Novel Reporter System Monitoring IL-18 Specific Signaling Can Be Applied to High-Throughput Screening
Abstract
:1. Introduction
2. Results
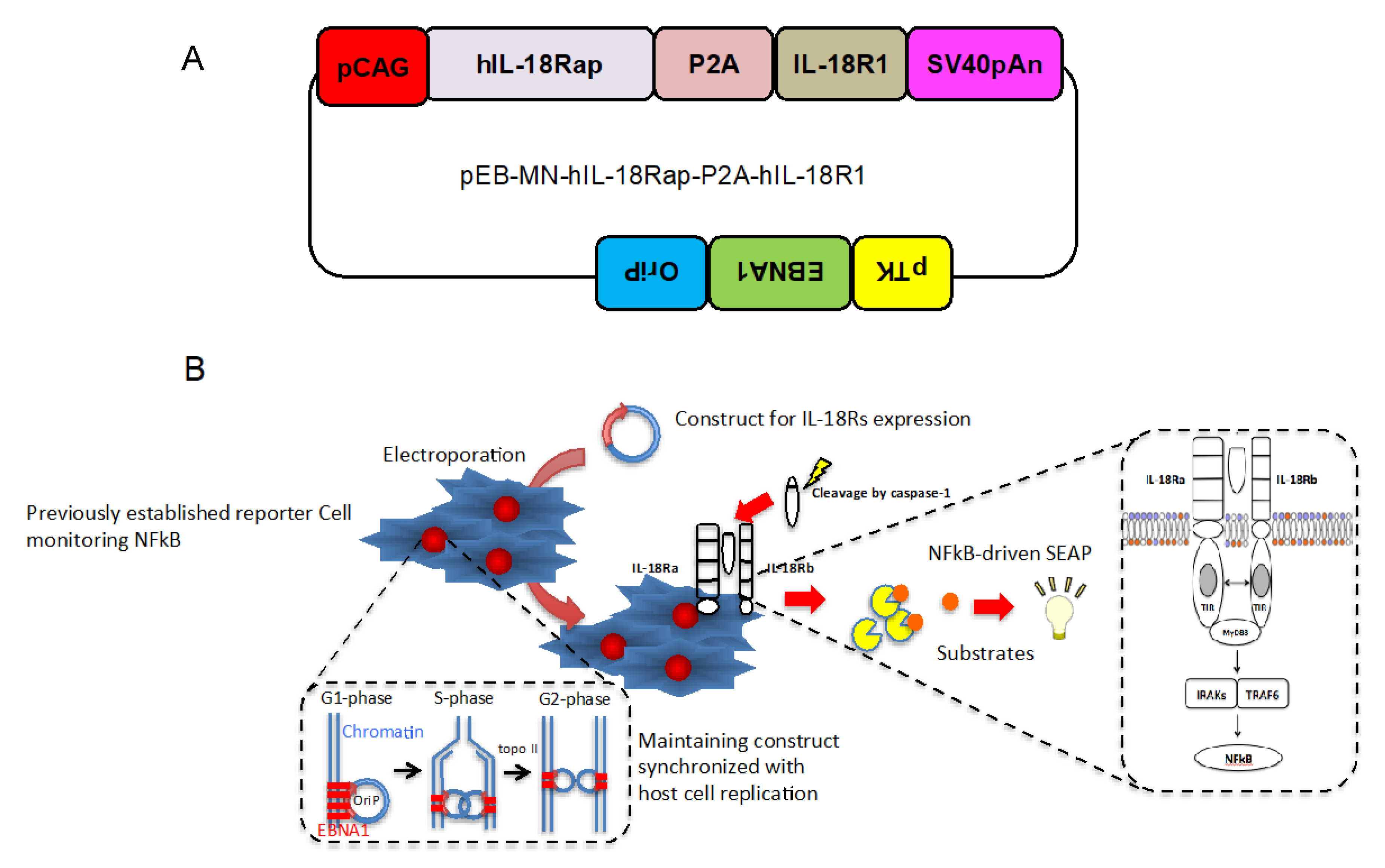
2.1. Design of Construct Stably and Simultaneously Expressing Two Cognate Human IL-18 Receptor Subunits
2.2. Establishment of Cells Specifically Monitor IL-18- Induced NFkB Activation
2.3. Human and Mouse IL-18-Induced NFkB Activation and Their Affinities
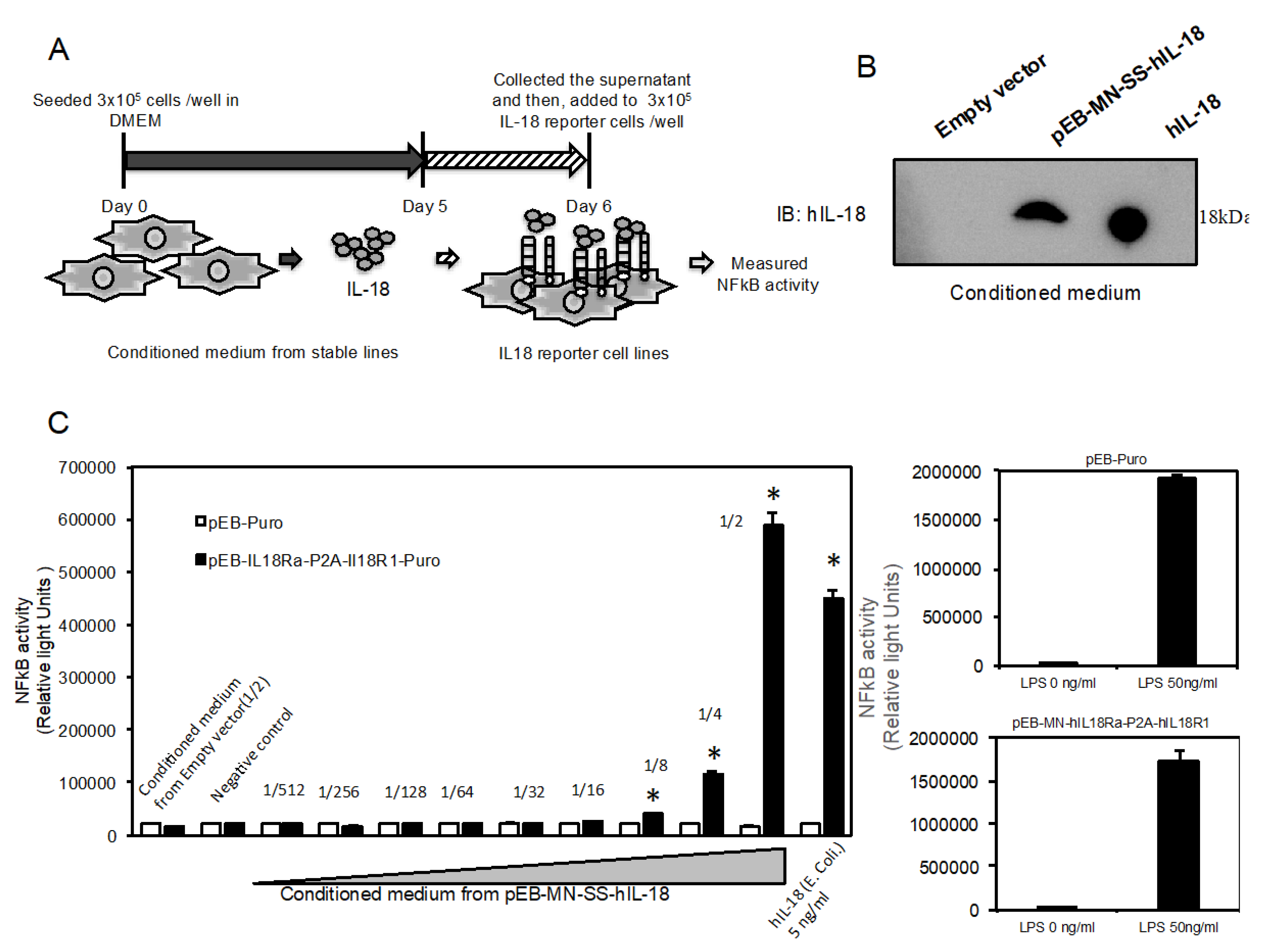
2.4. Novel Reporter Assay can Validate IL-18-Specific NFkB Activity in Supernatant
2.5. High-Throughput Screening in the Novel Reporter Cells Using Natural Product and Synthetic Compound Libraries
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plasmid Construction
4.3. Cell Culture and Electroporation
4.4. SEAP Assay with Chemilluminascence
4.5. Western Blotting
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Serrano, I.; Luque, A.; Aran, J.M. Exploring the Immunomodulatory Moonlighting Activities of Acute Phase Proteins for Tolerogenic Dendritic Cell Generation. Front. Immunol. 2018, 9, 892. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Salmikangas, P.; Kinsella, N.; Chamberlain, P. Chimeric Antigen Receptor T-Cells (CAR T-Cells) for Cancer Immunotherapy—Moving Target for Industry? Pharm. Res. 2018, 35, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, A.; Shimizu, K.; Kurata, R.; Cui, X.; Isagawa, T.; Harada, M.; Nagai, J.; Yoshida, Y.; Ozaki, K.I.; Takeda, N.; et al. Establishment of Novel Cells Stably Secreting Various Human IL-18 Recombinant Proteins. Curr. Pharm. Biotechnol. 2019, 20, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.R.; Yadav, R.; Rossi, R.J.; Ruby, C.E.; Weinberg, A.D.; Aguila, H.L.; Vella, A.T. IL-18 Bridges Innate and Adaptive Immunity through IFN-γ and the CD134 Pathway. J. Immunol. 2006, 177, 234–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Okamura, H.; Nagata, K.; Komatsu, T.; Tamura, T. Purification of a factor which provides a costimulatory signal for gamma interferon production. Infect. Immun. 