Production, Characterization and Immunomodulatory Activity of an Extracellular Polysaccharide from Rhodotorula mucilaginosa YL-1 Isolated from Sea Salt Field
Abstract
1. Introduction
2. Results
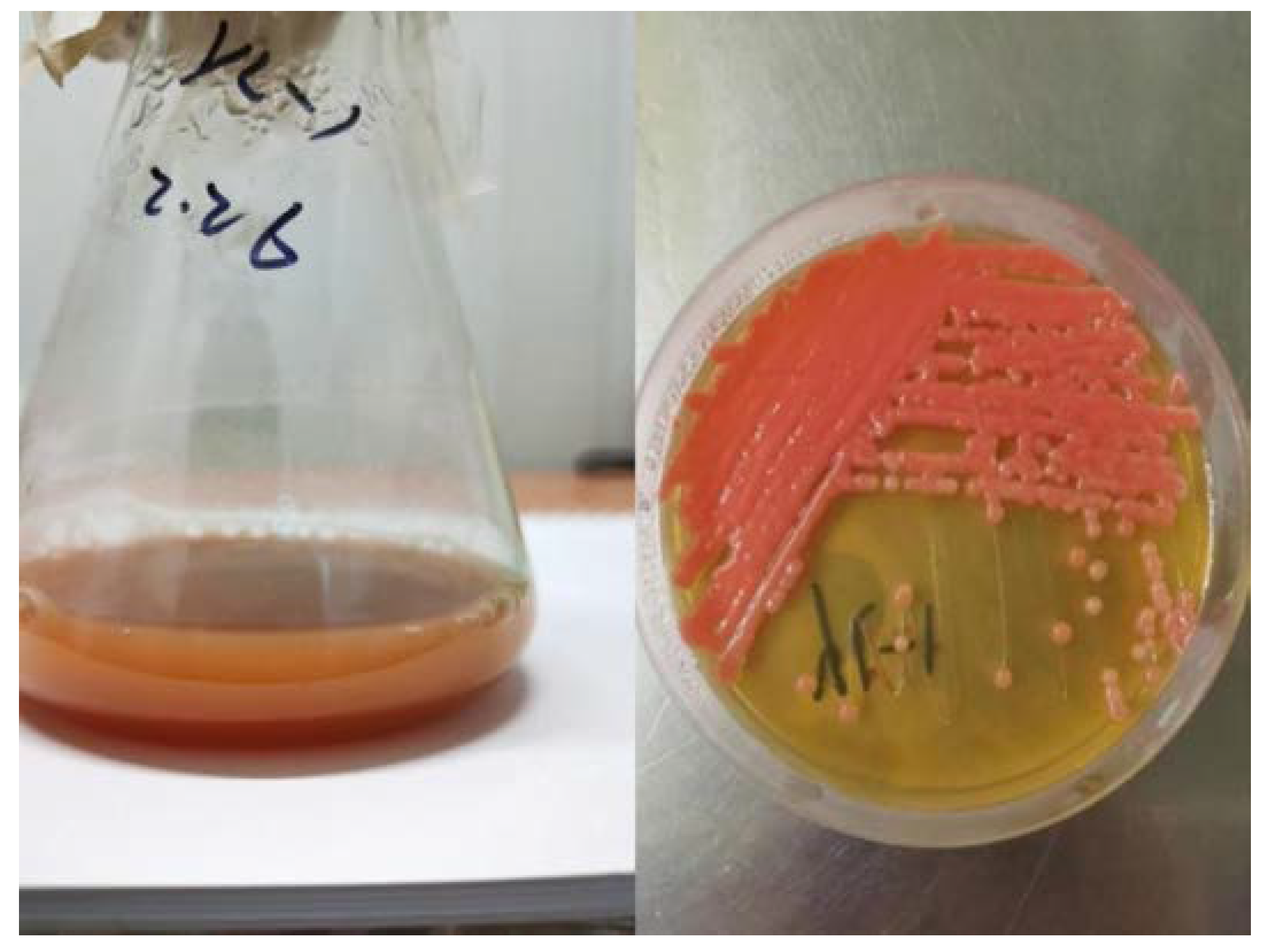
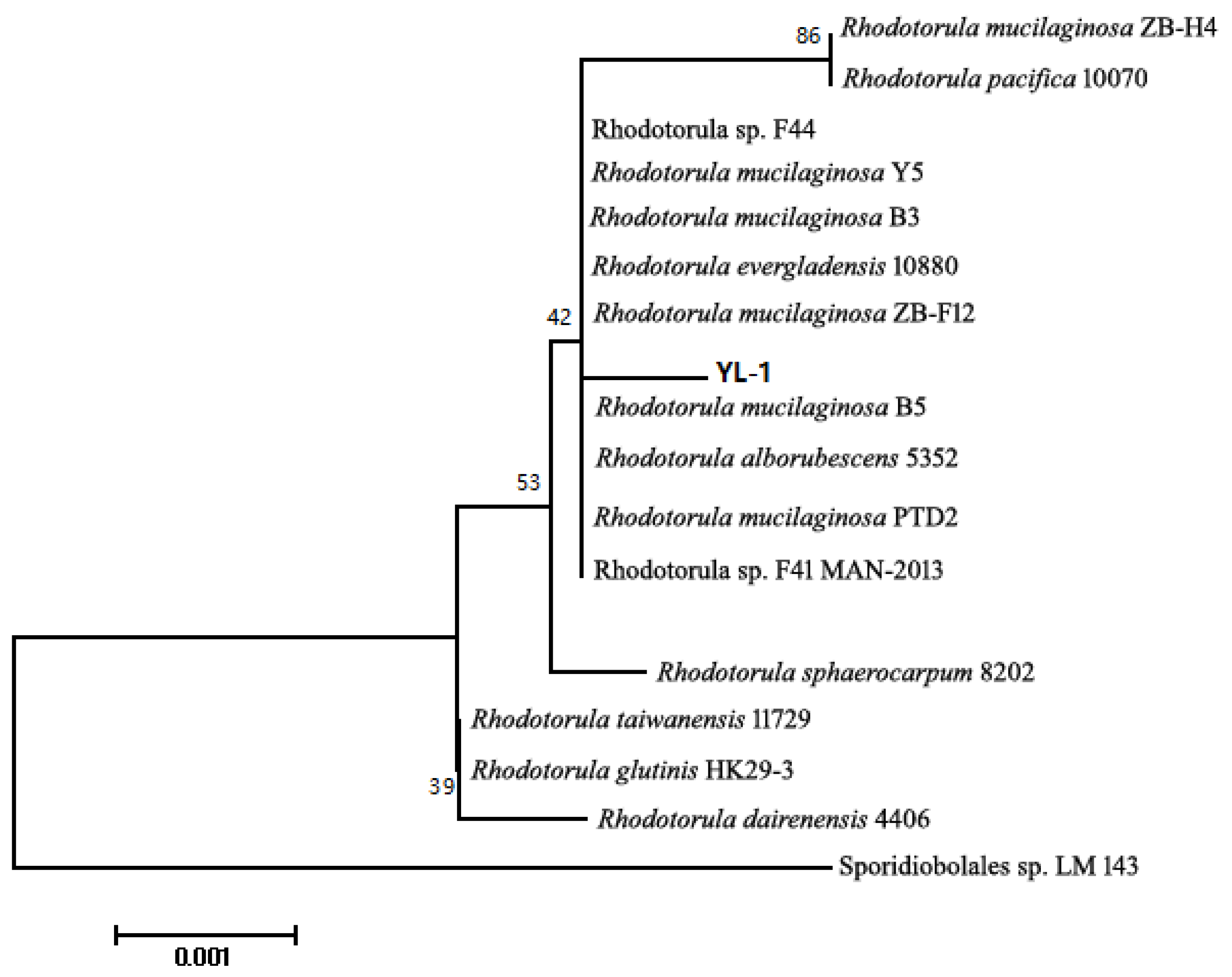
2.1. Identification of Strain and Phylogenetic Tree
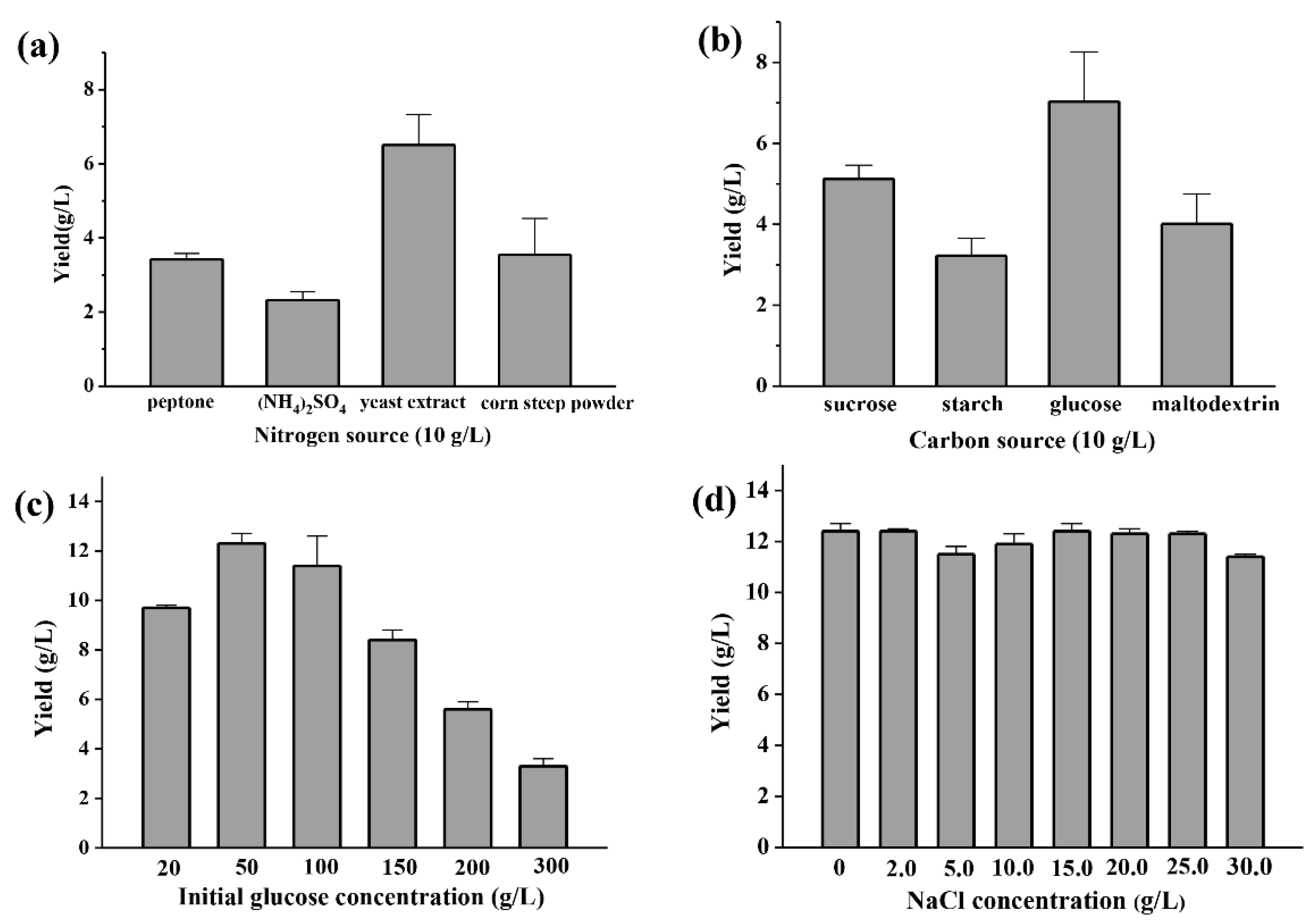
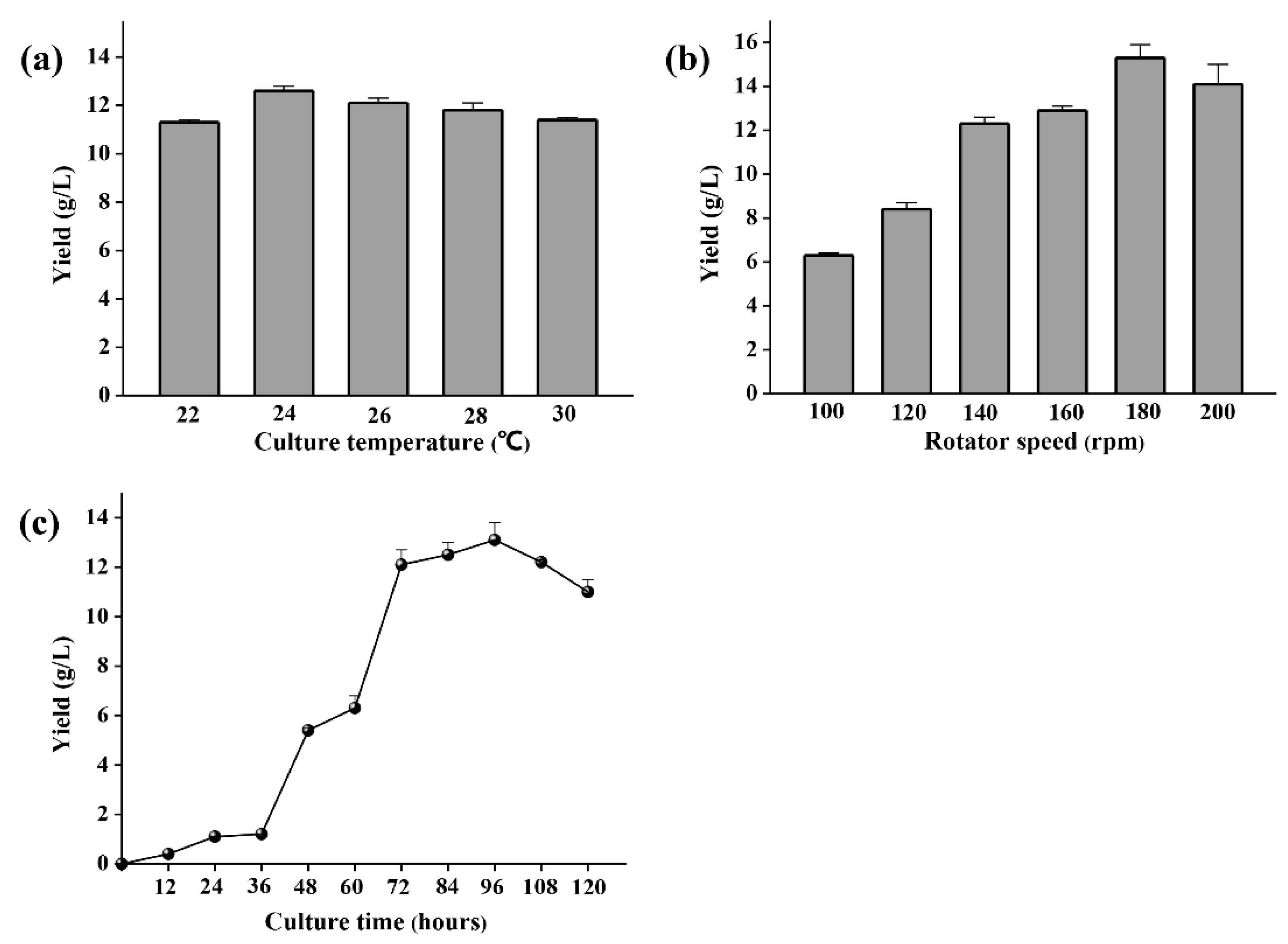
2.2. Polysaccharide Production Optimization
2.3. Purification and Structural Characterization of YL-1 Polysaccharide
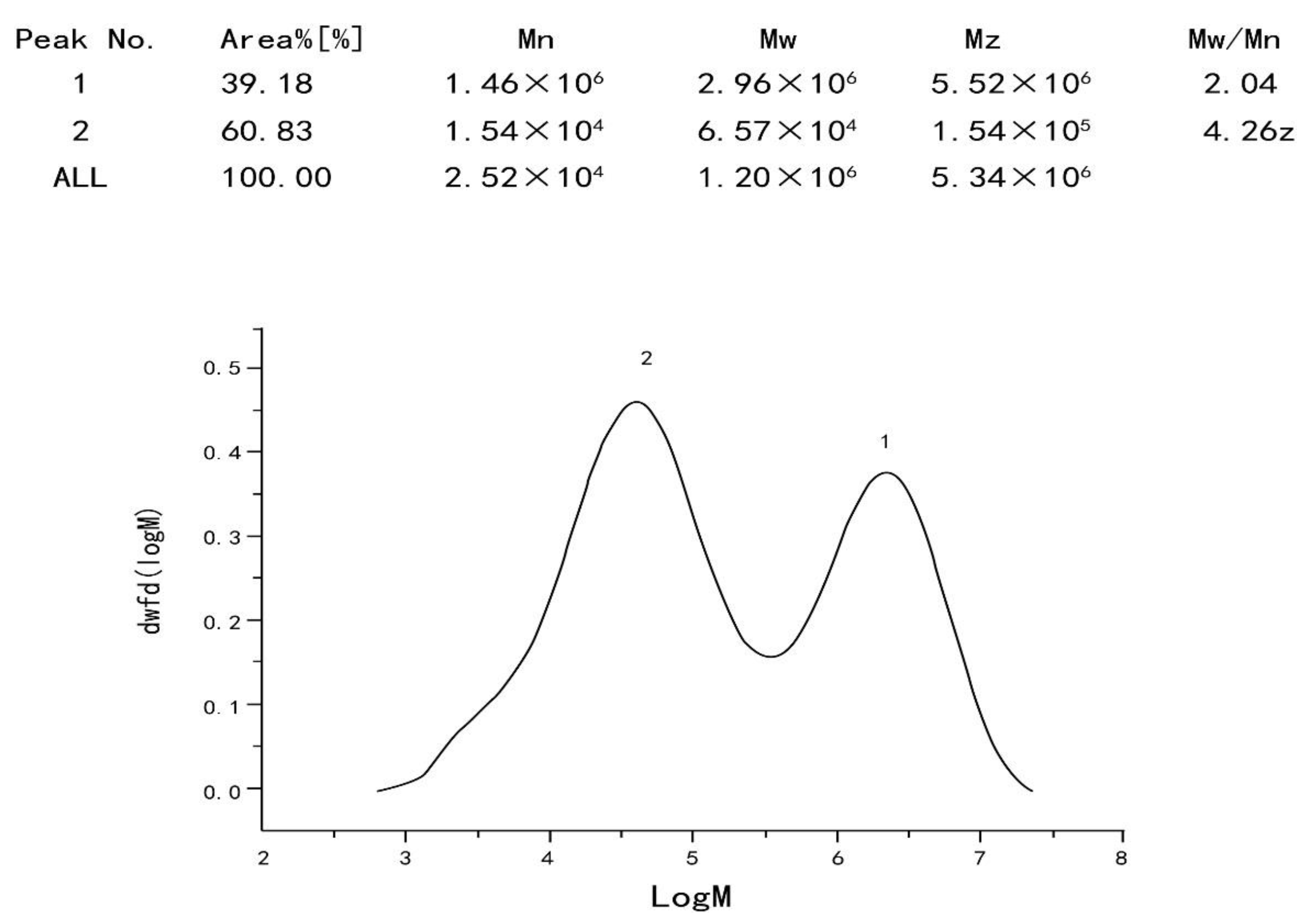
2.3.1. Determination of Homogeneity and Molecular Weight of Polysaccharide
2.3.2. Monosaccharide Composition Analysis
2.3.3. Sulfate Content Determination
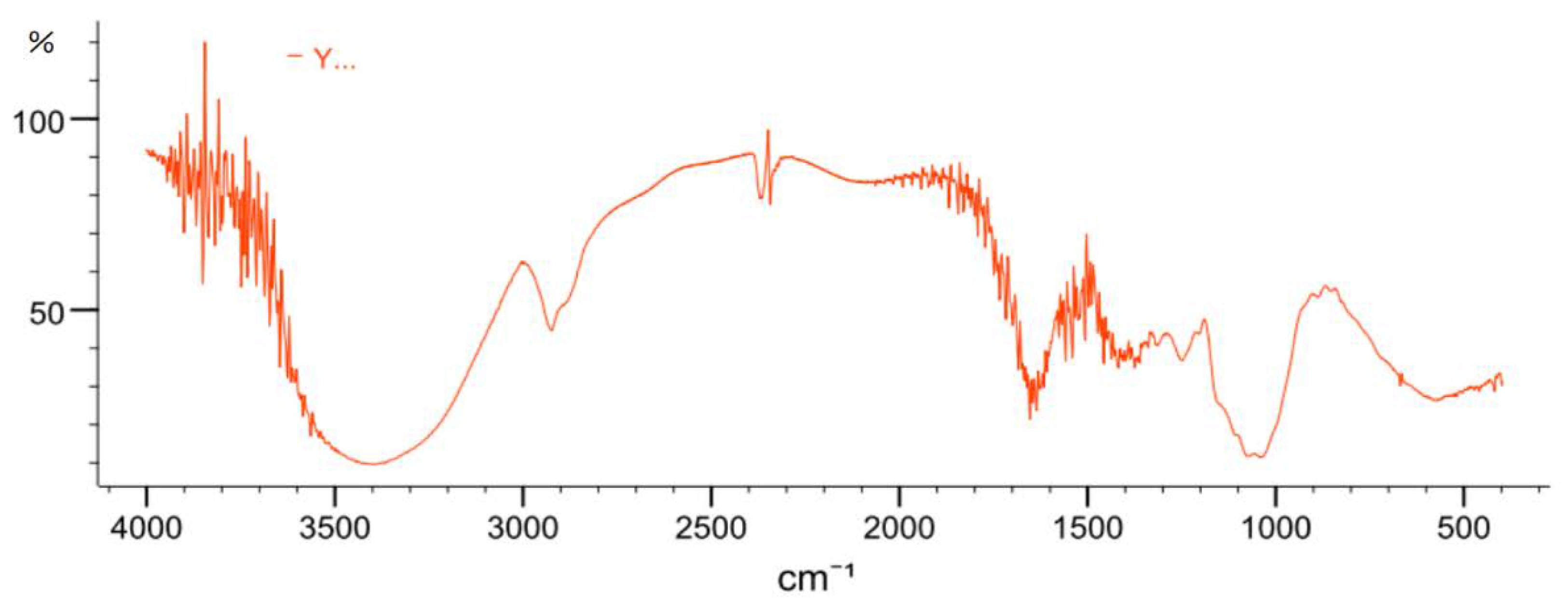
2.3.4. FT-IR Spectrometric Analysis
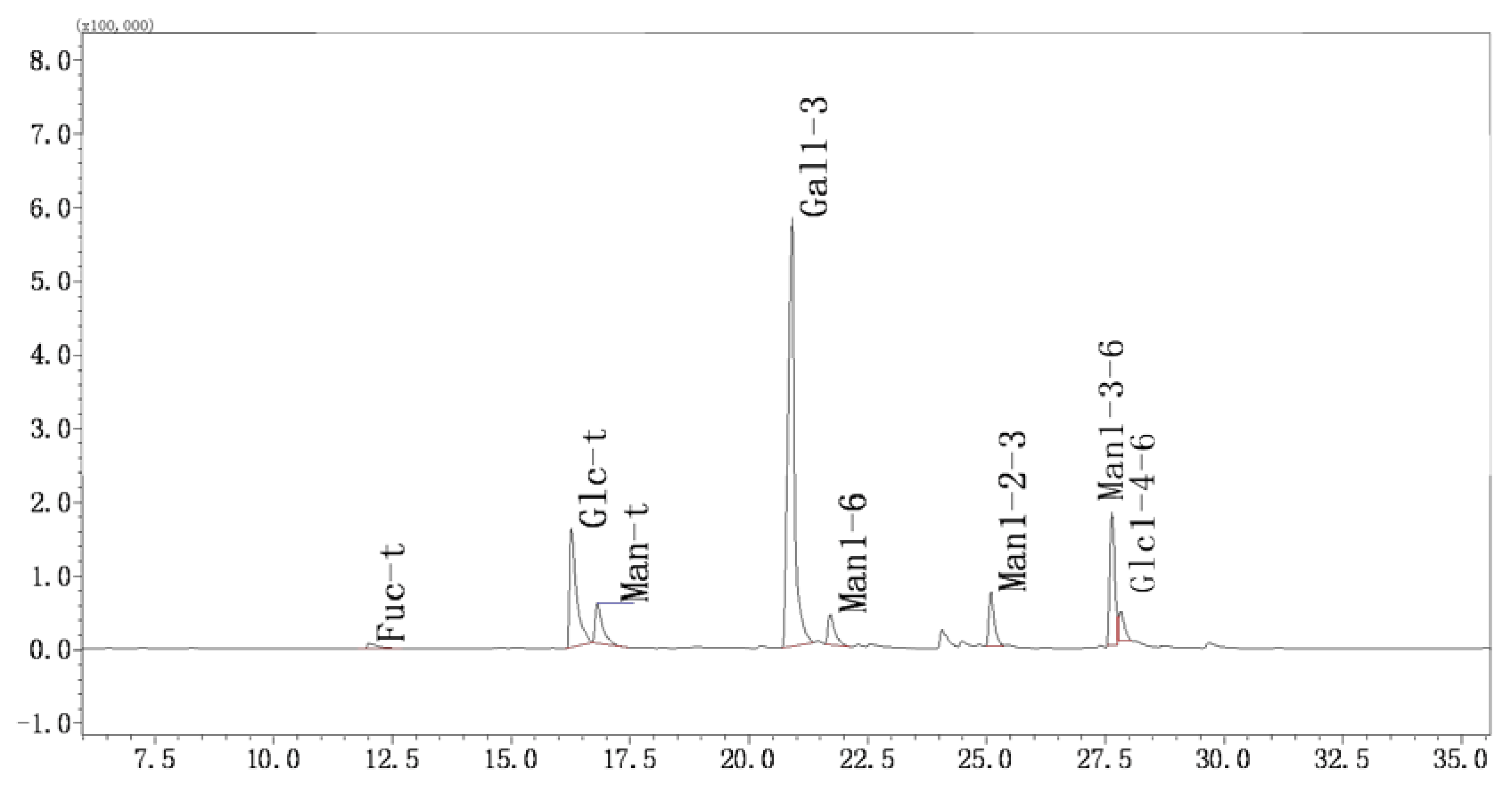
2.3.5. Methylation and GC-MS Analysis
2.4. Bioactivity Test of Polysaccharide on RAW264.7 Cell Lines
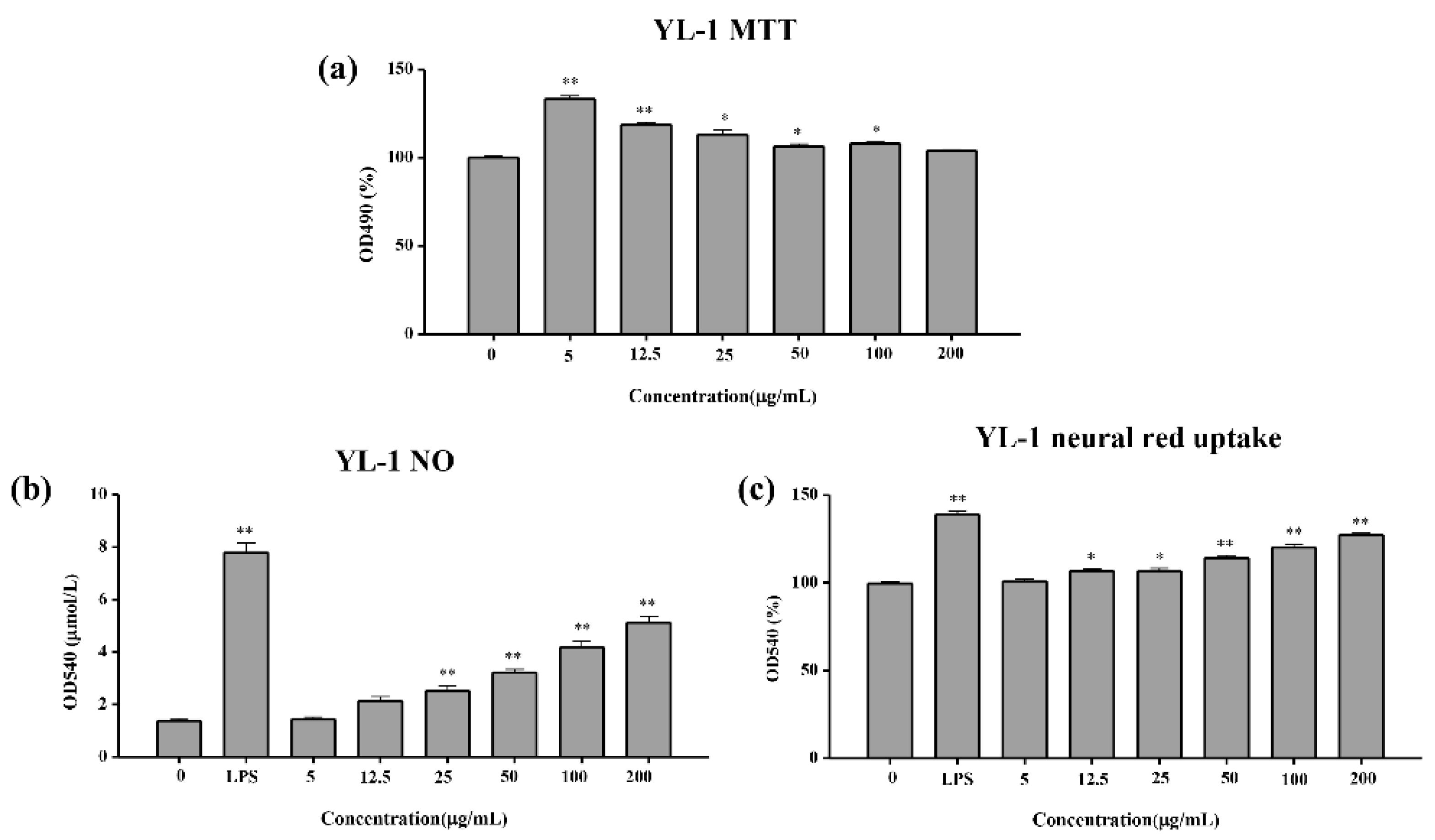
2.4.1. MTT Test of the Cell Viability
2.4.2. NO Release Test
2.4.3. Neutral Red Uptake Test
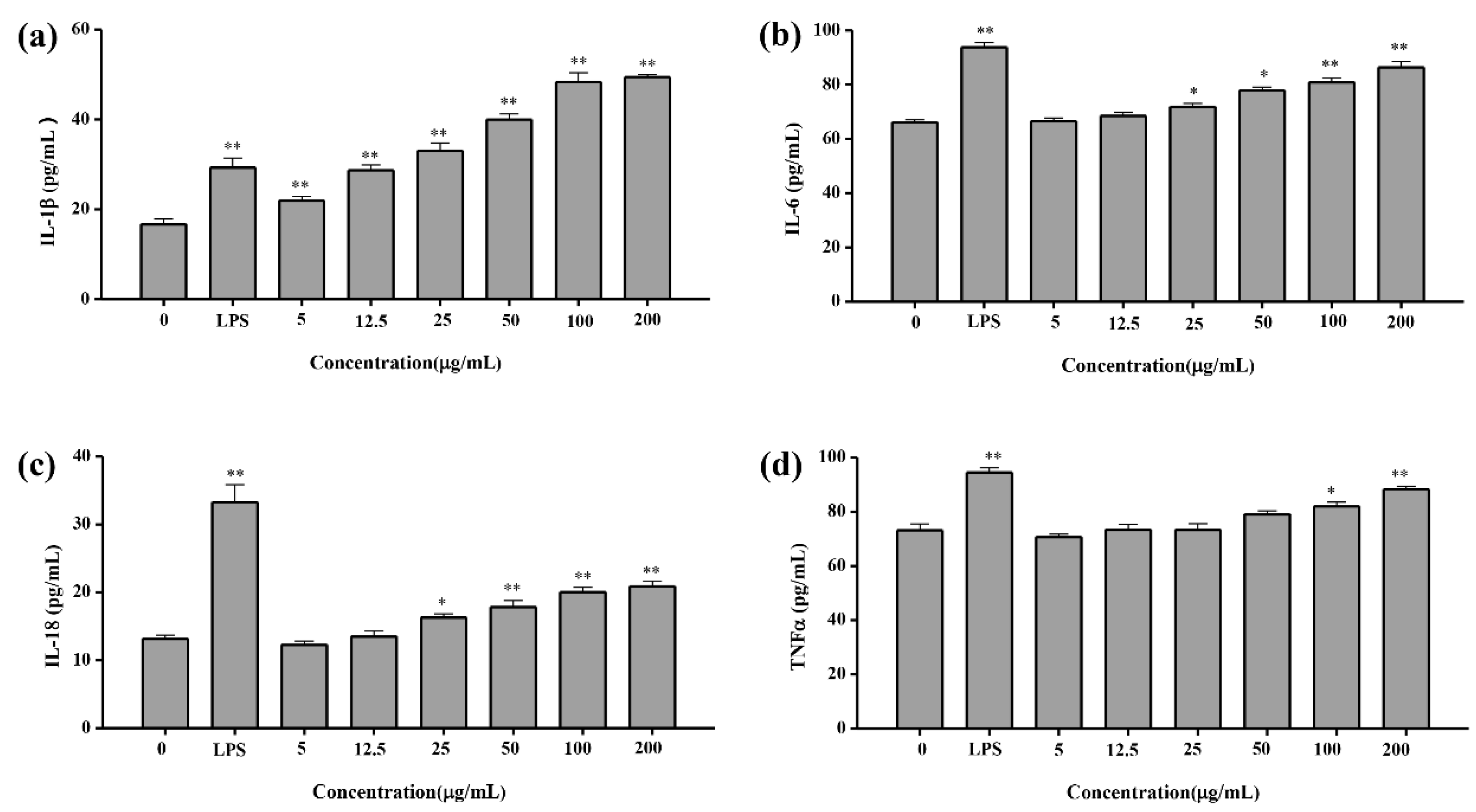
2.4.4. Cytokine Concentration Test
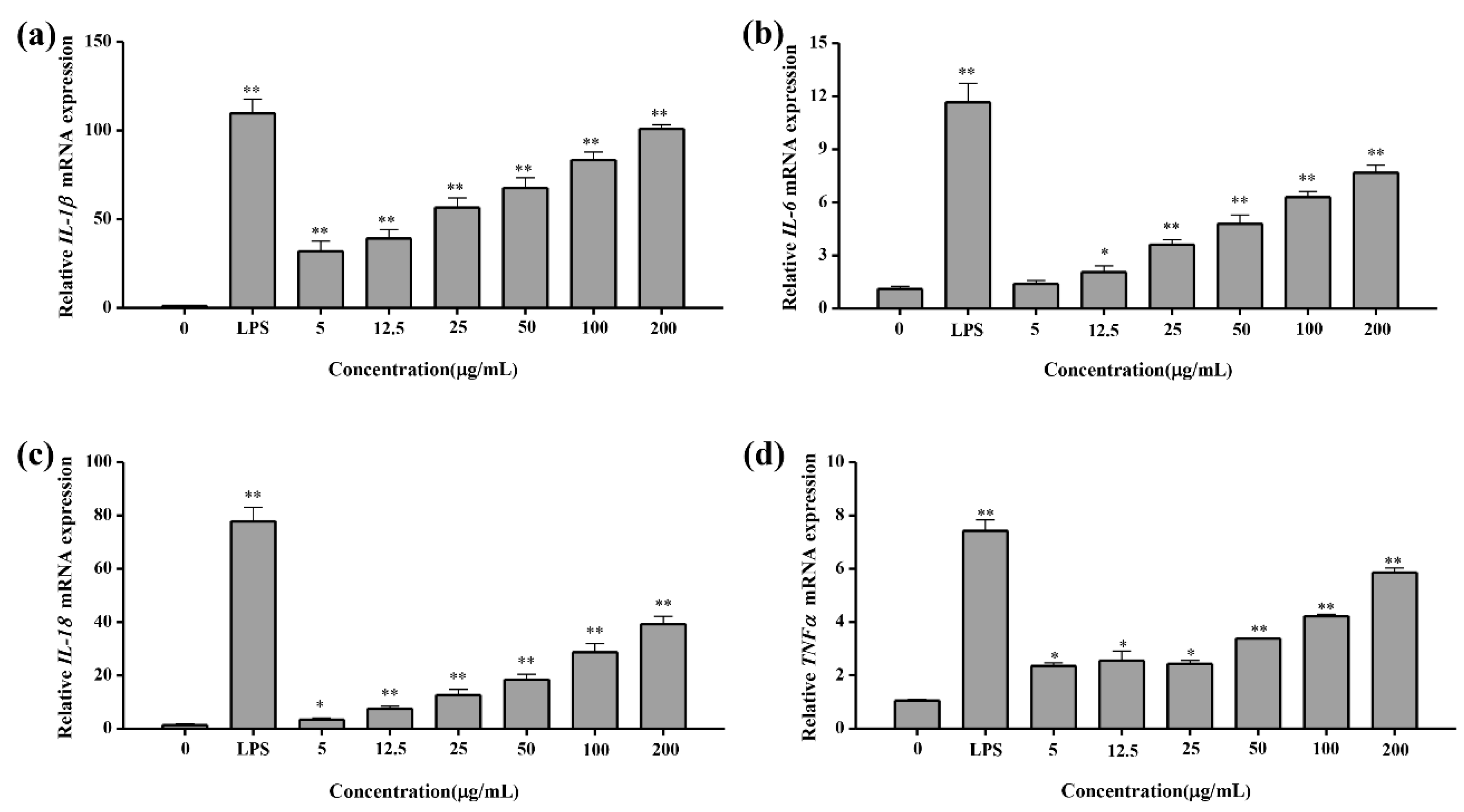
2.4.5. Q-PCR Test Result of Cytokines
