Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications
Abstract
1. Introduction
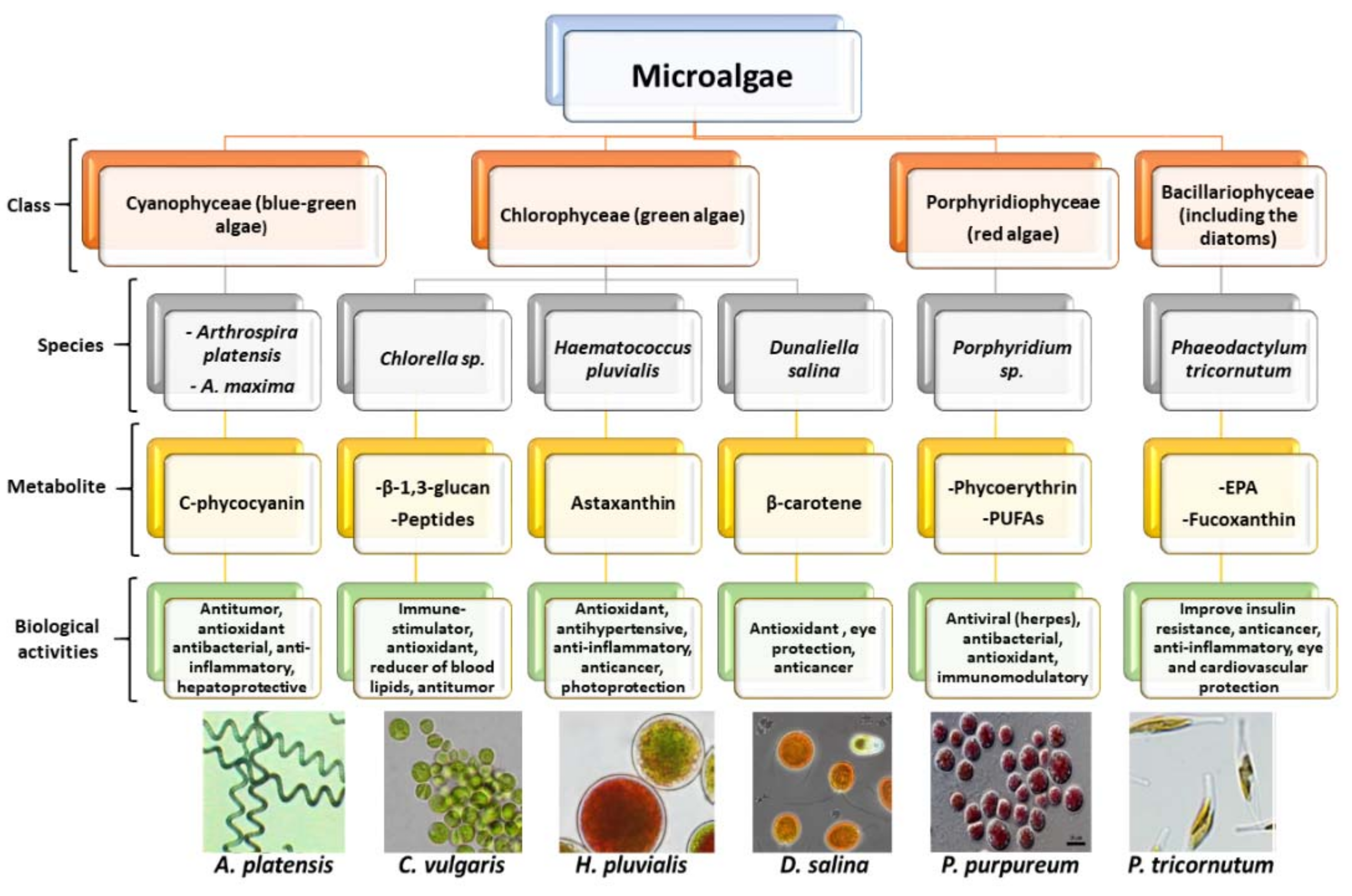
2. Microalgae
Biochemical Composition
3. Encapsulation
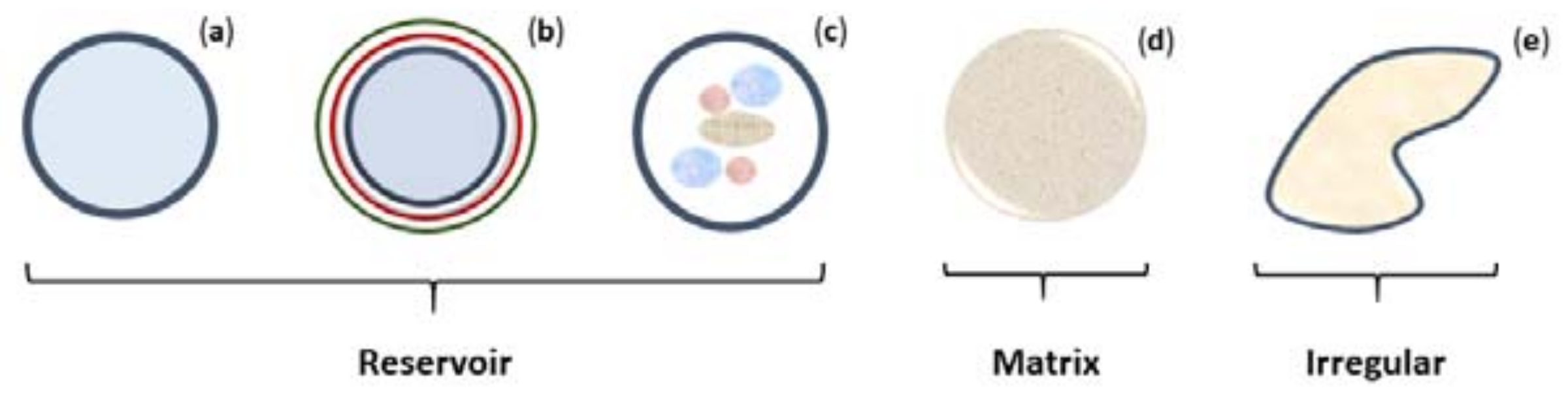
3.1. Structure and Composition
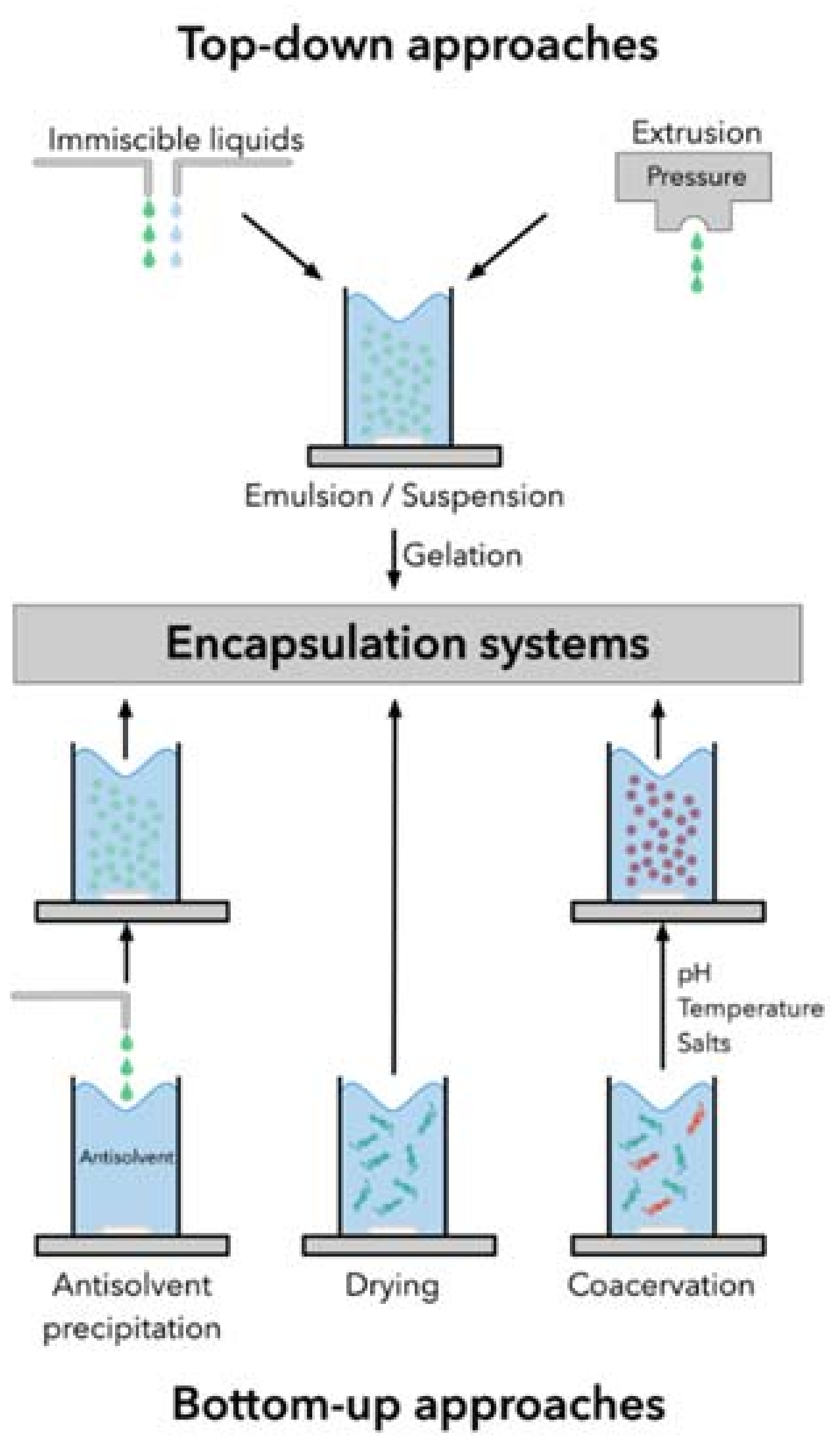
3.2. Encapsulation Techniques
4. Microalgae Encapsulation
4.1. Functional Foods
4.2. Pharmaceutical
4.3. Cosmetics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Borowitzka, M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- De Morais, M.G.; Vaz, B.D.S.; De Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “Healthy” Foods-Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Biotechnol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Sousa, I.; Raymundo, A.; Bandarra, N.M. Microalgae in novel food products. In Food Chemistry Research Developments; Papadopoulos, K.N., Ed.; Nova Science: New York, NY, USA, 2008; p. 36. ISBN 9781604562620. [Google Scholar]
- Vaz, B.D.S.; Moreira, J.B.; De Morais, M.G.; Costa, J.A.V. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.H.; Show, P.L. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 1–2. [Google Scholar] [CrossRef]
- Singh, S.; Kate, B.N.; Banecjee, U.C. Bioactive compounds from cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef]
- Christaki, E.; Florou-Paneri, P.; Bonos, E. Microalgae: A novel ingredient in nutrition. Int. J. Food Sci. Nutr. 2011, 62, 794–799. [Google Scholar] [CrossRef]
- Dominguez, H. Functional Ingredients from Algae for Foods and Nutraceuticals, 1st ed.; Woodhead Publishing Limited: Cambridge, MA, USA, 2013. [Google Scholar]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Pinto, M.D.S. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2012, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Singh, H. Nanotechnology applications in functional foods; Opportunities and challenges. Prev. Nutr. Food Sci. 2016, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of active ingredients in functional foods: From research stage to commercial food products. Trends Food Sci. Technol. 2018, 78, 167–179. [Google Scholar] [CrossRef]
- Kuang, S.S.; Oliveira, J.C.; Crean, A.M. Microencapsulation as a tool for incorporating bioactive ingredients into food. Crit. Rev. Food Sci. Nutr. 2010, 50, 951–968. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Sanna, V. Impact of nanotechnology on the delivery of natural products for cancer prevention and therapy. Mol. Nutr. Food Res. 2016, 60, 1330–1341. [Google Scholar] [CrossRef]
- Bux, F. Biotechnological Applications of Microalgae: Biodiesel and Value-Added Products, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2013. [Google Scholar]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I.S. Microalgae taxonomy and breeding. Biofuel Crop. Prod. Physiol. Genet. 2013, 44–53. [Google Scholar]
- Raja, R.; Hemaiswarya, S.; Kumar, N.A.; Sridhar, S.; Rengasamy, R. A perspective on the biotechnological potential of microalgae. Crit. Rev. Microbiol. 2008, 34, 77–88. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chang, C.; Chen, J.; Cao, F.; Zhao, J.; Zheng, Y.; Zhu, J. Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll. 2019, 96, 510–517. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguin, O.; Polle, J.E.W. Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Res. 2016, 15, 164–178. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Colla, L.M.; Oliveira Reinehr, C.; Reichert, C.; Costa, J.A.V. Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour. Technol. 2007, 98, 1489–1493. [Google Scholar] [CrossRef]
- Nuhu, A.A. Spirulina (Arthrospira): An Important Source of Nutritional and Medicinal Compounds. J. Mar. Biol. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Zaid, A.A.A.; Hammad, D.M.; Sharaf, E.M. Antioxidant and anticancer activity of Spirulina platensis water extracts. Int. J. Pharmacol. 2015, 11, 846–851. [Google Scholar] [CrossRef]
- Benedetti, S.; Benvenuti, F.; Pagliarani, S.; Francogli, S.; Scoglio, S.; Canestrari, F. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004, 75, 2353–2362. [Google Scholar] [CrossRef]
- Scoglio, S.; Lo Curcio, V.; Catalani, S.; Palma, F.; Battistelli, S.; Benedetti, S. Inhibitory effects of Aphanizomenon flos-aquae constituents on human UDP-glucose dehydrogenase activity. J. Enzym. Inhib. Med. Chem. 2016, 31, 1492–1497. [Google Scholar] [CrossRef]
- Kim, D.Y.; Vijayan, D.; Praveenkumar, R.; Han, J.I.; Lee, K.; Park, J.Y.; Chang, W.S.; Lee, J.S.; Oh, Y.K. Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus. Bioresour. Technol. 2016, 199, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Biology of microalgae. In Microalgae in Health and Disease Prevention; Levine, I., Fleurence, J., Eds.; Elsevier: London, UK, 2018; pp. 23–72. ISBN 9780128114056. [Google Scholar]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, F. Biology and industrial applications of Chlorella: Advances and prospects. In Microalgae Biotechnology; Posten, C., Chen, S.F., Eds.; Springer: Cham, Swizerland, 2015; pp. 1–35. [Google Scholar]
