Oxygenated Theonellastrols: Interpretation of Unusual Chemical Behaviors Using Quantum Mechanical Calculations and Stereochemical Reassignment of 7α-Hydroxytheonellasterol
Abstract
1. Introduction
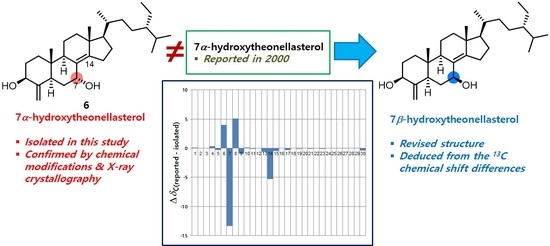
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. 13C Chemical Shift Calculations
3.5. IL-6 Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Kho, E.; Imagawa, D.K.; Rohmer, M.; Kashman, Y.; Djerassi, C. Sterols in marine invertebrates. 22. Isolation and structure elucidation of conicasterol and theonellasterol, two new 4-methylene sterols from the Red Sea sponges Theonella conica and Theonella swinhoei. J. Org. Chem. 1981, 46, 1836–1839. [Google Scholar] [CrossRef]
- Carmely, S.; Kashman, Y. Structure of swinholide-A, a new macrolide from the marine sponge Theonella swinhoei. Tetrahedron Lett. 1985, 26, 511–514. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tanaka, J.; Katori, T.; Kitagawa, I. Marine natural products. XXIII: Three new cytotoxic dimeric macrolides, Swinholides B and C and Isoswinholide A, congeners of Swinholide A, from the Okinawan marine sponge Theonella swinhoei. Chem. Pharm. Bull. 1990, 38, 2960–2966. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Ishibashi, M.; Sasaki, T.; Kobayashi, J.I. New congeners of swinholides from the okinawan marine sponge Theonella sp. J. Chem. Soc. Perkin Trans. 1 1991, 3185–3188. [Google Scholar] [CrossRef]
- Dumdei, E.J.; Blunt, J.W.; Munro, M.H.; Pannell, L.K. Isolation of calyculins, calyculinamides, and swinholide H from the New Zealand deep-water marine sponge Lamellomorpha strongylata. J. Org. Chem. 1997, 62, 2636–2639. [Google Scholar] [CrossRef]
- Youssef, D.T.; Mooberry, S.L. Hurghadolide A and Swinholide I, Potent Actin-Microfilament Disrupters from the Red Sea Sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 154–157. [Google Scholar] [CrossRef]
- Marino, S.D.; Festa, C.; D’Auria, M.V.; Cresteil, T.; Debitus, C.; Zampella, A. Swinholide J, a potent cytotoxin from the marine sponge Theonella swinhoei. Mar. Drugs 2011, 9, 1133–1141. [Google Scholar] [CrossRef]
- Sinisi, A.; Calcinai, B.; Cerrano, C.; Dien, H.A.; Zampella, A.; D’Amore, C.; Renga, B.; Fiorucci, S.; Taglialatela-Scafati, O. Isoswinholide B and swinholide K, potently cytotoxic dimeric macrolides from Theonella swinhoei. Bioorg. Med. Chem. 2013, 21, 5332–5338. [Google Scholar] [CrossRef]
- Sakai, R.; Higa, T.; Kashman, Y. Misakinolide-A, an antitumor macrolide from the marine sponge Theonella sp. Chem. Lett. 1986, 15, 1499–1502. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kondo, K.; Ishibashi, M.; Walchli, M.R.; Nakamura, T. Theonezolide A: A novel polyketide natural product from the Okinawan marine sponge Theonella sp. J. Am. Chem. Soc. 1993, 115, 6661–6665. [Google Scholar] [CrossRef]
- Kondo, K.; Ishibashi, M.; Kobayashi, J. Isolation and structures of Theonezolides B and C from the Okinawan marine sponge Theonella sp. Tetrahedron 1994, 50, 8355–8362. [Google Scholar] [CrossRef]