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Reagents
4.3. Production Optimization of Polysaccharides
4.4. Extraction and Purification of Extracellular Polysaccharides
4.5. Structure Characterization of Purified Polysaccharide
4.5.1. Determination of Homogeneity and Molecular Weight
4.5.2. Monosaccharide Composition Analysis
4.5.3. Sulfate Content Determination
4.5.4. FT-IR Spectrometric Analysis
4.5.5. Methylation and GC-MS Analysis
4.6. Immunomodulatory Activity of Purified Polysaccharide
4.6.1. Cell Viability
4.6.2. Immunomodulatory Activity of Cytokines
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar]
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef]
- Wei, Y.; Li, F.; Li, L.; Huang, L.; Li, Q. Genetic and Biochemical Characterization of an Exopolysaccharide With in vitro Antitumoral Activity Produced by Lactobacillus fermentum YL-11. Front. Microbiol. 2019, 10, 2898. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Xu, R.H.; Li, P.L. Expolysaccharides from Bifidobacterium animalis RH activates RAW 264.7 macrophages through toll-like receptor 4. Food Agric. Immunol. 2017, 28, 149–161. [Google Scholar] [CrossRef]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of Surface Exopolysaccharides from Bifidobacterium and Lactobacillus within the Intestinal Environment. Front. Microbiol. 2018, 11, 2426. [Google Scholar] [CrossRef]
