, J = 7.2 Hz, 1H, CH=), 5.11–5.04 (m, 6H, 6 × CH=), 4.93 (d, J = 6.9 Hz, 2H, NCH2), 4.60 (d, J = 7.3 Hz, 2H, NCH2), 2.14–2.01 (m, 16H, 8 × CH2), 1.99–1.93 (m, 8H, 4 × CH2), 1.83 (s, 3H, CH3), 1.81 (s, 3H, CH3), 1.67 (s, 6H, 2 × CH3), 1.59 (s, 6H, 2 × CH3), 1.584 (s, 3H, CH3), 1.579 (s, 6H, 2 × CH3), 1.56 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 157.4 (C, C-4), 151.0 (C, C-7a), 148.4 (CH, C-6), 142.7 (C=), 141.1 (C=), 135.9 (C=), 135.6 (C=), 135.13 (C= and CH, C-3), 135.09 (C=), 131.43 (C=), 131.41 (C=), 124.50 (CH=), 124.48 (CH=), 124.3 (CH=), 124.2 (CH=), 123.7 (CH=), 123.5 (CH=), 118.4 (CH=), 118.3 (CH=), 105.9 (C, C-3a), 45.4 (NCH2), 43.2 (NCH2), 39.86 (CH2), 39.85 (CH2), 39.80 (CH2), 39.78 (CH2), 39.7 (CH2), 39.6 (CH2), 26.9 (2 × CH2), 26.73 (CH2), 26.71 (CH2), 26.38 (CH2), 26.35 (CH2), 25.9 (2 × CH3), 17.8 (2 × CH3), 16.8 (CH3), 16.7 (CH3), 16.2 (2 × CH3), 16.1 (2 × CH3); IR (film from CH2Cl2): νmax 3392, 2975, 2937, 1699 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C45H69N4O 681.5466; Found 681.5470 (Δ = 0.6 ppm); HRESIMS/MS (40 eV) m/z (%): 341.01647 (72), 281.0500 (20), 266.9983 (21), 221.0823 (24), 207.0312 (25), 147.0648 (32), 137.0451 (100).
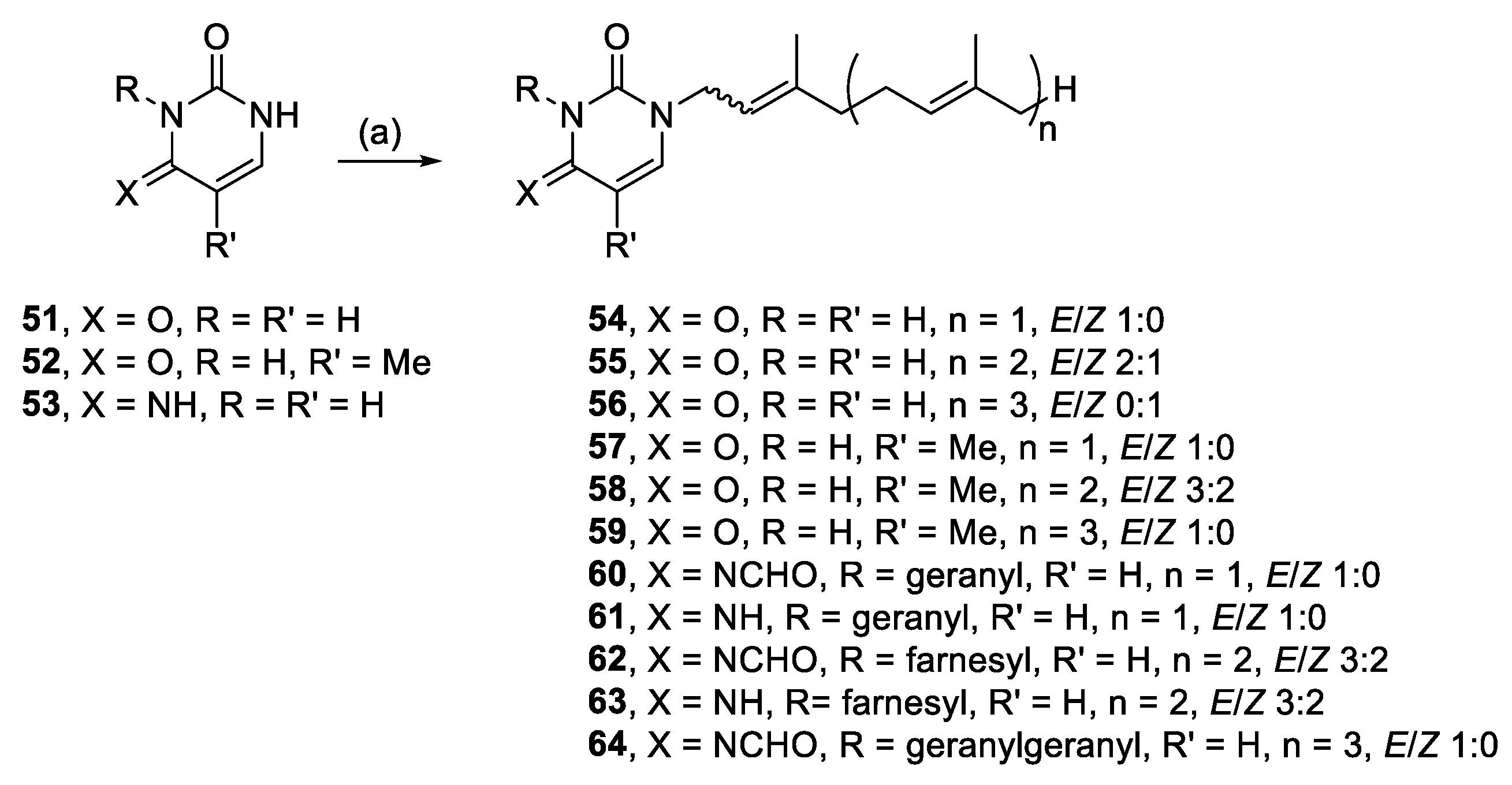
3.2.26. (E)-1-(3,7-Dimethylocta-2,6-dien-1-yl)pyrimidine-2,4(1H,3H)-dione (54)
Uracil (0.53 mmol, 59.9 mg), K2CO3 (0.51 mmol, 68.9 mg) and geranyl bromide (0.65 mmol, 141.1 mg) in DMF (2 mL) at 50 °C for 21 h yielded 54, after modified work up and modified purification—the reaction was filtered, H2O (6 mL) was added to the filtrate and stored in the fridge until precipitate formed. The isolated solid was recrystallised from PE, 15.8 mg (12%), white crystals. 1H NMR (500 MHz, CDCl3): δ 8.42 (br s, 1H, NH), 7.16 (d, J = 7.9 Hz, 1H, H-6), 5.68 (dd, J = 7.9, 2.1 Hz, 1H, H-5), 5.22 (t, J = 7.3, 1H, CH=), 5.04 (t, J = 6.6 Hz, 1H, CH=), 4.35 (d, J = 7.3 Hz, 2H, NCH2), 2.11 (m, 4H, 2 × CH2), 1.75 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.60 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 163.4 (C, C-4), 150.9 (C, C-2), 143.7 (C=), 143.6 (CH, C-6), 132.4 (C=), 123.5 (CH=), 117.3 (CH=), 102.2 (CH, C-5), 45.2 (NCH2), 39.6 (CH2), 26.2 (CH2), 25.9 (CH3), 17.9 (CH3), 16.6 (CH3); IR (neat): νmax 3121, 2967, 2929, 2808, 1696, 1657 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C14H21N2O2 249.1598; Found 249.1595 (Δ = −1.2 ppm); HRESIMS/MS (40 eV) m/z (%): 113.0346 (100), 81.0700 (18), 70.0289 (7).
