Bioavailability of Orally Administered Active Lipid Compounds from four Different Greenshell™ Mussel Formats
Abstract
1. Introduction
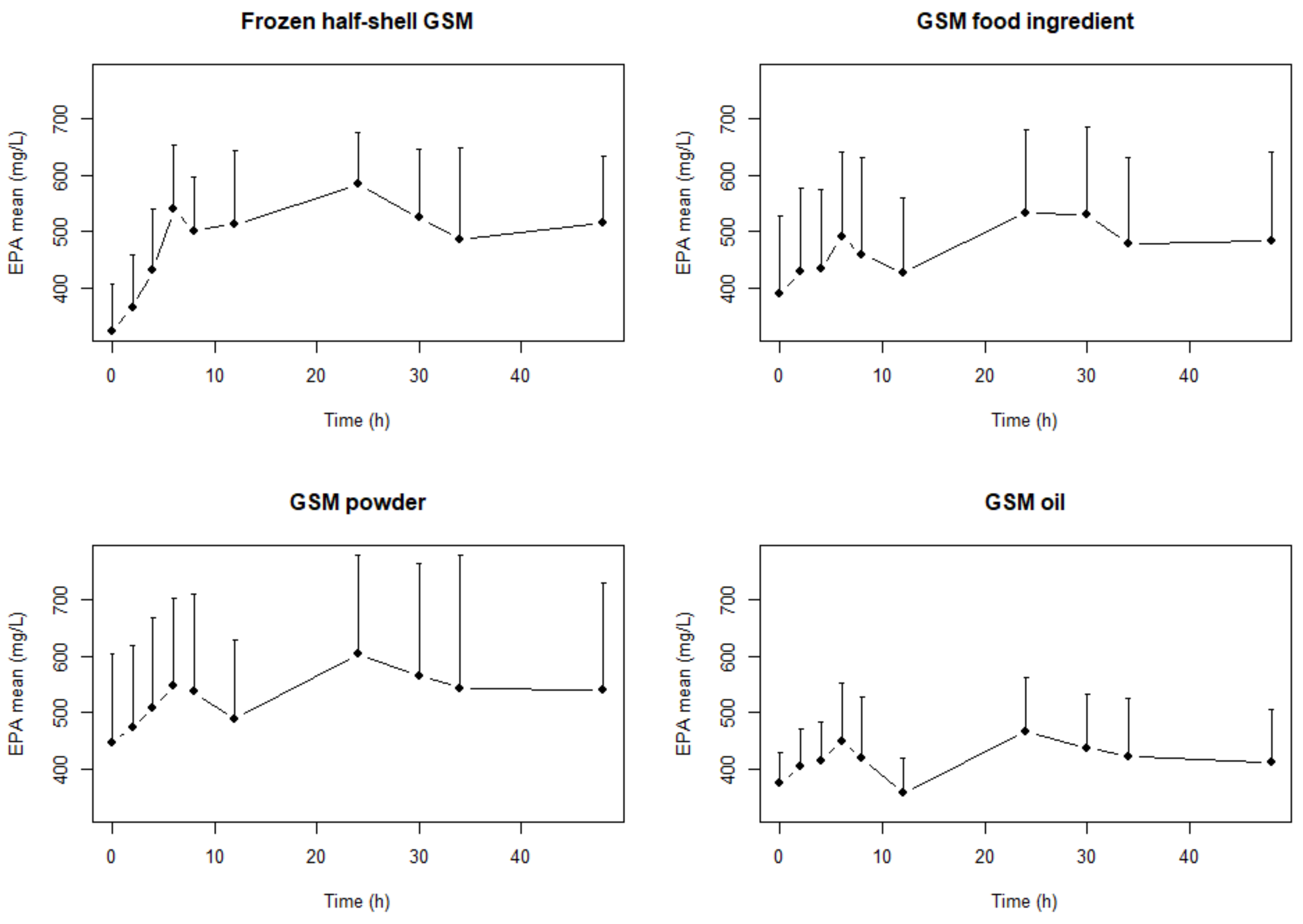
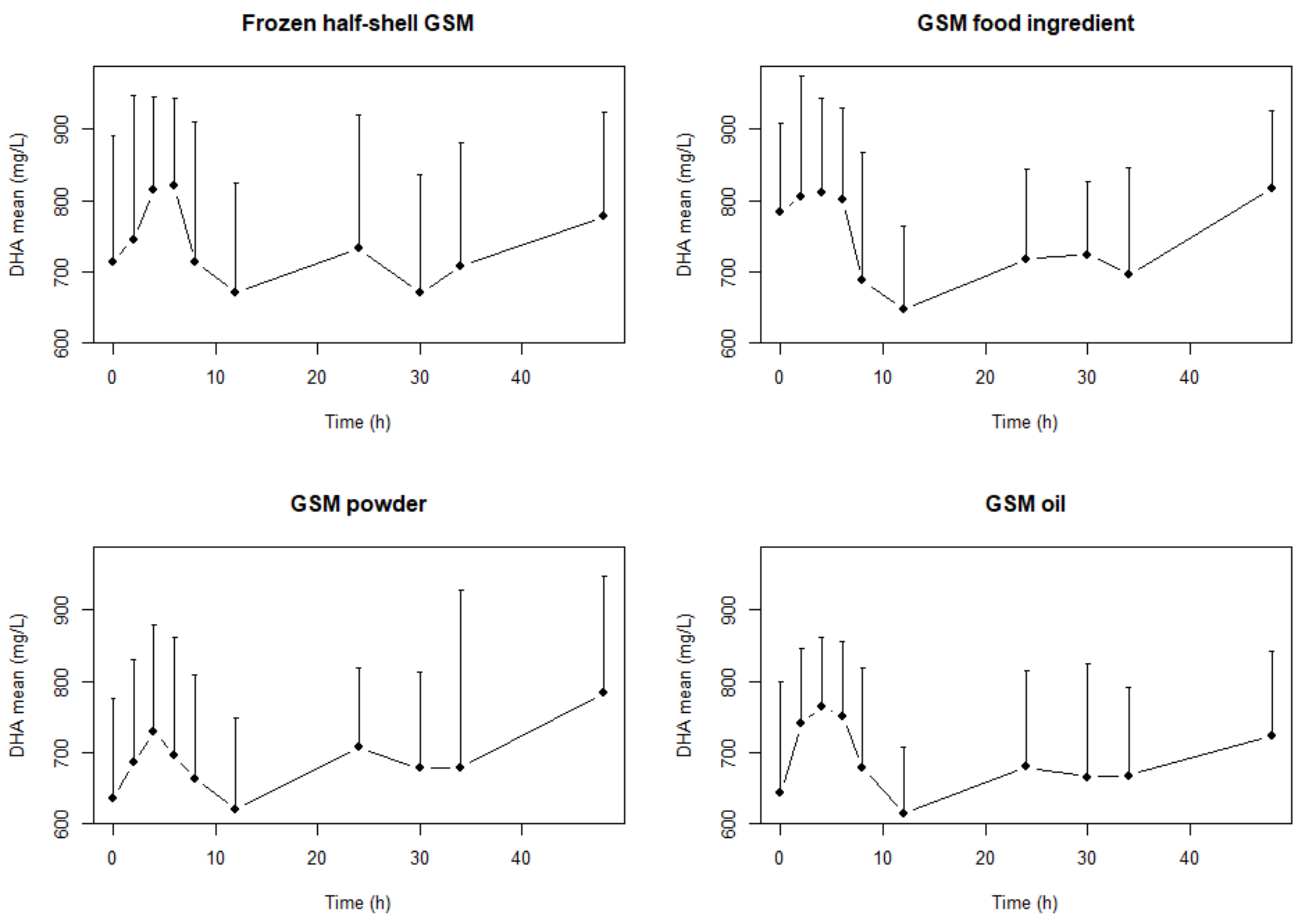
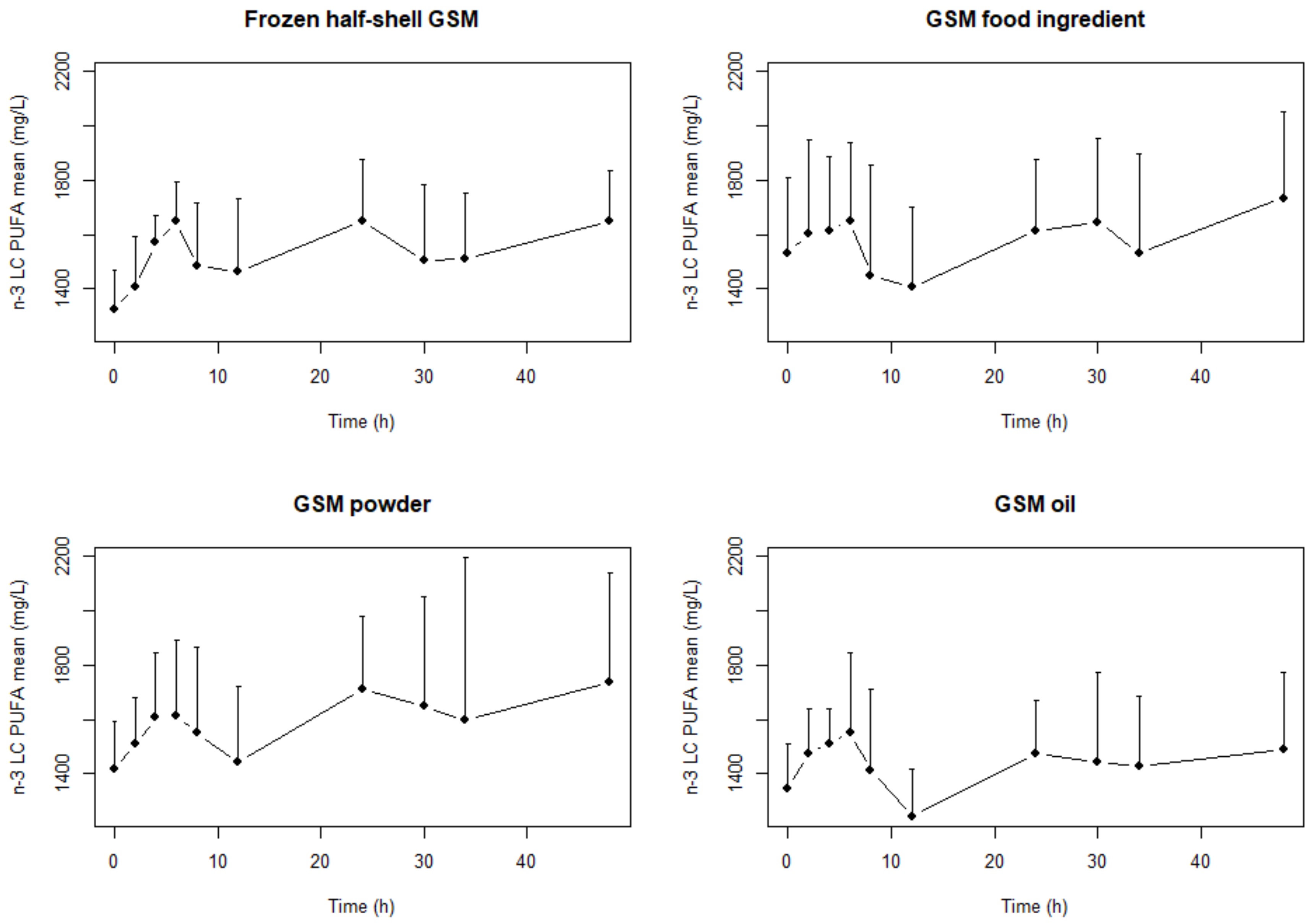
2. Results
3. Discussion
4. Materials and Methods
4.1. GSM Food Formats
- Frozen half-shell GSM—125 g Sanford (Blanched GSM, Orvida brand, Sanford, Havelock NZ, Lot # M177812907, production date 9 May 2018);
- GSM food ingredient—22.5 g Sanford (Batch L001, production 25 March 2018);
- GSM powder—22.5 g Enzaq (Batch L457–2, production 8 July 2018);
- GSM oil extract—2.3 g Pernatec oil (Waitaki Bioscience, production date 5 May 2018).
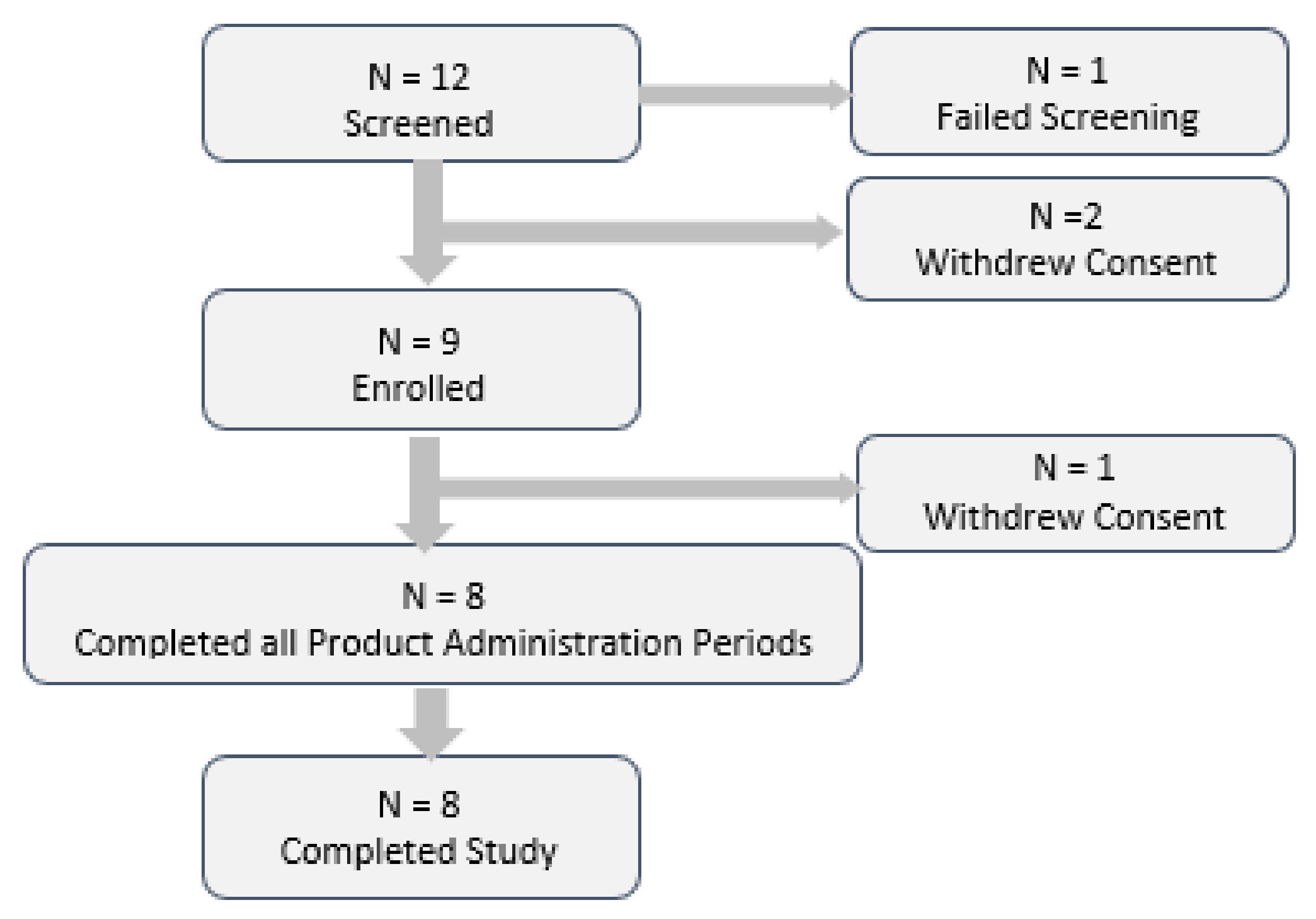
4.2. Clinical Study
4.3. Protocol Restrictions
- No prescription or over-the-counter medications, vitamins, minerals, or herbal supplements were permitted, from 2 weeks prior to first test product administration until study completion. Although medications required to treat adverse events were permissible with approval from the Investigator, no concomitant medications were used by any subjects during the study.
- Consumption of fish or other seafoods, or fish/seafood-containing products was not permitted, from two weeks prior to first test product administration until study completion.
- A fat-controlled diet was required, from 1 week prior to first test product administration until study completion.
- Alcohol was not permitted, from 48 h prior to each test product administration until the final PK sample collection in each administration period. A negative alcohol breath test was required at screening and prior to test product administration.
- Consumption of cigarettes or other nicotine-containing products was not permitted, from screening until study completion.
- Recreational drug use was not permitted, from screening until study completion. A negative urine drugs of abuse screen was a requirement for study entry.
- Water was not permitted, for one hour pre- and post-test product administration. Water was otherwise permitted ad libitum.
4.4. Assessment of Safety
4.5. Lipid Analysis
4.6. Pharmacokinetics Calculation and Statistical Analyses
- AUC0-t: The area under the plasma concentration-time curve, from time t = 0 to 12 and 48 h, calculated by the linear trapezoidal method.
- CMax: Maximum measured plasma concentration from time 0 to 12 and 48 h.
- TMax: Time of the maximum measured plasma concentration to 12 and 48 h.