1993, 61, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamura, H.; Tsutsi, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K.; et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. IL-18: A TH1-inducing, proinflammatory cyto- kine and new member of the IL-1 family. J. Allergy Clin. Immunol. 1999, 103, 11–24. [Google Scholar] [CrossRef]
- Torigoe, K.; Ushio, S.; Okura, T.; Kobayashi, S.; Taniai, M.; Kunikata, T.; Murakami, T.; Sanou, O.; Kojima, H.; Fujii, M.; et al. Purification and characterization of the human interleukin-18 receptor. J. Biol. Chem. 1997, 272, 25737–25742. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, K.; Tsutsui, H.; Kawai, T.; Takeda, K.; Nakanishi, K.; Takeda, Y.; Akira, S. Cutting edge: Generation of IL-18 receptor-deficient mice: Evidence for IL-1 receptor- related protein as an essential IL-18 binding receptor. J. Immunol. 1999, 162, 5041–5044. [Google Scholar]
- Dinarello, C.A. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int. Rev. Immunol. 1998, 16, 457–499. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Reid, J.; Pouliquen, I.; McHugh, S.; Abberley, L.; DeWall, S.; Taylor, A.; Tong, X.; Rocha Del Cura, M.; McKie, E. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single-dose antiinterleukin- 18 mAb GSK1070806 in healthy and obese subjects. Int. J. Clin Pharmacol. Ther. 2014, 52, 867–879. [Google Scholar] [CrossRef] [PubMed]
- McKie, E.A.; Reid, J.L.; Mistry, P.C.; DeWall, S.L.; Abberley, L.; Ambery, P.D.; Gil-Extremera, B. A Study to Investigate the Efficacy and Safety of an Anti-Interleukin-18 Monoclonal Antibody in the Treatment of Type 2 Diabetes Mellitus. PLoS ONE 2016, 11, e0150018. [Google Scholar] [CrossRef] [PubMed]
- Golab, J.; Stoklosa, T. Technology evaluation: SB-485232, GlaxoSmithKline. Curr. Opin. Mol. Ther. 2005, 7, 85–93. [Google Scholar]