- Rostami, Z.; Tabarsa, M.; You, S.; Rezaei, M. Structural characterization and RAW264.7 murine macrophage stimulating activity of a fucogalactoglucan from Colpomenia peregrina. J. Food Sci. Technol. 2018, 55, 4650–4660. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, H.; Zhao, J.; Sun, J.; Li, M.; Sun, X. Microwave-assisted extraction, characterization and immunomodulatory activity on RAW264.7 cells of polysaccharides from Trichosanthes kirilowii Maxim seeds. Int. J. Biol. Macromol. 2020, 164, 2861–2872. [Google Scholar] [CrossRef]
- Ye, J.; Chen, D.; Ye, Z.; Huang, Y.; Xiao, M. Fucoidan Isolated from Saccharina japonica Inhibits LPS-Induced Inflammation in Macrophages via Blocking NF-κB, MAPK and JAK-STAT Pathways. Mar. Drugs 2020, 18, 328. [Google Scholar] [CrossRef]
- Jiang, Z.; Okimura, T.; Yamaguchi, K.; Oda, T. The potent activity of sulfated polysaccharide, ascophyllan, isolated from Ascophyllum nodosum to induce nitric oxide and cytokine production from mouse macrophage RAW264.7 cells: Comparison between ascophyllan and fucoidan. Nitric Oxide 2011, 25, 407–415. [Google Scholar] [CrossRef]
