Microalgae with Immunomodulatory Activities
Abstract
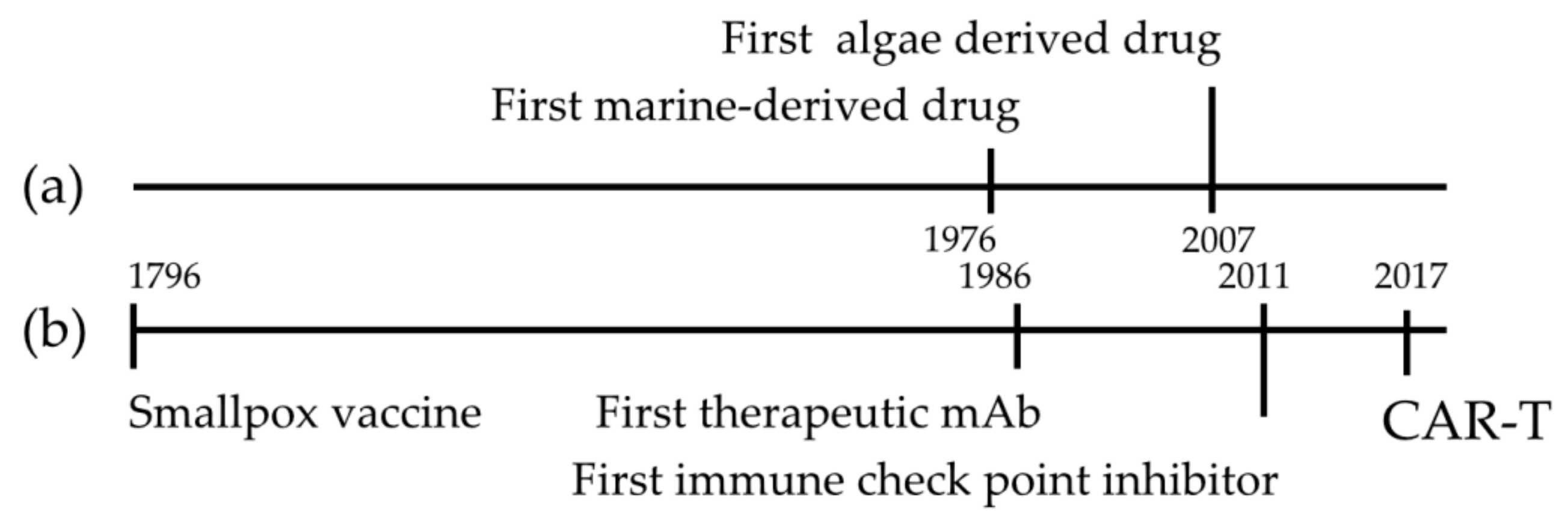
1. Introduction
- Vaccination: traditionally, vaccination stimulated immune response using inactivated biological agents. Recent vaccines consist of highly purified synthetic macromolecules combined with adjuvant agents. Adjuvant agents potentiate the immune response by activating the antigen-presenting cells (APCs) [32,33].
- CAR-T cells: Chimeric Antigen Receptor T-cell (CAR-T) immunotherapy consist in engineered T-cell redirected against a specific target. CAR-T therapy has shown a high rates of success (good outcome) against acute and chronic leukemia [41].
