Abstract
Tisochrysis lutea is a marine haptophyte rich in omega-3 polyunsaturated fatty acids (e.g., docosahexaenoic acid (DHA)) and carotenoids (e.g., fucoxanthin). Because of the nutraceutical applications of these compounds, this microalga is being used in aquaculture to feed oyster and shrimp larvae. In our earlier report, T. lutea organic crude extracts exhibited in vitro cytotoxic activity against human hepatocarcinoma (HepG2) cells. However, so far, the compound(s) accountable for the observed bioactivity have not been identified. Therefore, the aim of this study was to isolate and identify the chemical component(s) responsible for the bioactivity observed. Bioassay-guided fractionation through a combination of silica-gel column chromatography, followed by preparative thin layer chromatography (PTLC), led to the isolation of two diastereomers of a monoterpenoid lactone, namely, loliolide (1) and epi-loliolide (2), isolated for the first time in this species. The structural elucidation of both compounds was carried out by GC-MS and 1D (1H and 13C APT) and 2D (COSY, HMBC, HSQC-ed, and NOESY) NMR analysis. Both compounds significantly reduced the viability of HepG2 cells and were considerably less toxic towards a non-tumoral murine stromal (S17) cell line, although epi-loliolide was found to be more active than loliolide.
1. Introduction
In recent years, natural products derived from microalgal biomass produced at pilot scale have attracted great attention in drug discovery [1]. Because of their unique biochemical pathways and great adaptability to various environmental conditions such as high salinity, low or high temperatures, high light intensities, and a wide pH range, microalgae can be viable alternatives to terrestrial plants in producing highly valuable natural precursors with potential bioactivity [2]. For instance, Tisochrysis lutea (Haptophyta), formerly known as Isochrysis galbana T-ISO [3,4], is a marine unicellular flagellated microalga with a golden-brown phenotype, widely used as feed for early larval stages of mollusks, fish, and crustaceans in the aquaculture industry. Interestingly, its potential in food applications and as a source of high-value bioactive compounds has been considered [5]. More recently, it has been described that T. lutea is able to accumulate significant levels of n-3 polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid (DHA), and of xanthophylls such as fucoxanthin, which play an important role in the prevention and treatment of human diseases [6].
In addition, several isolated compounds have been reported to display a wide spectrum of medicinal properties. For example, crude polysaccharides of T. lutea have been evaluated concerning their immunomodulatory properties by the induction of IL-1 in murine macrophages [7]. Moreover, the anti-inflammatory activity of the galactosylglycerides and galactosylceramides has also been assayed [2]. T. lutea has exhibited promising therapeutic effects by inducing weight loss and decreasing glucose, triacylglycerol (TAG), and cholesterol levels in diabetic rats [8]. In addition, it contains antioxidants [9], compounds with anti-bacterial activity against multidrug-resistant Mycobacterium tuberculosis [10], as well as inhibitors of cyclooxygenase (COX-2) [11] and inhibitors of U937 human leukemic monocyte lymphoma cells proliferation [12]. In a previous study published by our group, the crude extracts of T. lutea displayed promising antioxidant properties, inhibition of acetylcholinesterase (AChE), and cytotoxicity against tumor cell lines (HepG2) [13].
Nevertheless, to the best of our knowledge, there are no reports on the isolation of compounds from T. lutea with cytotoxic activity against hepatocarcinoma HepG2 cells. Hepatocarcinoma is a prevalent cancer of the liver and occurs particularly in patients with underlying chronic liver disease and cirrhosis. It is the fourth leading cause of cancer mortality and third most common cause of cancer-related death worldwide after lung and gastric cancer [14,15]. Despite the existence of a few drugs for the treatment of liver cancer, patients suffer from hepatotoxicity (including liver failure) and drug resistance, which limit the successful outcome of the treatment in most cases [16]. Consequently, there is an increasing demand for finding new therapeutic drugs for the treatment and/or prevention of hepatocarcinoma [17]. Interestingly, marine algal crude extracts have recently been found to be a vital source of pharmaceutically valuable drugs for the treatment of numerous forms of tumors [18]. Hence, we report here for the first time the isolation and structural elucidation of two stereoisomers of a hydroxylated monoterpene lactone, namely, loliolide (1) and epi-loliolide (2), from T. lutea. The evaluation of their cytotoxic effects against human HepG2 cells is also discussed in comparison to those against a non-tumoral cell line (S17 cells).
