Determination of the Extraction, Physicochemical Characterization, and Digestibility of Sulfated Polysaccharides in Seaweed—Porphyra haitanensis
Abstract
1. Introduction
2. Results and Discussion
2.1. Single-Factor Experiments for the Extraction of Porphyra Polysaccharides
2.1.1. Effect of Extraction Temperature on the Yield of Polysaccharides
2.1.2. Effect of Extraction Time on the Yield of Polysaccharides
2.1.3. Effect of the Raw Material to Water Ratio on the Yield of Polysaccharides
2.2. Optimization of Polysaccharide Extraction
(R2 = 0.9563)
2.3. Analysis of the Interaction Effects between Two Factors
2.4. Physicochemical Properties of PHP
2.5. FT-IR Spectra of PHPs
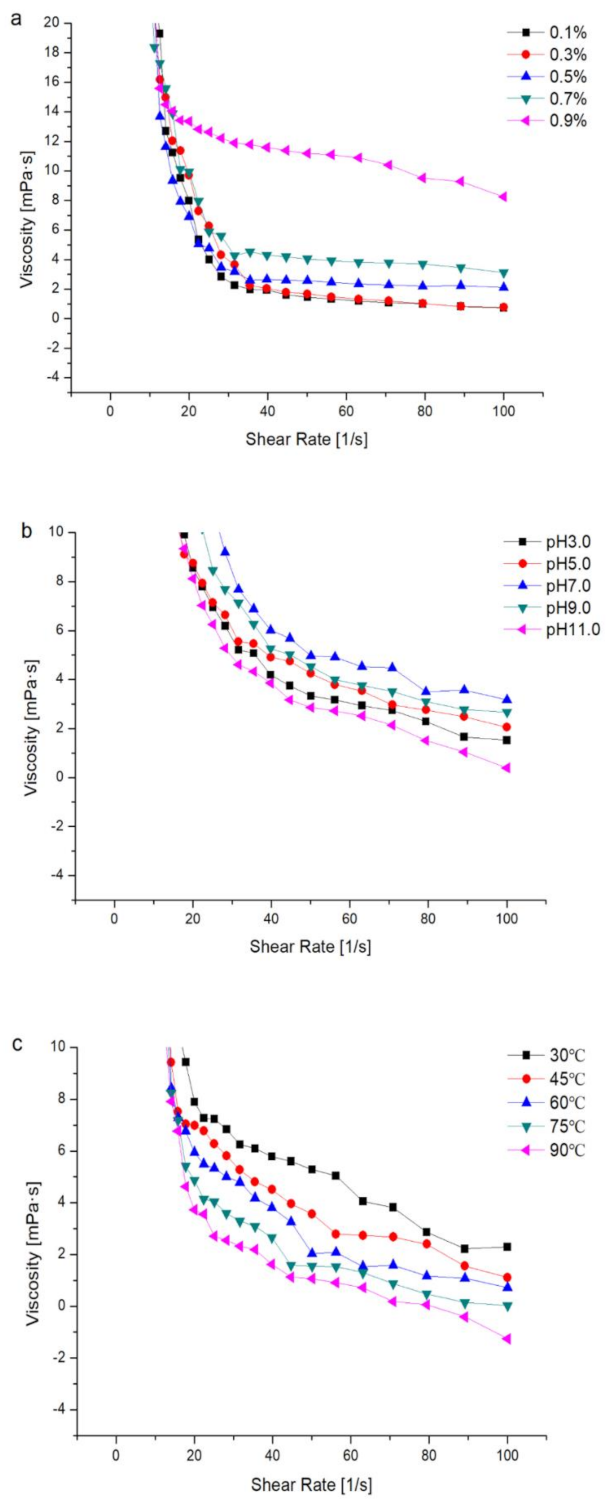
2.6. Rheological Analysis
2.6.1. Effects of Concentration on the Apparent Viscosity of the PHPs Solution
2.6.2. Effects of pH on the Apparent Viscosity of the PHPs Solution
2.6.3. Effects of Temperature on the Apparent Viscosity of the PHPs Solution
2.7. In Vitro Antioxidant Activity
2.8. The Digestibility of PHPs by Artificial Human Gastric Juice
2.9. Analysis of the Digestibility of PHPs by α-Amylase
3. Materials and Methods
3.1. Materials, Reagents, and Equipment
3.2. Extraction of PHPs
3.3. Experimental Design of RSM
3.4. Physicochemical Properties
3.4.1. General Analytical Methods
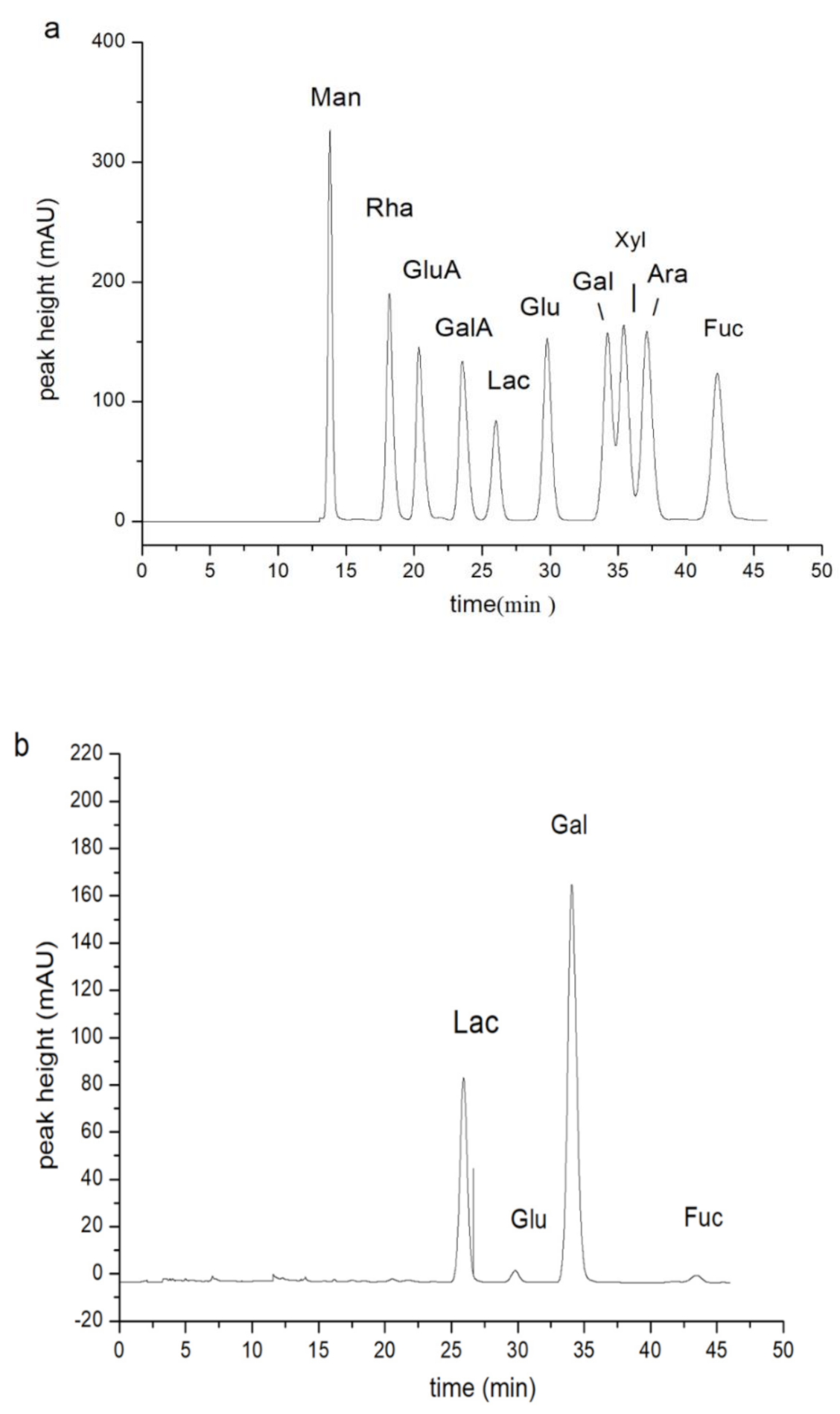
3.4.2. PHPs Composition
3.4.3. Determination of Molecular Weight
3.4.4. Fourier Transform Infrared Spectroscopy (FT-IR)
3.5. Rheological Properties
3.5.1. Effect of Concentration on Apparent Viscosity
3.5.2. Effect of pH on Apparent Viscosity
3.5.3. Effect of Temperature on Apparent Viscosity
3.6. In Vitro Antioxidant Activity
3.6.1. DPPH* Radical Scavenging Effect
3.6.2. Hydroxyl Free Radical (HO) Scavenging Effect
3.6.3. ABTS* Radical Scavenging Effect
3.7. Analysis of the Digestibility of PHPs by Artificial Human Gastric Juice
3.8. Analysis of the Digestibility of PHPs by α-Amylase
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, D.; Keesing, J.K.; Dong, Z.; Zhen, Y.; Di, B.; Shi, Y.; Fearns, P.; Shi, P. Recurrence of the world’s largest green-tide in 2009 in Yellow Sea, China: Porphyra yezoensis aquaculture rafts confirmed as nursery for macroalgal blooms. Mar. Pollut. Bull. 2010, 60, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, N.; Liu, X.; Zhao, Z.; Li, Z.; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, J.; Wang, S.; Xu, X. Porphyra Species: A Mini-Review of Its Pharmacological and Nutritional Properties. J. Med. Food 2016, 19, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, P.; Hao, C.; Zhang, X.