Amentadione from the Alga Cystoseira usneoides as a Novel Osteoarthritis Protective Agent in an Ex Vivo Co-Culture OA Model
Abstract
1. Introduction
2. Results
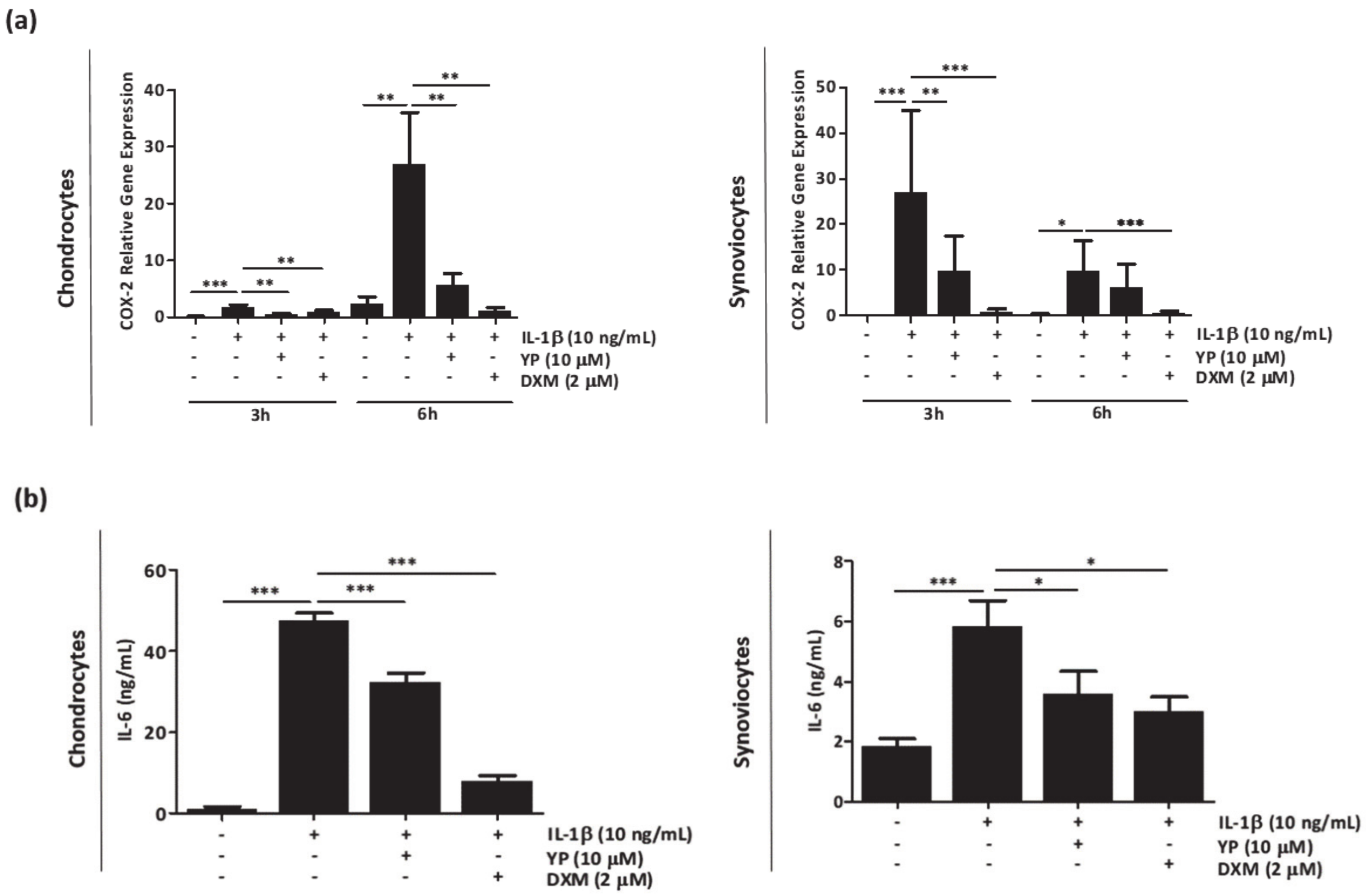
2.1. YP Acts as an Anti-Inflammatory Agent in the Articular OA Cell System Model
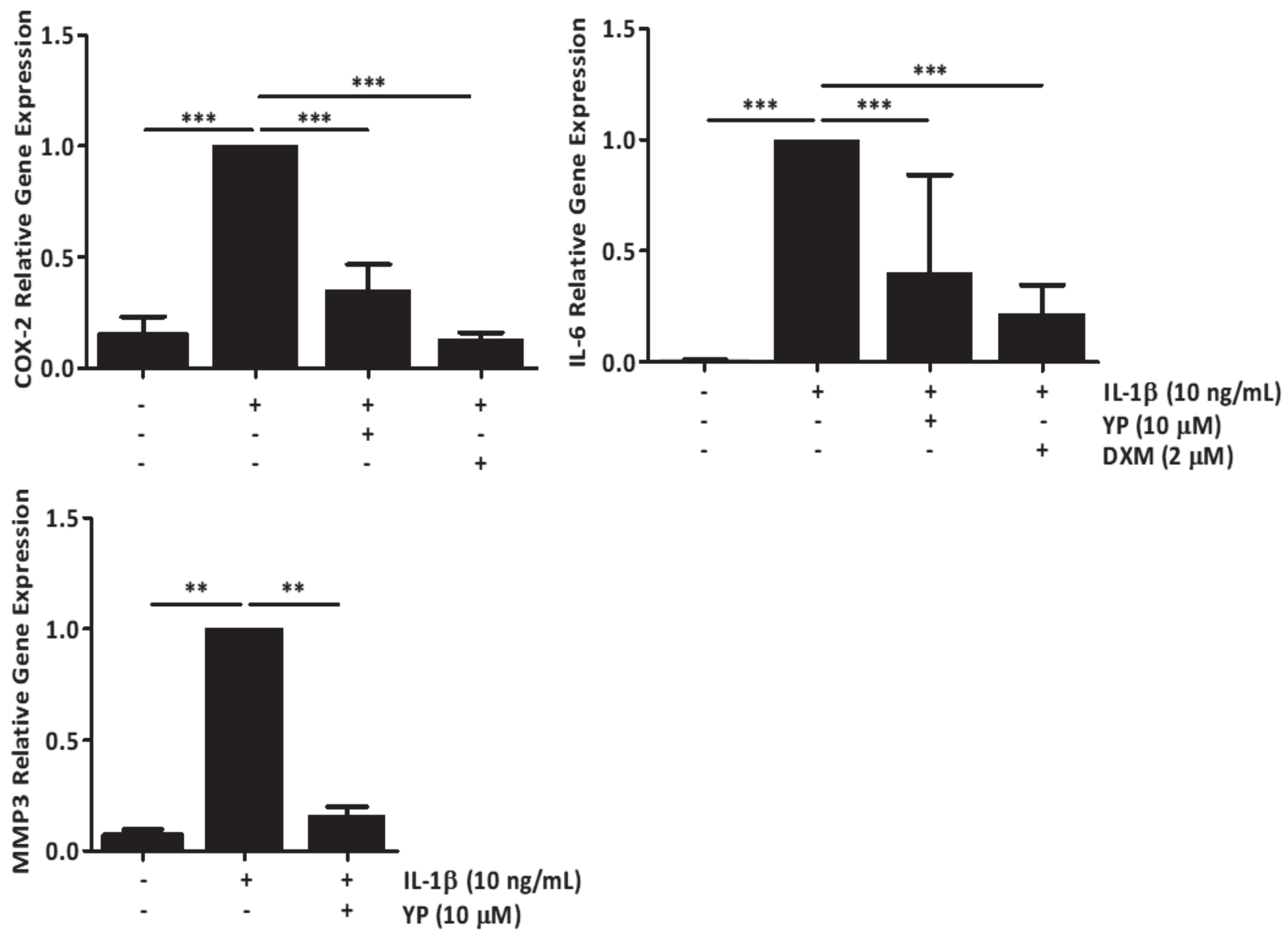
2.2. YP Modulates Cartilage Homeostasis under Mineralizing Conditions in an Ex-Vivo Cartilage Explant Model
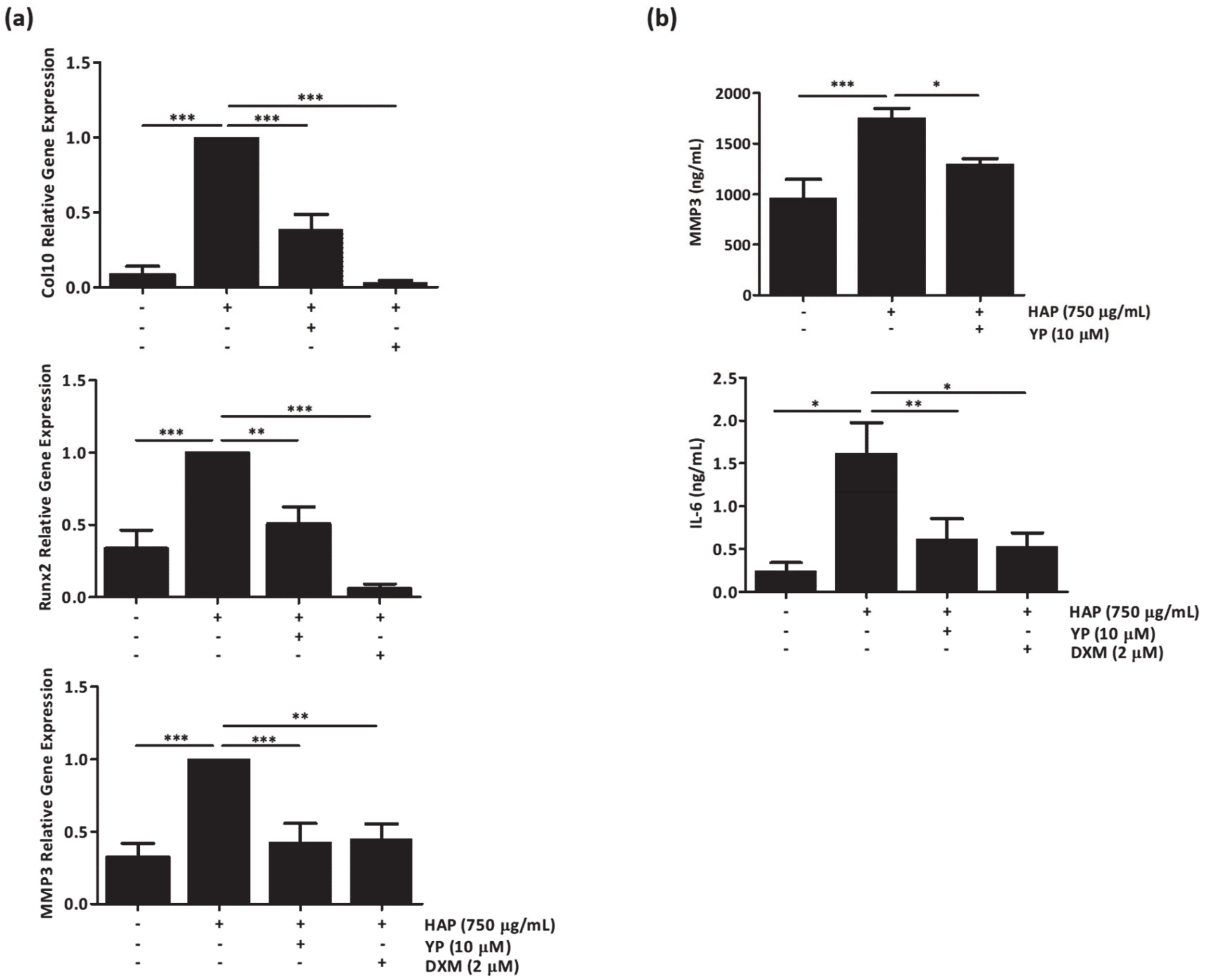
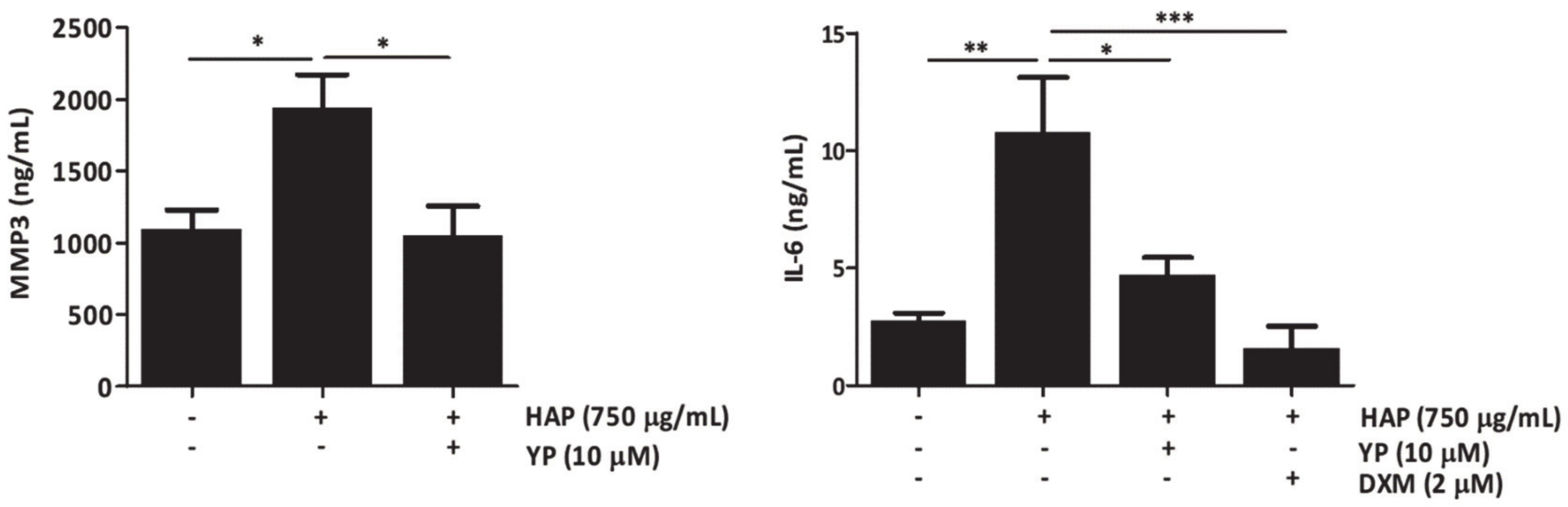
2.3. YP Function as a Protective Agent against Cartilage Deterioration under OA Promoting Conditions in an Explant-Based Co-Culture OA Model
2.4. YP Downregulates NF-kB Expression and Inhibits Ikbα Phosphorylation in Primary Chondrocyte Cells
3. Discussion
4. Materials and Methods
4.1. Isolation of Amentadione (YP)
4.2. Cell Culture
4.3. Inflammatory Assays in Monolayer Cells
4.4. Cell Proliferation
4.5. Cartilage Collection and Tissue Explants Preparation
4.6. Cartilage Explants Assays
4.7. Co-Culture Assays
4.8. RNA Extraction, cDNA Amplification and Quantitative Real-Time PCR (qPCR)
4.9. ELISA Assays
4.10. Histological Evaluation
4.11. Protein Extraction and Quantification
4.12. Electrophoresis and Western Blot
4.13. Determination of Total and Phosphorylated IkBα
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Moe, R.H.; Uhlig, T.; Kjeken, I.; Hagen, K.B.; Kvien, T.K.; Grotle, M. Multidisciplinary and multifaceted outpatient management of patients with osteoarthritis: Protocol for a randomised, controlled trial. BMC Musculoskelet. Disord. 2010, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2010, 81, 646–656. [Google Scholar]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis levels have recently doubled. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J. Osteoarthritis: Something is moving. Reumatol. Clin. 2014, 10, 4–5. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskeletal Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Sutton, S.; Clutterbuck, A.; Harris, P.; Gent, T.; Freeman, S.; Foster, N.; Barrett-Jolley, R.; Mobasher, A. The contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritis. Vet. J. 2009, 179, 10–24. [Google Scholar] [CrossRef]