2. Microalgae with Anti-Inflammatory Activity
3. Microalgae with Immunomodulatory Activity
4. Immunomodulatory Compounds from Microalgae
4.1. Sulfate Polysaccharides
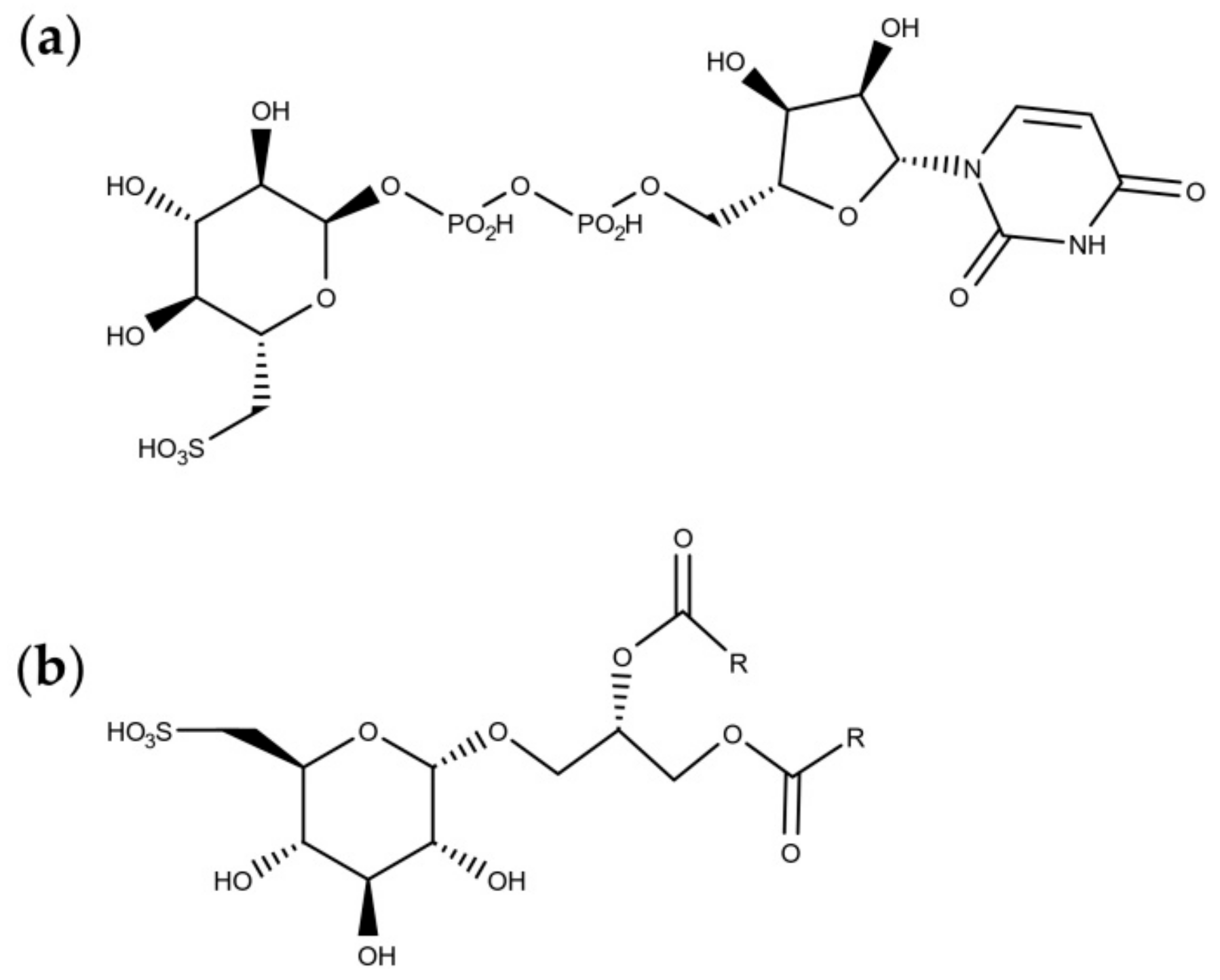
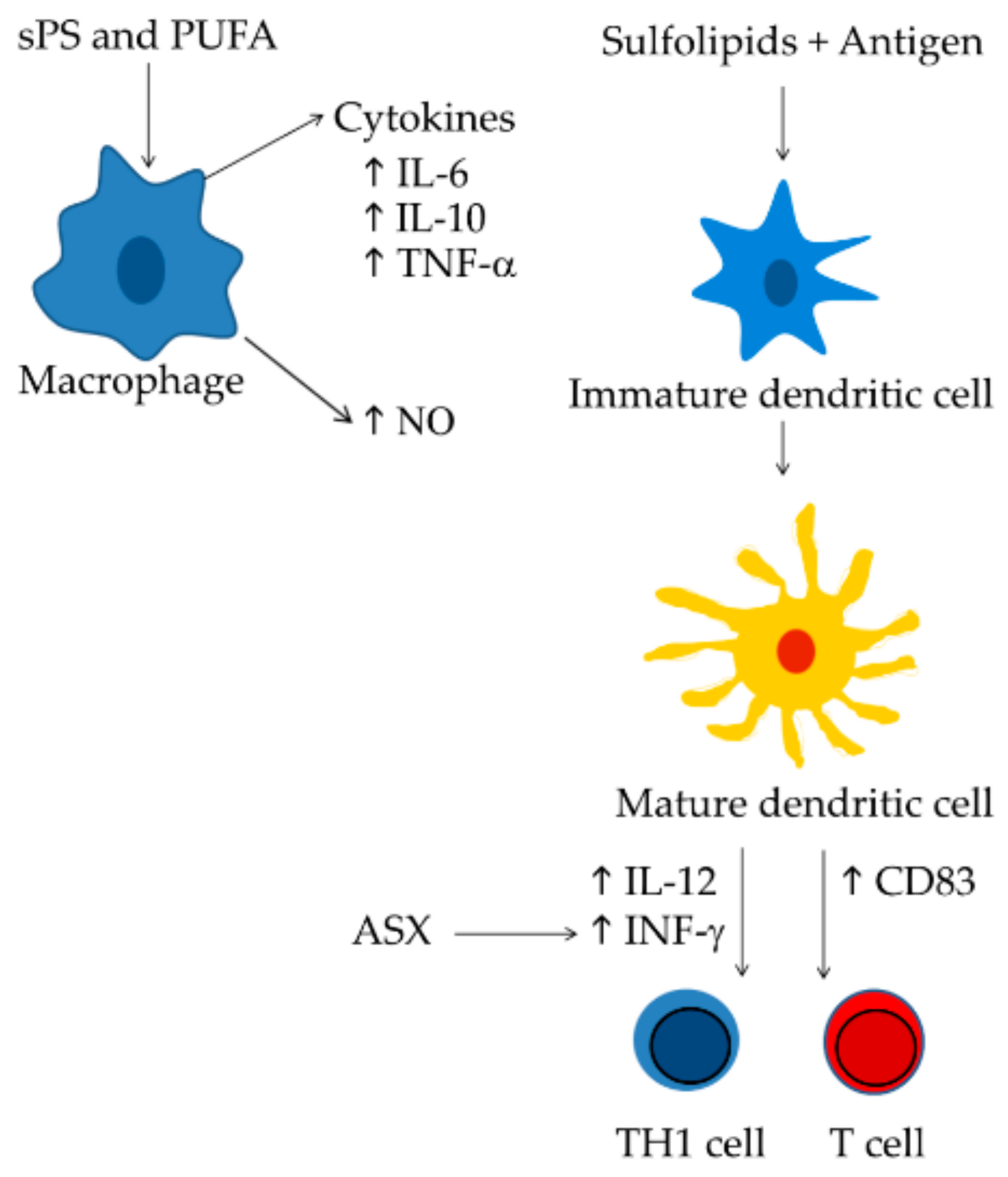
4.2. Sulfolipids
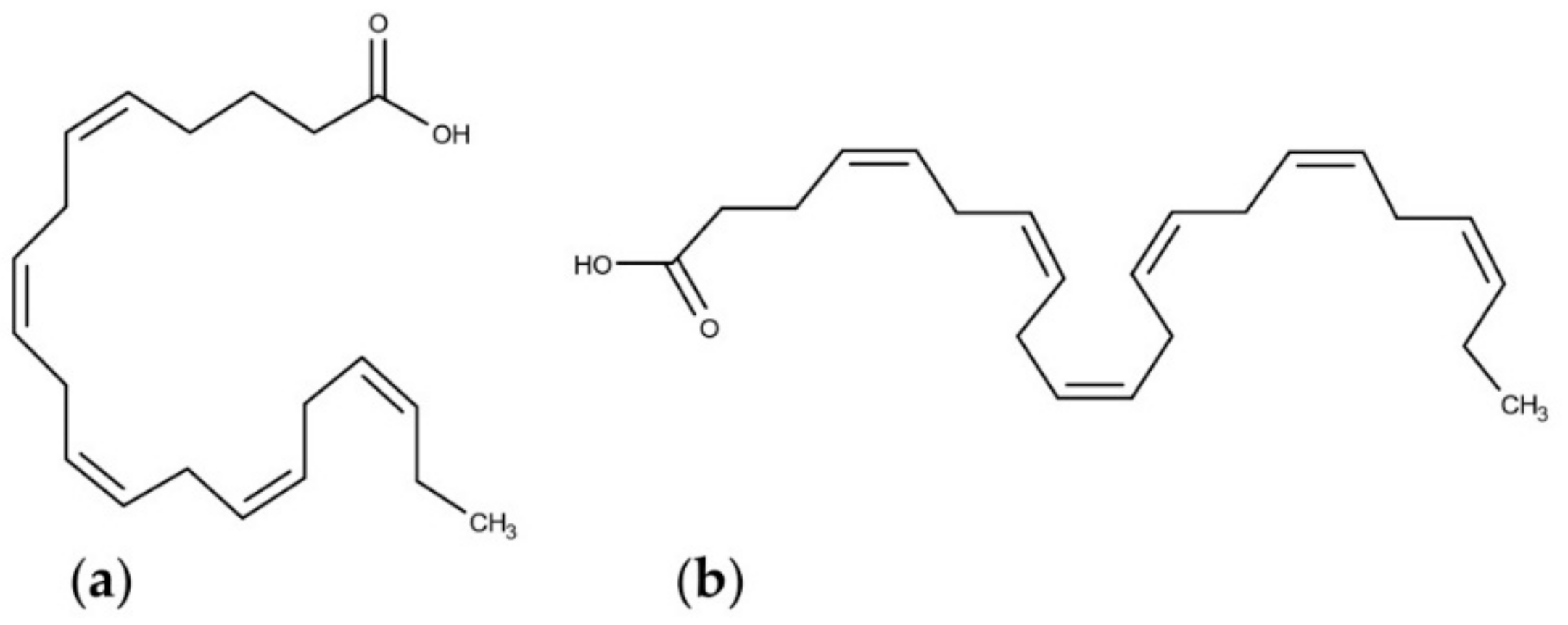
4.3. Polyunsaturated Fatty Acids (PUFAs)
4.4. Astaxanthin
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Martinez Andrade, K.A.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, L.; Wang, H.; Liu, S.; Yu, H.; Wang, X.; Li, R.; Liu, T.; Li, P. Partial Characterization, the Immune Modulation and Anticancer Activities of Sulfated Polysaccharides from Filamentous Microalgae Tribonema sp. Molecules 2019, 24, 322. [Google Scholar] [CrossRef] [PubMed]
- Martinez, K.A.; Lauritano, C.; Druka, D.; Romano, G.; Grohmann, T.; Jaspars, M.; Martin, J.; Diaz, C.; Cautain, B.; de la Cruz, M.; et al. Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium carterae. Mar. Drugs 2019, 17, 385. [Google Scholar] [CrossRef] [PubMed]
- Brillatz, T.; Lauritano, C.; Jacmin, M.; Khamma, S.; Marcourt, L.; Righi, D.; Romano, G.; Esposito, F.; Ianora, A.; Queiroz, E.F.; et al. Zebrafish-based identification of the antiseizure nucleoside inosine from the marine diatom Skeletonema marinoi. PLoS ONE 2018, 13, e0196195. [Google Scholar] [CrossRef]
- Montero-Lobato, Z.; Vazquez, M.; Navarro, F.; Fuentes, J.L.; Bermejo, E.; Garbayo, I.; Vilchez, C.; Cuaresma, M. Chemically-Induced Production of Anti-Inflammatory Molecules in Microalgae. Mar. Drugs 2018, 16, 487. [Google Scholar] [CrossRef]
- Rodriguez-Luna, A.; Avila-Roman, J.; Gonzalez-Rodriguez, M.L.; Cozar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Fucoxanthin-Containing Cream Prevents Epidermal Hyperplasia and UVB-Induced Skin Erythema in Mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef]