-E.; Cui, Z.-Q.; Guan, H.-S. In vitro inhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus. Antivir. Res. 2011, 92, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, W.; Li, X.; Lü, X.; Li, N.; Gao, X.; Song, J. Preparation and in vitro antioxidant activity of κ-carrageenan oligosaccharides and their oversulfated, acetylated, and phosphorylated derivatives. Carbohydr. Res. 2005, 340, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Wang, J.; Zhang, H.; Niu, X.; Li, P. Preparation of the different derivatives of the low-molecular-weight porphyran from Porphyra haitanensis and their antioxidant activities in vitro. Int. J. Biol. Macromol. 2009, 45, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Weiyun, Z.; Jianfeng, Z.; Hao, C.; Lijun, L.; Yayi, H. Effects of a polysaccharide from Porphyra yezoensis on Murin Immunocytes and Human Leukemia K562 Cells. Life Sci. Res. 2002, 6, 167–170. [Google Scholar]
- Qian, L.; Zhou, Y.; Ma, J.-X. Hypolipidemic effect of the polysaccharides from Porphyra yezoensis. Int. J. Biol. Macromol. 2014, 68, 48–49. [Google Scholar] [CrossRef]
- Meitian, X.; Junling, Y.; Haiying, L.; Fengxiang, T.; Chun, M.; Xianai, S.; Yanghao, G. Extraction of Porphyra haitanensis polysaccharides and its anti-influenza virus activity. J. Fuzhou Univ. 2003, 31, 631–635. [Google Scholar]
- Zhang, Z.; Zhang, Q.; Wang, J.; Song, H.; Zhang, H.; Niu, X. Chemical modification and influence of function groups on the in vitro-antioxidant activities of porphyran from Porphyra haitanensis. Carbohydr. Polym. 2010, 79, 290–295. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Zhou, G.; Lu, X.; Xu, Z.; Li, Z. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacol. Res. 2003, 48, 151–155. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, Q.; Qi, H.; Zhang, H.; Niu, X.; Xu, Z.; Li, Z. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. Int. J. Biol. Macromol. 2006, 38, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Zhao, J.; Wang, C.; Wei, M.; Dang, T.; Deng, Y.; Sun, J.; Song, S.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of the degradation products from Porphyra haitanensis polysaccharides. Process. Biochem. 2018, 74, 185–193. [Google Scholar] [CrossRef]
- Khan, B.M.; Qiu, H.-M.; Xu, S.-Y.; Liu, Y.; Cheong, K.-L. Physicochemical characterization and antioxidant activity of sulphated polysaccharides derived from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 145, 1155–1161. [Google Scholar] [CrossRef]