3.2.27. 1-((6E)-3,7,11-Trimethyldodeca-2,6,10-trien-1-yl)pyrimidine-2,4(1H,3H)-dione (55)
Uracil (0.49 mmol, 54.9 mg), K2CO3 (0.51 mmol, 69.9 mg) and farnesyl bromide (0.54 mmol, 150 mg) in DMF (2 mL) at 70 °C for 24 h yielded 55, after modified work up and modified purification as per 54, 38.6 mg (25%), white crystals. 3:2 E/Z, NMR data for major isomer: 1H NMR (600 MHz, CDCl3): δ 8.46 (s, 1H, NH), 7.16 (d, J = 7.9 Hz, 1H, H-6), 5.68 (dd J = 8.0, 2.2 Hz, 1H, H-5), 5.24–5.19 (m, 1H, CH=), 5.09–5.04 (m, 2H, 2 × CH=), 4.34 (d, J = 7.3 Hz, 2H, NCH2), 2.16–2.01 (m, 6H, 3 × CH2), 1.99–1.94 (m, 2H, CH2), 1.75 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.62 (s, 3H, CH3), 1.59 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 163.5 (C, C-4), 150.9 (C, C-2), 143.8 (C=), 143.6 (CH, C-6), 136.0 (C=), 131.6 (C=), 124.3 (CH=), 123.4 (CH=), 117.2 (CH=), 102.2 (CH, C-5), 45.3 (NCH2), 39.8 (CH2), 39.6 (CH2), 26.8 (CH2), 26.2 (CH2), 25.9 (CH3), 17.8 (CH3), 16.6 (CH3), 16.2 (CH3); IR (film from CH2Cl2): νmax 3054, 2979, 2930, 1683 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C19H29N2O2 317.2224; Found 317.2222 (Δ = −0.6 ppm); HRESIMS/MS (40 eV) m/z (%): 113.0340 (87), 81.0698 (100).
3.2.28. 1-((2Z,6E,10E)-3,7,11,15-Tetramethylhexadeca-2,6,10,14-tetraen-1-yl)pyrimidine-2,4(1H,3H)-dione (56)
Uracil (0.29 mmol, 32.7 mg), K2CO3 (0.36 mmol, 50.1 mg) and geranylgeranyl bromide (0.32 mmol, 111 mg) in DMF (1 mL) for 48 h yielded 56, 9.4 mg (31%), white solid. Rf = 0.23 (2:3 EA/PE); 1H NMR (500 MHz, CDCl3): δ 8.78 (s, 1H, NH), 7.15 (dd, J = 7.9, 0.8 Hz, 1H, H-6), 5.68 (dd, J = 7.9, 1.4 Hz, 1H, H-5), 5.22 (t, J = 7.2 Hz, 1H, CH=), 5.13–5.04 (m, 3H, 3 × CH=), 4.33 (d, J = 7.3 Hz, 2H, NCH2), 2.20–2.10 (m, 4H, 2 × CH2), 2.10–2.02 (m, 4H, 2 × CH2), 2.01–1.93 (m, 4H, 2 × CH2), 1.79 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.61 (s, 3H, CH3), 1.60 (s, 6H, 2 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 163.6 (C, C-4), 150.9 (C, C-2), 143.7 (C=), 143.6 (CH, C-6), 136.5 (C=), 135.3 (C=), 131.5 (C=), 124.5 (CH=), 124.1 (CH=), 123.1 (CH=), 118.1 (CH=), 102.2 (CH, C-5), 45.1 (NCH2), 39.9 (2 × CH2), 32.2 (CH2), 26.9 (CH2), 26.7 (CH2), 26.5 (CH2), 25.9 (CH3), 23.6 (CH3), 17.8 (CH3), 16.20 (CH3), 16.16 (CH3); IR (film from CH2Cl2): νmax 3425, 3197, 3054, 2970, 2930, 2875, 1686 cm−1; HRESIMS m/z: [M + H]+ Calcd. C24H37N2O2 385.2850; Found 385.2817 (Δ = −8.6 ppm); HRESIMS/MS (40 eV) m/z (%):113.0331 (100), 107.0389 (21).
