Influence of Low Salt Concentration on Growth Behavior and General Biomass Composition in Lyngbya purpurem (Cyanobacteria)
Abstract
1. Introduction
2. Results
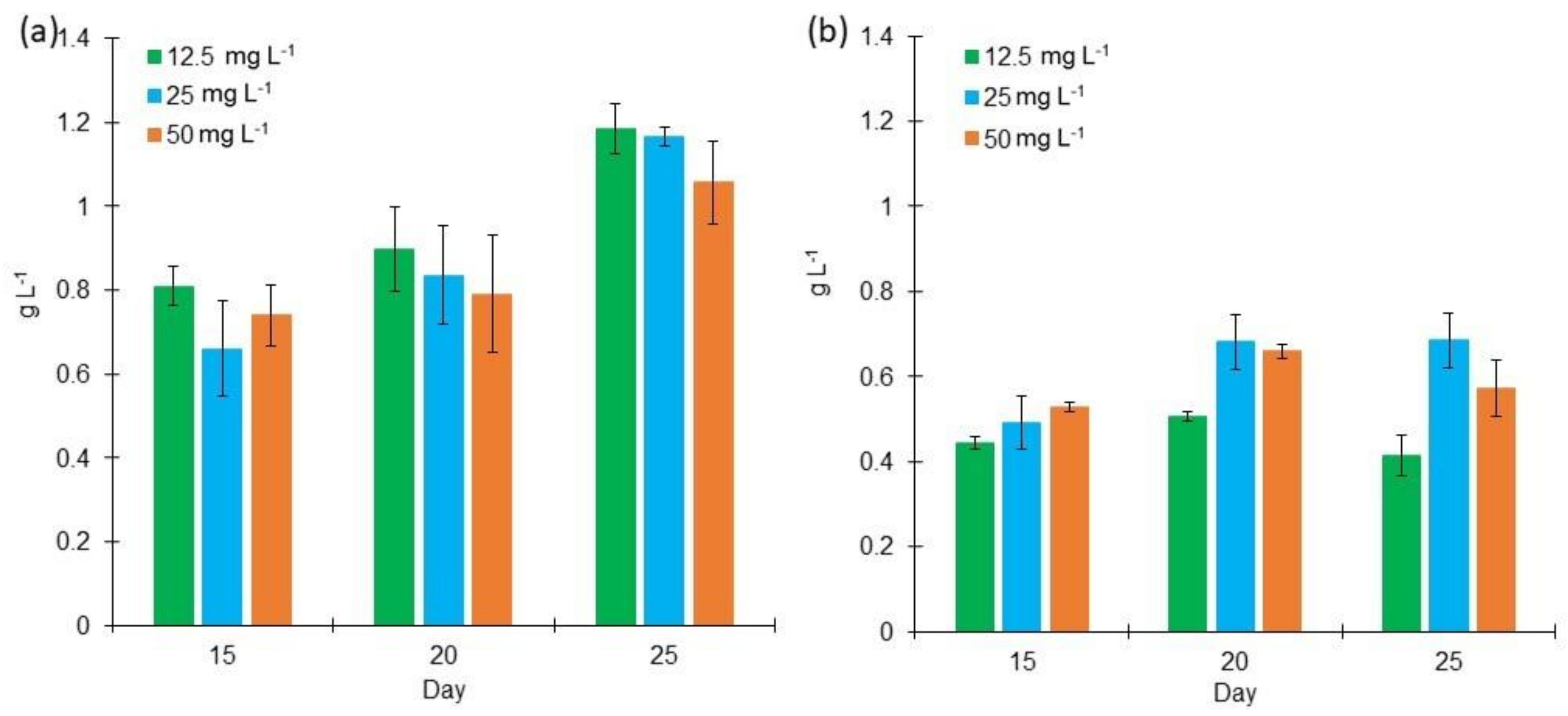
2.1. Cell Growth of L. purpurem at Different Low Salt Concentrations and Culture Media
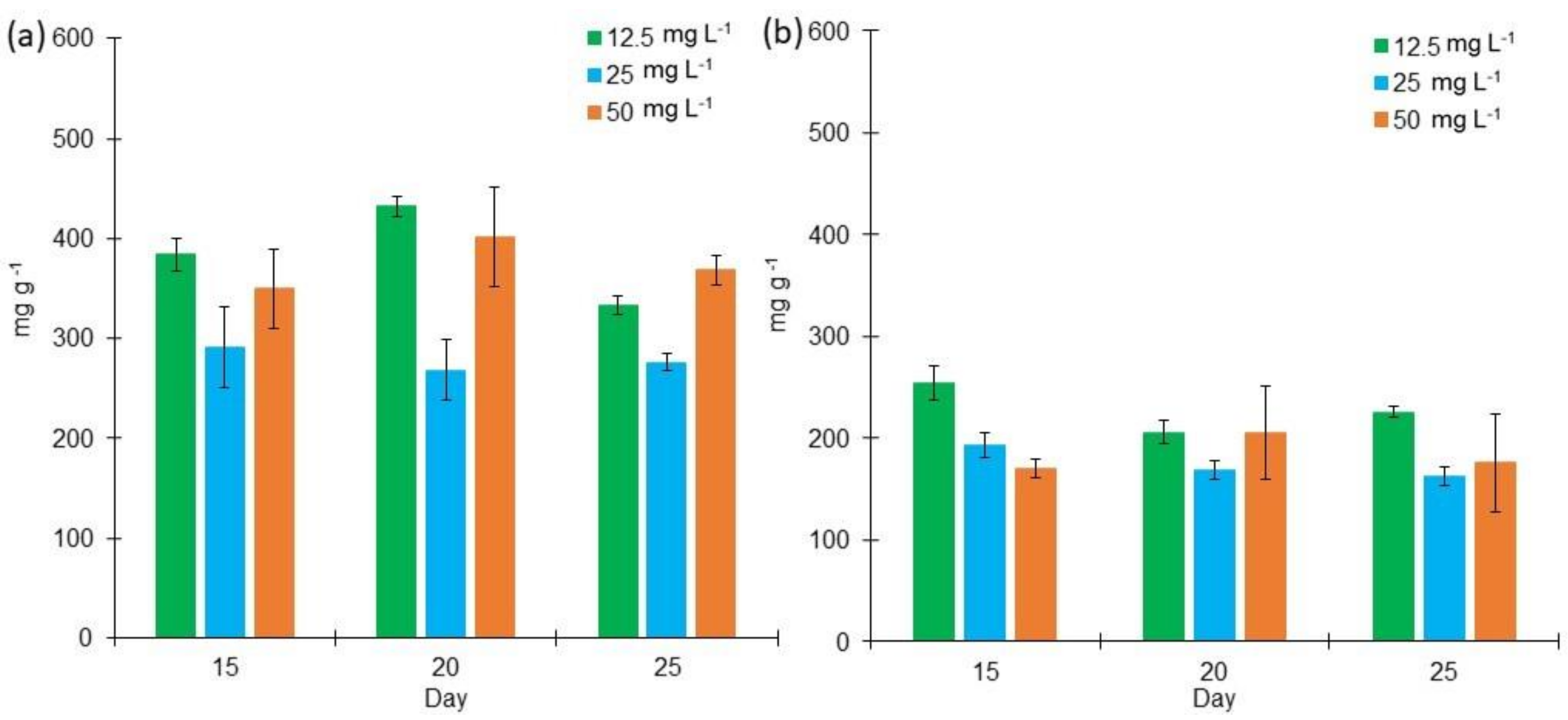
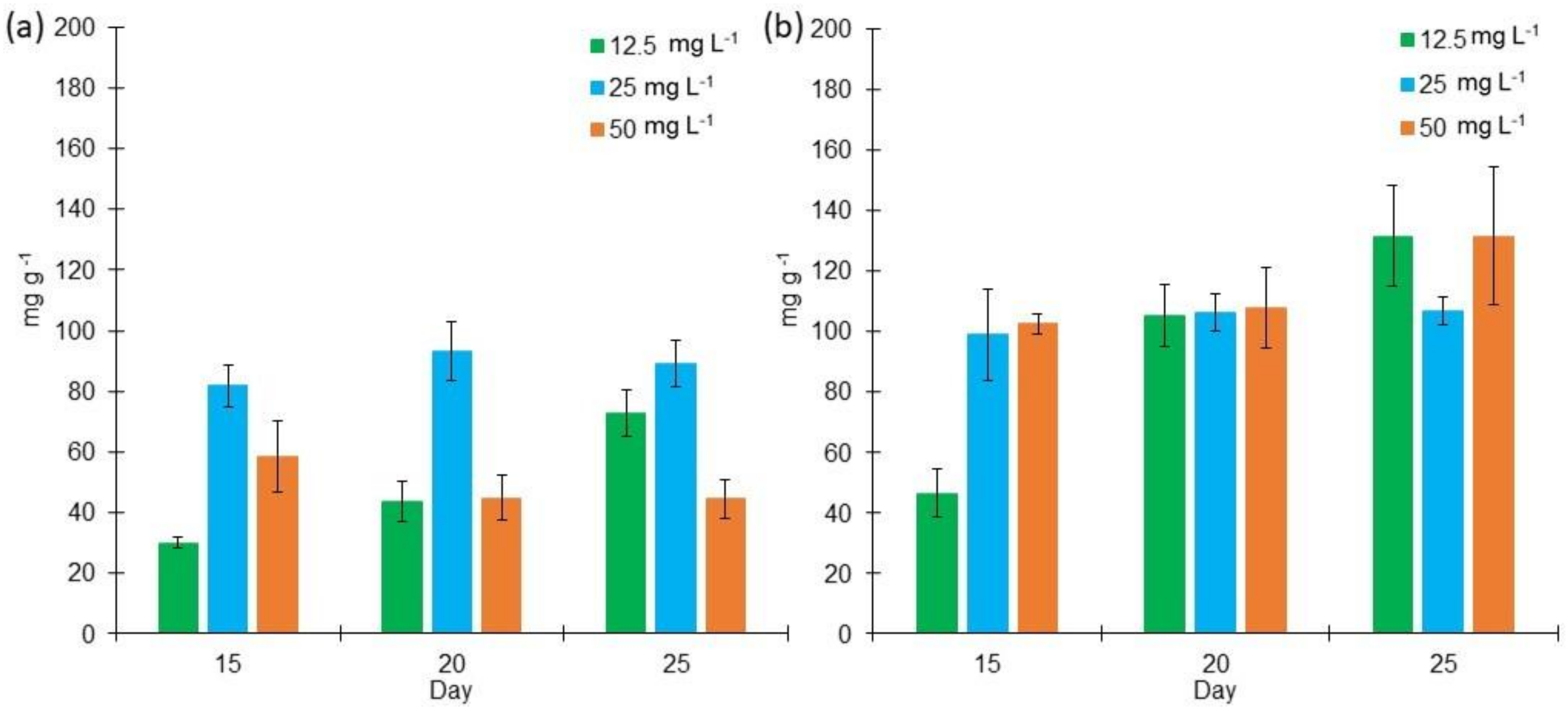
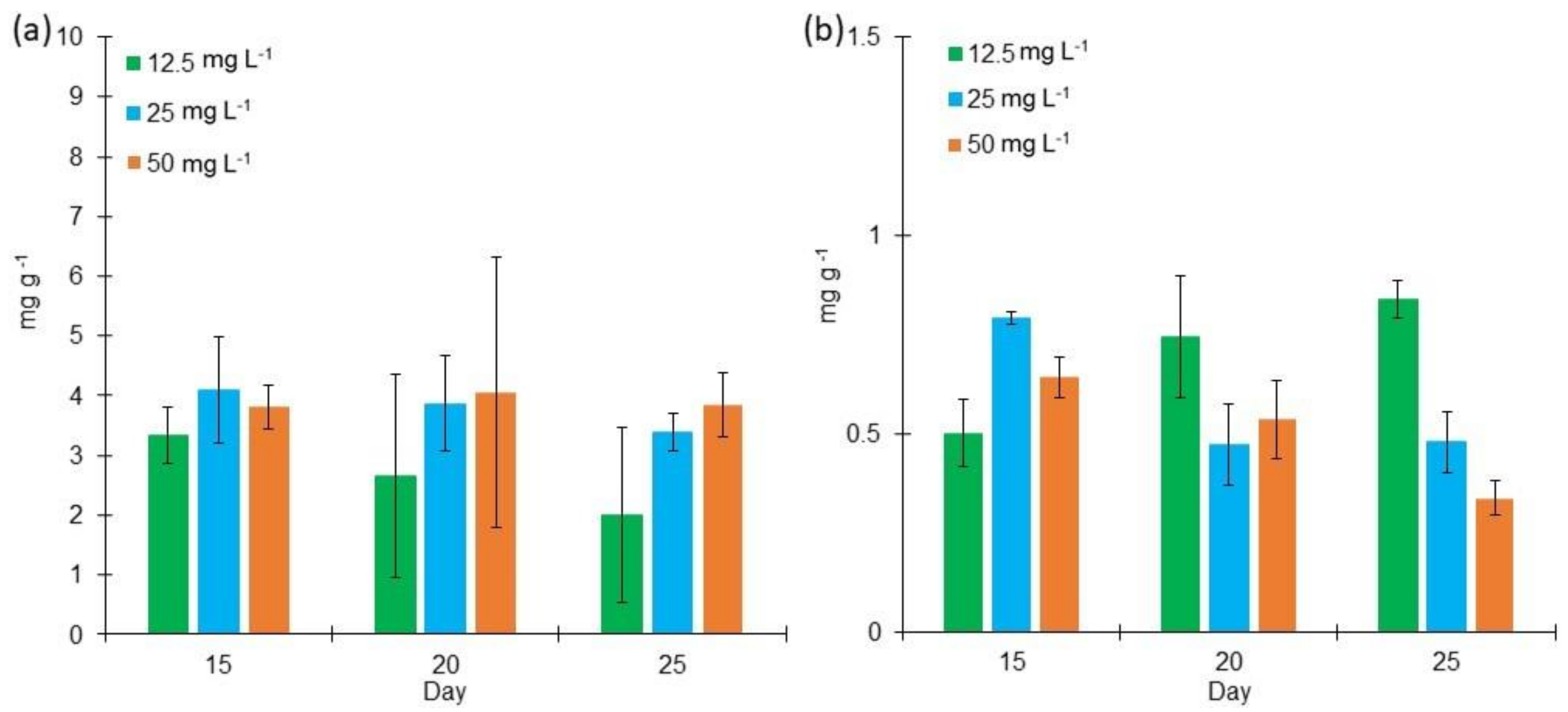
2.2. Proteins, Lipids, and Carbohydrates Content in L. purpurem’s Biomass at Different Low Salt Concentrations and Culture Media
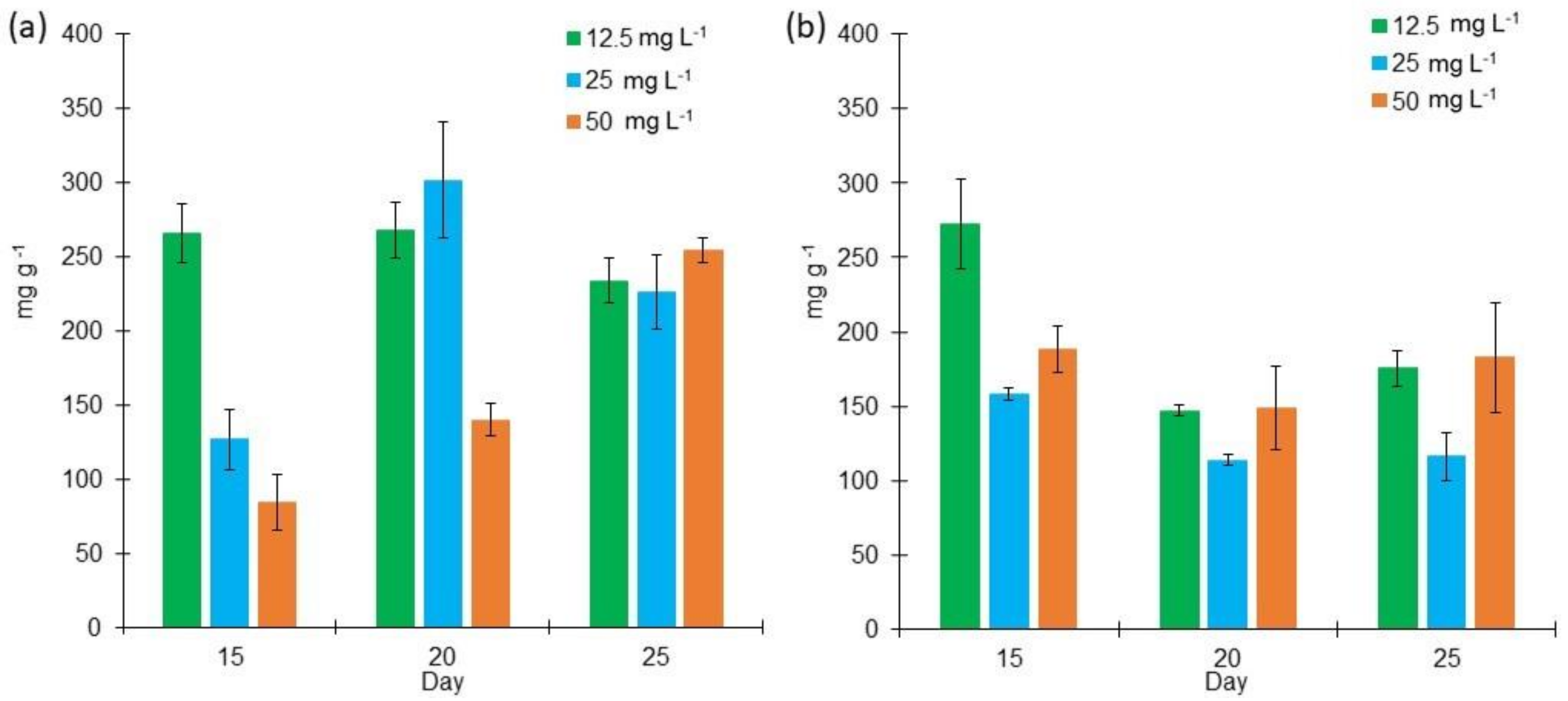
2.3. Pigments Behavior in L. purpurem’s Biomass Different Low Salt Concentrations and Culture Media
3. Discussion
3.1. Cell Growth of L. purpurem at Different Low Salt Concentrations and Culture Media
3.2. Protein, Lipid, and Carbohydrate Contents in L. purpurem’s Biomass at Low Salt Concentrations and Culture Media
3.3. Pigments Behavior in L. purpurem’s Biomass Different Low Salt Concentrations and Culture Media
4. Materials and Methods
4.1. Reagents and Equipment
4.2. Culture Conditions
4.3. Cell Growth of L. purpurem at Different Salt Concentrations and Culture Medium
4.4. Proteins, Lipids, and Carbohydrates Content in L. purpurem’s Biomass at Different Salt Concentrations and Culture Medium
4.5. Pigments Behavior in L. purpurem Biomass at Different Salt Concentrations and Culture Medium
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Engene, N.; Choi, H.; Esquenazi, E.; Rottacker, E.C.; Ellisman, M.H.; Dorrestein, P.C.; Gerwick, W.H. Underestimated biodiversity as a major explanation for the perceived rich secondary metabolite capacity of the cyanobacterial genus Lyngbya. Environ. Microbiol. 2011, 13, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Tristan, S.; Parra-Saldivar, R.; Iqbal, H.M.N.; Carrillo-Nieves, D. Bioinspired biomolecules: Mycosporine-like amino acids and scytonemin from Lyngbya sp. with UV-protection potentialities. J. Photochem. Photobiol. B Biol. 2019, 201, 111684. [Google Scholar] [CrossRef] [PubMed]
- Cowell, B.C.; Botts, P.S. Factors influencing the distribution, abundance and growth of Lyngbya wollei in central Florida. Aquat. Bot. 1994, 49, 1–17. [Google Scholar] [CrossRef]
- Bano, A.; Siddiqui, P.J.A. Characterization of five marine cyanobacterial species with respect to their pH and salinity requirements. Pakistan J. Bot. 2004, 36, 133–143. [Google Scholar]
- Manogar, P.; Vijayakumar, S.; Praseetha, P.K. Evaluation of antioxidant and neuroprotective activities of Lyngbya majuscula on human neural tissues. Gene Rep. 2020, 19, 100661. [Google Scholar] [CrossRef]
- Sameer Kumar, R.; Shakambari, G.; Ashokkumar, B.; Varalakshmi, P. Inhibition of advanced glycation end products formation and inflammation in C. elegans: Studies of potential of Lyngbya sp. against expression of stress related genes and Live cell imaging. Biocatal. Agric. Biotechnol. 2019, 17, 233–241. [Google Scholar] [CrossRef]