- t½: A measure of elimination, half-life is the time necessary for the concentration in the plasma to decrease by half.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aquaculture, N.Z. Mussel Export Stats; Aquaculture New Zealand: Nelson, New Zealand, 2020. [Google Scholar]
- Miller, M.R.; Perry, N.; Burgess, E.; Marshall, S. Regiospecific analyses of triacylglycerols of Hoki (Macruronus novaezelandiae) and Greenshell™ mussel (Perna canaliculus). J. Am. Oil Chem. Soc. 2011, 88, 509–515. [Google Scholar] [CrossRef]
- Miller, M.R.; Tian, H. Changes in proximate composition, lipid class and fatty acid profile in Greenshell™ mussels (Perna canaliculus) over an annual cycle. Aquac. Res. 2018, 49, 1153–1165. [Google Scholar] [CrossRef]
- Eason, C.T.; Adams, S.L.; Puddick, J.; Romanazzi, D.; Miller, M.R.; King, N.; Johns, S.; Forbes-Blom, E.; Hessian, P.A.; Stamp, L.K.; et al. Greenshell Mussels: A Review of Veterinary Trials and Future Research Directions. Vet. Sci. 2018, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Siriarchavatana, P.; Kruger, M.C.; Miller, M.R.; Tian, H.S.; Wolber, F.M. The Preventive Effects of Greenshell Mussel (Perna canaliculus) on Early-Stage Metabolic Osteoarthritis in Rats with Diet-Induced Obesity. Nutrients 2019, 11, 1601. [Google Scholar] [CrossRef] [PubMed]
- Backes, J.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C. Omega-3 fatty acids and inflammation: A perspective on the challenges of evaluating efficacy in clinical research. Prostaglandins Other Lipid Mediat. 2015, 116–117, 104–111. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the substantiation of health claims related to eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), docosapentaenoic acid (DPA) and maintenance of normal cardiac function (ID 504, 506, 516, 527, 538, 703, 1128, 1317, 1324, 1325). EFSA J. 2010, 8, 1–32. [Google Scholar] [CrossRef]
- Jamilian, M.; Hashemi Dizaji, S.; Bahmani, F.; Taghizadeh, M.; Memarzadeh, M.R.; Karamali, M.; Akbari, M.; Asemi, Z. A Randomized Controlled Clinical Trial Investigating the Effects of Omega-3 Fatty Acids and Vitamin E Co-Supplementation on Biomarkers of Oxidative Stress, Inflammation and Pregnancy Outcomes in Gestational Diabetes. Can. J. Diabetes 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Lane, K.E.; Li, W.L.; Smith, C.; Derbyshire, E. The bioavailability of an omega-3-rich algal oil is improved by nanoemulsion technology using yogurt as a food vehicle. Int. J. Food Sci. Technol. 2014, 49, 1264–1271. [Google Scholar] [CrossRef]
