Bioactive Metabolites of Marine Origin Have Unusual Effects on Model Membrane Systems
Abstract
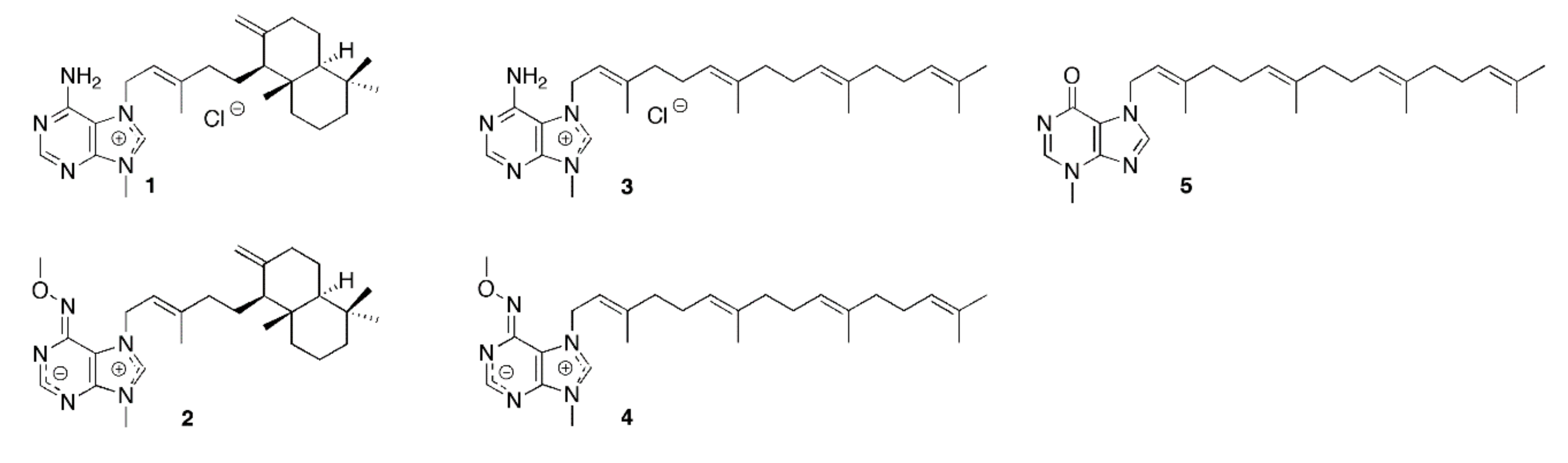
1. Introduction
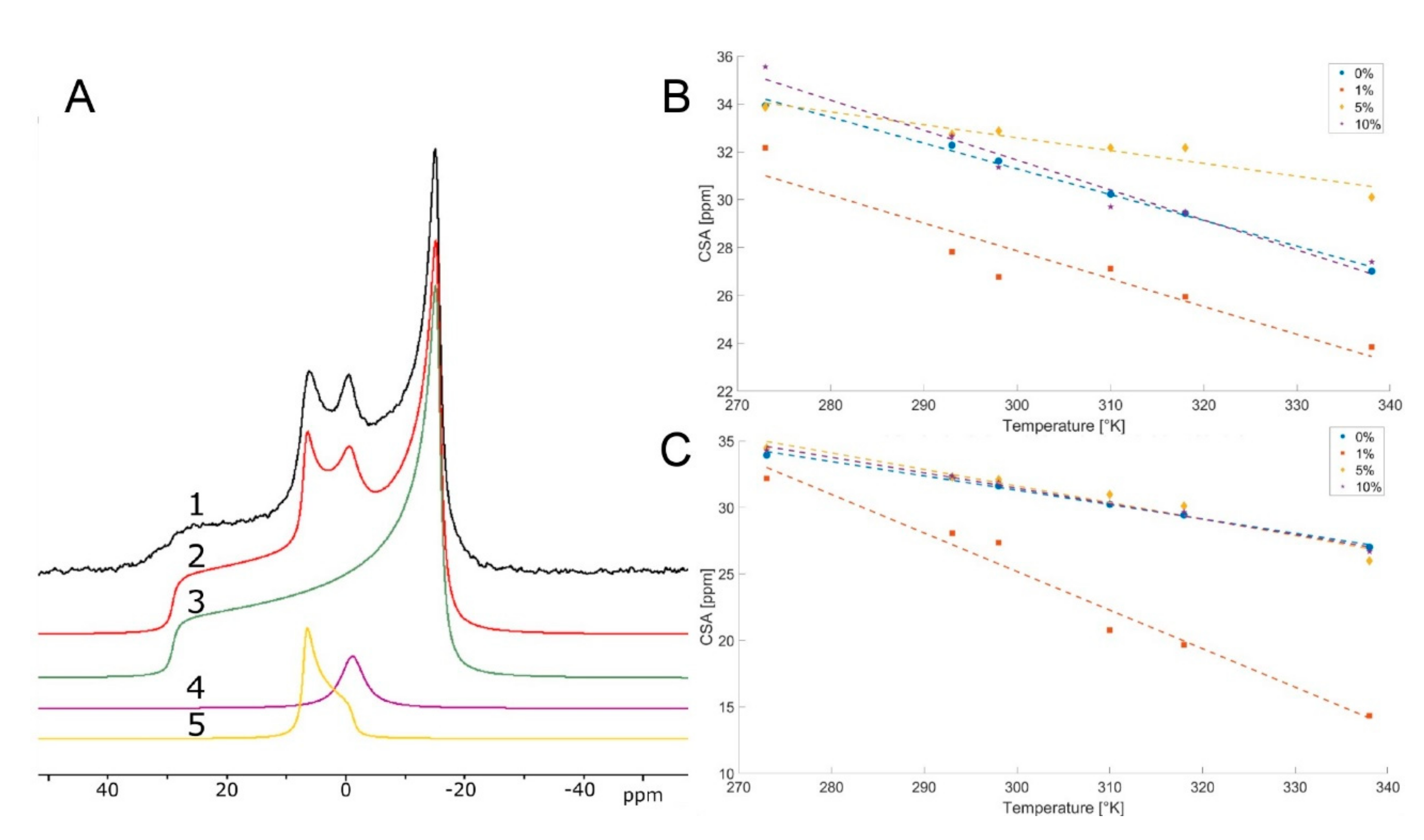
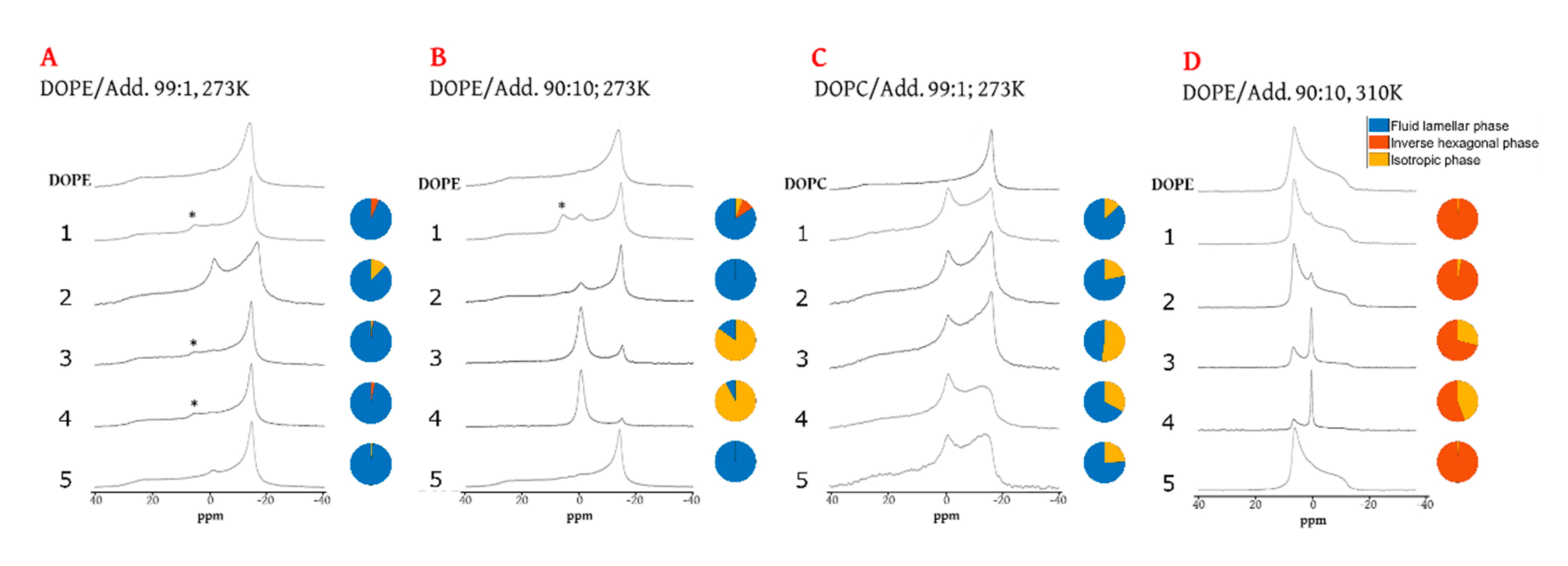
2. Results
3. Discussion
4. Material and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chellat, M.F.; Raguž, L.; Riedl, R. Targeting Antibiotic Resistance. Angew. Chem.-Int. Ed. 2016, 55, 6600–6626. [Google Scholar] [CrossRef]
- Zahreddine, H.; Borden, K.L.B. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Vila, J.; Moreno-Morales, J. Ballesté-Delpierre, C. Current landscape in the discovery of novel antibacterial agents. Clin. Microbiol. Infect. 2019. [Google Scholar] [CrossRef]
- Kållberg, C.; Salvesen Blix, H.; Laxminarayan, R. Challenges in Antibiotic R&D Calling for a Global Strategy Considering Both Short- And Long-Term Solutions. ACS Infect. Dis. 2019, 5, 1265–1268. [Google Scholar] [CrossRef]
- Van Blitterswijk, W.J.; Verheij, M. Anticancer mechanisms and clinical application of alkylphospholipids. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2013, 1831, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2009, 26, 170–244. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2009, 8, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; De Cicco, P.; Ianaro, A. New drugs from the sea: Pro-apoptotic activity of sponges and algae derived compounds. Mar. Drugs 2019, 17, 31. [Google Scholar] [CrossRef]
- Shinde, P.; Banerjee, A.; Mandhare, P. Marine natural products as source of new drugs: A patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 283–309. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef]
- Gundersen, L.-L. Synthesis and biological activities of marine terpene-adenine hybrids and synthetic analogs. Phytochem. Rev. 2013, 12, 467–486. [Google Scholar] [CrossRef]
- Sun, Z.H.; Cai, Y.H.; Fan, C.Q.; Tang, G.H.; Bin Luo, H.; Yin, S. Six new tetraprenylated alkaloids from the South China Sea gorgonian Echinogorgia pseudossapo. Mar. Drugs 2014, 12, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dong, J.; Chen, M.; Wang, K.; Tenney, H.; Crews, P. Bioactive secondary metabolites from the marine sponge genus Agelas. Mar. Drugs 2017, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- García, P.A.; Valles, D.; Díez, E.; Castro, M.