Pseudoceratonic Acid and Moloka’iamine Derivatives from the Red Sea Verongiid Sponge Pseudoceratina arabica
Abstract
1. Introduction
2. Results and Discussion
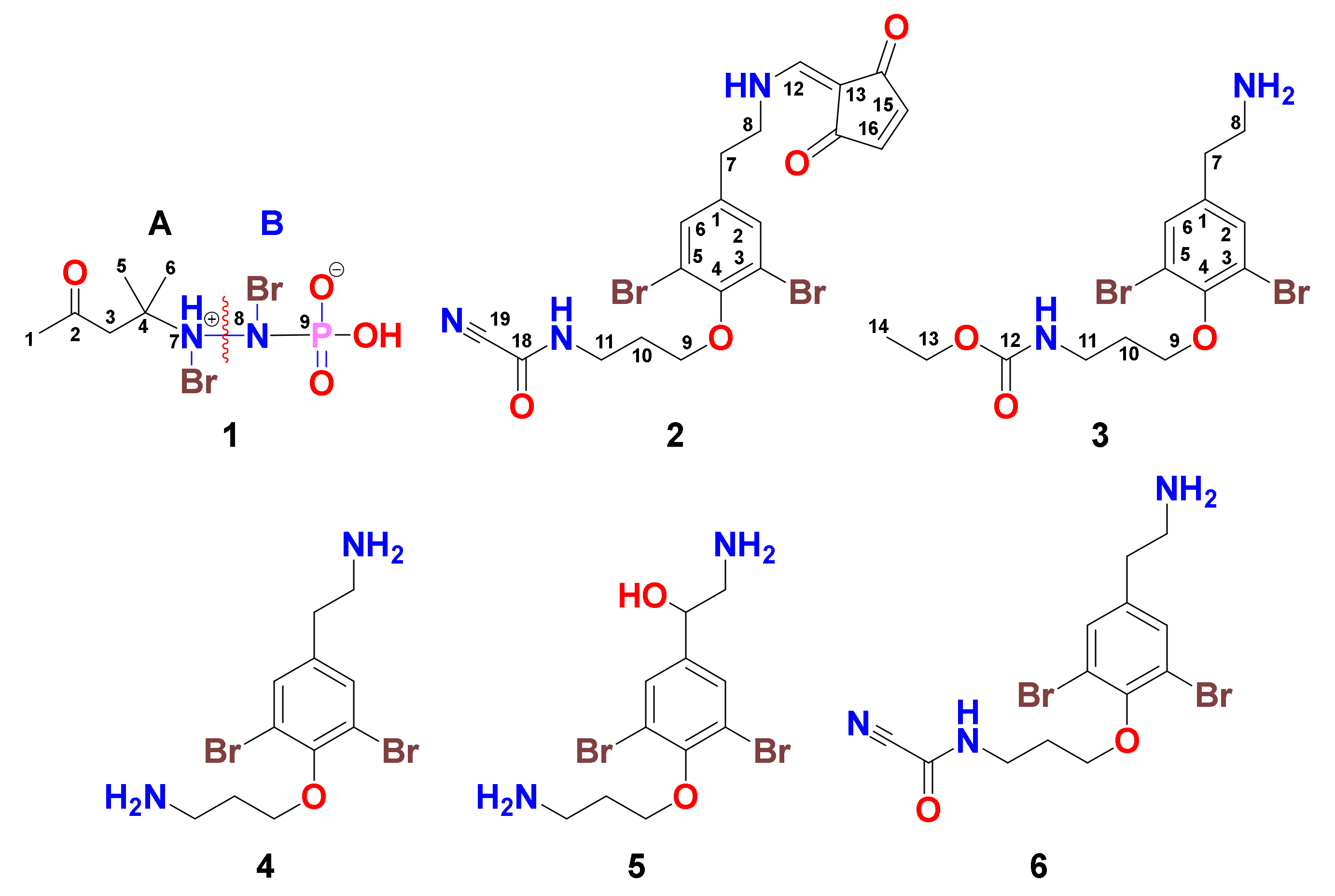
2.1. Purification of Compounds 1–6
2.2. Structural Determination of 1–6
3. Materials and Methods
3.1. General Experimental Procedures
3.2. The Sponge Pseudoceratina arabica
3.3. Purification of 1–6
Spectroscopic Data of the Compounds
Pseudoceratonic acid (1)
Ceratinine N (2)
Ceratinine O (3)
3.4. Biological Evaluation of the Compounds
Antimicrobial Evaluation of the Compounds
Disc Diffusion Assay
Determination of the MIC of the Compounds
3.5. Determination of the Cytotoxicity of the Compounds
3.5.1. Cell Lines and Cultures
3.5.2. MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakata, T.; Winzeler, E.A. Genomics, systems biology and drug development for infectious diseases. Mol. Biosyst. 2007, 3, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.T.; Scheuer, P.J.; Kelly-Borges, M. Biogenetically diverse, bioactive constituents of a sponge, order Verongida: Bromotyramines and sesquiterpene-shikimate derived metabolites. J. Org. Chem. 1993, 58, 6565–6569. [Google Scholar] [CrossRef]
- Kornprobst, J.-M. Encyclopedia of Marine Natural Products; Wiley-Blackwell: Weinheim, Germany, 2010; Volume 2, Chapter 19; pp. 796–805. [Google Scholar]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 37–175. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, J.; Hamann, M.T. The marine bromotyrosine derivatives. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Academic Press: San Diego, CA, USA, 2005; Volume 61, pp. 59–262. [Google Scholar]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Ceratinamine: An unprecedented antifouling cyanoformamide from the marine sponge Pseudoceratina purpurea. J. Org. Chem. 1996, 61, 2936–2937. [Google Scholar] [CrossRef] [PubMed]