- Li, J.; Hsu, H.C.; Mountz, J.D.; Allen, J.G. Unmasking Fucosylation: From Cell Adhesion to Immune System Regulation and Diseases. Cell Chem. Biol. 2018, 5, 25. [Google Scholar] [CrossRef]
- Rajani, P.S.; Seppo, A.E.; Järvinen, K.M. Immunologically Active Components in Human Milk and Development of Atopic Disease, With Emphasis on Food Allergy, in the Pediatric Population. Front. Pediatrics 2018, 6, 218. [Google Scholar] [CrossRef]
- Hong, Y.W.; Ying, T.J. Isolation, molecular characterization and antioxidant activity of a water-soluble polysaccharide extracted from the fruiting body of Termitornyces albuminosus (Berk.) Heim. Int. J. Biol. Macromol. 2019, 122, 115–126. [Google Scholar] [CrossRef]
- Damare, S.; Singh, P.; Raghukumar, S. Biotechnology of Marine Fungi. Prog. Mol. Subcell. Biol. 2012, 53, 277–297. [Google Scholar]
- Khot, M.; Ghosh, D. Lipids of Rhodotorula mucilaginosa IIPL32 with biodiesel potential: Oil yield, fatty acid profile, fuel properties. J. Basic Microbiol. 2017, 57, 345–352. [Google Scholar] [CrossRef]
- Leyton, A.; Flores, L.; Mäki-Arvela, P.; Lienque, M.E.; Shene, C. Macrocystis pyrifera source of nutrients for the production of carotenoids by a marine yeast Rhodotorula mucilaginosa. J. Appl. Microbiol. 2019, 127, 1069–1079. [Google Scholar] [CrossRef]
