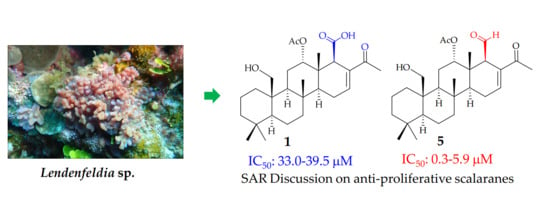
Probing Anti-Proliferative 24-Homoscalaranes from a Sponge Lendenfeldia sp.
Abstract
1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. MTT Cell Proliferative Assay
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fattorusso, E.; Magno, S.; Santacroce, C.; Sica, D. Scalarin, a new pentacyclic C-25 terpenoid from the sponge Cacospongia scalaris. Tetrahedron 1972, 28, 5993–5997. [Google Scholar] [CrossRef]
- Mo, S.; Krunic, A.; Pegan, S.D.; Franzblau, S.G.; Orjala, J. An antimicrobial guanidine-bearing sesterterpene from the cultured cyanobacterium Scytonema sp. J. Nat. Prod. 2009, 72, 2043–2045. [Google Scholar] [CrossRef] [PubMed]
- González, M.A. Scalarane sesterterpenoids. Curr. Bioact. Compd. 2010, 6, 178–206. [Google Scholar] [CrossRef]
- Marshall, L.A.; Winkler, J.D.; Griswold, D.E.; Bolognese, B.; Roshak, A.; Sung, C.-M.; Webb, E.F.; Jacobs, R. Effects of scalaradial, a type II phospholipase A2 inhibitor, on human neutrophil arachidonic acid mobilization and lipid mediator formation. J. Pharmacol. Exp. Ther. 1994, 268, 709–717. [Google Scholar]
- Fu, X.; Zeng, L.M.; Su, J.Y.; Pais, M.; Potier, P. Scalarane-type bishomosesterterpenes from the sponge Phyllospongia foliascens. J. Nat. Prod. 1992, 55, 1607–1613. [Google Scholar] [CrossRef]
- Jiménez, J.I.; Yoshida, W.Y.; Scheuer, P.J.; Lobkovsky, E.; Clardy, J.; Kelly, M. Honulactones: new bishomoscalarane sesterterpenes from the Indonesian sponge Strepsichordaia aliena. J. Org. Chem. 2000, 65, 6837–6840. [Google Scholar] [CrossRef]
- Chill, L.; Rudi, A.; Aknin, M.; Loya, S.; Hizi, A.; Kashman, Y. New sesterterpenes from Madagascan Lendenfeldia sponges. Tetrahedron 2004, 60, 10619–10626. [Google Scholar] [CrossRef]
- Cimino, G.; De Rosa, S.; De Stefano, S.; Sodano, G. The chemical defense of four Mediterranean nudibranchs. Comp. Biochem. Physiol. 1982, 73, 471–474. [Google Scholar] [CrossRef]