Á. Marine Alkylpurines: A promising group of bioactive marine natural products. Mar. Drugs 2018, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Proszenyák, Á.; Vermeersch, M.; Cos, P.; Maes, L.; Gundersen, L.L. Screening of agelasine D and analogs for inhibitory activity against pathogenic protozoa; identification of hits for visceral leishmaniasis and Chagas disease. Molecules 2009, 14, 279–288. [Google Scholar] [CrossRef]
- Vik, A.; Hedner, C.; Charnock, E.; Tangen, L.W.; Samuelsen, R.; Larsson, Ø.; Bohlin, L.; Gundersen, L.L. Antimicrobial and cytotoxic activity of agelasine and agelasimine analogs. Bioorganic Med. Chem. 2007, 15, 4016–4037. [Google Scholar] [CrossRef]
- Vik, A.; Hedner, C.; Charnock, E.; Samuelsen, R.; Larsson, Ø.; Gundersen, L.L.; Bohlin, L. (+)-Agelasine D: Improved synthesis and evaluation of antibacterial and cytotoxic activities. J. Nat. Prod. 2006, 69, 381–386. [Google Scholar] [CrossRef]
- Roggen, H.; Charnock, R.; Burman, C.; Felth, R.; Larsson, J.; Bohlin, L.; Gundersen, L.L. Antimicrobial and antineoplastic activities of agelasine analogs modified in the purine 2-position. Arch. Pharm. 2011, 344, 50–55. [Google Scholar] [CrossRef]
- Proszenyák, A.; Charnock, C.; Hedner, E.; Larsson, R.; Bohlin, L.; Gundersen, L.L. Synthesis, antimicrobial and antineoplastic activities for agelasine and agelasimine analogs with a β-cyclocitral derived substituent. Arch. Pharm. 2007, 340, 625–634. [Google Scholar] [CrossRef]
- Bakkestuen, A.K.; Gundersen, L.L.; Petersen, D.; Utenova, B.T.; Vik, A. Synthesis and antimycobacterial activity of agelasine E. and analogs. Org. Biomol. Chem. 2005, 3, 1025–1033. [Google Scholar] [CrossRef]
- Chamgordani, E.J.; Paulsen, J.; Gundersen, L.L. Selective N-7 alkylation of 3-methylhypoxanthine; the first synthesis of malonganenone J. Tetrahedron Lett. 2016, 57, 4926–4929. [Google Scholar] [CrossRef]