- Andersen, D.; Renshaw, J.C.; Wiebe, M.G. Rhodotorulic acid production by Rhodotorula mucilaginosa. Mycol. Res. 2003, 107, 949–956. [Google Scholar] [CrossRef]
- Chen, X.Q.; Zhao, W.; Xie, S.W.; Xie, J.J.; Zhang, Z.H.; Tian, L.X.; Liu, Y.J.; Niu, J. Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 90, 30–39. [Google Scholar] [CrossRef]
- Żymańczyk-Duda, E.; Dunal, N.; Brzezińska-Rodak, M.; Osiewała, A.; Olszewski, T.K.; Klimek-Ochab, M.; Serafin-Lewańczuk, M. First biological conversion of chiral heterophosphonate derivative—Scaling and paths of conversion discussion. Bioorg. Chem. 2019, 93, 102751. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Chen, S.; Wang, T.; Jiang, L.; Wang, M.; Wang, S.; Li, Z. Biochemical changes of polysaccharides and proteins within EPS under Pb(II) stress in Rhodotorula mucilaginosa. Ecotoxicol. Environ. Saf. 2019, 174, 484–490. [Google Scholar] [CrossRef]
- Garza-Gonzalez, M.T.; Perez, D.B.; Rodriguez, A.V.; Garcia-Gutierrez, D.I.; Zarate, X.; Cardenas, M.E.C.; Urraca-Botello, L.I.; Lopez-Chuken, U.J.; Trevino-Torres, A.L.; Cerino-Córdoba, F.D.J.; et al. Metal-Induced Production of a Novel Bioadsorbent Exopolysaccharide in a Native Rhodotorula mucilaginosa from the Mexican Northeastern Region. PLoS ONE 2016, 11, e0148430. [Google Scholar] [CrossRef]