- Manzo, E.; Cutignano, A.; Pagano, D.; Gallo, C.; Barra, G.; Nuzzo, G.; Sansone, C.; Ianora, A.; Urbanek, K.; Fenoglio, D.; et al. A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response. Sci. Rep. 2017, 7, 6286. [Google Scholar] [CrossRef]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef]
- Ugwu, C.U.; Aoyagi, H.; Uchiyama, H. Photobioreactors for mass cultivation of algae. Bioresour. Technol. 2008, 99, 4021–4028. [Google Scholar] [CrossRef]
- Chrismadha, T.; Borowitzka, M.A. Effect of Cell-Density and Irradiance on Growth, Proximate Composition and Eicosapentaenoic Acid Production of Phaeodactylum tricornutum Grown in a Tubular Photobioreactor. J. Appl. Phycol. 1994, 6, 67–74. [Google Scholar] [CrossRef]
- Guedes, A.C.; Meireles, L.A.; Amaro, H.M.; Malcata, F.X. Changes in Lipid Class and Fatty Acid Composition of Cultures of Pavlova lutheri, in Response to Light Intensity. J. Am. Oil Chem. Soc. 2010, 87, 791–801. [Google Scholar] [CrossRef]
- Hong, S.J.; Park, Y.S.; Han, M.A.; Kim, Z.H.; Cho, B.K.; Lee, H.; Choi, H.K.; Lee, C.G. Enhanced Production of Fatty Acids in Three Strains of Microalgae using a Combination of Nitrogen Starvation and Chemical Inhibitors of Carbohydrate Synthesis. Biotechnol. Bioprocess Eng. 2017, 22, 60–67. [Google Scholar] [CrossRef]
- Kamalanathan, M.; Pierangelini, M.; Shearman, L.A.; Gleadow, R.; Beardall, J. Impacts of nitrogen and phosphorus starvation on the physiology of Chlamydomonas reinhardtii. J. Appl. Phycol. 2016, 28, 1509–1520. [Google Scholar] [CrossRef]