- Abramson, S.B.; Attur, M.; Amin, A.R.; Clancy, R. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Curr. Rheumatol. Rep. 2001, 3, 535–541. [Google Scholar] [CrossRef]
- Rose, B.J.; Kooyman, D.L. A Tale of Two Joints: The Role of Matrix Metalloproteases in Cartilage Biology. Dis. Mark. 2016, 2016, 4895050. [Google Scholar] [CrossRef]
- Frallonardo, P.; Ramonda, R.; Peruzzo, L.; Scanu, A.; Galozzi, P.; Tauro, L.; Punzi, L.; Oliviero, F. Basic calcium phosphate and pyrophosphate crystals in early and late osteoarthritis: Relationship with clinical indices and inflammation. Clin. Rheumatol. 2018, 37, 2847–2853. [Google Scholar] [CrossRef] [PubMed]
- Conway, R.; McCarthy, G.M. Calcium-Containing Crystals and Osteoarthritis: An Unhealthy Alliance. Curr. Rheumatol. Rep. 2018, 20, 13. [Google Scholar] [CrossRef]
- Carlson, A.K.; McCutchen, C.N.; June, R.K. Mechanobiological implications of articular cartilage crystals Calcium. Curr. Opin. Rheumatol. 2017, 29, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C.; Altman, R.D.; April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthrit. Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef]
- Mobasheri, A. The future of osteoarthritis therapeutics: Targeted pharmacological therapy. Curr. Rheumatol. Rep. 2013, 15, 364. [Google Scholar] [CrossRef] [PubMed]
- Samvelyan, H.J.; Hughes, D.; Stevens, C.; Staines, K.A. Models of osteoarthritis: Relevance and new insights [published online ahead of print, 2020 Feb 15]. Calcif. Tissue Int. 2020. [Google Scholar] [CrossRef]