- Ma, W.; Chen, X.; Wang, B.; Lou, W.; Peng, T. Characterization, antioxidativity, and anti-carcinoma activity of exopolysaccharide extract from Rhodotorula mucilaginosa CICC 33013. Carbohydr. Polym. 2017, 181, 768–777. [Google Scholar] [CrossRef]
- Vazquez-Rodriguez, A.; Vasto-Anzaldo, X.G.; Perez, D.B.; Vázquez-Garza, E.; Chapoy-Villanueva, H.; García-Rivas, G.; Garza-Cervantes, J.A.; Gómez-Lugo, J.J.; Gomez-Loredo, A.E.; Gonzalez, M.T.G.; et al. Microbial Competition of Rhodotorula mucilaginosa UANL-001L and E. coli increase biosynthesis of Non-Toxic Exopolysaccharide with Applications as a Wide-Spectrum Antimicrobial. Sci. Rep. 2018, 8, 798. [Google Scholar] [CrossRef]
- Seveiri, R.M.; Hamidi, M.; Delattre, C.; Sedighian, H.; Pierre, G.; Rahmani, B.; Darzi, S.; Brasselet, C.; Karimitabar, F.; Razaghpoor, A.; et al. Characterization and Prospective Applications of the Exopolysaccharides Produced by Rhodosporidium babjevae. Adv. Pharm. Bull. 2020, 10, 254–263. [Google Scholar] [CrossRef]
| Retention Time (min) | Monosaccharide Composition | Relative Molar Ratio |
|---|---|---|
| 20.709 | rhamnose | 0.000 |
| 20.937 | fucose | 0.019 |