2. Results and Discussion
The extraction was first carried out sequentially using organic solvents with increasing polarity, such as hexane (Hex), dichloromethane (DCM), and acetone (Ace), in order to obtain an enriched bioactive extract. Later, the organic extracts of T. lutea were tested for their in vitro cytotoxic activity against HepG2 cells. In our study, dimethyl sulfoxide (DMSO) and the chemotherapeutic drug etoposide were used as negative and positive controls, respectively. Etoposide is a semisynthetic derivative of podophyllotoxin, which is well-known and widely used as an anti-cancer drug [19,20]. It is reported as a topoisomerase II inhibitor, leading to cell-cycle arrest followed by cell death [20]. The MTT colorimetric assay was employed to test the effect of crude extracts of T. lutea on mitochondrial metabolic activity (i.e., as an indicator of cell viability). It was found that the DCM extract was the most active compared to the hexane and acetone extracts, decreasing HepG2 cell viability down to 24.7% at a concentration of 125 µg/mL (Figure 1), resulting in an IC50 of 85.1 µg/mL (CI95% 73.1 to 99.1 µg/mL).
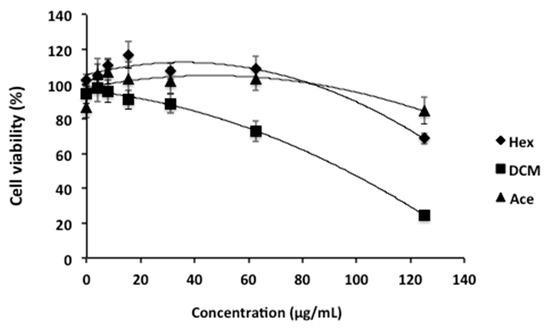
Figure 1.
Cytotoxicity of Tisochrysis lutea crude extracts of hexane (Hex), dichloromethane (DCM) and acetone (Ace) against HepG2 cell lines.
To identify the bioactive molecule(s) responsible for the cytotoxic properties, the active DCM extract was subjected to bioguided fractionation using silica-gel chromatography, which yielded five fractions named as F1 through F5 (Figure 2A). Each fraction was monitored by TLC in order to pool similar fractions. Fraction 5 was the most active towards HepG2 cells, and was further fractionated into fractions F5–1, F5–2, F5–3, and F5–4 by silica-gel column chromatography. These sub-fractions were re-tested at a concentration of 125 µg/mL to evaluate their cytotoxicity against the HepG2 cell line, as well as their selective index (SI) against non-tumoral cell lines sourced from murine bone marrow (S17). The SIs of two subfractions, namely, F5–3 and F5–4, were significantly higher (3.9 ± 0.2 and 5.3 ± 0.5, respectively) than those of F5–1 and F5–2 (SI < 2.0, see Figure 2B) [21,22]. The selectivity of a drug is a highly desirable feature, so that its toxicity is limited to the target (cancer) cells in order to avoid unwanted side effects [23] and also to increase the therapeutic concentration window. Therefore, the selectivity index value indicates the therapeutic potential of a drug; for instance, an SI > 1 means that the drug is less harmful or toxic for non-tumoral cells compared to tumoral cells and is safer for therapeutic applications. An SI > 5 is desired for molecules to be considered as potential drugs with low-toxicity as anti-cancer agents [24].
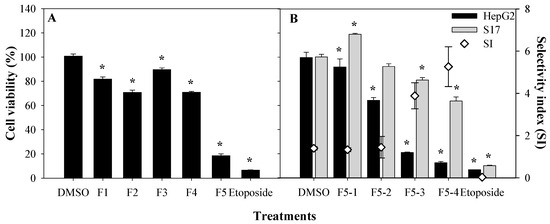
Figure 2.
Cell viabilities of fractions F1 to F5 (A) against HepG2 cells, and fractions F5–1 to F5–4 towards HepG2 and S17 cells, and the respective selectivity index (B). Etoposide was used as positive control. All samples were tested at a concentration of 125 µg/mL. Results are depicted in percentage (%) of cell viability, compared with a negative control (DMSO), tested at a concentration of 0.5% (v/v). Values show the mean ± SEM of at least three experiments (n = 9). Asterisks (*) indicate significant differences in cell viability between negative control and treated cell lines (p < 0.01).