- Saha, S.K.; McHugh, E.; Hayes, J.; Moane, S.; Walsh, D.; Murray, P. Effect of various stress-regulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresour. Technol. 2013, 128, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Jaime, L.; Rodríguez-Meizoso, I.; Cifuentes, A.; Santoyo, S.; Suarez, S.; Ibáñez, E.; Señorans, F.J. Pressurized liquids as an alternative process to antioxidant carotenoids’ extraction from Haematococcus pluvialis microalgae. LWT Food Sci. Technol. 2010, 43, 105–112. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a Carotenoid with Potential in Human Health and Nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.M.; Wosu, C.I.; Ben-Amotz, A.; Harvey, P.J. Potential of new isolates of Dunaliella salina for natural β-carotene production. Biology 2018, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008, 26, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Tafreshi, A.; Shariati, M. Dunaliella biotechnology: Methods and applications. J. Appl. Microbiol. 2009, 107, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Monte, J.; Ribeiro, C.; Parreira, C.; Costa, L.; Brive, L.; Casal, S.; Brazinha, C.; Crespo, J.G. Biorefinery of Dunaliella salina: Sustainable recovery of carotenoids, polar lipids and glycerol. Bioresour. Technol. 2020, 297, 10. [Google Scholar] [CrossRef]
- Peng, K.T.; Zheng, C.N.; Xue, J.; Chen, X.Y.; Yang, W.D.; Liu, J.S.; Bai, W.; Li, H.Y. Delta 5 fatty acid desaturase upregulates the synthesis of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum. J. Agric. Food Chem. 2014, 62, 8773–8776. [Google Scholar] [CrossRef]
- Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Recovery and Encapsualtion of Bioactive Extracts from Haematococcus Pluvialis and Phaedodactylum Tricornutum for food Applications. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 2319–2399. [Google Scholar]
- Zhao, C.; Wu, Y.; Yang, C.; Liu, B.; Huang, Y. Hypotensive, hypoglycaemic and hypolipidaemic effects of bioactive compounds from microalgae and marine micro-organisms. Int. J. Food Sci. Technol. 2015, 50, 1705–1717. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Custódio, L.; Soares, F.; Pereira, H.; Barreira, L.; Vizetto-Duarte, C.; Rodrigues, M.J.; Rauter, A.P.; Alberício, F.; Varela, J. Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: Possible application in the pharmaceutical and functional food industries. J. Appl. Phycol. 2014, 26, 151–161. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Rath, B. Bioactive Compounds From Microalgae And Cyanobacteria: Utility and Applications. Int. J. Pharm. Sci. Res. 2012, 3, 4123–4130. [Google Scholar]
- Huheihel, M.; Ishanu, V.; Tal, J.; Arad, S. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 2002, 50, 189–200. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different-molecular-weight polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Vicente-García, V.; Rios-Leal, E.; Calderón-Domínguez, G.; Cañizares-Villanueva, R.O.; Olvera-Ramírez, R. Detection, Isolation, and Characterization of Exopolysaccharide Produced by a Strain of Phormidium 94a Isolated from an Arid Zone of Mexico. Biotechnol. Bioeng. 2004, 85, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 1–29. [Google Scholar] [CrossRef]
- Lafarga, T. Cultured Microalgae and Compounds Derived Thereof for Food Applications: Strain Selection and Cultivation, Drying, and Processing Strategies. Food Rev. Int. 2019, 36, 1–26. [Google Scholar] [CrossRef]
- Patil, V.; Reitan, K.; Mortensen, L.; Källqvist, T.; Olsen, Y.; Vogt, G.; Gislerød, H. Microalgae as a source of polyunsaturated fatty acids for aquaculture. Curr. Top. Plant Biol. 2005, 6, 57–65. [Google Scholar]
- Gouveia, L.; Marques, A.E.; Sousa, J.M.; Moura, P.; Bandarra, N.M. Microalgae–source of natural bioactive molecules as functional ingredients. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 21–37. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, B.; Chen, W.Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Medina, A.R.; Grima, E.M.; Giménez, A.G.; González, M.J.I. Downstream processing of algal polyunsaturated fatty acids. Biotechnol. Adv. 1998, 16, 517–580. [Google Scholar] [CrossRef]
- Behrens, P.W.; Kyle, D.J. Microalgae as a Source of Fatty Acids. J. Food Lipids 1996, 3, 259–272. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Iskandarov, U.; Cohen, Z. LC-PUFA from photosynthetic microalgae: Occurrence, biosynthesis, and prospects in biotechnology. Appl. Microbiol. Biotechnol. 2011, 91, 905–915. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar] [CrossRef]
- Pignolet, O.; Jubeau, S.; Vaca-Garcia, C.; Michaud, P. Highly valuable microalgae: Biochemical and topological aspects. J. Ind. Microbiol. Biotechnol. 2013, 40, 781–796. [Google Scholar] [CrossRef]
- Hamed, I. The Evolution and Versatility of Microalgal Biotechnology: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Yen, H.W.; Hu, I.C.; Chen, C.Y.; Ho, S.H.; Lee, D.J.; Chang, J.S. Microalgae-based biorefinery—From biofuels to natural products. Bioresour. Technol. 2013, 135, 166–174. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, E.B.; Antoniosi Filho, N.R. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Fanning, K.; Schuhmann, H.; Netzel, M.; Schenk, P. Microalgae: A valuable Source of Natural Carotenoids with Potential Health Benefits. In Carotenoids Food Sources, Production and Health Benefits; Yamaguichi, M., Ed.; Nova Science: New York, NY, USA, 2013; p. 355. ISBN 978-1-62808-625-6. [Google Scholar]
- Huang, J.J.; Lin, S.; Xu, W.; Cheung, P.C.K. Occurrence and biosynthesis of carotenoids in phytoplankton. Biotechnol. Adv. 2017, 35, 597–618. [Google Scholar] [CrossRef] [PubMed]