| 21.42 | arabinose | 0.000 |
| 22.075 | xylose | 0.000 |
| 31.52 | mannose | 0.276 |
| 31.763 | glucose | 0.176 |
| 32.562 | galactose | 0.529 |
| RT | Methylated Sugar | Mass Fragments (m/z) | Molar Ratio | Type of Linkage |
|---|---|---|---|---|
| 12 | 2,3,4-Me3-Fucp | 43, 59, 72, 89, 101, 115, 117, 131, 175 | 1.17 | Fuc-(1→ |
| 16.26 | 2,3,4,6-Me4-Glcp | 43, 71, 87, 101, 117, 129, 145, 161, 205 | 15 | Glc-(1→ |
| 16.556 | 2,3,4,6-Me4-Manp | 43, 71, 87, 101, 117, 129, 145, 161, 205 | 5.51 | Man-(1→ |
| 22.216 | 2,4,6-Me3-Galp | 43, 87, 99, 101, 117, 129, 161, 173, 233 | 52.8 | →3)-Gal-(1→ |
| 22.608 | 2,3,4-Me3-Manp | 43, 71, 87, 99, 101, 117, 129, 159, 161 | 3.84 | →6)-Man-(1→ |
| 25.074 | 4,6-Me2-Manp | 43, 85, 87, 99, 101, 127, 129, 161, 201, 216 | 5.36 | →2,3)-Man-(1→ |
| 29.548 | 2,4-Me2-Manp | 43, 87, 117, 129, 159, 189, 233 | 12.38 | →3,6)-Man-(1→ |
| 27.311 | 2,3-Me2-Glcp | 43, 71, 85, 87, 99, 101, 117, 127, 159, 161, 201 | 3.94 | →4,6)-Glc-(1→ |
| Gene Name | Up Primers | Down Primers |
|---|---|---|
| TNF-α | 5-TGTCTACTGAACTTCGGGGTGAT-3 | 5-AACTGATGAGAGGGAGGCCAT-3 |
| IL-1β | 5-AGTTGACGGACCCCAAAAG-3 | 5-AGCTGGATGCTCTCATCAGG-3 |
| IL-6 | 5-GCTACCAAACTGGATATAATCAGGA-3 | 5-CCAGGTAGCTATGGTACTCCAGAA-3 |