- Rogers, S.D.; Paul, V.J. Chemical defenses of three Glossodoris nudibranchs and their dietary Hyrtios sponges. Mar. Ecol. Prog. Ser. 1991, 77, 221–232. [Google Scholar] [CrossRef]
- Avila, C.; Paul, V.J. Chemical ecology of the nudibranch Glossodoris pallida: Is the location of diet-derived metabolites important for defense? Mar. Ecol. Prog. Ser. 1997, 150, 171–180. [Google Scholar] [CrossRef]
- Kamel, H.N.; Kim, Y.B.; Rimoldi, J.M.; Fronczek, F.R.; Ferreira, D.; Slattery, M. Scalarane sesterterpenoids: Semisynthesis and biological activity. J. Nat. Prod. 2009, 72, 1492–1496. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Cambie, R.C.; Kernan, M.R. Scalarane sesterterpenes from Collospongia auris, a new thorectid sponge. Biochem. Syst. Ecol. 1990, 18, 349–357. [Google Scholar] [CrossRef]
- Gavagnin, M.; Mollo, E.; Docimo, T.; Guo, Y.-W.; Cimino, G. Scalarane metabolites of the nudibranch Glossodoris rufomarginata and its dietary sponge from the South China Sea. J. Nat. Prod. 2004, 67, 2104–2107. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Shaala, L.A.; Emara, S. Antimycobacterial scalarane-based sesterterpenes from the Red Sea sponge Hyrtios erecta. J. Nat. Prod. 2005, 68, 1782–1784. [Google Scholar] [CrossRef]
- Chang, L.C.; Otero-Quintero, S.; Nicholas, G.M.; Bewley, C.A. Phyllolactones A–E: New bishomoscalarane sesterterpenes from the marine sponge Phyllospongia lamellosa. Tetrahedron 2001, 57, 5731–5738. [Google Scholar] [CrossRef]
- Nam, S.-J.; Ko, H.; Shin, M.; Ham, J.; Chin, J.; Kim, Y.; Kim, H.; Shin, K.; Choi, H.; Kang, H. Farnesoid X- activated receptor antagonists from a marine sponge Spongia sp. Bioorg. Med. Chem. Lett. 2006, 16, 5398–5402. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Wells, R.J. Five new C26 tetracyclic terpenes from a sponge (Lendenfeldia sp.). Aust. J. Chem. 1982, 35, 51–59. [Google Scholar] [CrossRef]