- Skjånes, K.; Rebours, C.; Lindblad, P. Potential for green microalgae to produce hydrogen, pharmaceuticals and other high value products in a combined process. Crit. Rev. Biotechnol. 2013, 33, 172–215. [Google Scholar] [CrossRef] [PubMed]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential microalgae derived pharmaceutical and biological reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae–nutritious, sustainable aqua- and animal feed source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhao, X.Q.; Yen, H.W.; Ho, S.H.; Cheng, C.L.; Lee, D.J.; Bai, F.W.; Chang, J.S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef]
- Williams, P.J.L.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef]
- Bule, M.H.; Ahmed, I.; Maqbool, F.; Bilal, M.; Iqbal, H.M.N. Microalgae as a source of high-value bioactive compounds. Front. Biosci. Sch. 2018, 10, 197–216. [Google Scholar]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- McClements, D.J. Delivery by Design (DbD): A Standardized Approach to the Development of Efficacious Nanoparticle- and Microparticle-Based Delivery Systems. Compr. Rev. Food Sci. Food Saf. 2018, 17, 200–219. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Fang, Z.; Bhesh, B. Encapsulation of polyphenols—a review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Onwulata, C.I. Encapsulation of New Active Ingredients. Annu. Rev. Food Sci. Technol. 2012, 3, 183–204. [Google Scholar] [CrossRef]
- Dias, D.R.; Botrel, D.A.; de Fernandes, R.V.B.; Borges, S.V. Encapsulation as a tool for bioprocessing of functional foods. Curr. Opin. Food Sci. 2017, 13, 31–37. [Google Scholar] [CrossRef]
- Augustin, M.A.; Hemar, Y. Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem. Soc. Rev. 2009, 38, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef]
- Sonawane, S.H.; Bhanvase, B.A.; Sivakumar, M. Encapsulation of Active Molecules and Their Delivery System, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Alexander, A.; Ajazuddin; Patel, R.J.; Saraf, S.; Saraf, S. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Control. Release 2016, 241, 110–124. [Google Scholar] [CrossRef]
- Gao, G.H.; Li, Y.; Lee, D.S. Environmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapy. J. Control. Release 2013, 169, 180–184. [Google Scholar] [CrossRef]
- Van Tran, V.; Moon, J.Y.; Lee, Y.C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Rashidi, L.; Khosravi-Darani, K. The applications of nanotechnology in food industry. Crit. Rev. Food Sci. Nutr. 2011, 51, 723–730. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Tanver Rahman, M.R.; Van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zhang, G.H. Review on microencapsulated phase change materials (MEPCMs): Fabrication, characterization and applications. Renew. Sustain. Energy Rev. 2011, 15, 3813–3832. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Mansour, H.M.; Sohn, M.; Al-Ghananeem, A.; DeLuca, P.P. Materials for Pharmaceutical Dosage Forms: Molecular Pharmaceutics and Controlled Release Drug Delivery Aspects. Int. J. Mol. Sci. 2010, 11, 3298–3322. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, P. Impact of wall material physicochemical characteristics on the stability of encapsulated phytochemicals: A review. Food Res. Int. 2019, 107, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.J.R.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Rodríguez, S.A.G.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Pillai, O.; Panchagnula, R. Polymers in Drug Delivery. Curr. Opin. Chem. Biol. 2001, 5, 447–451. [Google Scholar] [CrossRef]
- Nesterenko, A.; Alric, I.; Silvestre, F.; Durrieu, V. Vegetable proteins in microencapsulation: A review of recent interventions and their effectiveness. Ind. Crops Prod. 2013, 42, 469–479. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano Struct. Nano Obj. 2019, 20, 100397. [Google Scholar] [CrossRef]
- De Vos, P.; Faas, M.M.; Spasojevic, M.; Sikkema, J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- de Simões, L.S.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, Ó.L. Micro- and nano bio-based delivery systems for food applications: In vitro behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.G.; Castro, G.R. Novel technologies for the encapsulation of bioactive food compounds. Curr. Opin. Food Sci. 2016, 7, 78–85. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. [Google Scholar] [CrossRef]
- Chan, H.K.; Kwok, P.C.L. Production methods for nanodrug particles using the bottom-up approach. Adv. Drug Deliv. Rev. 2011, 63, 406–416. [Google Scholar] [CrossRef]
- Tolve, R.; Galgano, F.; Caruso, M.C.; Tchuenbou-Magaia, F.L.; Condelli, N.; Favati, F.; Zhang, Z. Encapsulation of health-promoting ingredients: Applications in foodstuffs. Int. J. Food Sci. Nutr. 2016, 67, 888–918. [Google Scholar] [CrossRef]
- Rodríguez, J.; Martín, M.J.; Ruiz, M.A.; Clares, B. Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res. Int. 2016, 83, 41–59. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Moschakis, T.; Biliaderis, C.G. Complex Coacervation as a Novel Microencapsulation Technique to Improve Viability of Probiotics Under Different Stresses. Food Bioprocess Technol. 2014, 7, 2767–2781. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, H.; Hu, X.; Bao, S.; Huang, H. Synthesis and release studies of microalgal oil-containing microcapsules prepared by complex coacervation. Colloids Surfaces B Biointerfaces 2012, 89, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ach, D.; Briançon, S.; Broze, G.; Puel, F.; Rivoire, A.; Galvan, J.M.; Chevalier, Y. Formation of microcapsules by complex coacervation. Can. J. Chem. Eng. 2015, 93, 183–191. [Google Scholar] [CrossRef]