- Sharma, N.; Srivastava, P.; Sharma, A.; Nishad, D.K.; Karwasra, R.; Khanna, K.; Kakkar, D.; Bhatnagar, A. Potential applications of Abelmoschus moschatus polysaccharide as colon release tablets-Rheology and gamma scintigraphic study. J. Drug Deliv. Sci. Technol. 2020, 57, 101632. [Google Scholar] [CrossRef]
- Covis, R.; Desbrières, J.; Marié, E.; Durand, A. Dilational rheology of air/water interfaces covered by nonionic amphiphilic polysaccharides. Correlation with stability of oil-in-water emulsions. Colloids Surf. A: Physicochem. Eng. Asp. 2014, 441, 312–318. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Yang, F.; Sun, H.; Zhou, X.; Guo, Y.; Zhang, M. Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohydr. Polym. 2015, 125, 232–240. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Martera, G.; Goni, I.; Villanueva-Suárez, M.J.; Cuenca, A.R. Chemical structure and molecular weight influence the in vitro fermentability of polysaccharide extracts from the edible seaweeds Himathalia elongata and Gigartina pistillata. Food Hydrocoll. 2018, 83, 348–354. [Google Scholar] [CrossRef]
- Braga, M.E.; Moreschi, S.; Meireles, M.A.A. Effects of supercritical fluid extraction on Curcuma longa L. and Zingiber officinale R. starches. Carbohydr. Polym. 2006, 63, 340–346. [Google Scholar] [CrossRef]
- Chen, C.; You, L.-J.; Abbasi, A.M.; Fu, X.; Liu, R.H. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydr. Polym. 2015, 130, 122–132. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Zhang, J.; Wang, Z. Optimization for the extraction of polysaccharides from Gentiana scabra Bunge and their antioxidant in vitro and anti-tumor activity in vivo. J. Taiwan Inst. Chem. Eng. 2014, 45, 1126–1132. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Artificial neural network and response surface methodology modeling in mass transfer parameters predictions during osmotic dehydration of Carica papaya L. Alex. Eng. J. 2013, 52, 507–516. [Google Scholar] [CrossRef]
- Xie, J.-H.; Shen, M.-Y.; Xie, M.-Y.; Nie, S.-P.; Chen, Y.; Li, C.; Huang, D.-F.; Wang, Y.-X. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohydr. Polym. 2012, 89, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Xue, Y.-T. Optimization of microwave assisted extraction, chemical characterization and antitumor activities of polysaccharides from porphyra haitanensis. Carbohydr. Polym. 2019, 206, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Y.; Aweya, J.J.; Li, N.; Deng, R.-Y.; Chen, W.-Y.; Tang, J.; Cheong, K.