- Berry, J.P.; Gantar, M.; Gawley, R.E.; Wang, M.; Rein, K.S. Pharmacology and toxicology of pahayokolide A, a bioactive metabolite from a freshwater species of Lyngbya isolated from the Florida Everglades. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004, 139, 231–238. [Google Scholar] [CrossRef]
- Kumar, M.; Tripathi, M.K.; Srivastava, A.; Gour, J.K.; Singh, R.K.; Tilak, R.; Asthana, R.K. Cyanobacteria, Lyngbya aestuarii and Aphanothece bullosa as antifungal and antileishmanial drug resources. Asian Pac. J. Trop. Biomed. 2013, 3, 458–463. [Google Scholar] [CrossRef]
- Sharma, N.K.; Rai, A.K.; Stal, L.J.; Díez, B.; Ininbergs, K. Ecological importance of cyanobacteria. In Cyanobacteria: An Economic Perspective; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Nasseri, A.T.; Rasoul-Amini, S.; Morowvat, M.H.; Ghasemi, Y. Single cell protein: Production and process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Song, B.H.; Lee, D.H.; Kim, B.C.; Ku, S.H.; Park, E.J.; Kwon, I.H.; Kim, K.H.; Kim, K.J. Photodynamic therapy using chlorophyll-a in the treatment of acne vulgaris: A randomized, single-blind, split-face study. J. Am. Acad. Dermatol. 2014, 71, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Dashwood, R.; Negishi, T.; Hayatsu, H.; Breinholt, V.; Hendricks, J.; Bailey, G. Chemopreventive properties of chlorophylls towards aflatoxin B1: A review of the antimutagenicity and anticarcinogenicity data in rainbow trout. Carcinogenesis 1999, 20, 1919–1926. [Google Scholar] [CrossRef]
- Negishi, T.; Arimoto, S.; Nishizaki, C.; Hayatsu, H. Inhibitory effect of chlorophyll on the genotoxicity of. Carcinogenesis 1989, 10, 145–149. [Google Scholar] [CrossRef]
- Cherng, S.; Cheng, S.; Tarn, A.; Chou, T. Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macroophages. Life Sci. 2007, 81, 1431–1435. [Google Scholar] [CrossRef]
- Nagaoka, S.; Shimizu, K.; Kaneko, H.; Shibayama, F.; Morikawa, K.; Kanamaru, Y.; Otsuka, A.; Hirahashi, T.; Kato, T. Nutrient Interactions and Toxicity A Novel Protein C-Phycocyanin Plays a Crucial Role in the Hypocholesterolemic Action of Spirulina platensis Concentrate in Rats. Am. Soc. Nutr. 2005, 2425–2430. [Google Scholar]
- Sekar, S. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Sonani, R.R.; Singh, N.K.; Kumar, J.; Thakar, D.; Madamwar, D. Concurrent purification and antioxidant activity of phycobiliproteins from Lyngbya sp. A09DM: An antioxidant and anti-aging potential of phycoerythrin in Caenorhabditis elegans. Process Biochem. 2014, 49, 1757–1766. [Google Scholar] [CrossRef]
- Eriksen, N.T. Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef]