- Ichiba, T.; Scheuer, P.J.; Kelly-Borges, M. Three bromotyrosine derivatives, one terminating in an unprecedented diketocyclopentenylidene enamine. J. Org. Chem. 1993, 58, 4149–4150. [Google Scholar] [CrossRef]
- Badr, J.M.; Shaala, L.A.; Abou-Shoer, M.I.; Tawfik, M.A.; Habib, A.M. Bioactive brominated metabolites from the Red Sea sponge Pseudoceratina arabica. J. Nat. Prod. 2008, 71, 1472–1474. [Google Scholar] [CrossRef]
- Takada, N.; Watanabe, R.; Suenaga, K.; Yamada, K.; Ueda, K.; Kita, M.; Uemura, D. Zamamistatin, a significant antibacterial bromotyrosine derivative, from the Okinawan sponge Pseudoceratina purpurea. Tetrahedron Lett. 2001, 42, 5265–5267. [Google Scholar] [CrossRef]
- Jang, J.; van Soest, R.W.M.; Fusetani, N.; Matsunaga, S. Pseudoceratins A and B, antifungal bicyclic bromotyrosine-derived metabolites from the marine sponge Pseudoceratina purpurea. J. Org. Chem. 2007, 72, 1211–1217. [Google Scholar] [CrossRef]
- Kobayashi, J.; Tsuda, M.; Agemi, K.; Shigemori, H.; Ishibashi, M.; Sasaki, T.; Mikami, Y. Purealidins B and C, new bromotyrosine alkaloids from the Okinawan marine sponge Psammaplysilla purea. Tetrahedron 1991, 47, 6617–6622. [Google Scholar] [CrossRef]
- Dai, J.; Parrish, S.; Yoshida, W.; Yip, M.; Turkson, J.; Kelly, M.; Williams, P. Bromotyrosine-derived metabolites from an Indonesian marine sponge in the family Aplysinellidae (Order Verongiida). Bioorg. Med. Chem. Lett. 2016, 26, 499–504. [Google Scholar] [CrossRef]