- Lai, Y.-Y.; Chen, L.-C.; Wu, C.-F.; Lu, M.-C.; Wen, Z.-H.; Wu, T.-Y.; Fang, L.-S.; Wang, L.-H.; Wu, Y.-C.; Sung, P.-J. New cytotoxic 24-homoscalarane sesterterpenoids from the sponge Ircinia felix. Int. J. Mol. Sci. 2015, 16, 21950–21958. [Google Scholar] [CrossRef]
- Hochlowski, J.E.; Faulkner, D.J.; Bass, L.S.; Clardy, J. Metabolites of the dorid nudibranch Chromodoris sedan. J. Org. Chem. 1983, 48, 1738–1740. [Google Scholar] [CrossRef]
- Harinantenaina, L.; Brodie, P.J.; Maharavo, J.; Bakary, G.; TenDyke, K.; Shen, Y.; Kingston, D.G.I. Antiproliferative homoscalarane sesterterpenes from two Madagascan sponges. Bioorg. Med. Chem. 2013, 21, 2912–2917. [Google Scholar] [CrossRef]
- Bergquist, P.R. A revision of the supraspecific classification of the orders Dictyoceratida, Dendroceratida, and Verongida (class Demospongiae). N. Z. J. Zool. 1980, 7, 443–503. [Google Scholar] [CrossRef]

 ) and heteronuclear multiple bond correlation (HMBC) (
) and heteronuclear multiple bond correlation (HMBC) ( ) of 1–4.
) of 1–4.
 ) and heteronuclear multiple bond correlation (HMBC) (
) and heteronuclear multiple bond correlation (HMBC) ( ) of 1–4.
) of 1–4.
 ) of 1–4.
) of 1–4.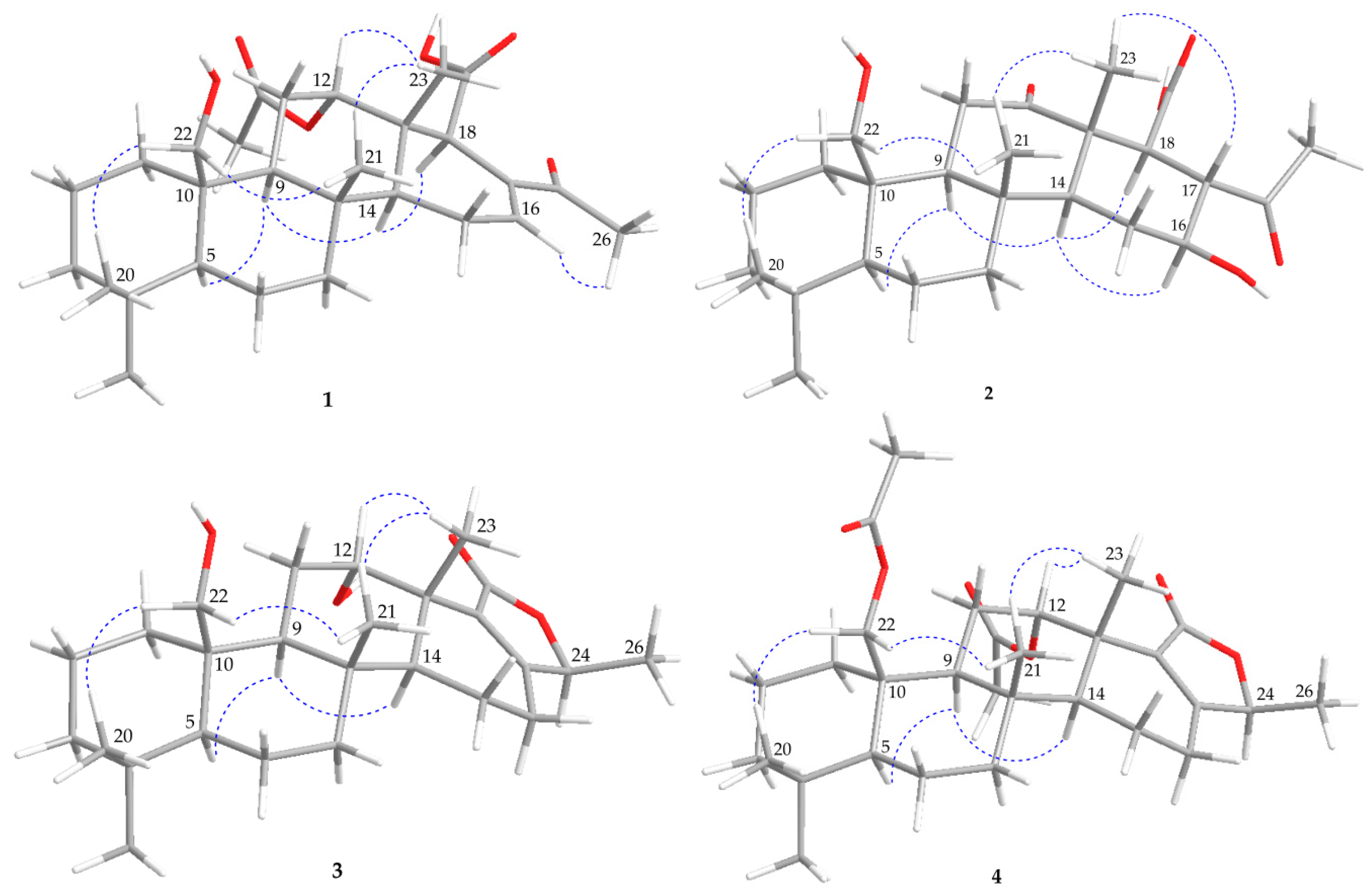
| C/H | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) a | δC Multiple b | δH (J in Hz) c | δC Multiple d | |
| 1 | 2.12 m; 0.48 ddd (12.8, 12.8, 3.2) | 34.4, CH2 | 2.13 m; 0.80 m | 33.8, CH2 |
| 2 | 1.50 m | 17.8, CH2 | 1.44 m; 1.65 m | 17.9, CH2 |
| 3 | 1.19 m; 1.43 m | 41.6, CH2 | 1.18 m; 1.43 m | 41.4, CH2 |
| 4 | 33.0, C | 33.0, C | ||
| 5 | 0.94 m | 56.8, CH | 0.95 br d (12.6) | 56.8, CH |
| 6 | 1.43 m | 18.3, CH2 | 1.53 m | 18.2, CH2 |
| 7 | 1.03 m; 1.82 ddd (12.8, 3.2, 3.2) | 42.2, CH2 | 0.96 m; 1.91 ddd (13.2, 3.6, 3.6) | 42.1, CH2 |
| 8 | 37.8, C | 38.7, C | ||
| 9 | 1.50 m | 49.3, CH | 1.26 m | 62.8, CH |
| 10 | 41.7, C | 42.8, C | ||
| 11 | 2.07 m | 24.9, CH2 | 2.62 dd (12.6, 1.8); 3.34 dd (14.4, 12.6) | 39.0, CH2 |
| 12 | 4.77 br s | 75.7, CH | 221.9, C | |
| 13 | 38.9, C | 52.8, C | ||