- Schlichting, N.; Dehne, T.; Mans, K.; Endres, M.; Stuhlmüller, B.; Sittinger, M.; Kaps, C.; Ringe, J. Suitability of porcine chondrocyte micromass culture to model osteoarthritis in vitro. Mol. Pharm. 2014, 11, 2092–2105. [Google Scholar] [CrossRef] [PubMed]
- Suroowan, S.; Mahomoodally, F. Herbal products for common auto-inflammatory disorders-novel approaches. Comb. Chem. High Throughput Screen. 2018, 21, 161–174. [Google Scholar] [CrossRef]
- Henrotin, Y.; Mobasheri, A. Natural products for promoting joint health and managing osteoarthritis. Curr. Rheumatol. Rep. 2018, 20, 72. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical supplements in the management and prevention of osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef]
- Stoppoloni, D.; Politi, L.; Leopizzi, M.; Gaetani, S.; Guazzo, R.; Basciani, S.; Moreschini, O.; De Santi, M.; Scandurra, R.; Scotto d’Abusco, A. Effect of glucosamine and its peptidyl-derivative on the production of extracellular matrix components by human primary chondrocytes. Osteoarth. Cart. 2015, 23, 103–113. [Google Scholar] [CrossRef]
- Paul, A.T.; Gohil, V.M.; Bhutani, K.K. Modulating TNF-alpha signaling with natural products. Drug Discov. Today 2006, 11, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tang, W.; Bidigare, R.R. Terpenoids as therapeutic drugs and pharmaceutical agents. Natural Products. In Natural Products: Drug Discovery and Therapeutic Medicine; Zhang, L., Demain, A.L., Eds.; Humana Press: Totowa, NJ, USA, 2017; pp. 197–227. [Google Scholar] [CrossRef]
- De los Reyes, C.; Zbakh, H.; Motilva, V.; Zubía, E. Antioxidant and anti-inflammatory meroterpenoids from the brown alga Cystoseira usneoides. J. Nat. Prod. 2013, 76, 621–629. [Google Scholar] [CrossRef] [PubMed]
- De los Reyes, C.; Ortega, M.J.; Zbakh, H.; Motilva, V.; Zubía, E. Cystoseira usneoides: A brown alga rich in antioxidant and anti-inflammatory meroditerpenoids. J. Nat. Prod. 2016, 79, 395–405. [Google Scholar] [CrossRef]