- Lauritano, C.; De Luca, D.; Amoroso, M.; Benfatto, S.; Maestri, S.; Racioppi, C.; Esposito, F.; Ianora, A. New molecular insights on the response of the green alga Tetraselmis suecica to nitrogen starvation. Sci. Rep. 2019, 9, 3336. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Rohit, M.V.; Chiranjeevi, P.; Chandra, R.; Navaneeth, B. Heterotrophic microalgae cultivation to synergize biodiesel production with waste remediation: Progress and perspectives. Bioresour. Technol. 2015, 184, 169–178. [Google Scholar] [CrossRef]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibanez, E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.P.; Morant-Manceau, A.; Schoefs, B. The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef]
- Lauritano, C.; Ferrante, M.I.; Rogato, A. Marine natural products from microalgae: An -omics overview. Mar. Drugs 2019, 17, 269. [Google Scholar] [CrossRef]
- Mishra, A.; Medhi, K.; Malaviya, P.; Thakur, I.S. Omics approaches for microalgal applications: Prospects and challenges. Bioresour. Technol. 2019, 29, 121890. [Google Scholar] [CrossRef]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Oh, C.; Choi, Y.U.; Yoon, K.T.; Kang, D.H.; Qian, Z.J.; Choi, I.W.; Jung, W.K. Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem. Toxicol. 2012, 50, 3336–3342. [Google Scholar] [CrossRef]
- Kong, Z.-L.; Kao, N.-J.; Hu, J.-Y.; Wu, C.-S. Fucoxanthin-Rich Brown Algae Extract Decreases Inflammation and Attenuates Colitis-associated Colon Cancer in Mice. J. Food Nutr. Res. 2016, 4, 137–147. [Google Scholar]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wan, H.; Wang, R.; Hao, D. Sulfated polysaccharides from Phaeodactylum tricornutum: Isolation, structural characteristics, and inhibiting HepG2 growth activity in vitro. PeerJ 2019, 7, e6409. [Google Scholar] [CrossRef] [PubMed]