- Zhu, Q.; Pan, Y.; Jia, X.; Li, J.; Zhang, M.; Yin, L. Review on the Stability Mechanism and Application of Water-in-Oil Emulsions Encapsulating Various Additives. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1660–1675. [Google Scholar] [CrossRef]
- Freitas, S.; Merkle, H.P.; Gander, B. Microencapsulation by solvent extraction/evaporation: Reviewing the state of the art of microsphere preparation process technology. J. Control. Release 2005, 102, 313–332. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.B.; McGinity, J.W. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 1997, 28, 25–42. [Google Scholar] [CrossRef]
- Taylor, T.M.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Liposomal nanocapsules in food science and agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, X.; Zhang, T.; Song, Y.; She, Z.; Li, J.; Deng, Y. Progress involving new techniques for liposome preparation. Asian J. Pharm. Sci. 2014, 9, 176–182. [Google Scholar] [CrossRef]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar]
- Vieira, M.V.; Oliveira, S.M.; Amado, I.R.; Fasolin, L.H.; Vicente, A.A.; Pastrana, L.M.; Fuciños, P. 3D printed functional cookies fortified with Arthrospira platensis: Evaluation of its antioxidant potential and physical-chemical characterization. Food Hydrocoll. 2020, 107, 105893. [Google Scholar] [CrossRef]
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Vidal, D.; Fito, P. Functional foods development: Trends and technologies. Trends Food Sci. Technol. 2011, 22, 498–508. [Google Scholar] [CrossRef]
- Freitas, A.C.; Rodrigues, D.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C. Marine biotechnology advances towards applications in new functional foods. Biotechnol. Adv. 2012, 30, 1506–1515. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Dewapriya, P.; Kim, S. Marine microorganisms: An emerging avenue in modern nutraceuticals and functional foods. Food Res. Int. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation Into Innovative Food Products With Potential Health Benefits. Front. Nutr. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Li, D.; Chen, W.; Zhang, K.; Chen, S. Preparation of stable microcapsules from disrupted cell of Haematococcus pluvialis by spray drying. Int. J. Food Sci. Technol. 2016, 51, 1834–1843. [Google Scholar] [CrossRef]
- Kittikaiwan, P.; Powthongsook, S.; Pavasant, P.; Shotipruk, A. Encapsulation of Haematococcus pluvialis using chitosan for astaxanthin stability enhancement. Carbohydr. Polym. 2007, 70, 378–385. [Google Scholar] [CrossRef]
- Machado, F.R.S.; Reis, D.F.; Boschetto, D.L.; Burkert, J.F.M.; Ferreira, S.R.S.; Oliveira, J.V.; Burkert, C.A.V. Encapsulation of astaxanthin from Haematococcus pluvialis in PHBV by means of SEDS technique using supercritical CO2. Ind. Crops Prod. 2014, 54, 17–21. [Google Scholar] [CrossRef]
- Machado, F.R.S.; Trevisol, T.C.; Boschetto, D.L.; Burkert, J.F.M.; Ferreira, S.R.S.; Oliveira, J.V.; Burkert, C.A.V. Technological process for cell disruption, extraction and encapsulation of astaxanthin from Haematococcus pluvialis. J. Biotechnol. 2016, 218, 108–114. [Google Scholar] [CrossRef]
- Bustamante, A.; Masson, L.; Velasco, J.; Del Valle, J.M.; Robert, P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: Kinetic stability of astaxanthin and alpha-tocopherol. Food Chem. 2016, 190, 1013–1021. [Google Scholar] [CrossRef]
- Vakarelova, M.; Zanoni, F.; Lardo, P.; Rossin, G.; Mainente, F.; Chignola, R.; Menin, A.; Rizzi, C.; Zoccatelli, G. Production of stable food-grade microencapsulated astaxanthin by vibrating nozzle technology. Food Chem. 2017, 221, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Alarcón, C.; Inostroza-Riquelme, M.; Torres-Gallegos, C.; Araya, C.; Miranda, M.; Sánchez-Caamaño, J.C.; Moreno-Villoslada, I.; Oyarzun-Ampuero, F.A. Protection of astaxanthin from photodegradation by its inclusion in hierarchically assembled nano and microstructures with potential as food. Food Hydrocoll. 2018, 83, 36–44. [Google Scholar] [CrossRef]
- Tachaprutinun, A.; Udomsup, T.; Luadthong, C.; Wanichwecharungruang, S. Preventing the thermal degradation of astaxanthin through nanoencapsulation. Int. J. Pharm. 2009, 374, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.F.; Chen, Y.C.; Chen, R.N.; Chen, L.C.; Ho, H.O.; Tsung, Y.H.; Sheu, M.T.; Liu, D.Z. Improving the stability of astaxanthin by microencapsulation in calcium alginate beads. PLoS ONE 2016, 11, e0153685. [Google Scholar] [CrossRef]
- Bustos-Garza, C.; Yáñez-Fernández, J.; Barragán-Huerta, B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Niizawa, I.; Espinaco, B.Y.; Zorrilla, S.E.; Sihufe, G.A. Natural astaxanthin encapsulation: Use of response surface methodology for the design of alginate beads. Int. J. Biol. Macromol. 2019, 121, 601–608. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, S.; Gu, K.; Zhang, N. Preparation of astaxanthin-loaded liposomes: Characterization, storage stability and antioxidant activity. CyTA J. Food 2018, 16, 607–618. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Stability of astaxanthin-loaded nanostructured lipid carriers in beverage systems. J. Sci. Food Agric. 2018, 98, 511–518. [Google Scholar] [CrossRef]
- Shrestha, S.; Sadiq, M.B.; Anal, A.K. Culled banana resistant starch-soy protein isolate conjugate based emulsion enriched with astaxanthin to enhance its stability. Int. J. Biol. Macromol. 2018, 120, 449–459. [Google Scholar] [CrossRef]