- Zbakh, H.; Talero, E.; Avila, J.; Alcaide, A.; de los Reyes, C.; Zubía, E.; Motilva, V. The algal meroterpene 11-hydroxy-1′-O-methylamentadione ameloriates dextran sulfate sodium-induced colitis in mice. Mar. Drugs 2016, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Zbakh, H.; Zubía, E.; Reyes, C.; Calderón-Montaño, J.M.; López-Lázaro, M.; Motilva, V. Meroterpenoids from the brown alga Cystoseira usneoides as potential anti-inflammatory and lung anticancer agents. Mar. Drugs 2020, 18, 207. [Google Scholar] [CrossRef]
- Viegas, C.S.B.; Costa, R.M.; Santos, L.; Videira, P.A.; Silva, Z.; Araújo, N.; Macedo, A.L.; Matos, A.P.; Cees Vermeer, C.; Simes, D.C. Gla-rich protein function as an anti-inflammatory agent in monocytes/macrophages: Implications for calcification-related chronic inflammatory diseases. PLoS ONE 2017, 12, e0177829. [Google Scholar] [CrossRef]
- Cavaco, S.; Viegas, C.S.B.; Rafael, M.S.; Ramos, A.; Magalhães, J.; Blanco, F.J.; Vermeer, C.; Simes, D.C. Gla-rich protein is involved in the cross-talk between calcification and inflammation in osteoarthritis. Cell. Mol. Life Sci. 2016, 73, 1051–1065. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.V. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarth. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, P.M.; van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarth. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Santana, A.; Yavorskyy, A.; Loughran, S.T.; McCarthy, G.M.; McMahon, G.P. New approaches in the detection of calcium-containing microcrystals in synovial fluid. Bioanalysis 2011, 3, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Corr, E.M.; Cunningham, C.C.; Helbert, L.; McCarthy, G.M.; Dunne, A. Osteoarthritis-associated basic calcium phosphate crystals activate membrane proximal kinases in human innate immune cells. Arthritis Res. Ther. 2017, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Ea, H.K.; Nguyen, C.; Bazin, D.; Bianchi, A.; Guicheux, J.; Reboul, P.; Daudon, M.; Lioté, F. Articular cartilage calcification in osteoarthritis: Insights into crystal-induced stress. Arthritis. Rheum. 2011, 63, 10–18. [Google Scholar] [CrossRef]