- Tarhini, A.A.; Millward, M.; Mainwaring, P.; Kefford, R.; Logan, T.; Pavlick, A.; Kathman, S.J.; Laubscher, K.H.; Dar, M.M.; Kirkwood, J.M. Phase 2, randomized study of SB-485232, rhIL-18, in patients with previously untreated metastatic melanoma. Cancer 2009, 115, 859–868. [Google Scholar] [CrossRef]
- Simpkins, F.; Flores, A.; Chu, C.; Berek, J.S.; Lucci, J., 3rd; Murray, S.; Bauman, J.; Struemper, H.; Germaschewski, F.; Jonak, Z.; et al. Chemoimmunotherapy using pegylated liposomal Doxorubicin and interleukin-18 in recurrent ovarian cancer: A phase I dose-escalation study. Cancer Immunol. Res. 2013, 1, 168–178. [Google Scholar] [CrossRef] [Green Version]
- Doyle, S.L.; López, F.J.; Celkova, L.; Brennan, K.; Mulfaul, K.; Ozaki, E.; Kenna, P.F.; Kurali, E.; Hudson, N.; Doggett, T.; et al. IL-18 Immunotherapy for Neovascular AMD: Tolerability and Efficacy in Nonhuman Primates. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5424–5430. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.R.; Li, L.H.; Park, H.J.; Park, J.H.; Lee, K.Y.; Kim, M.K.; Shin, B.A.; Choi, S.Y.; Thiel, V. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines Zebrafish and mice. PLoS ONE 2011, 6, e18556. [Google Scholar] [CrossRef] [Green Version]
- Chng, J.; Wang, T.; Nian, R.; Lau, A.; Hoi, K.M.; Ho, S.C.; Gagnon, P.; Bi, X.; Yang, Y. Cleavage efficient 2A peptides for high level monoclonal antibody expression in CHO cells. MAbs 2015, 7, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Kurata, R.; Kumagai, A.; Cui, X.; Harada, M.; Nagai, J.; Yoshida, Y.; Ozaki, K.I.; Tanaka, Y.; Yonezawa, T. Establishment of Novel Reporter Cells Stably Maintaining Transcription Factor-driven Human Secreted Alkaline Phosphatase Expression. Curr. Pharm. Biotechnol. 2018, 19, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Konishi, K.; Tanabe, F.; Taniguchi, M.; Yamauchi, H.; Tanimoto, T.; Ikeda, M.; Orita, K.; Kurimoto, M. A simple and sensitive bioassay for the detection of human interleukin-18/interferon-γ-inducing factor using human myelomonocytic KG-1 cells. J. Immunol. Meth. 1997, 209, 187–191. [Google Scholar] [CrossRef]