- Bertolin, T.E.; Guarienti, C.; Farias, D.; Souza, F.T.; Gutkoski, L.C.; Colla, L.M. Antioxidant effecct of phycocianin on dried-salted fish. Ciênc. Agrotec. 2011, 35, 751–757. [Google Scholar] [CrossRef][Green Version]
- Raja, R.; Hemaiswarya, S.; Kumar, N.A.; Sridhar, S.; Rengasamy, R. A perspective on the biotechnological potential of microalgae. Crit. Rev. Microbiol. 2008, 34, 77–88. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.G.; Ducat, D.C. Engineering cyanobacteria as photosynthetic feedstock factories. Photosynth. Res. 2015, 123, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.P.; Klisch, M.; Vaishampayan, A.; Häder, D.P. Biochemical and spectroscopic characterization of the cyanobacterium Lyngbya sp. inhabiting mango (Mangifera indica) trees: Presence of an ultraviolet-absorbing pigment, scytonemin. Acta Protozool. 1999, 38, 291–298. [Google Scholar]
- Rath, J.; Mandal, S.; Prasad, S. Salinity induced synthesis of UV-screening compound scytonemin in the cyanobacterium Lyngbya aestuarii. J. Photochem. Photobiol. B Biol. 2012, 115, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singh, P.; Tripathi, J.; Srivastava, A.; Tripathi, M.K.; Ravi, A.K.; Asthana, R.K. Identification and structure elucidation of antimicrobial compounds from Lyngbya aestuarii and Aphanothece bullosa. Cell. Mol. Biol. 2014, 60, 82–89. [Google Scholar]
- Entzeroth, M.; Mead, D.J.; Patterson, G.M.L.; Moore, R.E. A herbicidal fatty acid produced by Lyngbya aestuarii. Phytochemistry 1985, 24, 2875–2876. [Google Scholar] [CrossRef]
- Klein, D.; Braekman, J.C.; Daloze, D.; Hoffmann, L.; Castillo, G.; Demoulin, V. Madangolide and Laingolide A, Two Novel Macrolides from Lyngbya bouillonii (Cyanobacteria). J. Nat. Prod. 1999, 62, 934–936. [Google Scholar] [CrossRef]
- MacMillan, J.B.; Ernst-Russell, M.A.; de Ropp, J.S.; Molinski, T.F. Lobocyclamides A−C, Lipopeptides from a Cryptic Cyanobacterial Mat Containing Lyngbya confervoides. J. Org. Chem. 2002, 67, 8210–8215. [Google Scholar] [CrossRef]
- Matthew, S.; Ross, C.; Paul, V.J.; Luesch, H. Pompanopeptins A and B, new cyclic peptides from the marine cyanobacterium Lyngbya confervoides. Tetrahedron 2008, 64, 4081–4089. [Google Scholar] [CrossRef]
- Schaeffer, D.J.; Krylov, V.S. Anti-HIV Activity of Extracts and Compounds from Algae and Cyanobacteria. Ecotoxicol. Environ. Saf. 2000, 45, 208–227. [Google Scholar] [CrossRef] [PubMed]
- Mynderse, J.; Moore, R.; Kashiwagi, M.; Norton, T. Antileukemia activity in the Osillatoriaceae: Isolation of Debromoaplysiatoxin from Lyngbya. Science 1977, 196, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Rein, K.S. New Peptides Isolated from Lyngbya Species: A Review. Mar. Drugs 2010, 8, 1817–1837. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Ross, C.; Paul, V.J.; Matthew, S.; Luesch, H. Dragonamides C and D, Linear Lipopeptides from the Marine Cyanobacterium Brown Lyngbya polychroa. J. Nat. Prod. 2008, 71, 887–890. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Andrianasolo, E.H.; Shin, W.K.; Goeger, D.E.; Yokochi, A.; Schemies, J.; Jung, M.; France, D.; Cornell-Kennon, S.; Lee, E.; et al. Structural and Synthetic Investigations of Tanikolide Dimer, a SIRT2 Selective Inhibitor, and Tanikolide seco -Acid from the Madagascar Marine Cyanobacterium Lyngbya majuscula. J. Org. Chem. 2009, 74, 5267–5275. [Google Scholar] [CrossRef]
- Orjala, J.; Nagle, D.G.; Hsu, V.; Gerwick, W.H. Antillatoxin: An Exceptionally Ichthyotoxic Cyclic Lipopeptide from the Tropical Cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 1995, 117, 8281–8282. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Suyama, T.L.; Engene, N.; Wingerd, J.S.; Matainaho, T.; Gerwick, W.H. Apratoxin D, a Potent Cytotoxic Cyclodepsipeptide from Papua New Guinea Collections of the Marine Cyanobacteria Lyngbya majuscula and Lyngbya sordida. J. Nat. Prod. 2008, 71, 1099–1103. [Google Scholar] [CrossRef]
- Kothari, A.; Vaughn, M.; Garcia-Pichel, F. Comparative genomic analyses of the cyanobacterium, Lyngbya aestuarii BL J, a powerful hydrogen producer. Front. Microbiol. 2013, 4, 363. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumari, S.; Rastogi, R.P.; Singh, K.L.; Sinha, R.P. Photoprotective and biotechnological potentials of cyanobacterial sheath pigment, scytonemin. Afr. J. Biotechnol. 2010, 9, 580–588. [Google Scholar] [CrossRef][Green Version]
- Hoiczyk, E.; Hansel, A. Cyanobacterial cell walls: News from an unusual prokaryotic envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef]
- Balskus, E.P.; Case, R.J.; Walsh, C.T. The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities. FEMS Microbiol. Ecol. 2011, 77, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Rajneesh, R.; Sonker, A.S.; Kannaujiya, V.K.; Sinha, R.P. Isolation and partial purification of scytonemin and mycosporine-like amino acids from biological crusts. J. Chem. Pharm. Res. 2015, 7, 362–371. [Google Scholar]
- Constante, A.; Pillay, S.; Ning, H.; Vaidya, U.K. Utilization of algae blooms as a source of natural fibers for biocomposite materials: Study of morphology and mechanical performance of Lyngbya fibers. Algal Res. 2015, 12, 412–420. [Google Scholar] [CrossRef]
- Kushwaha, D.; Srivastava, N.; Prasad, D.; Mishra, P.K.; Upadhyay, S.N. Biobutanol production from hydrolysates of cyanobacteria Lyngbya limnetica and Oscillatoria obscura. Fuel 2020, 271, 117583. [Google Scholar] [CrossRef]
- King, L.J. A List of Myxophyceae from Wayne County, Indiana. Proc. Indiana Acad. Sci. 1942, 52, 71–81. [Google Scholar]
- Gomont, M. Monographie des Oscillariées (Nostocacées homocystées). Ann. Sci. Nat. Bot. Ser. 1892, 15, 263–368. [Google Scholar]