3.2.29. (E)-1-(3,7-Dimethylocta-2,6-dien-1-yl)-5-methylpyrimidine-2,4-(1H,3H)-dione (57)
Thymine (0.48 mmol, 61.0 mg), K2CO3 (0.50 mmol, 69.4 mg) and geranyl bromide (0.65 mmol, 141.1 mg) in DMF (2 mL) for 20 h yielded 57, after modified work up and modified purification as per 54, 6.8 mg (5%), white crystals. 1H NMR (600 MHz, CDCl3): δ 8.20 (br s, 1H, NH), 6.95 (d, J = 1.2 Hz, 1H, H-6), 5.21 (t, J = 7.1 Hz, 1H, CH=), 5.05 (t, J = 6.9 Hz, 1H, CH=), 4.32 (d, J = 7.3 Hz, 2H, NCH2), 2.14–2.05 (m, 4H, 2 × CH2), 1.92 (s, 3H,C(5)CH3), 1.75 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.60 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 163.9 (C, C-4), 150.8 (C, C-2), 142.9 (C=), 139.7 (CH, C-6), 132.3 (C=), 123.5 (CH=), 117.7 (CH=), 110.7 (C, C-5), 45.1 (NCH2), 39.6 (CH2), 26.3 (CH2), 25.9 (CH3), 17.9 (CH3), 16.6 (CH3), 12.6 (C(5)CH3); IR (film from CH2Cl2): νmax 3152, 2975, 2919, 2830, 1685, 1645 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C15H23N2O2 263.1754; Found 263.1752 (Δ = −0.8 ppm); HRESIMS/MS (10 eV) m/z (%): 128.0524 (7), 127.0501 (100), 81.07 (19).
3.2.30. 5-Methyl-1-((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)pyrimidine-2,4(1H,3H)-dione (58)
Thymine (0.49 mmol, 62.4 mg), K2CO3 (0.51 mmol, 70.5 mg) and farnesyl bromide (0.62 mmol, 176.9 mg) in DMF (2 mL) at 70 °C for 24 h yielded 58, after modified work up and modified purification as per 54, 31.9 mg (20%), white crystals. 3:2 E/Z, NMR data for major isomer: 1H NMR (600 MHz, CDCl3): δ 8.41 (br s, 1H, NH), 6.95 (s, 1H, H-6), 5.24–5.19 (m, 1H, CH=), 5.11–5.01 (m, 2H, 2 × CH=), 4.32 (d, J = 7.5 Hz, 2H, NCH2), 2.14–2.02 (m, 6H, 3 × CH2), 1.98–1.95 (m, 2H, CH2), 1.91 (s, 3H, C(5)CH3), 1.76 (s, 3H, CH3), 1.67 (s, 6H, 2 × CH3), 1.59 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 164.0 (C, C-4), 150.9 (C, C-2), 142.9 (C=), 139.7 (CH, C-6), 135.9 (C=), 131.6 (C=), 124.3 (CH=), 123.4 (CH=), 117.7 (CH=), 110.7 (C, C-5), 45.1 (NCH2), 39.8 (CH2), 39.6 (CH2), 26.8 (CH2), 26.2 (CH2), 25.9 (CH3), 17.8 (CH3), 16.7 (CH3), 16.2 (CH3), 12.6 (C(5)CH3); IR (film from CH2Cl2): νmax 3177, 3052, 2966, 2927, 1665 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C20H31N2O2 331.2380; Found 331.2372 (Δ = −2.4 ppm); HRESIMS/MS (40 eV) m/z (%): 127.0487 (100), 110.0219 (46).
3.2.31. 5-Methyl-1-((2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)pyrimidine-2,4(1H,3H)-dione (59)
Thymine (0.31 mmol, 38.7 mg), K2CO3 (0.36 mmol, 49.6 mg) and geranylgeranyl bromide (0.32 mmol, 111 mg) in DMF (1 mL) for 24 h yielded 59, 10.1 mg (11%), waxy white solid. Rf = 0.22 (2:3 EA/PE); 1H NMR (500 MHz, CDCl3): δ 8.67 (br s, 1H, NH), 6.95 (d, J = 1.1 Hz, 1H, H-6), 5.21 (t, J = 6.7 Hz, 1H, CH=), 5.12–5.04 (m, 3H, 3 × CH=), 4.32 (d, J = 7.1 Hz, 2H, NCH2), 2.16–2.02 (m, 8H, 4 × CH2), 2.01–1.94 (m, 4H, 2 × CH2), 1.91 (s, 3H, C(5)CH3), 1.76 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.60 (s, 6H, 2 × CH3), 1.59 (s, 3H, CH3); IR (film from CH2Cl2): νmax 3427, 3176, 3043, 2968, 2925, 2855, 1668 cm−1; 13C{1H} NMR (150 MHz, CDCl3): δ 164.1 (C, C-4), 151.0 (C, C-2), 143.0 (C=), 139.7 (CH, C-6), 135.9 (C=), 135.2 (C=), 131.4 (C=), 124.5 (CH=) 124.2 (CH=), 123.5 (CH=), 117.7 (CH=), 110.8 (C, C-5), 45.1 (NCH2), 39.9 (CH2), 39.8 (CH2), 39.7 (CH2), 26.9 (CH2), 26.7 (CH2), 26.3 (CH2), 25.9 (CH3), 17.8 (CH3), 16.7 (CH3), 16.21 (CH3), 16.15 (CH3), 12.6 (C(5)CH3); HRESIMS m/z: [M + H]+ Calcd. for C25H39N2O2 399.3006; Found 399.2995 (Δ = −2.8 ppm); HRESIMS/MS m/z (20 eV) (%): 399.2980 (8), 283.2625 (13), 127.0501 (100).