- Nakano, A.; Kawahara, S.; Akamatsu, S.; Morokuma, K.; Nakatani, M.; Iwabuchi, Y.; Takahashi, K.; Ishihara, J.; Hatakeyama, S. β-Isocupreidine–hexafluoroisopropyl acrylate method for asymmetric Baylis–Hillman reactions. Tetrahedron 2006, 62, 381–389. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.Y.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]



| Clone No. | S/B | S/N | Z’-Factor | Conc. of IL-18 | Number of Sample | |
|---|---|---|---|---|---|---|
| 384 well | 1 | 157.6 | 2680.8 | 0.842 | 300 ng/mL | n = 3 |
| 2 | 94.0 | 1006.7 | 0.910 | 300 ng/mL | n = 4 | |
| 1 | 171.6 | 2921.1 | 0.968 | 100 ng/mL | n = 3 | |
| 2 | 26.5 | 442.6 | 0.745 | 5 ng/mL | n = 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurata, R.; Shimizu, K.; Cui, X.; Harada, M.; Isagawa, T.; Semba, H.; Ishihara, J.; Yamada, K.; Nagai, J.; Yoshida, Y.; et al. Novel Reporter System Monitoring IL-18 Specific Signaling Can Be Applied to High-Throughput Screening. Mar. Drugs 2020, 18, 60. https://doi.org/10.3390/md18010060
Kurata R, Shimizu K, Cui X, Harada M, Isagawa T, Semba H, Ishihara J, Yamada K, Nagai J, Yoshida Y, et al. Novel Reporter System Monitoring IL-18 Specific Signaling Can Be Applied to High-Throughput Screening. Marine Drugs. 2020; 18(1):60. https://doi.org/10.3390/md18010060
Chicago/Turabian StyleKurata, Riho, Kenji Shimizu, Xiaofeng Cui, Masamitsu Harada, Takayuki Isagawa, Hiroaki Semba, Jun Ishihara, Koji Yamada, Jun Nagai, Yasuhiro Yoshida, and et al. 2020. "Novel Reporter System Monitoring IL-18 Specific Signaling Can Be Applied to High-Throughput Screening" Marine Drugs 18, no. 1: 60. https://doi.org/10.3390/md18010060
APA StyleKurata, R., Shimizu, K., Cui, X., Harada, M., Isagawa, T., Semba, H., Ishihara, J., Yamada, K., Nagai, J., Yoshida, Y., Takeda, N., Maemura, K., & Yonezawa, T. (2020). Novel Reporter System Monitoring IL-18 Specific Signaling Can Be Applied to High-Throughput Screening. Marine Drugs, 18(1), 60. https://doi.org/10.3390/md18010060