- Cook, C.M.; Hallaraker, H.; Saebo, P.C.; Innis, S.M.; Kelley, K.M.; Sanoshy, K.D.; Berger, A.; Maki, K.C. Bioavailability of long chain omega-3 polyunsaturated fatty acids from phospholipid-rich herring roe oil in men and women with mildly elevated triacylglycerols. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 17–24. [Google Scholar] [CrossRef]
- Kohler, A.; Sarkkinen, E.; Tapola, N.; Niskanen, T.; Bruheim, I. Bioavailability of fatty acids from krill oil, krill meal and fish oil in healthy subjects--a randomized, single-dose, cross-over trial. Lipids Health Dis. 2015, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific Opinion on the substantiation of health claims related to docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and brain, eye and nerve development (ID 501, 513, 540), maintenance of normal brain function (ID 497, 501, 510, 513, 519, 521, 53. EFSA J. 2011, 9, 1–30. [Google Scholar] [CrossRef]
- Abbott, K.A.; Burrows, T.L.; Acharya, S.; Thota, R.N.; Garg, M.L. DHA-enriched fish oil reduces insulin resistance in overweight and obese adults. Prostaglandins Leukot. Essent. Fat. Acids 2020, 159, 102154. [Google Scholar] [CrossRef]
- Sugasini, D.; Yalagala, P.C.R.; Goggin, A.; Tai, L.M.; Subbaiah, P.V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: Lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 2019, 74, 108231. [Google Scholar] [CrossRef] [PubMed]
- Arellanes, I.C.; Choe, N.; Solomon, V.; He, X.; Kavin, B.; Martinez, A.E.; Kono, N.; Buennagel, D.P.; Hazra, N.; Kim, G.; et al. Brain delivery of supplemental docosahexaenoic acid (DHA): A randomized placebo-controlled clinical trial. EBioMedicine 2020, 59, 102883. [Google Scholar] [CrossRef]
- Das, U.N. Bioactive Lipids in COVID-19-Further Evidence. Arch. Med. Res. 2020. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Scarsi, C.; Levesque, A.; Lisi, L.; Navarra, P. The free fractions of circulating docosahexaenoic acid and eicosapentenoic acid as optimal end-point of measure in bioavailability studies on n-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2015, 96, 11–16. [Google Scholar] [CrossRef]
- Gibson, S.L.M.; Gibson, R.G. The treatment of arthritis with a lipid extract of Perna canaliculus: A randomized trial. Complement. Ther. Med. 1998, 6, 122–126. [Google Scholar] [CrossRef]
- Lau, C.S.; Chiu, P.K.Y.; Chu, E.M.Y.; Cheng, I.Y.W.; Tang, W.M.; Man, R.Y.K.; Halpern, G.M. Treatment of knee osteoarthritis with Lyprinol®, lipid extract of the green-lipped mussel-A double-blind placebo-controlled study. Prog. Nutr. 2004, 6, 17–31. [Google Scholar]