3.2.32. N-(-1,3-Bis((E)-3,7-dimethylocta-2,6-dien-1-yl)-2-oxo-2,3-dihydropyrimidin-4(1H)-ylidene)formamide (60) and 1,3-bis((E)-3,7-dimethylocta-2,6-dien-1-yl)-4-imino-3,4-dihydropyrimidin-2(1H)-one (61)
Cytosine (1.0 mmol, 112.4 mg), K2CO3 (1.0 mmol, 152.0 mg) and geranyl bromide (1.1 mmol, 238.5 mg) in DMF (4 mL) for 27 h yielded 60 and 61.
Compound 60: 4.5 mg (2%), pale-yellow oil; Rf = 0.40 (1:1 EA/PE); 1H NMR (600 MHz, CDCl3): δ 9.14 (s, 1H, HC=O), 7.10 (d, J = 7.9 Hz, 1H, H-6), 6.4 (d, J = 7.9 Hz, 1H, H-5), 5.25 (t, J = 7.0 Hz, 1H, CH=), 5.22 (t, J = 7.0 Hz, 1H, CH=), 5.08–5.02 (m, 2H, 2 × CH=), 4.75 (d, J = 7.1 Hz, 2H, NCH2), 4.39 (d, J = 7.2 Hz, 2H, NCH2), 2.15–2.03 (m, 6H, 3 × CH2), 2.02–1.97 (m, 2H, CH2), 1.82 (s, 3H, CH3), 1.74 (s, 3H, CH3), 1.68 (s, 3H, CH3), 1.66 (s, 3H, CH3), 1.60 (s, 3H, CH3), 1.58 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 172.8 (HC=O), 159.8 (C, C-4), 150.5 (C, C-2), 144.3 (C=), 141.2 (C=), 140.8 (CH, C-6), 132.4 (C=), 131.7 (C=), 124.1 (CH=), 123.5 (CH=), 117.4 (CH=), 117.0 (CH=), 97.4 (CH, C-5), 46.6 (NCH2), 42.0 (NCH2), 39.8 (CH2), 39.6 (CH2), 26.6 (CH2), 26.2 (CH2), 25.89 (CH3), 25.85 (CH3), 17.89 (CH3), 17.85 (CH3), 16.8 (CH3), 16.7 (CH3); IR (film from CH2Cl2): νmax 3306, 2964, 2915, 2854, 1654, 1684, 1403 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C25H38N3O2 412.2959; Found 412.2985 (Δ = 6.3 ppm); HRESIMS/MS m/z (10 eV) (%): 412.0949 (11), 276.1708 (35), 140.0447 (100).
Compound 61: 18.3 mg (9%), pale-yellow oil; Rf = 0.19 (1:2 EA/PE); 1H NMR (600 MHz, CDCl3): δ 6.59 (d, J = 7.9 Hz, 1H, H-6), 5.53 (d, J = 7.9 Hz, 1H, H-5), 5.23 (t, J = 6.3 Hz, 1H, CH=), 5.21–5.16 (m, 1H, CH=), 5.09–5.02 (m, 2H, 2 × CH=), 4.62 (d, J = 6.4 Hz, 2H, NCH2), 4.25 (d, J = 7.2 Hz, 2H, NCH2), 2.14–2.03 (m, 6H, 3 × CH2), 2.01–1.97 (m, 2H, CH2), 1.80 (s, 3H, CH3), 1.71 (s, 3H, CH3), 1.66 (s, 3H, CH3), 1.65 (s, 3H, CH3), 1.58 (s, 3H, CH3), 1.57 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 158.2 (C, C-4), 151.3 (C, C-2), 142.3 (C=), 139.6 (C=), 135.2 (CH, C-6), 132.1 (C=), 131.6 (C=), 124.2 (CH=), 123.7 (CH=), 118.6 (CH=), 118.3 (CH=), 102.0 (CH, C-5), 45.7 (NCH2), 40.5 (NCH2), 39.8 (CH2), 39.6 (CH2), 26.6 (CH2), 26.3 (CH2), 25.83 (CH3), 25.81 (CH3), 17.9 (CH3), 17.8 (CH3), 16.7 (CH3), 16.5 (CH3); IR (film from CH2Cl2): νmax 3305, 3083, 2966, 2915, 2855, 1651 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C24H38N3O 384.3009; Found 384.3023 (Δ = 3.6 ppm); HRESIMS/MS m/z (20 eV) (%): 384.3018 (0.1), 248.1764 (100), 113.0529 (0.09).
3.2.33. N-(-2-Oxo-1,3-bis((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-2,3-dihydropyrimidin-4(1H)-ylidene)formamide (62) and 4-imino-1,3-bis((6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-3,4-dihydropyrimidin-2(1H)-one (63)
Cytosine (1.0 mmol, 114.8 mg), K2CO3 (1.5 mmol, 204.4 mg) and farnesyl bromide (1.2 mmol, 341 mg) in DMF (2 mL) for 25 h yielded 62 and 63.