- Liu, G.; Hu, M.; Zhao, Z.; Lin, Q.; Wei, D.; Jiang, Y. Enhancing the stability of astaxanthin by encapsulation in poly (l-lactic acid) microspheres using a supercritical anti-solvent process. Particuology 2019, 44, 54–62. [Google Scholar] [CrossRef]
- Boonlao, N.; Shrestha, S.; Sadiq, M.B.; Anal, A.K. Influence of whey protein-xanthan gum stabilized emulsion on stability and in vitro digestibility of encapsulated astaxanthin. J. Food Eng. 2020, 272, 109859. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.; Wang, Y.; Xue, C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018, 260, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Salatti-Dorado, J.A.; García-Gómez, D.; Rodriguez-Ruiz, V.; Gueguen, V.; Pavon-Djavid, G.; Rubio, S. Multifunctional green supramolecular solvents for cost-effective production of highly stable astaxanthin-rich formulations from Haematococcus pluvialis. Food Chem. 2019, 279, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Shu, G.; Kobayashi, I.; Nakajima, M.; Barrow, C.J. Formulation and characterization of monodisperse O/W emulsions encapsulating astaxanthin extracts using microchannel emulsification: Insights of formulation and stability evaluation. Colloids Surf. B Biointerfaces 2017, 157, 355–365. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.; Zhang, X.; Zhang, G.; Zhu, H. Astaxanthin from Haematococcus pluvialis Microencapsulated by Spray Drying: Characterization and Antioxidant Activity. JAOCS J. Am. Oil Chem. Soc. 2019, 96, 93–102. [Google Scholar] [CrossRef]
- Khalid, N.; Shu, G.; Holland, B.J.; Kobayashi, I.; Nakajima, M.; Barrow, C.J. Formulation and characterization of O/W nanoemulsions encapsulating high concentration of astaxanthin. Food Res. Int. 2017, 102, 364–371. [Google Scholar] [CrossRef]
- Shen, Q.; Quek, S.Y. Microencapsulation of astaxanthin with blends of milk protein and fiber by spray drying. J. Food Eng. 2014, 123, 165–171. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Rico, L.G.; Badolato, G.G.; Schubert, H. Production of O/W Emulsions Containing Astaxanthin by Repeated Premix Membrane Emulsification. Food Eng. Phys. Prop. 2005, 70, 117–123. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.; Guan, L.; Zhang, Y.; Dang, Q.; Dong, P.; Li, J.; Liang, X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017, 227, 9–15. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C.P. Physico-chemical stability of astaxanthin nanodispersions prepared with polysaccharides as stabilizing agents. Int. J. Food Sci. Nutr. 2013, 64, 744–748. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.; Sun, X.; Zhang, S.; Wang, S.; Feng, F.; Wang, D.; Xu, Y. Fabrication and Characterization of β-Lactoglobulin-Based Nanocomplexes Composed of Chitosan Oligosaccharides as Vehicles for Delivery of Astaxanthin. J. Agric. Food Chem. 2018, 66, 6717–6726. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, F.; Vakarelova, M.; Zoccatelli, G. Development and Characterization of Astaxanthin-Containing Whey Protein-Based Nanoparticles. Mar. Drugs 2019, 17, 627. [Google Scholar] [CrossRef]
- Sarada, R.; Vidhyavathi, R.; Usha, D.; Ravishankar, G.A. An efficient method for extraction of astaxanthin from green alga Haematococcus pluvialis. J. Agric. Food Chem. 2006, 54, 7585–7588. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yan, X.; Sun, L.; Yang, T.; Hu, X.; He, Z.; Liu, F.; Liu, X. Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends Food Sci. Technol. 2019, 91, 354–361. [Google Scholar] [CrossRef]
- Koo, S.Y.; Mok, I.K.; Pan, C.H.; Kim, S.M. Preparation of Fucoxanthin-Loaded Nanoparticles Composed of Casein and Chitosan with Improved Fucoxanthin Bioavailability. J. Agric. Food Chem. 2016, 64, 9428–9435. [Google Scholar] [CrossRef] [PubMed]
- Leach, G.; Oliveira, G.; Morais, R. Production of a carotenoid-rich product by alginate entrapment and fluid-bed drying of Dunaliella salina. J. Sci. Food Agric. 1998, 76, 298–302. [Google Scholar] [CrossRef]
- Leach, G.; Oliveira, G.; Morais, R. Spray-drying of Dunaliella salina to produce a β-carotene rich powder. J. Ind. Microbiol. Biotechnol. 1998, 20, 82–85. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Spray-drying microencapsulation of β-carotene contents in powdered Dunaliella salina biomass. Int. J. Pharm. Clin. Res. 2016, 8, 1533–1536. [Google Scholar]
- Jafari, Y.; Sabahi, H.; Rahaie, M. Stability and loading properties of curcumin encapsulated in Chlorella vulgaris. Food Chem. 2016, 211, 700–706. [Google Scholar] [CrossRef]
- Pu, C.; Tang, W. Encapsulation of lycopene in Chlorella pyrenoidosa: Loading properties and stability improvement. Food Chem. 2017, 235, 283–289. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X. Separation, antitumor activities, and encapsulation of polypeptide from Chlorella pyrenoidosa. Biotechnol. Prog. 2013, 29, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Bhattacharjee, P.; Satpati, G.G.; Pal, R. Spray dried extract of Phormidium valderianum as a promising source of natural antioxidant. Int. J. Food Sci. 2014, 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- de Bonilla-Ahumada, F.J.; Khandual, S.; del Lugo-Cervantes, E.C. Microencapsulation of algal biomass (Tetraselmis chuii) by spray-drying using different encapsulation materials for better preservation of beta-carotene and antioxidant compounds. Algal Res. 2018, 36, 229–238. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT Food Sci. Technol. 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.; Jha, A.; Rasane, P.; Gautam, A.K. Optimization of a process for high fibre and high protein biscuit. J. Food Sci. Technol. 2015, 52, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Bolanho, B.C.; Egea, M.B.; Jácome, A.L.M.; Campos, I.; de Carvalho, J.C.M.; Danesi, E.D.G. Antioxidant and nutritional potential of cookies enriched with Spirulina platensis and sources of fibre. J. Food Nutr. Res. 2014, 53, 171–179. [Google Scholar]