- Arai, M.; Yamano, Y.; Setiawan, A.; Kobayashi, M. Identification of the target protein of agelasine D, a marine sponge diterpene alkaloid, as an anti-dormant mycobacterial substance. ChemBioChem 2014, 15, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Wu, H.; Ohizumi, Y.; Hirata, Y. Agelasine-A, -B, -C and -D, novel bicyclic diterpenoids with a 9-methyladeninium unit possessing inhibitory effects on na,K-atpase from the okinawa sea sponge Agelas sp.1). Tetrahedron Lett. 1984, 25, 2989–2992. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nakamura, H.; Wu, H.; Kobayashi, J.; Ohizumi, Y. Mode of inhibition of brain Na+,K+-ATPase by agelasidines and agelasines from a sea sponge. Arch. Biochem. Biophys. 1987, 259, 179–184. [Google Scholar] [CrossRef]
- Fischer, W.; Urban, N.; Immig, K.; Franke, H.; Schaefer, M. Natural compounds with P2X7 receptor-modulating properties. Purinergic Signal. 2014, 10, 313–326. [Google Scholar] [CrossRef]
- Pimentel, A.A.; Felibertt, P.; Sojo, F.; Colman, L.; Mayora, A.; Silva, M.L.; Rojas, H.; Dipolo, R.; Suarez, A.I.; Compagnone, R.S.; et al. The marine sponge toxin agelasine B increases the intracellular Ca2+ concentration and induces apoptosis in human breast cancer cells (MCF-7). Cancer Chemother. Pharmacol. 2012, 69, 71–83. [Google Scholar] [CrossRef]
- Zhang, J.R.; Li, P.L.; Tang, X.L.; Qi, X.; Li, G.Q. Cytotoxic tetraprenylated alkaloids from the South China sea gorgonian Euplexaura robusta. Chem. Biodivers. 2012, 9, 2218–2224. [Google Scholar] [CrossRef]
- Cockburn, I.L.; Pesce, E.R.; Pryzborski, J.M.; Davies-Coleman, M.T.; Clark, P.G.K.; Keyzers, R.A.; Stephens, L.L.; Blatch, G.L. Screening for small molecule modulators of Hsp70 chaperone activity using protein aggregation suppression assays: Inhibition of the plasmodial chaperone PfHsp70-1. Biol. Chem. 2011, 2, 431–438. [Google Scholar] [CrossRef]
- Furse, S.; Brooks, N.J.; Woscholski, R.; Gaffney, P.R.J.; Templer, R.H. Pressure-dependent inverse bicontinuous cubic phase formation in a phosphatidylinositol 4-phosphate/phosphatidylcholine system. Chem. Data Collect. 2016, 4, 15–20. [Google Scholar] [CrossRef]
- Escribá, P.V. Membrane-lipid therapy: A historical perspective of membrane-targeted therapies-From lipid bilayer structure to the pathophysiological regulation of cells. Biochim. Biophys. Acta-Biomembr. 2017, 1859, 1493–1506. [Google Scholar] [CrossRef]