- Ford, P.W.; Gustafson, K.R.; McKee, T.C.; Shigematsu, N.; Maurizi, L.K.; Pannell, L.K.; Williams, D.E.; Dilip de Silva, E.; Lassota, P.; Allen, T.M. Papuamides A−D, HIV-Inhibitory and Cytotoxic Depsipeptides from the Sponges Theonella mirabilis and Theonella swinhoei Collected in Papua New Guinea. J. Am. Chem. Soc. 1999, 121, 5899–5909. [Google Scholar] [CrossRef]
- Fusetani, N.; Matsunaga, S.; Matsumoto, H.; Takebayashi, Y. Bioactive marine metabolites. 33. Cyclotheonamides, potent thrombin inhibitors, from a marine sponge Theonella sp. J. Am. Chem. Soc. 1990, 112, 7053–7054. [Google Scholar] [CrossRef]
- Nakao, Y.; Matsunaga, S.; Fusetani, N. Three more cyclotheonamides, C, D, and E, potent thrombin inhibitors from the marine sponge Theonella swinhoei. Bioorg. Med. Chem. 1995, 3, 1115–1122. [Google Scholar] [CrossRef]
- Hamada, T.; Matsunaga, S.; Yano, G.; Fusetani, N. Polytheonamides A and B, Highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J. Am. Chem. Soc. 2005, 127, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Fusetani, N. Theonellamides A−E, cytotoxic bicyclic peptides, from a marine sponge Theonella sp. J. Org. Chem. 1995, 60, 1177–1181. [Google Scholar] [CrossRef]
- Wada, S.-I.; Matsunaga, S.; Fusetani, N.; Watabe, S. Theonellamide F, a bicyclic peptide marine toxin, induces formation of vacuoles in 3Y1 rat embryonic fibroblast. Mar. Biotechnol. 1999, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Umeyama, A.; Shoji, N.; Enoki, M.; Arihara, S. Swinhosterols A−C, 4-Methylene Secosteroids from the Marine Sponge Theonella swinhoei. J. Nat. Prod. 1997, 60, 296–298. [Google Scholar] [CrossRef]
- Festa, C.; De Marino, S.; D’Auria, M.V.; Bifulco, G.; Renga, B.; Fiorucci, S.; Petek, S.; Zampella, A. Solomonsterols A and B from Theonella swinhoei. The first example of C-24 and C-23 sulfated sterols from a marine source endowed with a PXR agonistic activity. J. Med. Chem. 2011, 54, 401–405. [Google Scholar] [CrossRef]
- Gong, J.; Sun, P.; Jiang, N.; Riccio, R.; Lauro, G.; Bifulco, G.; Li, T.-J.; Gerwick, W.H.; Zhang, W. New steroids with a rearranged skeleton as (h) P300 inhibitors from the sponge Theonella swinhoei. Org. Lett. 2014, 16, 2224–2227. [Google Scholar] [CrossRef]
- Li, J.; Tang, H.; Kurtán, T.; Mándi, A.; Zhuang, C.-L.; Su, L.; Zheng, G.-L.; Zhang, W. Swinhoeisterols from the South China Sea Sponge Theonella swinhoei. J. Nat. Prod. 2018, 81, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, Y.-Y.; Tang, J.; Sun, F.; Lin, H.-W. New 4-methylidene sterols from the marine sponge Theonella swinhoei. Fitoterapia 2018, 127, 279–285. [Google Scholar] [CrossRef]
- De Marino, S.; Ummarino, R.; D’Auria, M.V.; Chini, M.G.; Bifulco, G.; D’Amore, C.; Renga, B.; Mencarelli, A.; Petek, S.; Fiorucci, S. 4-Methylenesterols from Theonella swinhoei sponge are natural pregnane-X-receptor agonists and farnesoid-X-receptor antagonists that modulate innate immunity. Steroids 2012, 77, 484–495. [Google Scholar] [CrossRef]