- Szechinski, J.; Zawadzki, M. Measurement of pain relief resulting from the administration of Perna canaliculus lipid complex PCSO-524™ as compared to fish oil for treating patients who suffer from osteoarthritis of knee and/or hip joints. Reumatologia 2011, 49, 244–252. [Google Scholar]
- Emelyanov, A.; Fedoseev, G.; Krasnoschekova, O.; Abulimity, A.; Trendeleva, T.; Barnes, P.J. Treatment of asthma with lipid extract of New Zealand green-lipped mussel: A randomised clinical trial. Eur. Respir. J. 2002, 20, 596–600. [Google Scholar] [CrossRef]
- Lello, J.; Liang, A.; Robinson, E.; Leutenegger, D.; Wheat, A. Treatment Of Children’s Asthma With A Lipid Extract Of The New Zealand Green Lipped Mussel (Perna Canaliculus) (Lyprinol®)-A Double Blind, Randomised Controlled Trial In Children With Moderate To Severe Chronic Obstructive Asthma. Internet J. Asthma Allergy Immunol. 2012, 8, 1–12. [Google Scholar]
- Baum, K.; Telford, R.D.; Cunningham, R.B. Marine oil dietary supplementation reduces delayed onset muscle soreness after a 30 km run. Open Access J. Sports Med. 2013, 4, 109–115. [Google Scholar] [CrossRef]
- Mickleborough, T.D.; Sinex, J.A.; Platt, D.; Chapman, R.F.; Hirt, M. The effects PCSO-524(R), a patented marine oil lipid and omega-3 PUFA blend derived from the New Zealand green lipped mussel (Perna canaliculus), on indirect markers of muscle damage and inflammation after muscle damaging exercise in untrained men: A randomized, placebo controlled trial. J. Int. Soc. Sports Nutr. 2015, 12, 10. [Google Scholar] [CrossRef]
- Siriarchavatana, P.; Kruger, M.C.; Miller, M.R.; Tian, H.; Wolber, F.M. Consumption of greenshell mussel increases acquisition of lean mass and bone mineral density in rats. In Proceedings of the World Congress on Oil & Fats International Convention Centre, Sydney, Australia, 9–12 February 2020. [Google Scholar]
- Sanguansri, L.; Augustin, M.A.; Lockett, T.J.; Abeywardena, M.Y.; Royle, P.J.; Mano, M.T.; Patten, G.S. Bioequivalence of n-3 fatty acids from microencapsulated fish oil formulations in human subjects. Br. J. Nutr. 2015, 113, 822–831. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; Kralovec, J.; Bailey-Hall, E.; Smeberg, V.; Stark, J.G.; Salem, N., Jr. Similar eicosapentaenoic acid and docosahexaenoic acid plasma levels achieved with fish oil or krill oil in a randomized double-blind four-week bioavailability study. Lipids Health Dis. 2015, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Schneider, I.; Schuchardt, J.P.; Meyer, H.; Hahn, A. Effect of gastric acid resistant coating of fish oil capsules on intestinal uptake of eicosapentaenoic acid and docosahexaenoic acid. J. Funct. Foods 2011, 3, 129–133. [Google Scholar] [CrossRef]