- Yan, M.; Liu, B.; Jiao, X.; Qin, S. Preparation of phycocyanin microcapsules and its properties. Food Bioprod. Process. 2014, 92, 89–97. [Google Scholar] [CrossRef]
- Kurniasih, R.A.; Purnamayati, L.; Amalia, U.; Dewi, E.N. Formulation and characterization of phycocyanin microcapsules within maltodextrin-alginate. Agritech 2018, 38, 23–29. [Google Scholar] [CrossRef]
- Pan-utai, W.; Iamtham, S. Physical extraction and extrusion entrapment of C-phycocyanin from Arthrospira platensis. J. King Saud Univ. Sci. 2019, 31, 1535–1542. [Google Scholar] [CrossRef]
- Machado, A.R.; Assis, L.M.; Costa, J.A.V.; Badiale-Furlong, E.; Motta, A.S.; Micheletto, Y.M.S.; Souza-Soares, L.A. Application of sonication and mixing for nanoencapsulation of the cyanobacterium Spirulina platensis in liposomes. Int. Food Res. J. 2015, 22, 96–101. [Google Scholar]
- Machado, A.R.; Pinheiro, A.C.; Vicente, A.A.; Souza-Soares, L.A.; Cerqueira, M.A. Liposomes loaded with phenolic extracts of Spirulina LEB-18: Physicochemical characterization and behavior under simulated gastrointestinal conditions. Food Res. Int. 2019, 120, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.C.; Fernandes, I.P.; Barros, L.; Fernandes, Â.; José Alves, M.; Calhelha, R.C.; Pereira, C.; Barreira, J.C.M.; Manrique, Y.; Colla, E.; et al. Spray-dried Spirulina platensis as an effective ingredient to improve yogurt formulations: Testing different encapsulating solutions. J. Funct. Foods 2019, 60, 103424. [Google Scholar] [CrossRef]
- Bezerra, P.Q.M.; de Matos, M.F.R.; Ramos, I.G.; Magalhães-Guedes, K.T.; Druzian, J.I.; Costa, J.A.V.; Nunes, I.L. Innovative functional nanodispersion: Combination of carotenoid from Spirulina and yellow passion fruit albedo. Food Chem. 2019, 285, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Rajmohan, D.; Bellmer, D. Characterization of Spirulina-alginate beads formed using ionic gelation. Int. J. Food Sci. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Zen, C.K.; Tiepo, C.B.V.; da Silva, R.V.; Reinehr, C.O.; Gutkoski, L.C.; Oro, T.; Colla, L.M. Development of functional pasta with microencapsulated Spirulina: Technological and sensorial effects. J. Sci. Food Agric. 2020, 100, 2018–2026. [Google Scholar] [CrossRef]
- Faieta, M.; Corradini, M.G.; Di Michele, A.; Ludescher, R.D.; Pittia, P. Effect of Encapsulation Process on Technological Functionality and Stability of Spirulina Platensis Extract. Food Biophys. 2020, 15, 50–63. [Google Scholar] [CrossRef]
- Schmatz, D.A.; da Mastrantonio, D.J.S.; Costa, J.A.V.; De Morais, M.G. Encapsulation of phycocyanin by electrospraying: A promising approach for the protection of sensitive compounds. Food Bioprod. Process. 2020, 119, 206–215. [Google Scholar] [CrossRef]
- Seyed Yagoubi, A.; Shahidi, F.; Mohebbi, M.; Varidi, M.; Golmohammadzadeh, S. Preparation, characterization and evaluation of physicochemical properties of phycocyanin-loaded solid lipid nanoparticles and nanostructured lipid carriers. J. Food Meas. Charact. 2018, 12, 378–385. [Google Scholar] [CrossRef]
- Dewi, E.N.; Kurniasih, R.A.; Purnamayanti, L. Physical properties of Spirulina phycocyanin microencapsulated with maltodextrin and carrageenan. Philipp. J. Sci. 2018, 147, 201–207. [Google Scholar]
- Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Rostro-Alanis, M.; Cuéllar-Bermúdez, S.P.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; García-Pérez, J.S.; Chandra, R.; Parra-Saldívar, R. Advancement of green process through microwave-assisted extraction of bioactive metabolites from Arthrospira Platensis and bioactivity evaluation. Bioresour. Technol. 2017, 224, 618–629. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar]
- Bajpai, V.K.; Shukla, S.; Kang, S.M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 1–23. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, V.; Yadav, S.K. Nanotechnology: A tool to enhance therapeutic values of natural plant products. Trends Med. Res. 2012, 7, 34–42. [Google Scholar]
- Nootem, J.; Chalorak, P.; Meemon, K.; Mingvanish, W.; Pratumyot, K.; Ruckthong, L.; Srisuwannaket, C.; Niamnont, N. Electrospun cellulose acetate doped with astaxanthin derivatives from Haematococcus pluvialis for in vivo anti-aging activity. RSC Adv. 2018, 8, 37151–37158. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chang, C.C.; Lin, S.-T.; Chyau, C.-C.; Peng, R.Y. Improved hepatoprotective effect of liposome-encapsulated astaxanthin in lipopolysaccharide-induced acute hepatotoxicity. Int. J. Mol. Sci. 2016, 17, 1128. [Google Scholar] [CrossRef] [PubMed]
- Hardiningtyas, S.D.; Wakabayashi, R.; Kitaoka, M.; Tahara, Y.; Minamihata, K.; Goto, M.; Kamiya, N. Mechanistic investigation of transcutaneous protein delivery using solid-in-oil nanodispersion: A case study with phycocyanin. Eur. J. Pharm. Biopharm. 2018, 127, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, W.; Dou, X.; Nan, D.; He, G. Astaxanthin Encapsulated in Biodegradable Calcium Alginate Microspheres for the Treatment of Hepatocellular Carcinoma In Vitro. Appl. Biochem. Biotechnol. 2019, 191, 511–527. [Google Scholar] [CrossRef]