- De Marino, S.; Ummarino, R.; D’Auria, M.V.; Chini, M.G.; Bifulco, G.; Renga, B.; D’Amore, C.; Fiorucci, S.; Debitus, C.; Zampella, A. Theonellasterols and conicasterols from Theonella swinhoei. Novel marine natural ligands for human nuclear receptors. J. Med. Chem. 2011, 54, 3065–3075. [Google Scholar] [CrossRef] [PubMed]
- Chini, M.G.; Jones, C.R.; Zampella, A.; D’Auria, M.V.; Renga, B.; Fiorucci, S.; Butts, C.P.; Bifulco, G. Quantitative NMR-derived interproton distances combined with quantum mechanical calculations of 13C chemical shifts in the stereochemical determination of conicasterol F, a nuclear receptor ligand from Theonella swinhoei. J. Org. Chem. 2012, 77, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Sepe, V.; Ummarino, R.; D’Auria, M.V.; Chini, M.G.; Bifulco, G.; Renga, B.; D’Amore, C.; Debitus, C.; Fiorucci, S.; Zampella, A. Conicasterol E, a small heterodimer partner sparing farnesoid X receptor modulator endowed with a pregnane X receptor agonistic activity, from the marine sponge Theonella swinhoei. J. Med. Chem. 2012, 55, 84–93. [Google Scholar] [CrossRef]
- Qureshi, A.; Faulkner, D.J. 7α-Hydroxytheonellasterol, a cytotoxic 4-methylene sterol from the Philippines sponge Theonella swinhoei. J. Nat. Prod. 2000, 63, 841–842. [Google Scholar] [CrossRef]
- Guo, J.-K.; Chiang, C.-Y.; Lu, M.-C.; Chang, W.-B.; Su, J.-H. 4-Methylenesterols from a Sponge Theonella swinhoei. Mar. Drugs 2012, 10, 1536–1544. [Google Scholar] [CrossRef]
- Sepe, V.; Ummarino, R.; D’Auria, M.V.; Taglialatela-Scafati, O.; Marino, S.D.; D’Amore, C.; Renga, B.; Chini, M.G.; Bifulco, G.; Nakao, Y. Preliminary structure-activity relationship on theonellasterol, a new chemotype of FXR antagonist, from the marine sponge Theonella swinhoei. Mar. Drugs 2012, 10, 2448–2466. [Google Scholar] [CrossRef]
- Pu, J.-X.; Huang, S.-X.; Ren, J.; Xiao, W.-L.; Li, L.-M.; Li, R.-T.; Li, L.-B.; Liao, T.-G.; Lou, L.-G.; Zhu, H.-J. Isolation and Structure Elucidation of Kadlongilactones C−F from Kadsura longipedunculata by NMR Spectroscopy and DFT Computational Methods. J. Nat. Prod. 2007, 70, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.; Jansma, M.J.; Hoye, T.R. Case study of empirical and computational chemical shift analyses: Reassignment of the relative configuration of phomopsichalasin to that of diaporthichalasin. J. Nat. Prod. 2012, 75, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Mun, B.; Wang, W.; Kim, H.; Hahn, D.; Yang, I.; Won, D.H.; Kim, E.-H.; Lee, J.; Han, C.; Kim, H. Cytotoxic 5α, 8α-epidioxy sterols from the marine sponge Monanchora sp. Arch. Pharm. Res. 2015, 38, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.L.; Gopichand, Y.; Schmitz, F.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. 26. Isolation and structure elucidation of nine new 5α, 8α-epidoxy sterols from four marine organisms. J. Org. Chem. 1981, 46, 3860–3866. [Google Scholar] [CrossRef]