- Tarazona, G.; Santamaría, G.; Cruz, P.G.; Fernández, R.; Pérez, M.; Martínez-Leal, J.F.; Rodríguez, J.; Jiménez, C.; Cuevas, C. Cytotoxic anomoian B and aplyzanzine B, new bromotyrosine alkaloids from Indonesian sponges. ACS Omega 2017, 2, 3494–3501. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Sulaiman, M.; Khedr, A.; El Sayed, K.A. Bioactive alkaloids from the Red Sea marine Verongid sponge Pseudoceratina arabica. Tetrahedron 2015, 71, 7837–7841. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A. Cytotoxic psammaplysin analogues from the Verongid Red Sea sponge Aplysinella species. Biomolecules 2019, 9, 841. [Google Scholar] [CrossRef]
- Mudianta, I.W.; Skinner-Adams, T.; Andrews, K.T.; Davis, R.A.; Hadi, T.A.; Hayes, P.Y.; Garson, M.J. Psammaplysin derivatives from the Balinese marine sponge Aplysinella strongylata. J. Nat. Prod. 2012, 75, 2132. [Google Scholar] [CrossRef]
- Yang, X.; Davis, R.A.; Buchanan, M.S.; Duffy, S.; Avery, V.M.; Camp, D.; Quinn, R.J. Antimalarial bromotyrosine derivatives from the Australian marine sponge Hyattella sp. J. Nat. Prod. 2010, 73, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, S.I.; Ohno, T.; Hokari, R.; Ishiyama, A.; Iwatsuki, M.; Ōmura, S.; Kobayashi, J.; Kubota, T. Ceratinadins E and F, new bromotyrosine alkaloids from an Okinawan marine sponge Pseudoceratina sp. Mar. Drugs 2018, 16, 463. [Google Scholar] [CrossRef]