| 14 | 1.31 m | 52.2, CH | 1.21 m | 58.0, CH |
| 15 | 2.27 m | 23.3, CH2 | 1.65 m; 1.94 ddd (12.6, 4.2, 1.8) | 30.1, CH2 |
| 16 | 6.90 br s | 139.7, CH | 3.53 ddd (10.8, 10.8, 4.8) | 72.7, CH |
| 17 | 137.9, C | 3.22 dd (12.0, 10.8) | 54.8, CH | |
| 18 | 3.79 br s | 48.0, CH | 3.18 d (12.0) | 51.3, CH |
| 19 | 0.75 s | 21.9, CH3 | 0.76 s | 21.7, CH3 |
| 20 | 0.86 s | 33.8, CH3 | 0.87 s | 33.7, CH3 |
| 21 | 1.16 s | 16.1, CH3 | 1.30 s | 16.4, CH3 |
| 22 | 3.85 d (11.6); 4.02 d (11.6) | 62.9, CH2 | 3.93 dd (11.4, 1.2); 4.08 d (11.4) | 62.7, CH2 |
| 23 | 0.96 s | 15.5, CH3 | 1.34 s | 15.3, CH3 |
| 24 | 199.3, C | 212.6, C | ||
| 25 | 175.1, C | 172.4, C | ||
| 26 | 2.29 s | 25.3, CH3 | 2.40 s | 33.4, CH |
| OAc-12 | 170.4, C | |||
| 2.14 s | 21.5, CH3 | |||
| C/H | 3 | 4 | ||
|---|---|---|---|---|
| δH (J in Hz) a | δC Multiple b | δH (J in Hz) c | δC Multiple d | |
| 1 | 2.16 m; 0.80 m | 34.0, CH2 | 2.01 m; 0.52 ddd (13.8, 13.8, 3.0) | 34.7, CH2 |
| 2 | 1.63 m | 18.4, CH2 | 1.63 m | 18.2, CH2 |
| 3 | 1.18 m; 1.43 m | 41.7, CH2 | 1.15 ddd (9.0, 9.0, 4.2); 1.43 m | 41.5, CH2 |
| 4 | 33.0, C | 33.0, C | ||
| 5 | 1.05 m | 56.8, CH | 1.01 dd (13.8, 3.6) | 57.1, CH |
| 6 | 1.56 m; 1.91 m | 16.9, CH2 | 1.56 m; 1.93 m | 17.0, CH2 |
| 7 | 1.10 m; 1.89 m | 42.0, CH2 | 1.11 ddd (12.0, 12.0, 4.2); 1.92 m | 41.9, CH2 |
| 8 | 37.6, C | 37.5, C | ||
| 9 | 1.56 m | 52.3, CH | 1.26 m | 53.2, CH |
| 10 | 41.8, C | 40.2, C | ||
| 11 | 1.89 m; 2.18 m | 27.1, CH2 | 1.99 m; 2.20 m | 23.3, CH2 |
| 12 | 4.60 br s | 69.9, CH | 5.54 t (3.0) | 73.8, CH |
| 13 | 40.2, C | 38.4, C | ||
| 14 | 1.60 m | 50.0, CH | 1.55 m | 51.2, CH |
| 15 | 2.18 m; 2.35 m | 24.1, CH2 | 2.23 m; 2.39 m | 24.0, CH2 |
| 16 | 1.46 m; 1.56 m | 18.0, CH2 | 1.60 m | 18.0, CH2 |
| 17 | 165.2, C | 163.6, C | ||
| 18 | 133.5, C | 132.6, C | ||
| 19 | 0.78 s | 21.8, CH3 | 0.83 s | 21.9, CH3 |
| 20 | 0.86 s | 33.9, CH3 | 0.89 s | 33.7, CH3 |
| 21 | 1.08 s | 16.3, CH3 | 0.98 s | 16.4, CH3 |