The structures of the isolated bioactive compounds present in the subfractions F5–3 and F5–4 were elucidated using spectral analysis, namely, GC-MS, 1H and 13C NMR. Firstly, subfractions F5–3 and F5–4 were analyzed by GC-MS (Figure 3). Each subfraction contained only one major peak, with retention times of 17.98 min and 18.80 min, respectively (Figure 3A,B). However, the compounds that were found as being the most abundant component of each subfraction had the same molecular ion with m/z 196 [M+], which we tentatively identified as isomers of loliolide by comparing the mass spectral data with the NIST library (Figure 3C–E) and spectrometric data reported elsewhere [25]. The retention factor (Rf) of the two molecules found in subfractions F5–3 and F5–4 were also compared by thin-layer chromatography (TLC), using hexane and ethyl acetate (20:80, v/v) as a mobile solvent system. This experiment showed two different spots at 0.43 and 0.34, respectively. To further elucidate the structures of both compounds, 1D (1H and 13C Attached Proton Test (APT) and 2D (COSY, HMBC, HSQC-ed, and NOESY) NMR spectral analyses were carried out. The chemical shifts and structural assignments (Table 1) were similar to those of loliolide (1) and epi-loliolide (2) (Figure 4) upon comparing with data previously reported in the literature on similar compounds synthesized chemically or found in brown algae [26,27,28,29].
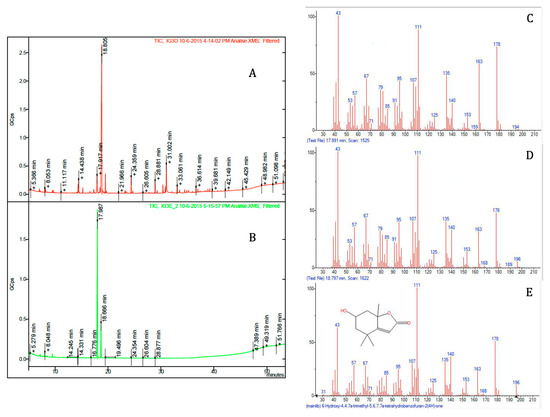
Figure 3.
GC-MS chromatograms of sub-fractions F5–3 (A) and F5–4 (B), and mass spectra of the major peaks of both sub-fractions, at 17.991 min (C) and 18.797 min (D) and their identification by comparison with the spectrum at the NIST library (E).

Table 1.
Chemical shifts (1H and 13C NMR) of loliolide and epi-loliolide.
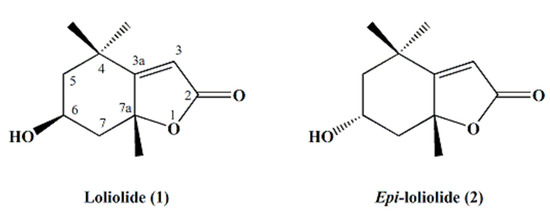
Figure 4.
Structures of loliolide (1) and epi-loliolide (2).
These two carotenoid-derived metabolites are monoterpene lactones, also classified as norisoprenoids or apocarotenoids [28,30,31], which have often been isolated from various sources such as plants, algae, and other marine organisms [25,32,33,34]. These types of metabolites are produced from the carotenoids fucoxanthin, zeaxanthin, and violaxanthin upon photo-oxidation or thermal degradation, among other catabolic pathways [33,34]. Carotenoids are well-known dietary supplements used as ingredients in food and cosmeceutical formulations (e.g., as colorants), and perform versatile roles in human health, including neuroprotection and the prevention and/or treatment of macular degeneration, inflammatory and rheumatoid arthritis, cataracts, cancer, diabetes, and coronary artery conditions, among others [35]. Both pigments, fucoxanthin and zeaxanthin, have been reported in Tisochrysis lutea [36], which might explain the presence of those monoterpene lactone diastereomers in this marine microalga. Indeed, carotenoids are highly prone to oxidation under different environments, such as light, heat, air, or the presence of transition metals or radicals. These conditions may lead to reactions of isomerization, rearrangement, oxidative cleavage, and/or combinations thereof, resulting in the production of carotenoid catabolites such as norisoprenoids and/or apocarotenoids, including loliolides [37,38]. Interestingly, it has been suggested that the degradation of carotenoids to these smaller molecules might be the reason why these pigments show various biological activities, rather than the original carotenoids themselves. For instance, in a previous report from our laboratory, we presented evidence that another zeaxanthin-derived metabolite (isololiolide), an epimer of loliolide, had significant cytotoxic activity against liver cancer cells (HepG2), while no toxicity was detected for non-tumoral cells derived from MRC-5 (Medical Research Council cell strain 5) of lungs and HFF-1 human fibroblasts [14]. Interestingly, although loliolide (1) isolated from T. lutea showed cytotoxicity towards HepG2 and S17 cell lines, this effect was lower than that of epi-loliolide (2); this result might be explained by a different configuration around C–6 and/or C–7a (Figure 3), which may account for the diverse bioactive properties of both loliolides [38]. Indeed, isomer-specific bioactivity is often found in nature—for example, the cis-isomer of β-carotene has gained more attention due to its higher bioavailability over the corresponding trans-isomer [39].