| IL-18 | 5-GCCATGTCAGAAGACTCTTGCGTC-3 | 5GTACAGTGAAGTCGGCCAAAGTTGTC-3 |
| Actin | 5-GATTACTGCTCTGGCTCCTAGC-3 | 5-GACTCATCGTACTCCTGCTTGC-3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Huang, L.; Zhang, Y.; Yan, Y. Production, Characterization and Immunomodulatory Activity of an Extracellular Polysaccharide from Rhodotorula mucilaginosa YL-1 Isolated from Sea Salt Field. Mar. Drugs 2020, 18, 595. https://doi.org/10.3390/md18120595
Li H, Huang L, Zhang Y, Yan Y. Production, Characterization and Immunomodulatory Activity of an Extracellular Polysaccharide from Rhodotorula mucilaginosa YL-1 Isolated from Sea Salt Field. Marine Drugs. 2020; 18(12):595. https://doi.org/10.3390/md18120595
Chicago/Turabian StyleLi, Haifeng, Lifeng Huang, Yunyi Zhang, and Yu Yan. 2020. "Production, Characterization and Immunomodulatory Activity of an Extracellular Polysaccharide from Rhodotorula mucilaginosa YL-1 Isolated from Sea Salt Field" Marine Drugs 18, no. 12: 595. https://doi.org/10.3390/md18120595
APA StyleLi, H., Huang, L., Zhang, Y., & Yan, Y. (2020). Production, Characterization and Immunomodulatory Activity of an Extracellular Polysaccharide from Rhodotorula mucilaginosa YL-1 Isolated from Sea Salt Field. Marine Drugs, 18(12), 595. https://doi.org/10.3390/md18120595