- Pina, I.C.; Gautschi, J.T.; Wang, G.; Sanders, M.L.; Schmitz, F.J.; France, D.; Cornell-Kennon, S.; Sambucetti, L.C.; Remiszewski, S.W.; Perez, L.B.; et al. Psammaplins from the sponge Pseudoceratina purpurea: Inhibition of both histone deacetylase and DNA methyltransferase. J. Org. Chem. 2003, 68, 3866–3873. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Pseudoceratidine: A new antifouling spermidine derivative from the marine sponge Pseudoceratina purpurea. Tetrahedron Lett. 1996, 37, 1439–1440. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Ceratinamides A and B: New antifouling dibromotyrosine derivatives from the marine sponge Pseudoceratina purpurea. Tetrahedron 1996, 52, 8181–8186. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; Sulaiman, M.; Behery, F.A.; Foudah, A.I.; El Sayed, K.A. Subereamolline A as a potent breast cancer migration, invasion and proliferation inhibitor and bioactive dibrominated alkaloids from the Red Sea sponge Pseudoceratina arabica. Mar. Drugs 2012, 10, 2492–2508. [Google Scholar] [CrossRef]
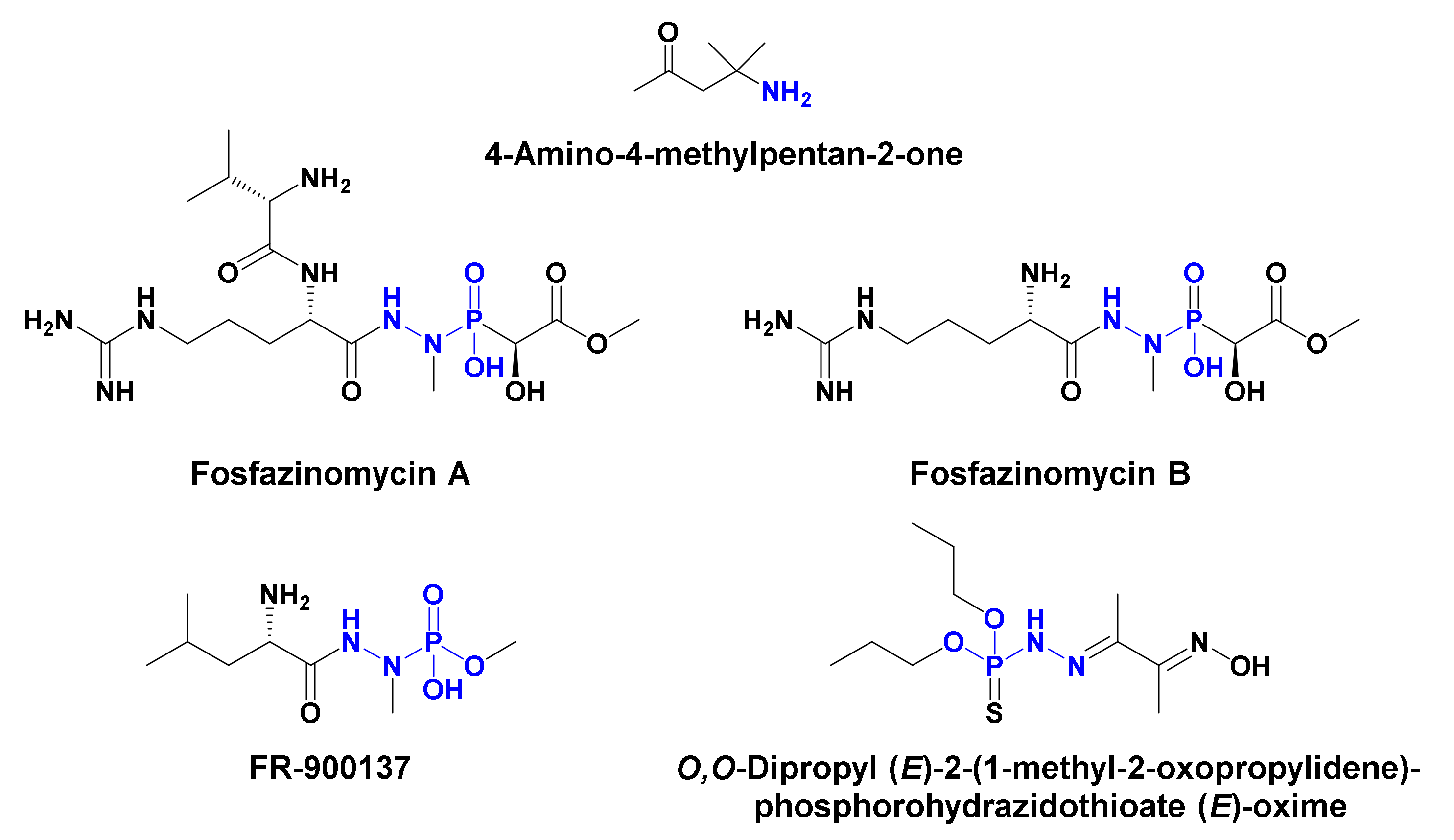
- Le Goff, G.; Ouazzani, J. Natural hydrazine-containing compounds: Biosynthesis, isolation, biological activities and synthesis. Bioorg. Med. Chem. 2014, 22, 6529–6544. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.M.; Sperry, J. Natural products containing a nitrogen–nitrogen bond. J. Nat. Prod. 2013, 76, 794–812. [Google Scholar] [CrossRef] [PubMed]
- Kafarski, P. Phosphonates: Their natural occurrence and physiological role. Open access peer-reviewed chapter. In Biological Role of Phosphorus; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Petkowski, J.J.; Bains, W.; Seager, S. Natural products containing ‘rare’ organophosphorus functional groups. Molecules 2019, 24, 866. [Google Scholar] [CrossRef] [PubMed]