- Shruthi, M.S.; Rajashekhar, M. Effect of salinity and pH on the growth and biomass production in the four species of estuarine cyanobacteria. J. Algal Biomass Utln 2014, 5, 29–36. [Google Scholar]
- Kim, J.H.; Choi, W.; Jeon, S.-M.; Kim, T.; Park, A.; Kim, J.; Heo, S.-J.; Oh, C.; Shim, W.-B.; Kang, D.-H. Isolation and characterization of Leptolyngbya sp. KIOST-1, a basophilic and euryhaline filamentous cyanobacterium from an open paddle-wheel raceway Arthrospira culture pond in Korea. J. Appl. Microbiol. 2015, 119, 1597–1612. [Google Scholar] [CrossRef]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P.K. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, V.P.; Prasad, S.M. NaCl-induced physiological and biochemical changes in two cyanobacteria Nostoc muscorum and Phormidium foveolarum acclimatized to different photosynthetically active radiation. J. Photochem. Photobiol. B Biol. 2015, 151, 221–232. [Google Scholar] [CrossRef]
- Patel, V.K.; Sundaram, S.; Patel, A.K.; Kalra, A. Characterization of Seven Species of Cyanobacteria for High-Quality Biomass Production. Arab. J. Sci. Eng. 2017, 43, 109–121. [Google Scholar] [CrossRef]
- Uma, V.S.; Gnanasekaran, D.; Lakshmanan, U.; Dharmar, P. Survey and isolation of marine cyanobacteria from eastern coast of India as a biodiesel feedstock. Biocatal. Agric. Biotechnol. 2020, 24, 101541. [Google Scholar] [CrossRef]
- Cui, J.; Sun, T.; Chen, L.; Zhang, W. Engineering salt tolerance of photosynthetic cyanobacteria for seawater utilization. Biotechnol. Adv. 2020, 43, 107578. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Song, J.Y.; Cho, S.M.; Kwon, H.C.; Pan, C.-H.; Park, Y.-I. Genomic Survey of Salt Acclimation-Related Genes in the Halophilic Cyanobacterium. Sci. Rep. 2020, 10, 676. [Google Scholar] [CrossRef]
- Cetin, A.K. Effect of nitrogen sources on growth, protein, total lipid amount and pigment content in Chlorella vulgaris. Fresen. Environ. Bull. 2019, 28, 3065–3072. [Google Scholar]
- Javad, M.; Farhadian, O.; Paykan, F.; Keramat, J. Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt. J. Aquat. Res. 2019, 46, 153–158. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Singh, A.; Tripathi, K.K.; Saxena, R.K.; Eriksson, K.E.L. Microorganisms as an alternative source of protein. Nutr. Rev. 1997, 55, 65–75. [Google Scholar] [CrossRef]
- Praveenkumar, R.; Shameera, K.; Mahalakshmi, G. Influence of nutrient deprivations on lipid accumulation in a dominant indigenous microalga Chlorella sp., BUM11008: Evaluation for biodiesel production. Biomass Bioenergy 2011, 37, 60–66. [Google Scholar] [CrossRef]
- Yenh, K.-L.; Chang, J.-S. Nitrogen starvation strategies and photobioreactor design for enhancing lipid production of a newly isolated microalga Chlorella vulgaris ESP-31: Implications for biofuels. Biotechnol. J. 2011, 6, 1358–1366. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; George, B.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Bioresource Technology Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2014, 156, 146–154. [Google Scholar] [CrossRef]