- Barros, M.P.; Pinto, E.; Colepicolo, P.; Pedersén, M. Astaxanthin and peridinin inhibit oxidative damage in Fe2+-loaded liposomes: Scavenging oxyradicals or changing membrane permeability? Biochem. Biophys. Res. Commun. 2001, 288, 225–232. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, C.A. Enhanced proliferation and differentiation of mesenchymal stem cells by astaxanthin-encapsulated polymeric micelles. PLoS ONE 2019, 14, e0216755. [Google Scholar] [CrossRef]
- Peng, C.H.; Chang, C.H.; Peng, R.Y.; Chyau, C.C. Improved membrane transport of astaxanthine by liposomal encapsulation. Eur. J. Pharm. Biopharm. 2010, 75, 154–161. [Google Scholar] [CrossRef]
- Fratter, A.; Biagi, D.; Cicero, A.F.G. Sublingual delivery of astaxanthin through a novel ascorbyl palmitate-based nanoemulsion: Preliminary data. Mar. Drugs 2019, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. In vitro antitumor potential of astaxanthin nanoemulsion against cancer cells via mitochondrial mediated apoptosis. Int. J. Pharm. 2019, 560, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Bianchini, R.D.; Lemos-Senna, E. Preparation and characterization of Haematococcus pluvialis carotenoid-loaded PLGA nanocapsules in a gel system with antioxidant properties for topical application. J. Drug Deliv. Sci. Tehnol. 2020, 102099. [Google Scholar] [CrossRef]
- Manconi, M.; Mura, S.; Manca, M.L.; Fadda, A.M.; Dolz, M.; Hernandez, M.J.; Casanovas, A.; Díez-Sales, O. Chitosomes as drug delivery systems for C-phycocyanin: Preparation and characterization. Int. J. Pharm. 2010, 392, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Pagnussatt, F.A.; De Lima, V.R.; Dora, C.L.; Costa, J.A.V.; Putaux, J.L.; Badiale-Furlong, E. Assessment of the encapsulation effect of phenolic compounds from Spirulina sp. LEB-18 on their antifusarium activities. Food Chem. 2016, 211, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Manconia, M.; Pendás, J.; Ledón, N.; Moreira, T.; Sinico, C.; Saso, L.; Fadda, A.M. Phycocyanin liposomes for topical anti-inflammatory activity: In-vitro in-vivo studies. J. Pharm. Pharmacol. 2009, 61, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Boutin, R.; Munnier, E.; Renaudeau, N.; Girardot, M.; Pinault, M.; Chevalier, S.; Chourpa, I.; Clément-Larosière, B.; Imbert, C.; Boudesocque-Delaye, L. Spirulina platensis sustainable lipid extracts in alginate-based nanocarriers: An algal approach against biofilms. Algal Res. 2019, 37, 160–168. [Google Scholar] [CrossRef]
- Jung, S.M.; Kim, D.S.; Ju, J.H.; Shin, H.S. Assessment of Spirulina-PCL nanofiber for the regeneration of dermal fibroblast layers. Vitr. Cell. Dev. Biol. Anim. 2013, 49, 27–33. [Google Scholar] [CrossRef]
- Yang, F.; Tang, Q.; Zhong, X.; Bai, Y.; Chen, T.; Zhang, Y.; Li, Y.; Zheng, W. Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int. J. Nanomed. 2012, 7, 835–844. [Google Scholar]
- Karakaş, C.Y.; Tekarslan Şahin, H.; İnan, B.; Özçimen, D.; Erginer, Y. In vitro cytotoxic activity of microalgal extracts loaded nano–micro particles produced via electrospraying and microemulsion methods. Biotechnol. Prog. 2019, 35, 1–8. [Google Scholar] [CrossRef]
- Zamani, H.; Rastegari, B.; Varamini, M. Antioxidant and anti-cancer activity of Dunaliella salina extract and oral drug delivery potential via nano-based formulations of gum Arabic coated magnetite nanoparticles. J. Drug Deliv. Sci. Technol. 2019, 54, 101278. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 2–14. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Nanocarriers for delivery of antioxidants on the skin. Cosmetics 2015, 2, 342–354. [Google Scholar] [CrossRef]
- Hu, F.; Liu, W.; Yan, L.; Kong, F.; Wei, K. Optimization and characterization of poly(lactic-co-glycolic acid) nanoparticles loaded with astaxanthin and evaluation of anti-photodamage effect in vitro. R. Soc. Open Sci. 2019, 6, 191184. [Google Scholar] [CrossRef]
- Sun, R.; Xia, N.; Xia, Q. Non-aqueous nanoemulsions as a new strategy for topical application of astaxanthin. J. Dispers. Sci. Technol. 2019, 41, 1–12. [Google Scholar] [CrossRef]
- Castangia, I.; Manca, M.L.; Catalán-Latorre, A.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Phycocyanin-encapsulating hyalurosomes as carrier for skin delivery and protection from oxidative stress damage. J. Mater. Sci. Mater. Med. 2016, 27, 1–10. [Google Scholar] [CrossRef]
- Hama, S.; Takahashi, K.; Inai, Y.; Shiota, K.; Sakamoto, R.; Yamada, A.; Tsuchiya, H.; Kanamura, K.; Yamashita, E.; Kogure, K. Protective Effects of Topical Application of a Poorly Soluble Antioxidant Astaxanthin Liposomal Formulation on Ultraviolet-Induced Skin Damage. J. Pharm. Sci. 2012, 101, 2909–2916. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Cho, M.K.; Shim, K.H.; Kim, H.J.; Song, H.G.; Shin, H.S. Development of a Spirulina extract/alginate-imbedded pcl nanofibrous cosmetic patch. J. Microbiol. Biotechnol. 2017, 27, 1657–1663. [Google Scholar] [CrossRef]
- AstaPure EyeQ: Natural Asthaxanthin for Eyes and Brain. Algaltecnologies Inc. Available online: https://www.algatech.com/algatech-product/astapure-natural-astaxanthin/ (accessed on 4 December 2020).
- Baiz Astaxanthin. Zibo Baiz Biotechnology-Co. Ltd. Available online: http://www.baiz-biotech.com/index.php?id=26 (accessed on 4 December 2020).