Compound 62: 11.3 mg (3%), pale-yellow oil; Rf = 0.16 (1:4 EA/PE); 3:2 E/Z, data for major isomer: 1H NMR (600 MHz, CDCl3): δ 9.13 (s, 1H, HC=O), 7.1 (d, J = 7.8 Hz, 1H, H-6), 6.39 (d, J = 7.8 Hz, 1H, H-5), 5.28–5.20 (m, 2H, 2 × CH=), 5.12–5.04 (m, 4H, 4 × CH=), 4.75 (d, J = 6.8 Hz, 2H, NCH2), 4.39 (d, J = 7.4 Hz, 2H, NCH2), 2.15–1.92 (m, 16H, 8 × CH2), 1.83 (s, 3H, CH3), 1.75 (s, 3H, CH3), 1.67 (s, 6H, 2 × CH3), 1.60 (s, 3H, CH3), 1.59 (s, 6H, 2 × CH3), 1.57 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 172.8 (C, HC=O), 159.8 (C, C-4), 150.5 (C, C-2), 144.3 (C=), 141.3 (C=), 140.8 (CH, C-6), 136.0 (C=), 135.4 (C=), 131.6 (C=), 131.4 (C=), 124.5 (CH=), 124.3 (CH=), 124.0 (CH=), 123.3 (CH=), 117.4 (CH=), 116.9 (CH=), 97.4 (CH, C-5), 46.6 (NCH2), 42.0 (NCH2), 39.84 (CH2), 39.82 (2 × CH2), 39.7 (CH2), 26.9 (CH2), 26.8 (CH2), 26.5 (CH2), 26.2 (CH2), 25.9 (2 × CH3), 17.852 (CH3), 17.846 (CH3), 16.8 (CH3), 16.7 (CH3), 16.22 (CH3), 16.16 (CH3); IR (film from CH2Cl2): νmax 2964, 2915, 2854, 1650, 1450 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C35H54N3O2 548.4211; Found 548.4233 (Δ = 4.0 ppm).
Compound 63: 96.4 mg (31%), pale-yellow oil; Rf = 0.19 (1:1 EA/PE); 3:2 E/Z, data for major isomer: 1H NMR (600 MHz, CDCl3): δ 6.59 (d, J = 7.9 Hz, 1H, H-6), 5.53 (d, J = 7.7 Hz, 1H, H-5), 5.24 (t, J = 5.7 Hz, 1H, CH=), 5.20 (t, J = 6.8 Hz, 1H, CH=), 5.13–5.04 (m, 4H, 4 × CH=), 4.63 (d, J = 6.3 Hz, 2H, NCH2), 4.26 (d, J = 7.2 Hz, 2H, NCH2), 2.15–1.91 (m, 16H, 8 × CH2), 1.81 (s, 3H, CH3), 1.72 (s, 3H, CH3), 1.68 (s, 9H, 3 × CH3), 1.59 (s, 9H, 3 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 158.2 (C, C-4), 151.4 (C, C-2), 142.3 (C=), 139.6 (C=), 135.7 (C=), 135.1 (C=), 134.9 (CH, C-6), 131.6 (C=), 131.4 (C=), 124.5 (CH=), 124.3 (CH=), 124.1 (CH=), 123.6 (CH=), 118.7 (CH=), 118.4 (CH=), 102.2 (CH, C-5), 45.7 (NCH2), 40.4 (NCH2), 39.84 (CH2), 39.83 (CH2), 39.77 (CH2), 39.65 (CH2), 26.9 (CH2), 26.8 (CH2), 26.6 (CH2), 26.3 (CH2), 25.0 (2 × CH3), 17.9 (2 × CH3), 16.7 (CH3), 16.6 (CH3), 16.2 (CH3), 16.1 (CH3); IR (film from CH2Cl2): νmax 3306, 2964, 2915, 2854 1654 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C34H54N3O 520.4261; Found 520.4269 (Δ = 1.5 ppm); HRESIMS/MS m/z (20 eV) (%): 316.2386 (21), 112.0511 (100).
3.2.34. N-(-2-Oxo-1,3-bis((2E,6E,10E)-3,7,11,15-tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-2,3-dihydropyrimidin-4(1H)-ylidene)formamide (64)
Cytosine (0.41 mmol, 45.7mg), K2CO3 (0.84 mmol, 116.4 mg), and geranylgeranyl bromide (0.42 mmol, 148.4 mg) in DMF (2 mL) for 25 h yielded 64, 6.3 mg (3%), pale-yellow oil. Rf = 0.50 (1:1 EA/PE); 1H NMR (600 MHz, CDCl3): δ 9.13 (s, 1H, HC=O), 7.09 (d, J = 7.9 Hz, 1H, H-6), 6.39 (d, J = 7.9 Hz, 1H, H-5), 5.26 (t, J = 7.0 Hz, 1H, CH=), 5.24 (t, J = 7.1 Hz, 1H, CH=), 5.12–5.04 (m, 6H, 6 × CH=), 4.75 (d, J = 6.8 Hz, 2H, NCH2), 4.39 (d, J = 7.3 Hz, 2H, NCH2), 2.17–1.94 (m, 24H, 12 × CH2), 1.83 (s, 3H, CH3), 1.75 (s, 3H, CH3), 1.67 (s, 6H, 2 × CH3), 1.60 (s, 9H, 3 × CH3), 1.59 (s, 6H, 2 × CH3), 1.58 (s, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 172.8 (HC=O), 159.8 (C, C-4), 150.5 (C, C-2), 144.3 (C=), 141.3 (C=), 140.8 (C, C-6), 136.1 (C=), 135.4 (C=), 135.3 (C=), 135.1 (C=), 131.44 (C=), 131.40 (C=), 124.53 (CH=), 124.48 (CH=), 124.4 (CH=), 124.1 (CH=), 124.0 (CH=), 123.4 (CH=), 117.4 (CH=), 116.9 (CH=), 97.4 (C, C-5), 46.6 (NCH2), 42.0 (NCH2), 39.87 (2 × CH2), 39.86 (CH2), 39.84 (CH2), 39.82 (CH2), 39.7 (CH2), 26.91 (CH2), 26.90 (CH2), 26.8 (CH2), 26.7 (CH2), 26.6 (CH2), 26.3 (CH2), 25.9 (2 × CH3), 17.8 (2 × CH3), 16.8 (CH3), 16.7 (CH3), 16.24 (CH3), 16.18 (CH3), 16.17 (CH3), 16.16 (CH3); IR (film from CH2Cl2): νmax 2964, 2916, 2852, 1622, 1537, 1452 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C45H70N3O2 684.5463; Found 684.5487 (Δ = 3.5 ppm); HRESIMS/MS (20 eV) m/z (%): 684.2009 (2), 412.2985 (13), 140.0471 (100).