-L. Microbial catabolism of Porphyra haitanensis polysaccharides by human gut microbiota. Food Chem. 2019, 289, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Y.; Chen, X.-Q.; Liu, Y.; Cheong, K.-L. Ultrasonic/microwave-assisted extraction, simulated digestion, and fermentation in vitro by human intestinal flora of polysaccharides from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 152, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Şen, M.; Erboz, E.N. Determination of critical gelation conditions of κ-carrageenan by viscosimetric and FT-IR analyses. Food Res. Int. 2010, 43, 1361–1364. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, W.; Bai, X.; Li, C.; Xiang, D. Rheological and physicochemical properties of polysaccharides extracted from stems of Dendrobium officinale. Food Hydrocoll. 2020, 103, 105706. [Google Scholar] [CrossRef]
- Niu, Y.; Li, N.; Xia, Q.; Hou, Y.; Xu, G. Comparisons of three modifications on structural, rheological and functional properties of soluble dietary fibers from tomato peels. LWT 2018, 88, 56–63. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Z.; Zhang, L.; Fang, Y.; Jiang, F.; Phillips, G.O. Structure and chain conformation of water-soluble heteropolysaccharides from Ganoderma lucidum. Carbohydr. Polym. 2011, 86, 844–851. [Google Scholar] [CrossRef]
- Ji, Y.-H.; Liao, A.-M.; Huang, J.-H.; Thakur, K.; Li, X.-L.; Hu, F.; Wei, Z.-J. The rheological properties and emulsifying behavior of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 137, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-Y.; Liao, A.-M.; Huang, J.-H.; Zhang, J.-G.; Thakur, K.; Wei, Z.-J. The rheological properties of differentially extracted polysaccharides from potatoes peels. Int. J. Biol. Macromol. 2019, 137, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, J.; Shi, X.; Song, H.; Zhang, J. In vitro antioxidant activities of acetylated, phosphorylated and benzoylated derivatives of porphyran extracted from Porphyra haitanensis. Carbohydr. Polym. 2009, 78, 449–453. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Yu, Y.; Liang, Y.-Z.; Chen, X. Purification, partial characterization and antioxidant activity of polysaccharides from Glycyrrhiza uralensis. Int. J. Biol. Macromol. 2015, 79, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Zhao, J.; Guo, Y.; Zhang, H.; Duan, M.; Wu, H. Extraction, purification, emulsifying property, hypoglycemic activity, and antioxidant activity of polysaccharides from comfrey. Ind. Crop. Prod. 2020, 146, 112183. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Fermentation and non-digestibility of Mangifera pajang fibrous pulp and its polysaccharides. J. Funct. Foods 2012, 4, 933–940. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, J.; Ren, X.; Li, B.; Zhang, Q. Extraction, preliminary characterization and in vitro antioxidant activity of polysaccharides from Oudemansiella radicata mushroom. Int. J. Biol. Macromol. 2018, 120, 1760–1769. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kawai, Y.; Seno, N.; Anno, K. A modified method for chondrosulfatase assay. Anal. Biochem. 1969, 32, 314–321. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Wang, J.; Shi, X.; Zhang, Z. Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC. Chin. J. Oceanol. Limnol. 2009, 27, 578–582. [Google Scholar] [CrossRef]
- Himmel, M.E.; Squire, P.G. High-performance size exclusion chromatography of sea worm chlorocruorin and other large proteins, viruses and polysaccharides on a TSK G5000 PW preparative column. J. Chromatogr. A 1981, 210, 443–452. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Liu, J.-P.; Huang, X.; Du, L.-P.; Shi, F.-L.; Dong, R.; Huang, X.-T.; Zheng, K.; Liu, Y.; Cheong, K.-L. Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT 2018, 90, 577–582. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takamura, H.; Matoba, T.; Terao, J. HPLC Method for Evaluation of the Free Radical-scavenging Activity of Foods by Using 1,1-Diphenyl-2-picrylhydrazyl. Biosci. Biotechnol. Biochem. 1998, 62, 1201–1204. [Google Scholar] [CrossRef]
- Rozi, P.; Abuduwaili, A.; Mutailifu, P.; Gao, Y.; Rakhmanberdieva, R.; Aisa, H.A.; Yili, A. Sequential extraction, characterization and antioxidant activity of polysaccharides from Fritillaria pallidiflora Schrenk. Int. J. Biol. Macromol. 2019, 131, 97–106. [Google Scholar] [CrossRef]
- Saqib, A.A.N.; Whitney, P.J. Differential behaviour of the dinitrosalicylic acid (DNS) reagent towards mono- and di-saccharide sugars. Biomass-Bioenergy 2011, 35, 4748–4750. [Google Scholar] [CrossRef]
- Wichienchot, S.; Jatupornpipat, M.; Rastall, R. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. 2010, 120, 850–857. [Google Scholar] [CrossRef]






| Number | A/(g/mL) | B/h | C/ °C | Y/% |
|---|---|---|---|---|
| 1 | 0.05 | 3.00 | 90.00 | 17.79 |
| 2 | 0.03 | 4.00 | 80.00 | 17.45 |
| 3 | 0.04 | 4.00 | 90.00 | 17.24 |
| 4 | 0.04 | 3.00 | 80.00 | 20.05 |
| 5 | 0.05 | 2.00 | 80.00 | 17.05 |
| 6 | 0.03 | 3.00 | 70.00 | 16.01 |
| 7 | 0.04 | 2.00 | 70.00 | 15.9 |
| 8 | 0.04 | 2.00 | 90.00 | 17.1 |
| 9 | 0.04 | 3.00 | 80.00 | 20.79 |