From a biological point of view, stereoisomers of carotenoid metabolites, such as loliolide, have been described as having various biological activities, such as potent germination inhibition and ant-repellence; they also showed immunosuppressive and anticholinesterase effects and antioxidant properties, and the ability to prevent H2O2-induced cell damage [40,41,42]. Loliolide was demonstrated to have antiapoptotic and antiscratching effects in human keratinocytes [43]. In addition, loliolide and isololiolide exhibited strong growth-inhibitory properties on cress and barnyard grass seedlings and anti-melanogenetic activity [30,32,44]. Therefore, the wide spectra of biological properties attributed to loliolide and its isomers [45,46,47,48,49,50] is a strong indication that further research is needed to fully understand the effects of these carotenoid catabolites on either animal, plant, or microbial cells.
3. Materials and Methods
3.1. Materials
Tisochrysis lutea biomass was procured from NECTON S.A. (Faro, Portugal) as a dark green powder material produced by lyophilization. Human hepatocellular carcinoma (HepG2) and murine bone marrow stromal (S17 cell) cell lines were kindly provided by Dr. Vera Marques and Dr. Nuno Santos, Center for Molecular and Structural Biomedicine (CBME), University of Algarve (Faro, Portugal), respectively. Hexane (Hex), methanol, ethyl acetate (EA), dichloromethane (DCM), acetone (Ace), and dimethyl sulfoxide (DMSO) from Fisher Scientific (Loughborough, UK) and 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Merck, Lisbon, Portugal) were purchased from VWR International (Lisbon, Portugal). TLC-plates ALUGRAM® Xtra Sil G/UV254, pre-coated with silica gel 60 (1 mm) and silica gel (70–130 mesh), were purchased from M/s Merck (Lisbon, Portugal). All the other chemicals used were of reagent grade.
3.2. Extract Preparation from Microalgae
Lyophilized biomass (50 g) of Tisochrysis lutea was dispersed in the chosen solvent and homogenized using a disperser IKA T10B Ultra-Turrax at room temperature. The extractions were made sequentially using 200 mL of hexane, dichloromethane, and acetone at room temperature to obtain crude extracts. The extractions were done in triplicate, filtered through Whatman filter paper n° 4, and the supernatants combined and concentrated under reduced pressure using a rotary evaporator, at 40 °C. All extracts were dissolved in DMSO at a concentration of 50 mg/mL, aliquoted, and stored at 4 °C until use.
3.3. Cellular Viability
Cell lines were maintained in culture media RPMI-1640 supplemented with glucose (1000 mg/mL), 10% fetal bovine serum (FBS), L-glutamine (2 mM), streptomycin (50 µg/mL), and penicillin (50 µg/mL). Both HepG2 and S17 cell lines were seeded on 96-well plates, incubated under 5.0% CO2 in humidified atmosphere at 37 °C overnight, and later treated with the extracts (hexane, DCM, and acetone) at concentrations ranging from 3.9 to 125 µg/mL for 72 h. Negative controls for cell lines were performed with DMSO at a maximum concentration of 0.5% (v/v). The MTT colorimetric assay was carried out to assess their effect on mitochondrial metabolic activity, as an indicator of cell viability [22]. Briefly, two hours before the completion of the incubation period, 20 µL of MTT (5 mg/mL in PBS) were added to each well and further incubated for 2 h at 37 °C. Absorbance was measured at 590 nm using a Multi-Mode Microplate Reader (BioTek Synergy TM 4, Winooski, VT, USA), and results were calculated as percent of cell viability and as IC50 values (µg/mL). Furthermore, the selective index (SI) of the subfractions (F5–1, F5–2, F5–3, and F5–4), and positive control etoposide was evaluated at a concentration of 125 µg/mL. The SI was calculated using the following equation: SI = VNT/VT, where VNT and VT represent the cell viability of non-tumoral cells (S17) and tumoral cells (HepG2), respectively, after exposure to the same concentration of extract or fraction [22].