- Betatene 7.5% N: Natural Mixed Carotenoids. BASF Personal Care and Nutrition. Available online: https://documents.basf.com/126c0e366f9dad9e74c100bcbbdd29d30ff76f07?response-content-disposition=inline (accessed on 4 December 2020).
| Core Substance | Coating Material | Encapsulation Technique/System | Application | Major Findings | Reference |
|---|---|---|---|---|---|
| Disrupted cells | Maillard reaction products | Spray dryer | Functional food |
| [143] |
| Homogenized cells | Chitosan | Immersion | Functional food |
| [144] |
| Astaxanthin or carotenoid extract | Polymerpoly(hydroxybutirate-co-hydroxyvalerat)(PHB) | Supercritical fluids (SEDS) | Functional food and pharmaceutical |
| [145,146] |
| Extract oleoresin | Capsul | Spray-dryer | Functional food |
| [147] |
| Astaxanthin-enriched oil | Sodium alginate and low-methoxyl pectin | Vibrating-nozzle extrusion technology | Functional food |
| [148] |
| Astaxanthin |
|
| Functional food |
| [149] |
| Lipid extract | Ulvan-pullulan | Electrospinning | Functional food |
| [51] |
| Astaxanthin |
| Polymeric nanospheres by solvent displacement | Functional food and pharmaceutical |
| [150] |
| Astaxanthin | Calcium-Alginate | Extrusion | Functional food and pharmaceutical |
| [151] |
| Astaxanthin oleoresin | Gum arabic and whey protein, alone or in combination with maltodextrin or inulin | Spray-dryer | Functional food |
| [152] |
| Astaxanthin oleoresin | Calcium-Alginate | External ionic gelation | Functional food |
| [153] |
| Astaxanthin |
| Liposomes | Functional food |
| [154] |
| Astaxanthin oleoresin |
| Nanostructured lipid carriers (NLCs) (melt-emulsification/ultrasonication technique) | Beverages (whey and non-alcoholic beer) |
| [155] |
| Astaxanthin oleoresin |
| Emulsification | Functional food and pharmaceutical |
| [156] |
| Astaxanthin | Poly (l-lactic acid) | Supercritical anti-solvent | Functional food and pharmaceutical |
| [157] |
| Astaxanthin oleoresin |
| Emulsification | Functional food |
| [158] |
| Esterified astaxanthin |
| Complex coacervation | Functional food and pharmaceutical |
| [159] |
| Astaxanthin oleoresin |
| Nanostructured lipid carriers (hot homogenization) | Functional food |
| [160] |
| Astaxanthin extract |
| Microchannel emulsification | Functional food and pharmaceutical |
| [161] |
| Astaxanthin oleoresin |
| Complex coacervation followed by spray dryer | Functional food |
| [162] |
| Astaxanthin |
| Nanoemulsion (high-pressure homogenization) | Functional beverages |
| [163] |
| Astaxanthin |
| Spray dryer | Functional food |
| [164] |
| Astaxanthin |
| Premix membrane emulsification | Functional food |
| [165] |
| Astaxanthin |
| Co-assembly | Functional food and pharmaceutical |
| [166] |
| Astaxanthin |
| Emulsification- solvent evaporation | Functional food |
| [167] |
| Astaxanthin |
| Spontaneous self-assembly | Functional food |
| [168] |
| Astaxanthin oleoresin | Whey protein concentrate | Emulsification-Solvent evaporation | Functional food |
| [169] |
| Core Substance | Coating Material | Encapsulation Technique/System | Application | Major Findings | Reference |
|---|---|---|---|---|---|
| Phycocyanin |
| Extrusion | Functional food |
| [184] |
| Phycocyanin |
| Spray dryer | Functional food |
| [185] |
| Phycocyanin | Calcium-Alginate | Ultrasonic and extrusion techniques | Functional food |
| [186] |
| A. platensis biomass | Purified soybean phosphatidylcholine | Liposomes | Functional food |
| [187] |
| Phenolic extracts | Rice and soybean lecithin | Liposomes | Functional food and pharmaceutical |
| [188] |
| A. platensis powder | Maltodextrin pure or crosslinked with citric acid | Spray dryer | Yoghurt |
| [189] |
| Carotenoid extract | Yellow passion fruit albedo flour | Solvent displacement method | Functional food |
| [190] |
| A. platensis powder | Alginate | Extrusion (internal and external ionic gelation) | Functional food |
| [191] |
| Arthorspira biomass | Calcium-Alginate | Spray dryer | Pasta |
| [192] |
| A. platensis aqueous extract | Calcium-Alginate | Vibrational extrusion | 3D printed cookies |
| [136] |
| A. platensis extract | Pure trehalose or with maltodextrin |
| Functional food |
| [193] |
| Phycocyanin | Polyvinyl alcohol | Electrospray | Functional food |
| [194] |
| Phycocyanin |
| Ultrasound-assisted high-shear homogenization | Functional food |
| [195] |
| Phycocyanin |
| Spray dryer | Functional food |
| [196] |
| Core Substance | Coating Material | Encapsulation Technique/System | Application | Major Findings | Reference |
|---|---|---|---|---|---|
| Astaxanthin rich-extract | Cellulose acetate (CA) | Electrospinning | Antiaging |
| [201] |
| Astaxanthin | Calcium alginate | Double emulsification | Hepato carcinoma |
| [204] |
| Astaxanthin | Egg-yolk phosphatidylcholine | Liposomes | Lipoperoxidation inhibition |
| [205] |
| Astaxanthin | Methoxypolyethyleneglycol-polycaprolactone (mPEG-PCL) copolymer | Micelles (self-assembly) | Proliferation and differentiation of human mesenchymal stem cells |
| [206] |
| Astaxanthin |
| Liposomes | Hepatoprotection |
| [202] |
| Astaxanthin |
| Liposomes | Antioxidant |
| [207] |
| Astaxanthin | Ascorbyl palmitate | Nanoemulsion | Sublingual drug delivery |
| [208] |
| Astaxanthin and α-tocopherol | Sodium caseinate | Nanoemulsion (spontaneous emulsification-ultrasonication) | Anticancer |
| [209] |
| Carotenoid-rich extract | Poly-lactide-co-glycolide | Polymeric nanocapsules by solvent displacement | Antioxidant |
| [210] |
| Core Substance | Coating Material | Encapsulation Technique/System | Application | Major Findings | Reference |
|---|---|---|---|---|---|
| C-Phycocyanin |
| Coated liposomes (chitosomes)/spray and freeze-dryer | Colonic drug delivery (anti-inflammatory) |
| [211] |
| Phenolic extract |
| Liposomes | Antifusarium |
| [212] |
| Phycocyanin |
| Solid-in-oil nanodispersion | Transdermal drug delivery |
| [203] |
| C-Phycocyanin from A. platensis |
| Liposomes | Topical anti-inflammatory |
| [213] |
| A. platensis fatty acids |
| Ultrasound emulsification | Anti-biofilm |
| [214] |
| Aqueous extract | Polycaprolactone | Electrospinning | Tissue engineering (scaffold) |
| [215] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. https://doi.org/10.3390/md18120644
Vieira MV, Pastrana LM, Fuciños P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Marine Drugs. 2020; 18(12):644. https://doi.org/10.3390/md18120644
Chicago/Turabian StyleVieira, Marta V., Lorenzo M. Pastrana, and Pablo Fuciños. 2020. "Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications" Marine Drugs 18, no. 12: 644. https://doi.org/10.3390/md18120644
APA StyleVieira, M. V., Pastrana, L. M., & Fuciños, P. (2020). Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Marine Drugs, 18(12), 644. https://doi.org/10.3390/md18120644