| 10 | 0.04 | 3.00 | 80.00 | 20.15 |
| 11 | 0.03 | 3.00 | 90.00 | 19.69 |
| 12 | 0.05 | 4.00 | 80.00 | 16.91 |
| 13 | 0.05 | 3.00 | 70.00 | 16.53 |
| 14 | 0.04 | 4.00 | 70.00 | 16.45 |
| 15 | 0.03 | 2.00 | 80.00 | 16.61 |
| Source | Sum of Squares | df | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| model | 35.32 | 9 | 3.92 | 12.15 | 0.0067 |
| A | 0.27 | 1 | 0.27 | 0.85 | 0.3995 |
| B | 0.24 | 1 | 0.24 | 0.75 | 0.4267 |
| C | 6.00 | 1 | 6.00 | 18.59 | 0.0076 |
| AB | 0.24 | 1 | 0.24 | 0.74 | 0.4280 |
| AC | 1.46 | 1 | 1.46 | 4.53 | 0.0865 |
| BC | 0.042 | 1 | 0.042 | 0.13 | 0.7331 |
| A2 | 5.73 | 1 | 5.73 | 17.75 | 0.0084 |
| B2 | 15.96 | 1 | 15.96 | 49.40 | 0.0009 |
| C2 | 9.20 | 1 | 9.20 | 28.49 | 0.0031 |
| Residual | 1.62 | 5 | 0.32 | ||
| Lack of Fit | 1.29 | 3 | 0.43 | 2.67 | 0.2840 |
| Pure error | 0.32 | 2 | 0.16 | ||
| Cor total | 36.93 | 14 |
| Protein a Content/(g/L) | Polysaccharide Purity b/% | Sulfate c/(mg/mL) | Molecular Weight d/Da | Monosaccharide Composition e (Molar Ratio) |
|---|---|---|---|---|
| Glucose Galactose Fucose | ||||
| 0.056 | 85 | 2.7 | 6.3 × 105 | 2.1 76.2 1 |
| Variable | Symbol | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| ratios of raw materials to water (g/mL) | X1 | 1:20 | 1:25 | 1:30 |
| extraction temperature /°C | X2 | 70 | 80 | 90 |
| extraction time/h | X3 | 2 | 3 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, M.; Jiang, Y.; Wang, C.; Yang, Q.; Jiang, X.; Zhu, C. Determination of the Extraction, Physicochemical Characterization, and Digestibility of Sulfated Polysaccharides in Seaweed—Porphyra haitanensis. Mar. Drugs 2020, 18, 539. https://doi.org/10.3390/md18110539
Dong M, Jiang Y, Wang C, Yang Q, Jiang X, Zhu C. Determination of the Extraction, Physicochemical Characterization, and Digestibility of Sulfated Polysaccharides in Seaweed—Porphyra haitanensis. Marine Drugs. 2020; 18(11):539. https://doi.org/10.3390/md18110539
Chicago/Turabian StyleDong, Mingshuang, Yanhui Jiang, Chun Wang, Qian Yang, Xiaolu Jiang, and Changliang Zhu. 2020. "Determination of the Extraction, Physicochemical Characterization, and Digestibility of Sulfated Polysaccharides in Seaweed—Porphyra haitanensis" Marine Drugs 18, no. 11: 539. https://doi.org/10.3390/md18110539
APA StyleDong, M., Jiang, Y., Wang, C., Yang, Q., Jiang, X., & Zhu, C. (2020). Determination of the Extraction, Physicochemical Characterization, and Digestibility of Sulfated Polysaccharides in Seaweed—Porphyra haitanensis. Marine Drugs, 18(11), 539. https://doi.org/10.3390/md18110539