- Nadra, I.; Mason, J.C.; Philippidis, P.; Florey, O.; Smythe, C.D.; McCarthy, G.M.; Landis, R.C.; Haskardet, D.O. Proinflammatory activation of macrophages by basic calcium phosphate crystals via proteinkinase C and MAP kinase pathways: A vicious cycle of inflammation and arterial calcification? Circ. Res. 2005, 96, 1248–1256. [Google Scholar] [CrossRef]
- Nasi, S.; So, A.; Combes, C.; Daudon, M.; Busso, N. Interleukin-6 and chondrocyte mineralisation act in tandem to promote experimental osteoarthritis. Ann. Rheum. Dis. 2016, 75, 1372–1379. [Google Scholar] [CrossRef]
- Jung, Y.K.; Han, M.S.; Park, H.R.; Lee, E.J.; Jang, J.A.; Kim, G.-W.; Lee, S.Y.; Moon, D.; Han, S. Calcium-phosphate complex increased during subchondral bone remodelling affects early stage osteoarthritis. Sci. Rep. 2018, 8, 487. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014, 2014, 19. [Google Scholar] [CrossRef]
- Ismail, H.M.; Yamamoto, K.; Vincent, T.L.; Nagase, H.; Troeberg, L.; Saklatvala, J. Interleukin-1 Acts via the JNK-2 Signaling Pathway to Induce Aggrecan Degradation by Human Chondrocytes. Arthritis Rheumatol. 2015, 67, 826–836. [Google Scholar] [CrossRef]
- Zweers, M.C.; de Boer, T.N.; van Roon, J.; Bijlsma, J.W.; Lafeber, F.P.; Mastbergen, S.C. Celecoxib: Considerations regarding its potential disease-modifying properties in osteoarthritis. Arthritis Res. Ther. 2011, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Aitken, D.; Laslett, L.L.; Pan, F.; Haugen, I.K.; Otahal, P.; Bellamy, N.; Bird, P.; Jones, G.A. Randomised double-blind placebo-controlled crossover trial of HUMira (adalimumab) for erosive hand Osteoarthritis-the HUMOR trial. Osteoarth. Cart. 2018, 26, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Carmi, Y.; Cohen, I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int. J. Cell Biol. 2016, 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Wu, D.; Zuo, Q.; Wang, Z.; Fan, W. Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes. Int. Orthop. 2013, 37, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Aravinthan, A.; Hossain, M.A.; Kim, B.; Kang, C.W.; Kim, N.S.; Hwang, K.C.; Kim, J.H. Ginsenoside Rb1 inhibits monoiodoacetate-induced osteoarthritis in postmenopausal rats through prevention of cartilage degradation. J. Ginseng Res. 2020, 1226–8453. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-Inflammatory Properties of curcumin, a major constituent of Curcuma Longa: A Review of Preclinical and Clinical Research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-kappaB signaling: Multiple angles to target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef]
- Olivotto, E.; Otero, M.; Marcu, K.B.; Goldring, M.B. Pathophysiology of osteoarthritis: Canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open. 2015, 1, e000061. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling pathways in osteoarthritic cartilage destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Amico, V.; Oriente, G.; Neri, P.; Piatelli, M.; Ruberto, G. Tetraprenyltoluquinols from the brown alga Cystoseira stricta. Phytochemistry 1987, 26, 1715–1718. [Google Scholar] [CrossRef]
- Burguera, E.F.; Vela, A.A.; Magalhães, J.; Meijide-Faílde, R.; Blanco, F.J. Effect of hydrogen sulfide sources on inflammation and catabolic markers on interleukin 1b-stimulated human articular chondrocytes. Osteoarth. Cartil. 2014, 22, 1026–1035. [Google Scholar] [CrossRef]
- Cillero, P.B.; Martin, M.; Arenas, J.; Lopez-Armada, M.J.; Blanco, F.J. Effect of nitric oxide on mitochondrial activity of human synovial cells. BMC Musculoskelet. Disord. 2011, 12, 42. [Google Scholar] [CrossRef]
- Single-step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [CrossRef]