- Bakrač, B.; Gutiérrez-Aguirre, I.; Podlesek, Z.; Sonnen, A.F.P.; Gilbert, R.J.C.; Maček, P.; Lakey, J.H.; Anderluh, G. Molecular determinants of sphingomyelin specificity of a eukaryotic pore-forming toxin. J. Biol. Chem. 2008, 283, 18665–18677. [Google Scholar] [CrossRef] [PubMed]
- Tejuca, M.; Dalla Serra, M.; Ferreras, M.; Lanio, M.E.; Menestrina, G. Mechanism of membrane permeabilization by sticholysin I, a cytolysin isolated from the venom of the sea anemone Stichodactyla helianthus. Biochemistry 1996, 35, 14947–14957. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, C.A.; Dalla Serra, M.; Potrich, C.; Bernhart, I.; Tejuca, M.; Martinez, D.; Pazos, F.; Lanio, M.E.; Menestrina, G. Effects of Lipid Composition on Membrane Permeabilization by Sticholysin I and II, Two Cytolysins of the Sea Anemone Stichodactyla helianthus. Biophys. J. 2001, 80, 2761–2774. [Google Scholar] [CrossRef]
- Wen, H.; Glomm, W.R.; Halskau, Ø. Cytotoxicity of bovine α-lactalbumin: Oleic acid complexes correlates with the disruption of lipid membranes. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 2691–2699. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nielsen, S.B.; Wilhelm, K.; Vad, B.; Schleucher, J.; Morozova-Roche, L.A.; Otzen, D. The interaction of equine lysozyme: Oleic acid complexes with lipid membranes suggests a cargo off-loading mechanism. J. Mol. Biol. 2010, 8, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Mulet, X.; Templer, R.H.; Woscholski, R.; Ces, O. Evidence that phosphatidylinositol promotes curved membrane interfaces. Langmuir 2008, 24, 8443–8447. [Google Scholar] [CrossRef] [PubMed]
- Furse, S.; Jakubec, M.; Rise, F.; Williams, H.E.; Rees, C.E.D.; Halskau, O. Evidence that Listeria innocua modulates its membrane’s stored curvature elastic stress, but not fluidity, through the cell cycle. Sci. Rep. 2017, 7, 8012. [Google Scholar] [CrossRef]
- Furse, S.; Brooks, N.J.; Seddon, A.M.; Woscholski, R.; Templer, R.H.; Tate, E.W.; Gaffney, P.R.J.; Ces, O. Lipid membrane curvature induced by distearoyl phosphatidylinositol 4-phosphate. Soft Matter 2012, 8, 3090–3093. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the phosphatidylcholines. Biochim. Biophys. Acta-Rev. Biomembr. 1998, 1376, 91–145. [Google Scholar] [CrossRef]