| 22 | 3.92 d (11.5); 4.05 d (11.5) | 63.0, CH2 | 4.15 dd (12.0, 1.2); 4.58 d (12.0) | 64.7, CH2 |
| 23 | 1.13 s | 21.7, CH3 | 1.19 s | 21.3, CH3 |
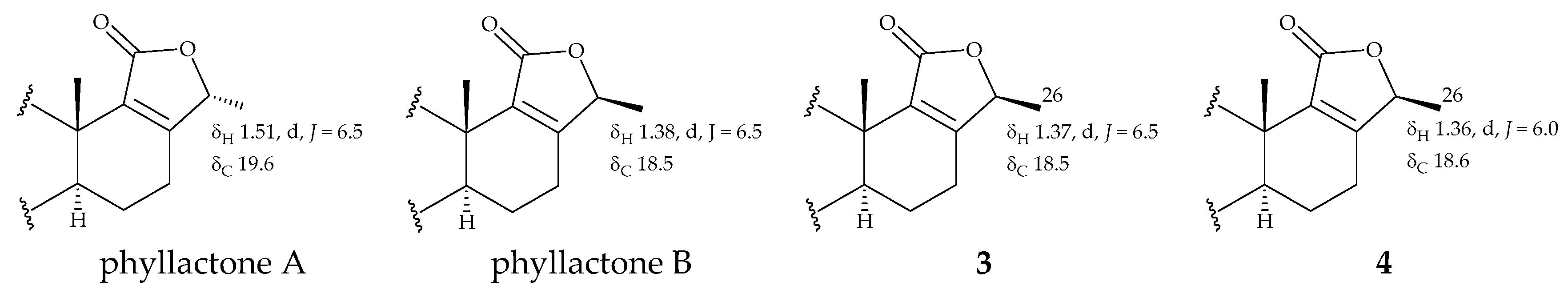
| 24 | 4.79 q (6.5) | 78.2, CH | 4.78 q (6.0) | 77.7, CH |
| 25 | 172.6, C | 171.1, C | ||
| 26 | 1.37 d (6.5) | 18.5, CH3 | 1.36 d (6.0) | 18.6, CH3 |
| OAc-12 | 169.9, C | |||
| 1.97 s | 21.2, CH3 | |||
| OAc-22 | 170.9, C | |||
| 2.07 s | 21.2, CH3 | |||
| Compound | Cell lines IC50 (μM) | |||
|---|---|---|---|---|
| MOLT-4 | K-562 | U-937 | SUP-T1 | |
| 1 | 39.54 | NA | NA | 33.02 |
| 2 | 34.93 | NA | NA | NA |
| 3 | 6.31 | 11.69 | 5.74 | 9.00 |
| 4 | 29.83 | NA | NA | NA |
| 5 | 0.31 | 3.04 | 2.35 | 5.90 |
| 6 | 5.67 | 9.71 | 6.97 | 12.33 |
| 7 | 1.49 | 1.04 | 5.88 | 7.49 |
| Doxorubicin a | 0.02 | 0.13 | 0.04 | 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, B.-R.; Lai, K.-H.; Chen, Y.-Y.; Su, J.-H.; Huang, Y.M.; Chen, Y.-H.; Lu, M.-C.; Yu, S.S.-F.; Duh, C.-Y.; Sung, P.-J. Probing Anti-Proliferative 24-Homoscalaranes from a Sponge Lendenfeldia sp. Mar. Drugs 2020, 18, 76. https://doi.org/10.3390/md18020076
Peng B-R, Lai K-H, Chen Y-Y, Su J-H, Huang YM, Chen Y-H, Lu M-C, Yu SS-F, Duh C-Y, Sung P-J. Probing Anti-Proliferative 24-Homoscalaranes from a Sponge Lendenfeldia sp. Marine Drugs. 2020; 18(2):76. https://doi.org/10.3390/md18020076
Chicago/Turabian StylePeng, Bo-Rong, Kuei-Hung Lai, You-Ying Chen, Jui-Hsin Su, Yusheng M. Huang, Yu-Hsin Chen, Mei-Chin Lu, Steve Sheng-Fa Yu, Chang-Yih Duh, and Ping-Jyun Sung. 2020. "Probing Anti-Proliferative 24-Homoscalaranes from a Sponge Lendenfeldia sp." Marine Drugs 18, no. 2: 76. https://doi.org/10.3390/md18020076
APA StylePeng, B.-R., Lai, K.-H., Chen, Y.-Y., Su, J.-H., Huang, Y. M., Chen, Y.-H., Lu, M.-C., Yu, S. S.-F., Duh, C.-Y., & Sung, P.-J. (2020). Probing Anti-Proliferative 24-Homoscalaranes from a Sponge Lendenfeldia sp. Marine Drugs, 18(2), 76. https://doi.org/10.3390/md18020076