- Rosenberg, L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J. Bone Joint Surg. Am. 1971, 53, 69–82. [Google Scholar] [CrossRef] [PubMed]
- López-Senra, E.; Casal-Beiroa, P.; López-Álvarez, M.; Serra, J.; González, P.; Valcarcel, J.; Vázquez, J.A.; Burguera, E.F.; Blanco, F.J.; Magalhães, J. Impact of prevalence ratios of chondroitin sulfate (CS)- 4 and -6-isomers derived from marine sources in cell proliferation and chondrogenic differentiation processes. Mar. Drugs 2020, 18, 94. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, N.; Viegas, C.S.B.; Zubía, E.; Magalhães, J.; Ramos, A.; Carvalho, M.M.; Cruz, H.; Sousa, J.P.; Blanco, F.J.; Vermeer, C.; et al. Amentadione from the Alga Cystoseira usneoides as a Novel Osteoarthritis Protective Agent in an Ex Vivo Co-Culture OA Model. Mar. Drugs 2020, 18, 624. https://doi.org/10.3390/md18120624
Araújo N, Viegas CSB, Zubía E, Magalhães J, Ramos A, Carvalho MM, Cruz H, Sousa JP, Blanco FJ, Vermeer C, et al. Amentadione from the Alga Cystoseira usneoides as a Novel Osteoarthritis Protective Agent in an Ex Vivo Co-Culture OA Model. Marine Drugs. 2020; 18(12):624. https://doi.org/10.3390/md18120624
Chicago/Turabian StyleAraújo, Nuna, Carla S. B. Viegas, Eva Zubía, Joana Magalhães, Acácio Ramos, Maria M. Carvalho, Henrique Cruz, João Paulo Sousa, Francisco J. Blanco, Cees Vermeer, and et al. 2020. "Amentadione from the Alga Cystoseira usneoides as a Novel Osteoarthritis Protective Agent in an Ex Vivo Co-Culture OA Model" Marine Drugs 18, no. 12: 624. https://doi.org/10.3390/md18120624
APA StyleAraújo, N., Viegas, C. S. B., Zubía, E., Magalhães, J., Ramos, A., Carvalho, M. M., Cruz, H., Sousa, J. P., Blanco, F. J., Vermeer, C., & Simes, D. C. (2020). Amentadione from the Alga Cystoseira usneoides as a Novel Osteoarthritis Protective Agent in an Ex Vivo Co-Culture OA Model. Marine Drugs, 18(12), 624. https://doi.org/10.3390/md18120624