- Ogita, T.; Gunji, S.; Fukazawa, Y.; Terahara, A.; Kinoshita, T.; Nagaki, H.; Beppu, T. The structures of fosfazinomycins A and B. Tetrahedron Lett. 1983, 24, 2283–2286. [Google Scholar] [CrossRef]
- Kuroda, Y.; Goto, T.; Okamoto, M.; Yamashita, M.; Iguchi, E.; Kohsaka, M.; Aoki, H.; Imanaka, H. FR-900137, a new antibiotic. I. Taxonomy and fermentation of the organism, and isolation and characterization of the antibiotic. J. Antibiot. 1980, 33, 272–279. [Google Scholar] [CrossRef]
- Kuroda, Y.; Tanaka, H.; Okamoto, M.; Goto, T.; Kohsaka, M.; Aoki, H.; Imanaka, H. FR-900137, a new antibiotic. II. Structure determination of FR-900137. J. Antibiot. 1980, 33, 280–283. [Google Scholar] [CrossRef]
- Alam, M.; Sanduja, R.; Hossain, M.B.; van der Helm, D. Gymnodinium breve toxins. 1. Isolation and x-ray structure of O,O-dipropyl (E)-2-(1-methyl-2-oxopropylidene)phosphorohydrazidothioate (E)-oxime from the red tide dinoflagellate Gymnodinium breve. J. Am. Chem. Soc. 1982, 104, 5232–5234. [Google Scholar] [CrossRef]
- Zheng, D.; Jiang, Y.; Han, L.; Lou, K.; Chen, Y.; Xu, L.; Huang, X. Chemical constituents from mycelium of a new streptomycete. Zhongguo Yaowu Huaxue Zazhi 2010, 20, 201–205. [Google Scholar]
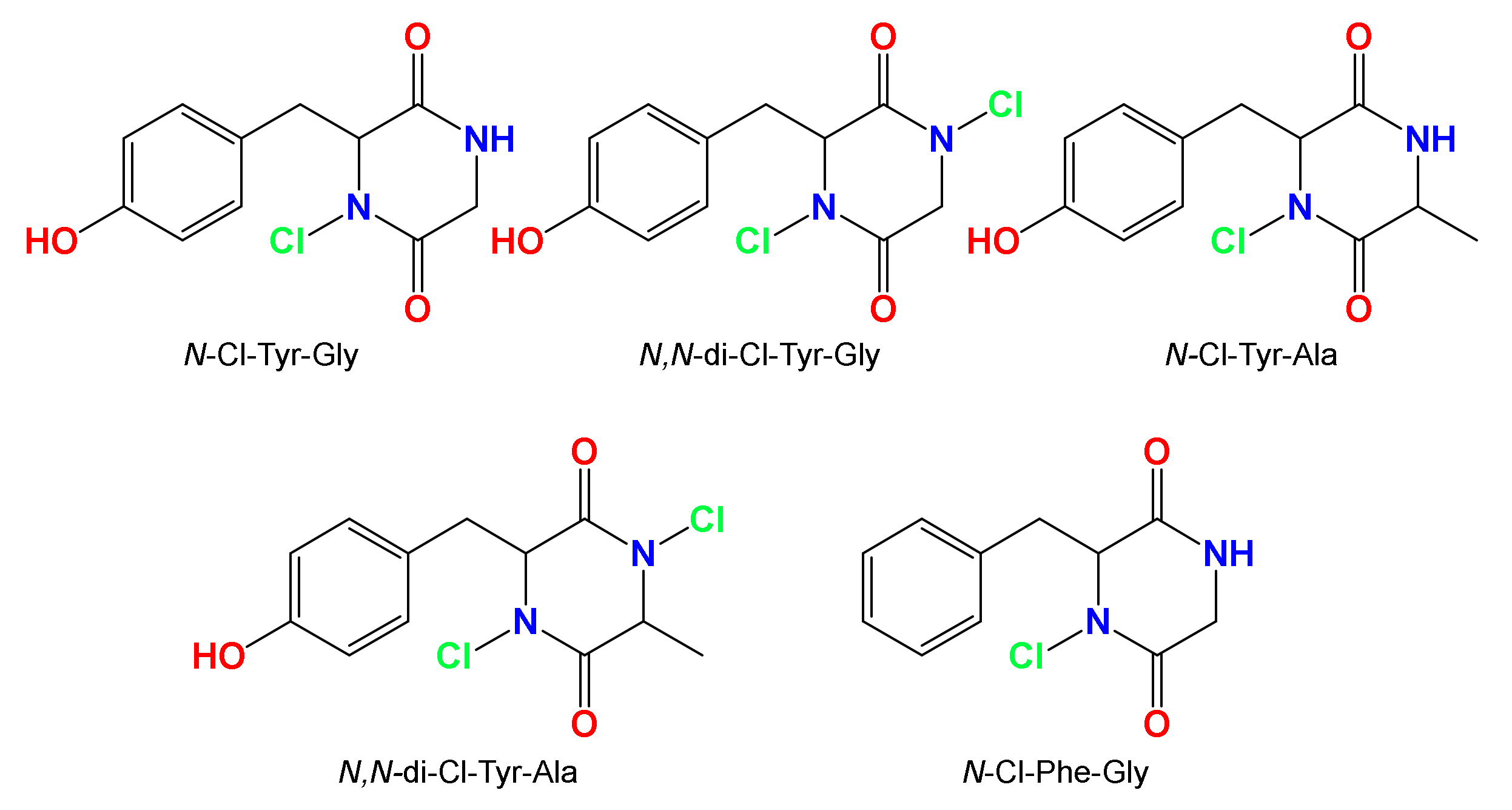
- Huang, G.; Jiang, P.; Li, X.-F. Mass spectrometry identification of N-chlorinated dipeptides in drinking water. Anal. Chem. 2017, 89, 4204–4209. [Google Scholar] [CrossRef]
- Abou-Shoer, M.I.; Shaala, L.A.; Youssef, D.T.A.; Badr, J.M.; Habib, A.M. Bioactive brominated metabolites from the Red Sea sponge Suberea mollis. J. Nat. Prod. 2008, 71, 1464–1467. [Google Scholar] [CrossRef]