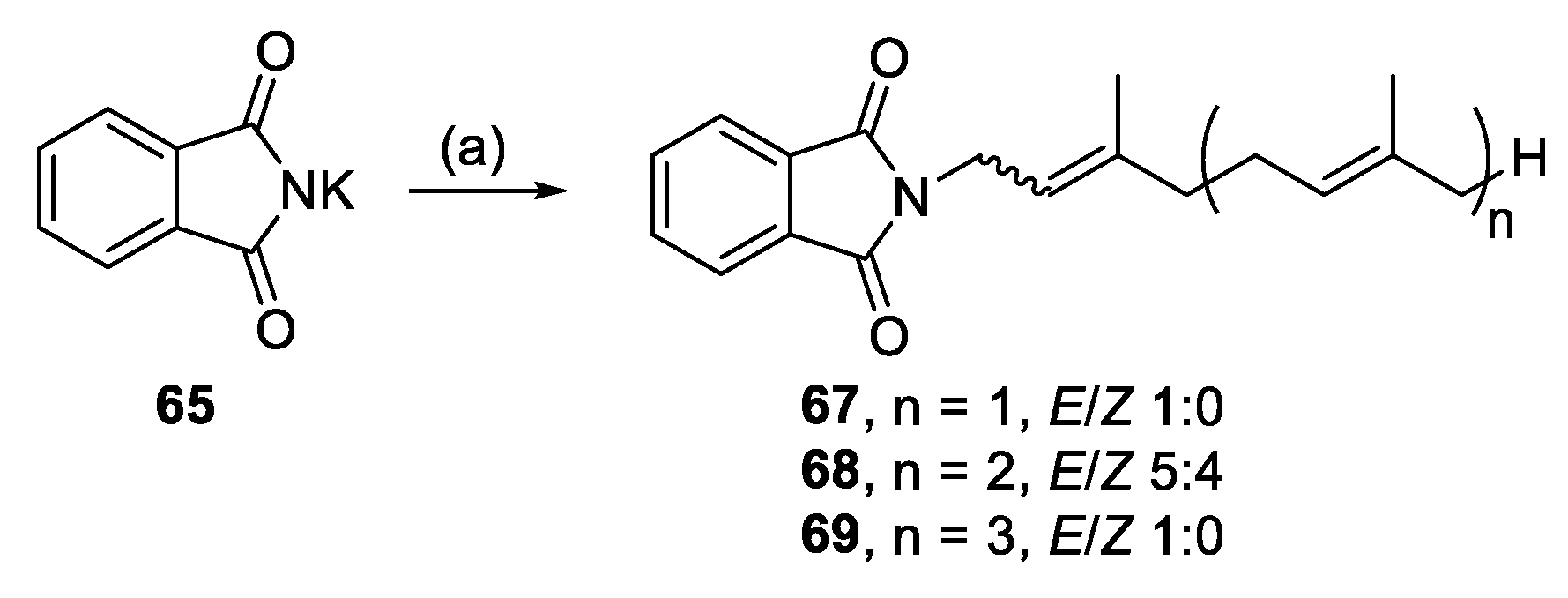
3.2.35. (E)-2-(3,7-Dimethylocta-2,6-dien-1-yl)isoindoline-1,3-dione (67)
Potassium phthalimide (2.6 mmol, 472.8 mg), K
2CO
3/Na
2CO
3 (1:1, 240 mg), and geranyl bromide (2.5 mmol, 543 mg) in DMF (10 mL) for 22 h yielded
67, additionally recrystallised from PE after chromatography, 170.2 mg (24%), white crystals. R
f = 0.59 (1:4 EA/PE);
1H and
13C NMR, and IR data previously reported [
56]; HRESIMS
m/
z: [M + H]
+ Calcd. for C
18H
22NO
2 284.1645; Found 284.1640 (Δ = −1.8 ppm); HRESIMS/MS (40 eV)
m/
z (%): 160.0385 (100), 133.0287 (47), 81.0697 (22).
3.2.36. 2-((6E)-3,7,11-Trimethyldodeca-2,6,10-trien-1-yl)isoindoline-1,3-dione (68)
Potassium phthalimide (2.9 mmol, 541.2 mg), K
2CO
3/Na
2CO
3 (1:1, 1.9 g) and farnesyl bromide (2.0 mmol, 570.5 mg) in DMF (10 mL) for 19 h yielded
68, 12.8 mg (2%), colourless oil. R
f = 0.23 (1:10 EA/PE); 5:4
E/
Z,
1H and
13C NMR data for the (2
E)-isomer previously reported [
57]; NMR data for (2
Z)-isomer:
1H NMR (500 MHz, CDCl
3): δ 7.84–7.81 (m, 2H, H-5), 7.71–7.68 (m, 2H, H-6), 5.30–5.24 (m, 1H, CH=), 5.11–5.01 (m, 2H, 2 × CH=), 4.27 (d,
J = 7.1 Hz, 2H, NCH
2), 2.30–2.24 (m, 2H, CH
2), 2.01–2.03 (m, 2H, CH
2), 2.03–1.97 (m, 2H, CH
2), 1.94–1.87 (m, 2H, CH
2), 1.82 (s, 3H, CH
3), 1.63 (s, 3H, CH
3), 1.56 (s, 6H, 2 × CH
3);
13C{
1H} NMR (150 MHz, CDCl
3): δ 168.3 (C, C-1), 140.8 (C=), 135.5 (C=), 133.9 (CH, C-6), 132.5 (C, C-4), 131.7 (C=), 124.6 (CH=), 124.4 (CH=), 123.3 (CH, C-5), 118.1 (CH=), 39.9 (CH
2), 32.1 (CH
2), 35.9 (NCH
2), 26.7 (CH
2), 26.2 (CH
2), 25.9 (CH
3), 23.5 (CH
3), 17.8 (CH
3), 16.1 (CH
3); IR (film from CH
2Cl
2): ν
max 2964, 2917, 2854, 1710 cm
−1; HRESIMS
m/
z: [M + H]
+ Calcd. for C
23H
30NO
2 352.2271; Found 352.2257 (Δ = −4.0 ppm); HRESIMS/MS (40 eV)
m/
z (%): 250.9685 (16), 160.0391(100).