- Koynova, R.; Caffrey, M. Phases and phase transitions of the hydrated phosphatidylethanolamines. Chem. Phys. Lipids 1994, 69, 1–34. [Google Scholar] [CrossRef]
- Totland, C.; Nerdal, W.; Steinkopf, S. Effects and Location of Coplanar and Noncoplanar PCB in a Lipid Bilayer: A Solid-State NMR Study. Environ. Sci. Technol. 2016, 50, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Dave, P.C.; Tiburu, E.K.; Damodaran, K.; Lorigan, G.A. Investigating Structural Changes in the Lipid Bilayer upon Insertion of the Transmembrane Domain of the Membrane-Bound Protein Phospholamban Utilizing 31P and 2H Solid-State NMR Spectroscopy. Biophys. J. 2004, 86, 1564–1573. [Google Scholar] [CrossRef][Green Version]
- Sampaio, J.L.; Gerl, M.J.; Klose, C.; Ejsing, C.S.; Beug, H.; Simons, K.; Shevchenko, A. Membrane lipidome of an epithelial cell line. Proc. Natl. Acad. Sci. USA 2011, 108, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef]
- Furse, S.; Liddell, S.; Ortori, C.A.; Williams, H.; Neylon, D.C.; Scott, D.J.; Barrett, D.A.; Gray, D.A. The lipidome and proteome of oil bodies from Helianthus annuus (common sunflower). J. Chem. Biol. 2013, 6, 63–76. [Google Scholar] [CrossRef]
- Ejsing, C.S.; Sampaio, J.L.; Surendranath, V.; Duchoslav, E.; Ekroos, K.; Klemm, R.W.; Simons, K.; Shevchenko, A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA 2009, 106, 2136–2141. [Google Scholar] [CrossRef]
- Furse, S.; Wienk, H.; Boelens, R.; De Kroon, A.I.P.M.; Killian, J.A. E. coli MG1655 modulates its phospholipid composition through the cell cycle. FEBS Lett. 2015, 589, 2726–2730. [Google Scholar] [CrossRef]
- Hui, S.W.; Stewart, T.P.; Yeagle, P.L.; Albert, A.D. Bilayer to non-bilayer transition in mixtures of phosphatidylethanolamine and phosphatidylcholine: Implications for membrane properties. Arch. Biochem. Biophys. 1981, 207, 227–240. [Google Scholar] [CrossRef]
- Chapman, D.; Owens, N.F.; Walker, D.A. Physical studies of phospholipids II. Monolayer studies of some synthetic 2,3-diacyl-dl-phosphatidylethanolamines and phosphatidylcholines containing trans double bonds. BBA-Biophys. Incl. Photosynth. 1966, 120, 148–155. [Google Scholar] [CrossRef]