- Shaala, L.A.; Youssef, D.T.A.; Alzughaibi, T.A.; Elhady, S.S. Antimicrobial chlorinated 3-phenylpropanoic acid derivatives from the Red Sea marine actinomycete Streptomyces coelicolor LY001. Mar. Drugs 2020, 18, 450. [Google Scholar] [CrossRef] [PubMed]
- Acar, J.F. The disc susceptibility test. In Antibiotics in Laboratory Medicine, Williams and Wilkins, Baltimore; Lorian, V., Ed.; Williams & Wilkins: Philadelphia, PA, USA, 1980; pp. 24–54. [Google Scholar]
- Kiehlbauch, J.A.; Hannett, G.E.; Salfinger, M.; Archinal, W.; Monserrat, C.; Carlyn, C. Use of the National Committee for Clinical Laboratory Standards Guidelines for Disk Diffusion Susceptibility Testing in New York State Laboratories. J. Clin. Microbiol. 2000, 38, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Khalifa, S.I.; Mesbah, M.K.; van Soest, R.W.M.; Youssef, D.T.A. Subereaphenol A, a new cytotoxic and antimicrobial dibrominated phenol from the Red Sea sponge Suberea mollis. Nat. Prod. Commun. 2008, 3, 219–222. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, approved standard 9th ed.; CLSI Documents M07-A9. West Valley Road, Suite 2500; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2007. [Google Scholar]
- Youssef, D.T.A.; Mooberry, S.L. Hurghadolide A and swinholide I, potent actin-microfilament disrupters from the Red Sea sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 154–157. [Google Scholar] [CrossRef]
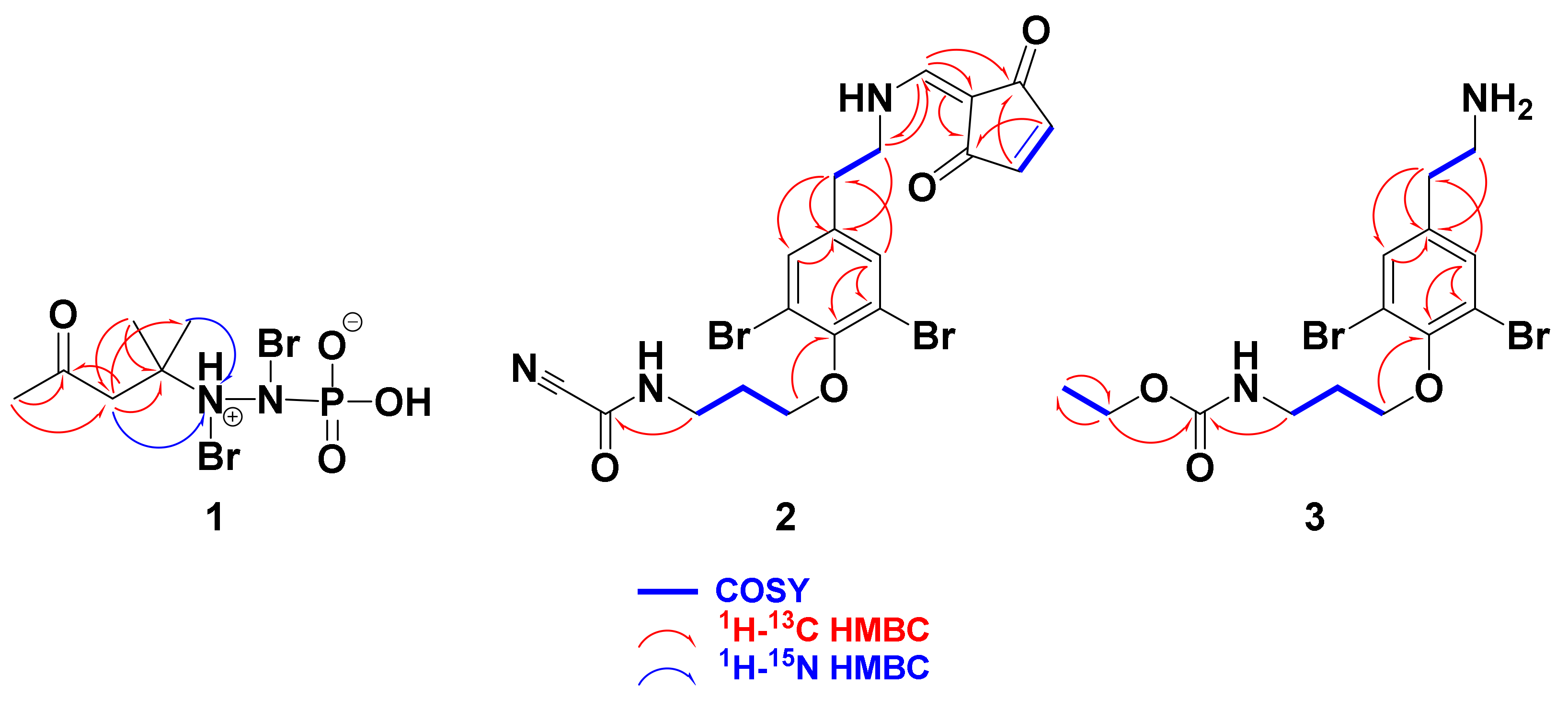
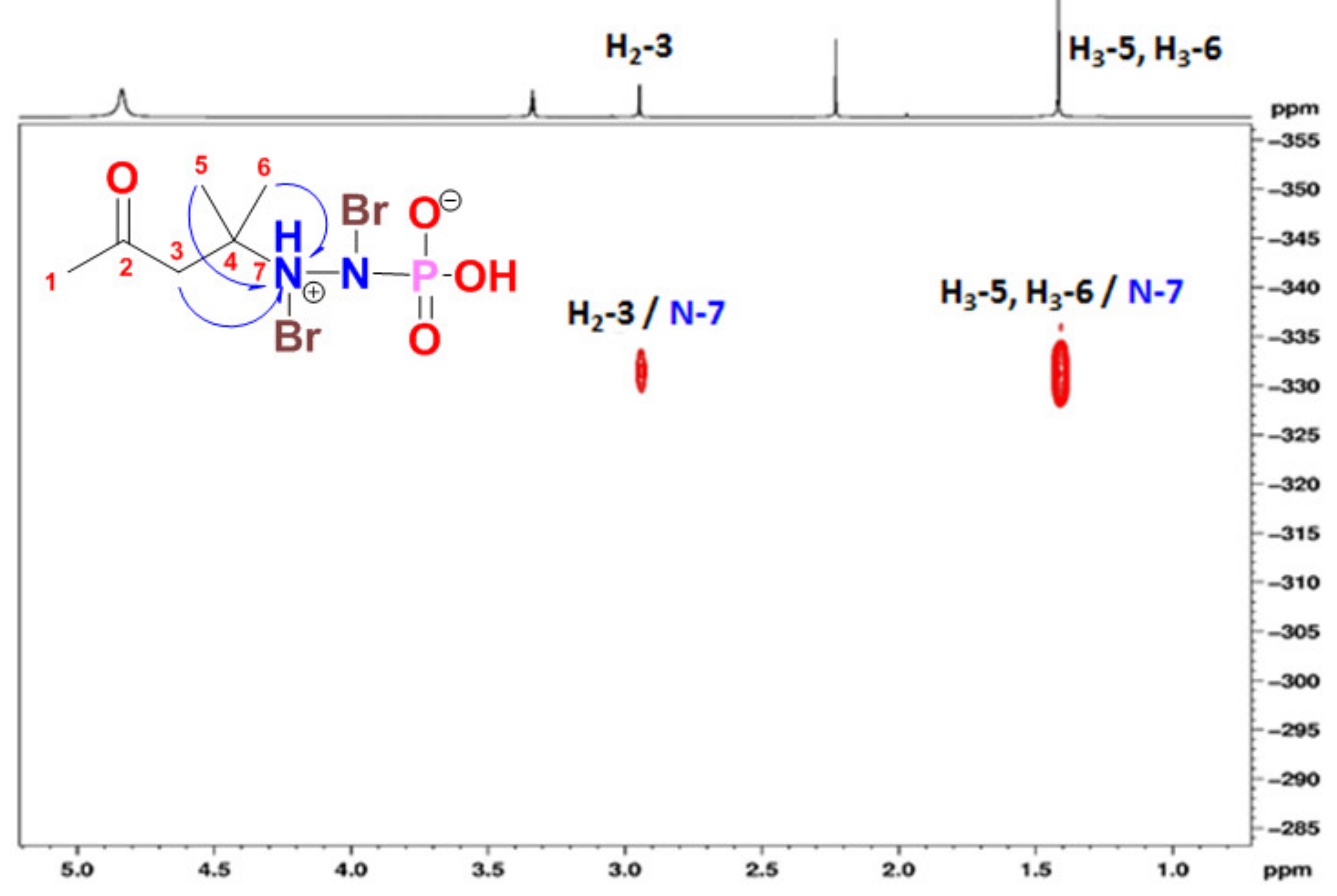
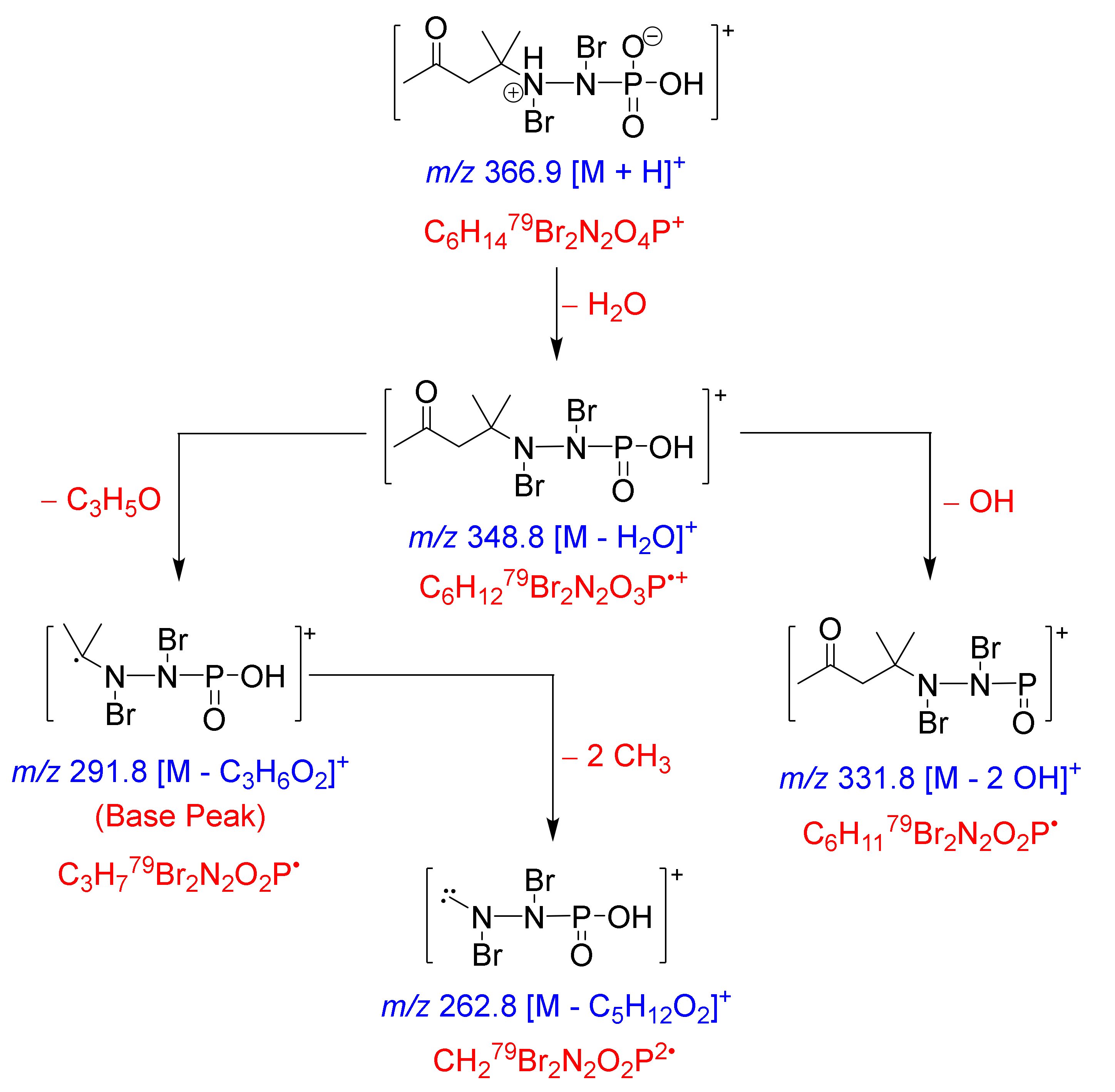
| No. | δC (mult.) | δN b | δH (mult.) | 1H-13C HMBC | 1H-15N HMBC |
|---|---|---|---|---|---|
| 1 | 31.0, CH3 | 2.23 (3H, s) | C-2, C-3 | ||
| 2 | 209.3, qC | ||||
| 3 | 50.7, CH2 | 2.91 (2H, s) | C-2, C-4, C-6 | N-7 | |