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Nanotechnology for increased micronutrient bioavailability. Trends Food Sci. Technol. 2014, 40, 168–182. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations--a comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Pearce, L.; Bettjeman, B.I. Detailed distribution of lipids in Greenshell mussel (Perna canaliculus). Nutrients 2014, 6, 1454–1474. [Google Scholar] [CrossRef]
- Visioli, F.; Rise, P.; Barassi, M.C.; Marangoni, F.; Galli, C. Dietary intake of fish vs. formulations leads to higher plasma concentrations of n-3 fatty acids. Lipids 2003, 38, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Elvevoll, E.O.; Barstad, H.; Breimo, E.S.; Brox, J.; Eilertsen, K.E.; Lund, T.; Olsen, J.O.; Osterud, B. Enhanced incorporation of n-3 fatty acids from fish compared with fish oils. Lipids 2006, 41, 1109–1114. [Google Scholar] [CrossRef]
- Stonehouse, W.; Pauga, M.R.; Kruger, R.; Thomson, C.D.; Wong, M.; Kruger, M.C. Consumption of salmon v. salmon oil capsules: Effects on n-3 PUFA and selenium status. Br. J. Nutr. 2011, 106, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Pottala, J.V.; Sands, S.A.; Jones, P.G. Comparison of the effects of fish and fish-oil capsules on the n-3 fatty acid content of blood cells and plasma phospholipids. Am. J. Clin. Nutr. 2007, 86, 1621–1625. [Google Scholar] [CrossRef]
- Pawlosky, R.; Hibbeln, J.; Lin, Y.H.; Salem, N. n-3 Fatty acid metabolism in women. Br. J. Nutr. 2003, 90, 993–994. [Google Scholar] [CrossRef] [PubMed]
- Ghasemifard, S.; Turchini, G.M.; Sinclair, A.J. Omega-3 long chain fatty acid “bioavailability”: A review of evidence and methodological considerations. Prog. Lipid Res. 2014, 56, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre-Delaunay, D.; Pachiaudi, C.; Laville, M.; Pousin, J.; Armstrong, M.; Lagarde, M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of C-13 DHA in phosphatidylcholine. J. Lipid Res. 1999, 40, 1867–1874. [Google Scholar]
- Davidson, M.H.; Johnson, J.; Rooney, M.W.; Kyle, M.L.; Kling, D.F. A novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: The ECLIPSE (Epanova (R) compared to Lovaza (R) in a pharmacokinetic single-dose evaluation) study. J. Clin. Lipidol. 2012, 6, 573–584. [Google Scholar] [CrossRef]