3.4. Bio-Guided Fractionation and Isolation of Anti-Tumoral Compounds
The DCM crude extract (DCM; 10.8 g) was subjected to a bioguided fractionation using silica-gel (70–130 mesh) column chromatography eluting with mixtures of hexane, ethyl acetate, and methanol of increasing polarity to obtain five fractions as follows: F1 (pure hexane), F2 (Hex:EA, 90:10, v/v), F3 (Hex:EA, 70:30 v/v), F4 (Hex:EA, 50:50 v/v), and F5 (Hex:EA, 70:30 v/v and EA:methanol, 80:20 v/v). After identification of the active fraction (F5, 2.44 g) this was again subjected to silica-gel column chromatographic fractionation to afford fractions F5–1, F5–2, F5–3, and F5–4, which were eluted using mixtures ranging from pure hexane to pure ethyl acetate, particularly fractions F5–3 and F5–4, which were obtained in a solvent system containing 40–50% hexane in ethyl acetate. The resulting active fractions (F5–3 and F5–4) were further purified by preparative TLC which yielded 29 mg of F5–3 (1) and 13 mg of F5–4 (2). These pure compounds were stored at 4 °C until further use, such as structural elucidation and biological activities evaluation.
3.5. Spectral and Chromatographic Analysis
Pure samples of loliolide (1) and epi-loliolide (2) were dissolved in deuterated chloroform (CDCl3, Sigma-Aldrich, Switzerland) and analyzed by NMR. 1H (400.1 MHz) and 13C (100.6 MHz) NMR were recorded on a Bruker Avance spectrometer (Wissembourg, France); chemical shifts were expressed in δ values and referenced to the residual CDCl3 peak (δH = 7.26 ppm and δC = 77.00 ppm); coupling constants were reported in hertz (Hz). Unequivocal assignments of all proton and carbon signals were achieved by 1D (1H, and 13C APT) and 2D (COSY, HMBC, HSQC-ed, and NOESY) NMR experiments. The NMR data obtained for both compounds were in accordance with previously reported data [25,26,27,28]. GC-MS analysis was performed using an Agilent 6890N Gas Chromatograph connected to Bruker GC-MS Triple Quad MS System (Model SCION 456-GC; Billerica, MA, USA) at 70 eV (m/z 33–1000; source at 230 °C and quadruple at 150 °C) in EI mode with a ZB-5 ms capillary column (30 m × 0.25 mm; 0.25 µm). The column temperature was initially maintained at 60 °C for 1 min, and gradually increased as follows: 60 °C to 120 °C at 30 °C/min, 120 °C to 250 °C at 4 °C/min, then 250 °C to 270 °C at 20 °C/min, and finally 270 °C to 300 °C at 2.5 °C/min, where it remained for 5 min. The carrier gas was helium at a flow rate of 1.0 mL/min, the inlet temperature was maintained at 300 °C and split-less mode was used.
4. Conclusions
Our results indicate that crude extracts and/or biomass of T. lutea can be a source of compounds for the prevention and treatment of human hepatocarcinoma. Two cytotoxic and selective compounds were isolated, loliolide (1) and epi-loliolide (2), which are likely to be degradation products of fucoxanthin and/or zeaxanthin. Therefore, comparative biological studies of pure carotenoids (e.g., fucoxanthin and zeaxanthin) and their respective catabolites are warranted in order to find a structure–activity relationship (SAR) of loliolide and its stereoisomers. A possible research avenue would include the modification of the hydroxyl group occurring in loliolides in order to generate various analogues and determine their bioactivities, which could further improve the usefulness of these lactones.
Author Contributions
Conceptualization, K.N.G., F.X.M., L.B., and J.V; Research plan, K.N.G., L.B., and J.V.; Validation, K.N.G., L.B., and J.V.; formal analysis, K.N.G., M.J.R., L.B., and J.V.; investigation, K.N.G., M.J.R., L.B., and J.V.; data curation, K.N.G., H.P., H.G., L.B., and J.V.; writing—original manuscript preparation, K.N.G., H.P., H.G., L.B., and J.V.; writing—review and editing, K.N.G., L.B., F.X.M., and J.V.; visualization, K.N.G; supervision, L.B., F.X.M., and J.V. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was funded by the Foundation for Science and Technology (FCT, Portugal) under the projects of the UID/Multi/04326/2019, UID/MAR/04292/2020 (MARE), UID/Multi/04046/2020 (BioISI) and from the grant of 0055 ALGARED +5E —INTERREG V-A España-Portugal. K.N.G is an auxiliary researcher (Decree-Law no. 57/2016 as amended by Law No. 57/2017) funded by University of Algarve. FCT also funded M.J.R (SFRH/BD/116604/2016) and H.P. (SFRH/BD/105541/2014).
Acknowledgments
We would like to acknowledge NECTON S.A, Portugal, for kindly supplying T. lutea biomass. We also thank Vera Marques and Nuno Santos, CBME, University of Algarve (Portugal) for providing the hepatocellular carcinoma (HepG2) and non-tumoral murine bone marrow stromal (S17) cell lines, respectively.