| 4 | 53.4, qC | ||||
| 5 | 26.0, CH3 | 1.42 (3H, s) | C-3, C-4 | N-7 | |
| 6 | 26.0, CH3 | 1.42 (3H, s) | C-3, C-4 | N-7 | |
| N-7 | −331.6 |
| No. | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| δC (Mult.) a | δH (Mult., J (Hz)) | HMBC (C#) | δC (Mult.) a | δH (Mult., J (Hz)) | HMBC (C#) | |
| 1 | 138.6 (qC) | 139.6 (qC) | ||||
| 2 | 134.8 (CH) | 7.45 (s) | C-1, C-3/5, C-4, C-7 | 134.3 (CH) | 7.46 (s) | C-1, C-3/5, C-4, C-7 |
| 3 | 119.3 (qC) | 119.1 (qC) | ||||
| 4 | 153.2 (qC) | 152.9 (qC) | ||||
| 5 | 119.3 (qC) | 119.1 (qC) | ||||
| 6 | 134.8 (CH) | 7.45 (s) | C-1, C-3/5, C-4, C-7 | 134.3 (CH) | 7.46 (s) | C-1, C-3/5, C-4, C-7 |
| 7 | 36.7 (CH2) | 2.86 (t, 6.6) | C-1, C-2/6, C-8 | 33.0 (CH2) | 2.92 (t, 6.6) | C-1, C-2/6, C-8 |
| 8 | 51.9 (CH2) | 3.61 (t, 6.6) | C-1, C-7, C-12 | 41.0 (CH2) | 3.13 (t, 6.6) | C-1, C-7 |
| 9 | 71.0 (CH2) | 4.02 (t, 6.6) | C-4, C-10, C-11 | 72.0 (CH2) | 4.01 (t, 6.6) | C-4, C-10, C-11 |
| 10 | 30.1 (CH2) | 2.08 (quin, 6.6) | C-9, C-11 | 31.3 (CH2) | 2.01 (quin, 6.6) | C-9, C-11 |
| 11 | 38.4 (CH2) | 3.56 (t, 6.6) | C-9, C-10, C-18 | 39.1 (CH2) | 3.42 (t, 6.6) | C-9, C-10, C-12 |
| 12 | 150.8 (CH) | 7.21 (s) | C-8, C-13, C-14, C-17 | 159.2 (qC) | ||
| 13 | 99.5 (qC) | 61.7 (CH2) | 4.05 (q, 6.6) | C-12, C-14 | ||
| 14 | 198.7 (qC) | 14.5 (CH3) | 1.22 (t, 6.6) | C-13 | ||
| 15 | 142.7 (CH) | 6.67 (d, 6.6) | C-13, C-14, C-16, C-17 | |||
| 16 | 142.9 (CH) | 6.73 (d, 6.6) | C-13, C-14, C-15, C-17 | |||
| 17 | 196.8 (qC) | |||||
| 18 | 145.0 (qC) | |||||
| 19 | 113.1 (qC) | |||||
| Compound | E. coli | S. aureus | C. albicans | |||
|---|---|---|---|---|---|---|