| AUC0–12 h | ||||||||||||
| AUC0–12h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | AUC0–12h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | AUC0–12h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | ||||
| Frozen half-shell GSM | EPA | 5407.0 | 4375.7 | 6681.5 | DHA | 8819 | 7457 | 10,429.9 | n-3 LC PUFA | 17,880.3 | 16,250.7 | 19,673.4 |
| GSM Food ingredient | 5046.2 | 3722.2 | 6841.2 | 8840.1 | 7570.1 | 10,323.2 | 18,071.9 | 15,091.8 | 21,640.5 | |||
| GSM Powder | 5821.7 | 4395.8 | 7710.3 | 7947.8 | 6771.1 | 9328.9 | 18,180.9 | 15,760.3 | 20,973.3 | |||
| GSM Oil extract | 4886.9 | 4137.2 | 5772.3 | 8340.8 | 7350.8 | 9464.0 | 17,305.3 | 15,516.0 | 19,300.8 | |||
| F | − | - | - | − | - | - | − | - | - | |||
| p-value | − | - | - | − | - | - | − | - | - | |||
| AUC0–48h | ||||||||||||
| AUC0–48h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | AUC0–48h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | AUC0–48h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | ||||
| Frozen half-shell GSM | EPA | 23,963.6 b | 19,489.7 | 29,464.6 | DHA | 34,167.4 | 28,805.8 | 40,526.9 | n-3 LC PUFA | 73,793.4 | 65,863 | 82,678.8 |
| GSM Food ingredient | 22,138.7 a,b | 17,126.5 | 28,617.8 | 34,549.4 | 30,361.7 | 39,314.6 | 74,412.4 | 63,442.4 | 87,279.2 | |||
| GSM Powder | 24,683.2 b | 18,464.3 | 32,996.6 | 32,574.5 | 27,579.2 | 38,474.6 | 75,639.8 | 62,800.7 | 91,103.7 | |||
| GSM Oil extract | 19,858.4 a | 16,821.8 | 23,443.2 | 32,194.5 | 28,017.1 | 36,994.9 | 68,033.5 | 60,115.2 | 76,994.7 | |||
| F | 4.840 | - | - | − | - | - | − | - | - | |||
| p-value | 0.013 | - | - | − | - | - | − | - | - | |||
| CMax0–12h | ||||||||||||
| CMax0–12h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | CMax0–12h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | CMax0–12h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | ||||
| Frozen half-shell GSM | EPA | 546.6c | 441.4 | 676.9 | DHA | 851 | 747.8 | 967.3 | n-3 LC PUFA | 1694 | 1578.5 | 1818.5 |
| GSM Food ingredient | 474.4 a,b | 359.7 | 625.7 | 834 | 724.1 | 959.3 | 1681 | 1439.6 | 1961.9 | |||
| GSM Powder | 539.0 b,c | 405.7 | 716 | 736 | 640.2 | 845.5 | 1659 | 1434 | 1918.5 | |||
| GSM Oil extract | 450.4 a | 372.1 | 545.1 | 780 | 711.2 | 855.6 | 1604.5 | 1414.2 | 1820.9 | |||
| F | 5.045 | - | - | − | - | - | − | - | - | |||
| p-value | 0.011 | - | - | − | - | - | − | - | - | |||