3.2.37. 2-((2E,6E,10E)-3,7,11,15-Tetramethylhexadeca-2,6,10,14-tetraen-1-yl)isoindoline-1,3-dione (69)
Following the general alkylation procedure, also previously published [
58], potassium phthalimide (0.30 mmol, 54.8 mg), K
2CO
3 (1.1 mmol, 155.6 mg) and geranylgeranyl bromide (0.32 mmol, 111 mg) yielded
69, 42.6 mg (45%), colourless oil. R
f = 0.31 (1:9 EA/PE);
1H NMR (500 MHz, CDCl
3): δ 7.83 (dd,
J = 5.3, 3.0 Hz, 1H, H-5), 7.69 (dd,
J = 5.4, 3.0 Hz, 1H, H-6), 5.27 (t,
J = 7.2 Hz, 1H, CH=), 5.13–5.02 (m, 3H, 3 × CH=), 4.28 (d,
J = 7.1 Hz, 2H, NCH
2), 2.11–1.89 (m, 12H, 6 × CH
2), 1.83 (s, 3H, CH
3), 1.67 (s, 3H, CH
3), 1.59 (s, 3H, CH
3), 1.57 (s, 6H, 2 × CH
3);
13C{
1H} NMR (150 MHz, CDCl
3): δ 168.2 (C, C-1), 140.8 (C=), 135.4 (C=), 134.9 (C=), 133.9 (CH, C-6), 132.4 (C, C-4), 131.3 (C=), 124.5 (CH=), 124.3 (CH=), 123.7 (CH=), 123.2 (CH, C-5), 118.0 (CH=), 39.8 (CH
2), 39.7 (CH
2), 39.6 (CH
2), 35.9 (NCH
2), 26.8 (CH
2), 26.7 (CH
2), 26.3 (CH
2), 25.8 (CH
3), 17.8 (CH
3), 16.5 (CH
3), 16.10 (CH
3), 16.07 (CH
3); IR (film from CH
2Cl
2): ν
max 3057, 2930, 1710 cm
−1; HRESIMS
m/
z: [M + H]
+ Calcd. for C
28H
38NO
2 420.2897; Found 420.2889 (Δ = −1.9 ppm).
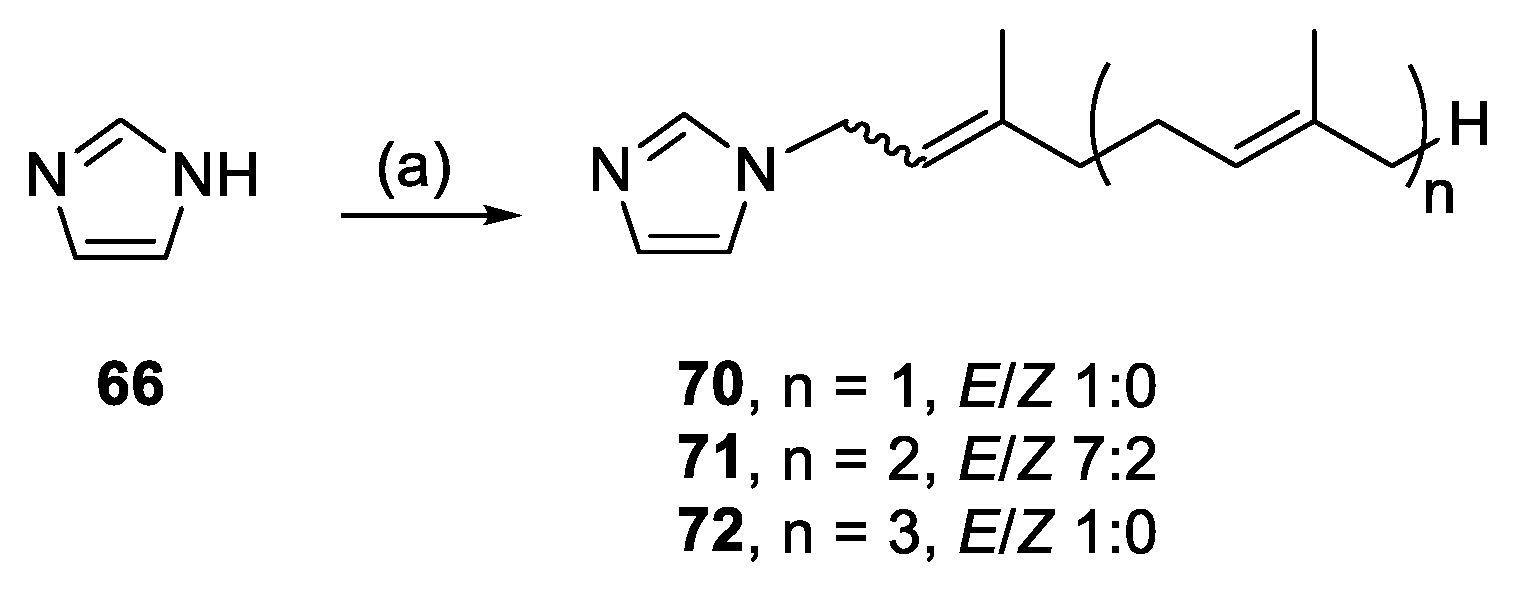
3.2.38. (E)-1-(3,7-Dimethylocta-2,6-dien-1-yl)-1H-imidazole (70)
Following the general alkylation procedure, also previously published [
59], imidazole (0.57 mmol, 38.7 mg), K
2CO
3 (0.66 mmol, 91.4 mg) and geranyl bromide (0.60 mmol, 130 mg) in DMF (2 mL) for 46 h yielded
70, 36.7 mg (32%), colourless oil. R
f = 0.20 (1:1 EA/PE);
1H NMR data reported previously [
60];
13C{
1H} NMR (120 MHz, CDCl
3): δ 141.7 (C=), 136.8 (CH, C-2), 132.2 (C=), 129.3 (CH, C-4), 123.6 (CH=), 118.7 (CH, C-5), 118.6 (CH=), 44.6 (NCH
2), 39.4 (CH
2), 26.2 (CH
2), 25.8 (CH
3), 17.8 (CH
3), 16.3 (CH
3); IR (film from CH
2Cl
2): ν
max 3110, 2966, 2916, 2855 cm
−1; HRESIMS
m/
z: [M + H]
+ Calcd. for C
13H
21N
2 205.1699; Found 205.1700 (Δ = 0.5 ppm); HRESIMS/MS (40 eV)
m/
z (%): 81.0716 (21), 79.0559 (18), 69.0470 (100).