Conflicts of Interest
The authors hereby declare that they have no conflict of interest. The above funding agencies had no participation in the design of research plan and execution of biological activities, or interpretation of data; in the writing of the manuscript; or in the determination of the results.
References
- Gilbert-López, B.; Mendiola, J.A.; Fontecha, J.; van den Broek, L.A.M.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Downstream processing of Isochrysis galbana: A step towards microalgal biorefinery†. Green Chem. 2015, 17, 4599–4609. [Google Scholar] [CrossRef]
- De los Reyes, C.; Ortega, M.J.; Rodríguez-Luna, A.; Talero, E.; Motilva, V.; Zubía, E. Molecular characterization and anti-inflammatory activity of galactosylglycerides and galactosylceramides from the microalga Isochrysis galbana. J. Agric. Food Chem. 2016, 64, 8783–8794. [Google Scholar] [CrossRef] [PubMed]
- Bendif, E.M.; Probert, I.; Schroeder, D.C.; de Vargas, C. On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta). J. Appl. Phycol. 2013, 25, 1763–1776. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Niccolai, A.; Biondi, N.; Rodolfi, L.; D’Ottavio, M.; D’Ambrosio, M.; Lodovici, M.; Tredici, M.R.; Luceri, C. Preliminary data on the dietary safety, tolerability and effects on lipid metabolism of the marine microalga Tisochrysis lutea. Algal Res. 2018, 34, 244–249. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Delbrut, A.; Albina, P.; Lapierre, T.; Pradelles, R.; Dubreucq, E. Fucoxanthin and polyunsaturated fatty acids co-extraction by a green process. Molecules 2018, 23, 874. [Google Scholar] [CrossRef]
- Yu, C.C.; Chen, H.W.; Chen, M.J.; Chang, Y.C.; Chien, S.C.; Kuo, Y.H.; Chao, L.K.P. Chemical composition and bioactivities of the marine alga Isochrysis galbana CCMP 1324 from Taiwan. Nat. Prod. Commun. 2010, 5, 1941–1944. [Google Scholar] [CrossRef]
- Nuño, K.; Villarruel-Lopez, A.; Puebla-Perez, A.M.; Romero-Velarde, E.; Puebla-Mora, A.G.; Ascencio, F. Effects of the marine microalgae Isochrysis galbana and Nannochloropsis oculata in diabetic rats. J. Funct. Foods 2013, 5, 106–115. [Google Scholar] [CrossRef]
- Yingying, S.; Hui, W.; Ganlin, G.; Yinfang, P.; Binlun, Y. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef]
- Prakash, S.; Sasikala, S.L.; Aldous, V.H.J. Isolation and identification of MDR-Mycobacterium tuberculosis and screening of partially characterised antimycobacterial compounds from chosen marine micro algae. Asian Pac. J. Trop. Med. 2010, 3, 655–661. [Google Scholar] [CrossRef]
- Bonfanti, C.; Cardoso, C.; Afonso, C.; Matos, J.; Garcia, T.; Tanni, S.; Bandarra, N.M. Potential of microalga Isochrysis galbana: Bioactivity and bioaccessibility. Algal Res. 2018, 29, 242–248. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Souissi, A.; Souissi, S.; Grad, T.; Lencel, P.; Greene, C.M.; Duin, S.; Dmitrenok, P.S.; Chizhov, A.O.; Shashkov, A.S.; et al. Chemical structure and biological activity of a highly branched (1→ 3, 1→ 6)-β-D-glucan from Isochrysis galbana. Carbohydr. Polym. 2014, 111, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Soares, F.; Pereira, H.; Barreira, L.; Vizetto-Duarte, C.; Rodrigues, M.J.; Amélia Pilar Rauter, A.M.; Alberício, F.; Varela, J. Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: Possible application in the pharmaceutical and functional food industries. J. Appl. Phycol. 2014, 26, 151–161. [Google Scholar] [CrossRef]
- Vizetto-Duarte, C.; Custódio, L.; Gangadhar, K.N.; Lago, J.H.G.; Dias, C.; Matos, A.M.; Rauter, A.P. Isololiolide, a carotenoid metabolite isolated from the brown alga Cystoseira tamariscifolia, is cytotoxic and able to induce apoptosis in hepatocarcinoma cells through caspase-3 activation, decreased Bcl-2 levels, increased p53 expression and PARP cleavage. Phytomedicine 2016, 23, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.-B. Dietary natural products for prevention and treatment of liver cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahmad, R.; Khan, M.A.; Upadhyay, S.; Husain, I.; Srivastava, A.N. Cytostatic and anti-tumor potential of ajwa date pulp against human hepatocellular carcinoma HepG2 cells. Sci. Rep. 2019, 9, 245. [Google Scholar] [CrossRef]