- Shishido, S.N.; Varahan, S.; Yuan, K.; Li, X.D.; Fleming, S.D. Humoral innate immune response and disease. Clin. Immunol. 2012, 144, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.; Schroder, K. Inflammasome signaling and regulation of interleukin-1 family cytokines. J. Exp. Med. 2019, 217, e20190314. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2019. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Ben-Aharon, O.; Magnezi, R.; Leshno, M.; Goldstein, D.A. Association of Immunotherapy With Durable Survival as Defined by Value Frameworks for Cancer Care. JAMA Oncol. 2018, 4, 326–332. [Google Scholar] [CrossRef]
- Moynihan, K.D.; Opel, C.F.; Szeto, G.L.; Tzeng, A.; Zhu, E.F.; Engreitz, J.M.; Williams, R.T.; Rakhra, K.; Zhang, M.H.; Rothschilds, A.M.; et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat. Med. 2016, 22, 1402–1410. [Google Scholar] [CrossRef]
- Zhu, E.F.; Gai, S.A.; Opel, C.F.; Kwan, B.H.; Surana, R.; Mihm, M.C.; Kauke, M.J.; Moynihan, K.D.; Angelini, A.; Williams, R.T.; et al. Synergistic innate and adaptive immune response to combination immunotherapy with anti-tumor antigen antibodies and extended serum half-life IL-2. Cancer Cell 2015, 27, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Rappuoli, R. From empiricism to rational design: A personal perspective of the evolution of vaccine development. Nat. Rev. Immunol. 2014, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Akinleye, A.; Rasool, Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J. Hematol. Oncol. 2019, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Morse, M.A. Technology evaluation: Ipilimumab, Medarex/Bristol-Myers Squibb. Curr. Opin. Mol. Ther. 2005, 7, 588–597. [Google Scholar]