| CMax48h | ||||||||||||
| CMax0–48h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | CMax0–48h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | CMax0–48h mg/L | 95.0% Lower CL for Mean | 95.0% Upper CL for Mean | ||||
| Frozen half-shell GSM | EPA | 594.7 c | 499.8 | 707.5 | DHA | 857 | 759.2 | 968.4 | n-3 LC PUFA | 1772 | 1623.6 | 1934 |
| GSM Food ingredient | 536.8 a,b | 421.5 | 683.7 | 852 | 749.1 | 968 | 1782 | 1509.3 | 2103.4 | |||
| GSM Powder | 593.3 b,c | 445.5 | 790.2 | 835 | 703.6 | 992 | 1832 | 1504.6 | 2230.6 | |||
| GSM Oil extract | 477.4 a | 395.1 | 576.8 | 806 | 713.9 | 909.2 | 1634 | 1431.5 | 1864.7 | |||
| F | 6.388 | - | - | − | - | - | − | - | - | |||
| p-value | 0.004 | - | - | − | - | - | − | - | - | |||
| TMax12h | ||||||||||||
| TMax (0–12 h) 25 percentile | TMax (0–12 h) Median | TMax (0–12 h) 75 percentile | TMax (0–12 h) 25 percentile | TMax (0–12 h) Median | TMax (0–12 h) 75 percentile | TMax (0–12 h) 25 percentile | TMax (0–12 h) Median | TMax (0–12 h) 75 percentile | ||||
| Frozen half-shell GSM | EPA | 6 | 6 | 8 | DHA | 2 | 4 | 6 | n-3 LC PUFA | 6 | 6 | 8 |
| GSM Food ingredient | 6 | 6 | 6 | 2 | 3 | 5 | 3 | 5 | 6 | |||
| GSM Powder | 5 | 6 | 7 | 4 | 4 | 6 | 4 | 5 | 7 | |||
| GSM Oil extract | 5 | 6 | 6 | 4 | 4 | 5 | 4 | 5 | 6 | |||
| F | - | − | - | - | − | - | - | − | - | |||
| p-value | - | − | - | - | − | - | - | − | - | |||
| TMax48h | ||||||||||||
| TMax (0–48 h) 25 percentile | TMax (0–48 h) Median | TMax (0–48 h) 75 percentile | TMax (0–48 h) 25 percentile | TMax (0–48 h) Median | TMax (0–48 h) 75 percentile | TMax (0–48 h) 25 percentile | TMax (0–48 h) Median | TMax (0–48 h) 75 percentile | ||||
| Frozen half-shell GSM | EPA | 12 | 24 | 24 | DHA | 2 | 4 | 6 | n-3 LC PUFA | 12 | 24 b | 48 |
| GSM Food ingredient | 24 | 30 | 30 | 2 | 4 | 6 | 15 | 27 b | 48 | |||
| GSM Powder | 24 | 24 | 30 | 4 | 29 | 48 | 24 | 30 b | 41 | |||
| GSM Oil extract | 5 | 15 | 24 | 4 | 4 | 18 | 4 | 5 a | 16 | |||
| F | - | − | - | - | − | - | - | − | - | |||
| p-value | - | − | - | - | − | - | - | 0.04 | - | |||
| GSM Oil Extract | Frozen Half-Shell GSM | GSM Food Ingredient | GSM Powder | |
|---|---|---|---|---|
| Sample size (g) | 2.3 | 125 | 22.5 | 22.5 |
| Proximate composition | Amount per serving (g) | |||
| Fat | 2.30 | 2.25 | 2.05 | 2.23 |
| Ash | 0.00 | 1.75 | 4.82 | 3.94 |
| Crude Protein | 0.00 | 17.79 | 11.12 | 10.44 |
| Carbohydrate | 0.00 | 5.25 | 3.85 | 5.06 |
| Fatty acid profile | Amount per serving (mg) | |||