| Inhibition Zone (mm) | MIC (µg/mL) | Inhibition Zone (mm) | MIC (µg/mL) | Inhibition Zone (mm) | MIC (µg/mL) | |
| 1 | 15 | 16 | 17 | 16 | 6 | 125 |
| 2 | 6 | 125 | 7 | 125 | 16 | 16 |
| 3 | 6 | 125 | 8 | 64 | 9 | 64 |
| 4 | 6 | 125 | 7 | 125 | 6 | 125 |
| 5 | NT | NT | NT | NT | NT | NT |
| 6 | 7 | 125 | 9 | 64 | 12 | 32 |
| Ciprofloxacin a | 30 | 0.25 | 22 | 0.5 | NT | NT |
| Ketoconazole b | NT | NT | NT | NT | 30 | 0.5 |
| Compound | IC50 (µM) | |||
|---|---|---|---|---|
| MDA-MB-231 | HeLa | HCT 116 | NHDF | |
| 1 | ≥10 | ≥10 | ≥10 | |
| 2 | 3.1 ± 0.29 | 2.3 ± 0.16 | 2.9 ± 0.18 | 3.5 ± 0.30 |
| 3 | ≥10 | ≥10 | 9.5 ± 0.85 | |
| 4 | ≥10 | ≥10 | ≥10 | |
| 5 | ≥10 | ≥10 | ≥10 | |
| 6 | ≥10 | ≥10 | ≥10 | |
| 5-FU b | 13.0 ± 0.30 | 12.3 ± 0.25 | 4.6 ± 0.23 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaala, L.A.; Youssef, D.T.A. Pseudoceratonic Acid and Moloka’iamine Derivatives from the Red Sea Verongiid Sponge Pseudoceratina arabica. Mar. Drugs 2020, 18, 525. https://doi.org/10.3390/md18110525
Shaala LA, Youssef DTA. Pseudoceratonic Acid and Moloka’iamine Derivatives from the Red Sea Verongiid Sponge Pseudoceratina arabica. Marine Drugs. 2020; 18(11):525. https://doi.org/10.3390/md18110525
Chicago/Turabian StyleShaala, Lamiaa A., and Diaa T. A. Youssef. 2020. "Pseudoceratonic Acid and Moloka’iamine Derivatives from the Red Sea Verongiid Sponge Pseudoceratina arabica" Marine Drugs 18, no. 11: 525. https://doi.org/10.3390/md18110525
APA StyleShaala, L. A., & Youssef, D. T. A. (2020). Pseudoceratonic Acid and Moloka’iamine Derivatives from the Red Sea Verongiid Sponge Pseudoceratina arabica. Marine Drugs, 18(11), 525. https://doi.org/10.3390/md18110525