- Yongmanitchai, W.; Ward, P. Growth of and Omega-3 Fatty Acid Production by Phaeodactylum tricornutum under Different Culture Conditions. Appl. Environ. Microbiol. 1991, 57, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Das, P.; Vishal, G.; Nagra, S. Factors affecting the induction of UV protectant and lipid productivity in Lyngbya for sequential biorefinery product recovery. Bioresour. Technol. 2019, 278, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Sharathchandra, K.; Rajashekhar, M. Total lipid and fatty acid composition in some freshwater cyanobacteria. J. Algal Biomass Util. 2011, 2, 83–97. [Google Scholar]
- Chandra, R.; Pons-Faudoa, F.P.; Parra Saldívar, R.; Rittmann, B.E. Effect of ultra-violet exposure on production of mycosporine-like amino acids and lipids by Lyngbya purpurem. Biomass Bioenergy 2020, 134, 105475. [Google Scholar] [CrossRef]
- Du, W.; Liang, F.; Duan, Y.; Tan, X.; Lu, X. Exploring the photosynthetic production capacity of sucrose by cyanobacteria. Metab. Eng. 2013, 19, 17–25. [Google Scholar] [CrossRef]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using High Performance Thin Layer Chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Arora, R.; Sudhakar, K.; Rana, R.S. Comparative study on the growth performance of Spirulina platensis on modifying culture media. Energy Rep. 2019, 5, 327–336. [Google Scholar] [CrossRef]
- Danesi, E.D.G.; De Carvalho, J.C.M.; Sato, S. An investigation of e ect of replacing nitrate by urea in the growth and production of chlorophyll by Spirulina platensis. Biomm 2002, 23, 261–269. [Google Scholar]
- Sudhir, P.; Pogoryelov, D.; Kovács, L.; Garab, G.; Murthy, S.D.S. The Effects of Salt Stress on Photosynthetic Electron Transport and Thylakoid Membrane Proteins in the Cyanobacterium Spirulina platensis. Biochem. Mol. Biol. 2005, 38, 481–485. [Google Scholar] [CrossRef]
- Setyoningrum, T.M.; Nur, M.M.A. Biocatalysis and Agricultural Biotechnology Optimization of C-phycocyanin production from S. platensis cultivated on mixotrophic condition by using response surface methodology. Biocatal. Agric. Biotechnol. 2015, 4, 603–607. [Google Scholar] [CrossRef]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J. Rapid quantification of microalgal lipids in aqueous medium by a simple colorimetric method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef] [PubMed]
- López-legarda, X.; Taramuel-gallardo, A.; Arboleda-, C. Comparison of methods using sulfuric acid for determination of total sugars. Rev. Cuba Quím. 2017, 29, 180–198. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Extraction of chlorophylls and carotenoids from dry and wet biomass of isolated Chlorella Thermophila: Optimization of process parameters and modelling by artificial neural network. Process Biochem. 2020, 96, 58–72. [Google Scholar] [CrossRef]