- Setiawan, I.; Blanchard, G.J. Ethanol-induced perturbations to planar lipid bilayer structures. J. Phys. Chem. B 2014, 118, 537–546. [Google Scholar] [CrossRef]
- Komatsu, H.; Okada, S. Ethanol-enhanced permeation of phosphatidylcholine/phosphatidylethanolamine mixed liposomal membranes due to ethanol-induced lateral phase separation. Biochim. Biophys. Acta-Biomembr. 1996, 1283, 73–79. [Google Scholar] [CrossRef]
- Dubovskii, P.V.; Lesovoy, D.M.; Dubinnyi, M.A.; Konshina, A.G.; Utkin, Y.N.; Efremov, R.G.; Arseniev, A.S. Interaction of three-finger toxins with phospholipid membranes: Comparison of S- and P-type cytotoxins. Biochem. J. 2005, 387, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.P.; Mcintyre, S.K.; Alam, T.M. Distinguishing Individual Lipid Headgroup Mobility and Phase Transitions in Raft-Forming Lipid Mixtures with 31 P. MAS NMR. Biophys. J. 2006, 90, 4248–4260. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, C.R.; Thiel, S.; Otzen, D.E. Protein-fatty acid complexes: Biochemistry, biophysics and function. FEBS J. 2013, 280, 1733–1749. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Strømland, Ø.; Halskau, Ø.; Stromland, O.; Halskau, O. Alpha-Lactalbumin:Oleic Acid Complex Spontaneously Delivers Oleic Acid to Artificial and Erythrocyte Membranes. J. Mol. Biol. 2015, 427, 3177–3187. [Google Scholar] [CrossRef] [PubMed]
- Chehimi, S.; Pons, A.-M.; Sablé, S.; Hajlaoui, M.-R.; Limam, F. Mode of action of thuricin S, a new class IId bacteriocin from Bacillus thuringiensis. Can. J. Microbiol. 2010, 56, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of “bug and drug”. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, H.; Hong, M. Distinguishing bicontinuous lipid cubic phases from isotropic membrane morphologies using 31P solid-state NMR spectroscopy. J. Phys. Chem. B. 2015, 119, 4993–5001. [Google Scholar] [CrossRef]
- Paulsen, B.; Fredriksen, K.A.; Petersen, D.; Maes, L.; Matheeussen, A.; Naemi, A.O.; Scheie, A.A.; Simm, R.; Ma, R.; Wan, B.; et al. Synthesis and antimicrobial activities of N 6 -hydroxyagelasine analogs and revision of the structure of ageloximes. Bioorganic Med. Chem. 2019, 27, 620–629. [Google Scholar] [CrossRef]