| C14:0 myristic acid | 111.3 | 97.5 | 81.8 | 87.1 |
| C16:0 palmitic acid | 326.2 | 289.4 | 308.0 | 348.4 |
| C16:1 palmitoleic acid | 146.5 | 128.1 | 137.5 | 151.5 |
| C18:0 stearic acid | 84.0 | 88.6 | 69.6 | 92.8 |
| C18:1n7 vaccenic acid | 58.6 | 50.4 | 50.5 | 64.4 |
| C18:1n9c oleic acid | 48.8 | 21.7 | 24.4 | 24.6 |
| C18:2n6c linoleic acid | 84.0 | 39.5 | 40.0 | 32.2 |
| C18:3n3 alpha linolenic acid (ALA) | 25.4 | 26.1 | 27.8 | 17.6 |
| C18:3n4 octadecatrienoic acid | 23.4 | 21.0 | 2.6 | 2.5 |
| C18:4n3 stearidonic acid (SDA) | 48.8 | 58.0 | 47.0 | 32.2 |
| C20:1 gadoleic acid | 37.1 | 31.2 | 36.5 | 41.7 |
| C20:4n6 arachidonic acid (AA) | 33.2 | 27.4 | 29.6 | 26.5 |
| C20:5n3 eicosapentaenoic acid (EPA) | 355.5 | 350.0 | 339.4 | 320.0 |
| C22:5n3 docosapentaenoic acid (DPA) | 25.4 | 23.6 | 24.4 | 28.4 |
| C22:6n3 docosahexaenoic acid (DHA) | 267.6 | 311.1 | 174.0 | 189.3 |
| ∑SFA | 554.1 | 509.8 | 493.4 | 567.8 |
| ∑MUFA | 291.0 | 234.7 | 251.5 | 285.3 |
| ∑PUFA | 879.5 | 880.5 | 718.3 | 684.8 |
| ∑Omega 3 | 727.9 | 776.0 | 622.2 | 595.7 |
| ∑Omega 6 | 140.6 | 91.5 | 77.4 | 69.7 |
| Lipid class | Amount per serving (mg) | |||
| Polar lipids (PL) | 1463.6 | 1530.0 | 14.3 | 46.8 |
| Sterols | 29.9 | 104.3 | 43.0 | 82.4 |
| Triacylglycerols (TAG) | 801.9 | 622.5 | 1951.3 | 2051.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, M.R.; Kruger, M.C.; Wynne, C.; Waaka, D.; Li, W.; Frampton, C.; Wolber, F.M.; Eason, C. Bioavailability of Orally Administered Active Lipid Compounds from four Different Greenshell™ Mussel Formats. Mar. Drugs 2020, 18, 524. https://doi.org/10.3390/md18110524
Miller MR, Kruger MC, Wynne C, Waaka D, Li W, Frampton C, Wolber FM, Eason C. Bioavailability of Orally Administered Active Lipid Compounds from four Different Greenshell™ Mussel Formats. Marine Drugs. 2020; 18(11):524. https://doi.org/10.3390/md18110524
Chicago/Turabian StyleMiller, Matthew R., Marlena C. Kruger, Chris Wynne, Devonie Waaka, Weili Li, Chris Frampton, Fran M. Wolber, and Charles Eason. 2020. "Bioavailability of Orally Administered Active Lipid Compounds from four Different Greenshell™ Mussel Formats" Marine Drugs 18, no. 11: 524. https://doi.org/10.3390/md18110524
APA StyleMiller, M. R., Kruger, M. C., Wynne, C., Waaka, D., Li, W., Frampton, C., Wolber, F. M., & Eason, C. (2020). Bioavailability of Orally Administered Active Lipid Compounds from four Different Greenshell™ Mussel Formats. Marine Drugs, 18(11), 524. https://doi.org/10.3390/md18110524