3.2.39. 1-((6E)-3,7,11-Trimethyldodeca-2,6,10-trien-1-yl)-1H-imidazole (71)
Following the general alkylation procedure, also previously published [
59], imidazole (0.51 mmol, 34.6 mg), K
2CO
3 (0.65 mmol, 90.4 mg) and farnesyl bromide (0.60 mmol, 171 mg) in DMF (2 mL) for 48 h yielded
71, 11.4 mg (8%), colourless oil. R
f = 0.10 (1:1 EA/PE); 7:2
E/
Z, NMR data for major isomer:
1H NMR (600 MHz, CDCl
3): δ 7.46 (s, 1H, H-2), 7.04 (s, 1H, H-4), 6.88 (s, 1H, H-5), 5.38–5.33 (m, 1H, CH=), 5.12–5.05 (m, 2H, 2 × CH=), 4.52 (d,
J = 7.1 Hz, 2H, NCH
2), 2.19–1.94 (m, 8H, 4 × CH
2), 1.75 (s, 3H, CH
3), 1.67 (s, 3H, CH
3), 1.60 (s, 3H, CH
3), 1.59 (s, 3H, CH
3);
13C{
1H} NMR (150 MHz, CDCl
3): δ 141.9 (C=) 136.8 (CH, C-2), 135.9 (C=), 131.5 (C=), 129.2 (CH, C-4), 124.4 (CH=), 123.5 (CH=), 118.8 (CH, C-5), 118.6 (CH=), 44.7 (NCH
2), 39.8 (CH
2), 39.5 (CH
2), 26.8 (CH
2), 26.3 (CH
2), 25.8 (CH
3), 17.8 (CH
3), 16.5 (CH
3), 16.2 (CH
3); IR (film from CH
2Cl
2): ν
max 2965, 2917, 2856 cm
−1; HRESIMS
m/
z: [M + H]
+ Calcd. for C
18H
29N
2 273.2325; Found 273.2326 (Δ = 0.4 ppm); HRESIMS/MS (20 eV)
m/
z (%): 81.0700 (45), 69.0457 (100).
3.2.40. 1-((2E,6E,10E)-3,7,11,15-Tetramethylhexadeca-2,6,10,14-tetraen-1-yl)-1H-imidazole (72)
Imidazole (0.30 mmol, 20.4 mg), K2CO3 (0.34 mmol, 46.4 mg) and geranylgeranyl bromide (0.32 mmol, 111 mg) in DMF (1 mL) for 48 h yielded 72, 16.5 mg (20%), colourless oil. Rf = 0.18 (2:1 EA/PE); 1H NMR (500 MHz, CDCl3): δ 7.47 (s, 1H, H-2), 7.05 (s, 1H, H-4), 6.89 (s, 1H, H-5), 5.36 (t, J = 6.7 Hz, 1H, CH=), 5.12–5.05 (m, 3H, 3 × CH=), 4.52 (d, J = 7.1 Hz, 2H, NCH2), 2.17–2.01 (m, 8H, 4 × CH2), 2.01–1.92 (m, 4H, 2 × CH2), 1.75 (s, 3H, CH3), 1.67 (s, 3H, CH3), 1.59 (s, 9H, 3 × CH3); 13C{1H} NMR (150 MHz, CDCl3): δ 142.0 (C=), 136.8 (CH, C-2), 135.9 (C=), 135.1 (C=), 131.4 (C=), 129.1 (CH, C-4), 124.5 (CH=), 124.2 (CH=), 123.5 (CH=), 118.8 (CH, C-5), 118.5 (CH=), 44.7 (NCH2), 39.9 (CH2), 39.8 (CH2), 39.5 (CH2), 26.9 (CH2), 26.7 (CH2), 26.3 (CH2), 25.8 (CH3), 17.8 (CH3), 16.5 (CH3), 16.2 (CH3), 16.1 (CH3); IR (film from CH2Cl2): νmax 3118, 2966, 2925 cm−1; HRESIMS m/z: [M + H]+ Calcd. for C23H37N2 341.2951; Found 341.2934 (Δ = −5.0 ppm); HRESIMS/MS (40 eV) m/z (%): 121.1001 (95), 109.1006 (58), 107.0849 (100).