| Compound | Bacteria MIC (µg/mL) | Fungi MIC (µg/mL) | Cancer Cells IC50 (µg/mL) | Protozoa IC50 (µg/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Streptococcus pyogenes | Enterococcus faecalis | Escherichia coli | Pseudomonas aeruginosa | Bacteroides fragilis | Bacteroides thetaiotaomicron | Mycobacterium tuberculosis | Candida krusei | U-937GTB | RPMI8226/s | CEM7S | ACHN | Plasmodium falciparum | Leishmania infantum | Trypanosoma cruzi | Trypanosoma bruceii | |
| 1 | 1 c | 2 c | 8 c | 8 c | 16 c | 16 c | 8–16 c | >6.25 c 92% at 6.25 µg/mL | – | 2.6 c | 1.8 c | 2.3 c | 11.8 c | 0.3 a | 1.5 a | 4.5 a | 0.9 a |
| 2 | 2 c | 2 c | 8 c | 8 c | 32 c | 4–8 c | 4–8 c | >6.25 c 96% at 6.25 µg/mL | – | 0.5 c | 0.1 c | 1.1 c | 3.7 c | 0.3 a | 0.6 a | 0.5 a | 0.3 a |
| 3 | 32 b | – | – | >32 d | – | – | – | >6.25 b 38% at 6.25 µg/mL | >16 b | 1.5 b | 1.3 b | 1.5 b | 9.6 b | – | – | – | – |
| 4 | 4 b | 4 b | 8 b | 16 b | >32 b | 8 b | 8 b | 3.13 b | 2.0 b | 0.7 b | 0.5 b | 0.9 b | 3.6 b | 0.1 a | 0.1 a | 0.1 a | 0.2 a |
| 5 | 32 d | – | – | >32d | – | – | – | – | – | – | – | – | – | – | 4.6 d | 0.9 d | 4.5 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubec, M.; Totland, C.; Rise, F.; Chamgordani, E.J.; Paulsen, B.; Maes, L.; Matheeussen, A.; Gundersen, L.-L.; Halskau, Ø. Bioactive Metabolites of Marine Origin Have Unusual Effects on Model Membrane Systems. Mar. Drugs 2020, 18, 125. https://doi.org/10.3390/md18020125
Jakubec M, Totland C, Rise F, Chamgordani EJ, Paulsen B, Maes L, Matheeussen A, Gundersen L-L, Halskau Ø. Bioactive Metabolites of Marine Origin Have Unusual Effects on Model Membrane Systems. Marine Drugs. 2020; 18(2):125. https://doi.org/10.3390/md18020125
Chicago/Turabian StyleJakubec, Martin, Christian Totland, Frode Rise, Elahe Jafari Chamgordani, Britt Paulsen, Louis Maes, An Matheeussen, Lise-Lotte Gundersen, and Øyvind Halskau. 2020. "Bioactive Metabolites of Marine Origin Have Unusual Effects on Model Membrane Systems" Marine Drugs 18, no. 2: 125. https://doi.org/10.3390/md18020125
APA StyleJakubec, M., Totland, C., Rise, F., Chamgordani, E. J., Paulsen, B., Maes, L., Matheeussen, A., Gundersen, L.-L., & Halskau, Ø. (2020). Bioactive Metabolites of Marine Origin Have Unusual Effects on Model Membrane Systems. Marine Drugs, 18(2), 125. https://doi.org/10.3390/md18020125