- Beer, S.; Eshel, A. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Mar. Freshw. Res. 1985, 36, 785–792. [Google Scholar] [CrossRef]


| Biomolecule | Beneficial Properties and Applications | Reference |
|---|---|---|
| General biomolecules of Lyngbya species | ||
| Chlorophyll a | Potential applications in the cosmetic, food, and pharmaceutical industries due to its anticarcinogenic and antimutagenic activity, and inhibitory effect on genotoxicity. It has been shown to reduce acne lesions and sebum levels, exhibiting antioxidant activity and protection against lipid oxidation at concentrations of 1mM L−1. | [12,13,14,15] |
| Phycocyanin | Applications as fluorescent agent in fluorescent probes; in food preservation by working as antioxidant; as natural pigment in food, beverages, cosmetics, and textiles; and as nutraceutical and pharmaceutical due to its anti-inflamatory, antioxidant, anticancerous, hepatoprotective, neuroprotective, and hypocholesterolemic activity. | [16,17,18,19,20,21] |
| Protein | Protein production from microbial biomass, or single-cell protein (SCP), as an alternative source of protein and other biomolecules rather than animal sources. SCP can be used in human nutrition as protein supplements, in animal breading and cattle fattening. Furthermore, in paper, leather processing, and other industries. | [10] |
| Lipids | Lipids produced by microorganisms can be used in the production of biodiesel and other biofuels. | [22,23] |
| Carbohydrates | Microbial carbohydrates can be used for biofuels production and food industry as an alternative source of carbohydrates production. | [24] |
| Specific biomolecules of Lyngbya species | ||
| Scytonemin and Mycosporine-like aminoacids | UV-absorbing compounds with potential applications in cosmeceuticals as sun-care and antiaging products, and photostabilizer additives in paints, varnishes, and plastics. They possess antioxidant activity and antiphotoaging effect on human skin cells. | [2,25,26] |
| Malyngolide | Antibacterial and antifungal activity | [27] |
| 2,5-dimethyldodecanoic acid | Herbicidal activity | [28] |
| Madangolide | Cytotoxicity against several cancer cell lines | [29] |
| Laingolide A | Cytotoxicity against several cancer cell lines | [29] |
| Lobocyclamide | Antifungal activity | [30] |
| Pompanopeptin A (PPPA) | Inhibitor of trypsin | [31] |
| Sulfolipids with different fatty acid esters | Anti-HIV activity | [32] |
| Debromoaplysiatoxin | Dermonecrotic activity and inflammatory | [33] |
| Carmabin A | Antimalarial activity | [34,35] |
| Curacin A | Cytotoxicity against several cancer cell lines | [34] |
| Tanikolide | Cytotoxicity against several cancer cell lines | [36] |