- Andrade, K.A.M.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef]
- Xie, B.S.; Zhao, H.C.; Yao, S.K.; Zhuo, D.X.; Jin, B.; Lv, D.C.; Wu, C.L.; Dai-Long Ma, D.L.; Ai, Z.L. Autophagy inhibition enhances etoposide-induced cell death in human hepatoma G2 cells. Int. J. Mol. Med. 2011, 27, 599–606. [Google Scholar] [CrossRef]
- Dostál, Z.; Kosina, P.; Mlejnek, P.; Kikalová, K.; Modrianský, M. Mifepristone potentiates etoposide toxicity in Hep G2 cells by modulating drug transport. Toxicol. In Vitro 2019, 54, 33–40. [Google Scholar] [CrossRef]
- Oh, S.H.; Ahn, J.; Kang, D.H.; Lee, H.Y. The effect of ultrasonificated extracts of Spirulina maxima on the anticancer activity. Mar. Biotechnol. 2011, 13, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime halophyte species from Southern Portugal as sources of bioactive molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef] [PubMed]
- Mencher, S.K.; Wang, L.G. Promiscuous drugs compared to selective drugs (promiscuity can be a virtue). BMC Clin. Pharmacol. 2005, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Segun, P.A.; Ogbole, O.O.; Ismail, F.M.D.; Nahar, L.; Evans, A.R.; Ajaiyeoba, E.O.; Sarker, S.D. Resveratrol derivatives from Commiphora africana (A. Rich.) Endl. display cytotoxicity and selectivity against several human cancer cell lines. Phytother. Res. 2019, 33, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Percot, A.; Yalcin, A.; Aysel, V.; Erdugan, H.; Dural, B.; Guven, K.C. Loliolide in marine algae. Nat. Prod. Res. 2009, 23, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Qin, J.J.; Cheng, X.R.; Yan, S.K.; Jin, H.Z.; Zhang, W.D. Five new sesquiterpene lactones from Inula hupehensis. Arch. Pharm. Res. 2013, 36, 1319–1325. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Vanderah, D.J.; Hollenbeak, K.H.; Enwall, C.E.L.; Gopichand, Y.; SenGupta, P.K.; Van der Helm, D. Metabolites from the marine sponge Tedania ignis. A new atisanediol and several known diketopiperazines. J. Org. Chem. 1983, 48, 3941–3945. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, R.-M.; Lin, X.-P.; Liu, Y.-H. Norisoprenoids from the brown alga Sargassum naozhouense Tseng et Lu. Molecules 2018, 23, 348. [Google Scholar] [CrossRef]
- Mori, K.; Khlebnikove, V. Carotenoids and Degraded Carotenoids, VIII – Synthesis of (+)-dihydroactinidiolide, (+)- and (−)-actinidiolide, (+)- and (−)-loliolide as well as (+)- and (−)-epiloliolide. Liebigs Ann. Chem. 1993, 1, 77–82. [Google Scholar] [CrossRef]
- Murata, M.; Nakai, Y.; Kawazu, K.; Ishizaka, M.; Kajiwara, H.; Abe, H.; Seo, S. Loliolide, a carotenoid metabolite, is a potential endogenous inducer of herbivore resistance. Plant Physiol. 2019, 179, 1822–1833. [Google Scholar] [CrossRef]
- Machida, K.; Kikuchi, M. Norisoprenoids from Viburnum dilatatum*. Phytochemistry 1996, 41, 1333–1336. [Google Scholar] [CrossRef]
- Ko, R.K.; Kang, M.C.; Kim, S.S.; Oh, T.H.; Kim, G.O.; Hyun, C.G.; Lee, N.H. Anti-melanogenesis constituents from the seaweed Dictyota coriacea. Nat. Prod. Commun. 2013, 8, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Repeta, D.J. Carotenoid diagenesis in recent marine sediments: II. Degradation of fucoxanthin to loliolide*. Geochimica et Cosmochimica Acta 1989, 53, 669–707. [Google Scholar] [CrossRef]
- Taylor, H.F.; Burden, R.S. Identification of plant growth inhibitors produced by photolysis of violaxanthin. Phytochemistry 1970, 9, 2217–2223. [Google Scholar] [CrossRef]