- Vien, L.T.; Hanh, T.T.H.; Hong, P.T.; Thanh, N.V.; Huong, T.T.; Cuong, N.X.; Nam, N.H.; Thung, D.C.; Kiem, P.V.; Minh, C.V. Polar steroid derivatives from the Vietnamese starfish Astropecten polyacanthus. Nat. Prod. Res. 2018, 32, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, F.; Park, Y.; Hong, J.; Lee, C.-O.; Kong, J.Y.; Shin, S.; Im, K.S.; Jung, J.H. Bioactive Sterols from the Starfish Certonardoa semiregularis. J. Nat. Prod. 2003, 66, 384–391. [Google Scholar] [CrossRef]
- Zhang, H.J.; Yi, Y.H.; Lin, H.W. Oxygenated 4-Methylidene Sterols from the South China Sea Sponge Theonella swinhoei. Helv. Chim. Acta 2010, 93, 1120–1126. [Google Scholar] [CrossRef]
- Sright, J.; McInnes, A.; Shimizu, S.; Smith, D.; Walter, J.; Idler, D.; Khalil, W. Identification of C-24 alkyl epimers of marine sterols by 13C nuclear magnetic resonance spectroscopy. Can. J. Chem. 1978, 56, 1898–1903. [Google Scholar] [CrossRef]











| Position | δexp (ppm) | δcalcd (ppm) | |||
|---|---|---|---|---|---|
| 5-I | 5-II | 5-III | 5-IV | ||
| C-7 | 28.3 | 31.7 | 35.5 | 31.8 | 30.9 |
| C-8 | 71.3 | 72.4 | 74.2 | 63.4 | 65.0 |
| C-9 | 55.3 | 51.6 | 55.1 | 47.2 | 50.0 |
| C-13 | 42.5 | 45.7 | 47.3 | 43.3 | 42.5 |
| C-14 | 75.9 | 76.4 | 75.1 | 80.0 | 72.0 |
| C-15 | 58.8 | 60.3 | 62.5 | 73.9 | 65.8 |
| C-16 | 32.3 | 25.7 | 27.6 | 38.7 | 38.8 |
| DP4+ | 100% | 0% | 0% | 0% | |
| R2 | 0.9908 | 0.9828 | 0.9772 | 0.9845 | |
| MAD a | 1.38 | 1.91 | 2.30 | 1.64 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, A.-Y.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Son, A.; Choi, C.; Lee, J. Oxygenated Theonellastrols: Interpretation of Unusual Chemical Behaviors Using Quantum Mechanical Calculations and Stereochemical Reassignment of 7α-Hydroxytheonellasterol. Mar. Drugs 2020, 18, 607. https://doi.org/10.3390/md18120607
Shin A-Y, Lee H-S, Lee Y-J, Lee JS, Son A, Choi C, Lee J. Oxygenated Theonellastrols: Interpretation of Unusual Chemical Behaviors Using Quantum Mechanical Calculations and Stereochemical Reassignment of 7α-Hydroxytheonellasterol. Marine Drugs. 2020; 18(12):607. https://doi.org/10.3390/md18120607
Chicago/Turabian StyleShin, A-Young, Hyi-Seung Lee, Yeon-Ju Lee, Jong Seok Lee, Arang Son, Changhoon Choi, and Jihoon Lee. 2020. "Oxygenated Theonellastrols: Interpretation of Unusual Chemical Behaviors Using Quantum Mechanical Calculations and Stereochemical Reassignment of 7α-Hydroxytheonellasterol" Marine Drugs 18, no. 12: 607. https://doi.org/10.3390/md18120607
APA StyleShin, A.-Y., Lee, H.-S., Lee, Y.-J., Lee, J. S., Son, A., Choi, C., & Lee, J. (2020). Oxygenated Theonellastrols: Interpretation of Unusual Chemical Behaviors Using Quantum Mechanical Calculations and Stereochemical Reassignment of 7α-Hydroxytheonellasterol. Marine Drugs, 18(12), 607. https://doi.org/10.3390/md18120607