| Antillatoxin | Sodium-channel-blocking activity | [37] |
| Apratoxin D | Cytotoxicity against several cancer cell lines | [38] |
| BG11 | Bold 3N | Production Yield | ||||
|---|---|---|---|---|---|---|
| Salinity | Incubation Time | Salinity | Incubation Time | |||
| Total biomass | Low | 25 | 1.18 ± 0.06 | g L−1 | ||
| Proteins | Low | 20 | 431.69 ± 9.5 | mg g−1 DW | ||
| Lipids | Low | 25 | 131.5 ± 22.81 | mg g−1 DW | ||
| Carbohydrates | Med | 20 | 301.45 ± 38.94 | mg g−1 DW | ||
| Chlorophyll a | Med | 15 | 4.09 ± 0.88 | mg g−1 DW | ||
| Phycocyanin | Med | 20 | 40.4 ± 2.23 | mg g−1 DW | ||
| Run | Blocks | Salinity | Day |
|---|---|---|---|
| 1 | 1 | Low | 15 |
| 2 | 1 | Low | 20 |
| 3 | 1 | Low | 25 |
| 4 | 1 | Medium | 15 |
| 5 | 1 | Medium | 20 |
| 6 | 1 | Medium | 25 |
| 7 | 1 | High | 15 |
| 8 | 1 | High | 20 |
| 9 | 1 | High | 25 |
| 10 | 2 | Low | 15 |
| 11 | 2 | Low | 20 |
| 12 | 2 | Low | 25 |
| 13 | 2 | Medium | 15 |
| 14 | 2 | Medium | 20 |
| 15 | 2 | Medium | 25 |
| 16 | 2 | High | 15 |
| 17 | 2 | High | 20 |
| 18 | 2 | High | 25 |
| 19 | 3 | Low | 15 |
| 20 | 3 | Low | 20 |
| 21 | 3 | Low | 25 |
| 22 | 3 | Medium | 15 |
| 23 | 3 | Medium | 20 |
| 24 | 3 | Medium | 25 |
| 25 | 3 | High | 15 |
| 26 | 3 | High | 20 |
| 27 | 3 | High | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Pacheco, I.Y.; Fuentes-Tristan, S.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Pedro-Carrillo, I.; Martínez-Prado, M.A.; Iqbal, H.M.N.; Parra-Saldívar, R. Influence of Low Salt Concentration on Growth Behavior and General Biomass Composition in Lyngbya purpurem (Cyanobacteria). Mar. Drugs 2020, 18, 621. https://doi.org/10.3390/md18120621
López-Pacheco IY, Fuentes-Tristan S, Rodas-Zuluaga LI, Castillo-Zacarías C, Pedro-Carrillo I, Martínez-Prado MA, Iqbal HMN, Parra-Saldívar R. Influence of Low Salt Concentration on Growth Behavior and General Biomass Composition in Lyngbya purpurem (Cyanobacteria). Marine Drugs. 2020; 18(12):621. https://doi.org/10.3390/md18120621
Chicago/Turabian StyleLópez-Pacheco, Itzel Y., Susana Fuentes-Tristan, Laura Isabel Rodas-Zuluaga, Carlos Castillo-Zacarías, Itzel Pedro-Carrillo, María Adriana Martínez-Prado, Hafiz M. N. Iqbal, and Roberto Parra-Saldívar. 2020. "Influence of Low Salt Concentration on Growth Behavior and General Biomass Composition in Lyngbya purpurem (Cyanobacteria)" Marine Drugs 18, no. 12: 621. https://doi.org/10.3390/md18120621
APA StyleLópez-Pacheco, I. Y., Fuentes-Tristan, S., Rodas-Zuluaga, L. I., Castillo-Zacarías, C., Pedro-Carrillo, I., Martínez-Prado, M. A., Iqbal, H. M. N., & Parra-Saldívar, R. (2020). Influence of Low Salt Concentration on Growth Behavior and General Biomass Composition in Lyngbya purpurem (Cyanobacteria). Marine Drugs, 18(12), 621. https://doi.org/10.3390/md18120621