- Schüler, L.M.; Gangadhar, K.N.; Duarte, P.; Placines, C.; Molina-Márquez, A.M.; Léon-Bañares, R.; Sousa, V.S.; Varela, J.; Barreira, L. Improvement of carotenoid extraction from a recently isolated, robust microalga, Tetraselmis sp. CTP4 (chlorophyta). Bioproc. Biosyst. Eng. 2020, 43, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Beuzenberg, V.; Goodwin, E.O.; Puddick, J.; Romanazzi, D.; Adams, S.L.; Packer, M.A. Optimising conditions for growth and xanthophyll production in continuous culture of Tisochrysis lutea using photobioreactor arrays and central composite design experiments. N. Z. J. Bot. 2017, 55, 64–78. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Nonier, M.-F.; De Gaulejac, N.V.; Vivas, N.; Vitry, C. Characterization of carotenoids and their degradation products in oak wood. Incidence on the flavour of wood. Comptes Rendus Chim. 2004, 7, 689–698. [Google Scholar] [CrossRef]
- Mallik, A.K.; Qiu, H.; Sawada, T.; Takafuji, M.; Ihara, H. Molecular-shape selectivity by molecular gel-forming compounds: Bioactive and shape-constrained isomers through the integration and orientation of weak interaction sites†. Chem. Commun. 2011, 47, 10341–10343. [Google Scholar] [CrossRef]
- Okada, N.; Shirata, K.; Niwano, M.; Koshino, H.; Uramoto, M. Immunosuppressive activity of a monoterpene from Eucommia ulmoides. Int. J. Plant Biochem. 1994, 37, 281–282. [Google Scholar] [CrossRef]
- Kumar, J.; Dhar, P.; Tayade, A.B.; Gupta, D.; Chaurasia, O.P.; Upreti, D.K.; Srivastava, R.B. Chemical composition and biological activities of Trans-Himalayan alga Spirogyra porticalis (Muell.) cleve. PLOS ONE 2015, 10, e0118255. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Jeong, S.Y.; Jung, H.A.; Choi, J.S.; Min, B.S.; Woo, M.H. Anticholinesterase and antioxidant constituents from Gloiopeltis furcate. Chem. Pharm. Bull. 2010, 58, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, D.S.; Kim, S.; Lorz, L.R.; Choi, E.; Lim, H.Y.; Yoon, K. Loliolide Presents Antiapoptosis and Antiscratching Effects in Human Keratinocytes. Int. J. Mol. Sci. 2019, 20, 651. [Google Scholar] [CrossRef]
- Islam, M.S.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and identification of two potential phytotoxic substances from the aquatic fern Marsilea crenata. J. Plant Biol. 2017, 60, 75–81. [Google Scholar] [CrossRef]
- Takasaki, M.; Tokuda, H.; Nishino, H.; Konoshima, T. Cancer chemopreventive agents (antitumor-promoters) from Ajuga decumbens. J. Nat. Prod. 1999, 62, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Neergaard, J.S.; Rasmussen, H.B.; Stafford, G.I.; Van Staden, J.V.; Jäger, A.K. Serotonin transporter affinity of (−)-loliolide, a monoterpene lactone from Mondia whitei. S. Afr. J. Bot. 2010, 76, 593–596. [Google Scholar] [CrossRef][Green Version]
- Yang, H.H.; Hwangbo, K.; Zheng, M.S.; Cho, J.H.; Son, J.K.; Kim, H.Y.; Kim, J.R. Inhibitory effects of (2)-loliolide on cellular senescence in human dermal fibroblasts. Arch. Pharm. Res. 2015, 38, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Liu, C.H.; Burnouf, T.; Wang, G.H.; Chang, S.P.; Jassey, A.; Yen, M.H. Activity-based and fraction-guided analysis of Phyllanthus urinaria identifies loliolide as a potent inhibitor of hepatitis C virus entry. Antivir. Res. 2016, 130, 58–68. [Google Scholar] [CrossRef]
- Lima, M.L.; Romanelli, M.M.; Borborema, S.E.T.; Johns, D.M.; Migotto, A.E.; Lago, J.H.G.; Tempone, A.G. Antitrypanosomal activity of isololiolide isolated from the marine hydroid Macrorhynchia philippina (Cnidaria, Hydrozoa). Bioorg. Chem. 2019, 89, 103002. [Google Scholar] [CrossRef]
- Machado, F.B.; Yamamoto, R.E.; Zanoli, K.; Nocchi, S.R.; Novello, C.R.; Schuquel, I.T.; Sakuragui, C.M.; Luftmann, H.; Nakamura, T.U.; Nakamura, C.V.; et al. Evaluation of the antiproliferative activity of the leaves from Arctium lappa by a bioassay-guided fractionation. Molecules 2012, 17, 1852–1859. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).