- Rebe, C.; Ghiringhelli, F. STAT3, a Master Regulator of Anti-Tumor Immune Response. Cancers (Basel) 2019, 11, 1280. [Google Scholar] [CrossRef]
- Kwek, S.S.; Cha, E.; Fong, L. Unmasking the immune recognition of prostate cancer with CTLA4 blockade. Nat. Rev. Cancer 2012, 12, 289–297. [Google Scholar] [CrossRef]
- Scott, A.M.; Allison, J.P.; Wolchok, J.D. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012, 12, 14. [Google Scholar]
- Shepard, H.M.; Phillips, G.L.; D Thanos, C.; Feldmann, M. Developments in therapy with monoclonal antibodies and related proteins. Clin. Med. 2017, 17, 220–232. [Google Scholar] [CrossRef]
- Lim, W.A.; June, C.H. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017, 168, 724–740. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and temperature effects on bioactivity in diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.O.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, e68. [Google Scholar] [CrossRef]
- Samarakoon, K.W.; Ko, J.Y.; Shah, M.M.R.; Lee, J.H.; Kang, M.C.; O-Nam, K.; Lee, J.B.; Jeon, Y.J. In vitro studies of anti-inflammatory and anticancer activities of organic solvent extracts from cultured marine microalgae. Algae 2013, 28, 111–119. [Google Scholar]
- Lavy, A.; Naveh, Y.; Coleman, R.; Mokady, S.; Werman, M.J. Dietary Dunaliella bardawil, a beta-carotene-rich alga, protects against acetic acid-induced small bowel inflammation in rats. Inflamm. Bowel Dis. 2003, 9, 372–379. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Ebrahimi-Mameghani, M.; Sadeghi, Z.; Abbasalizad Farhangi, M.; Vaghef-Mehrabany, E.; Aliashrafi, S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Beneficial effects of supplementation with microalgae Chlorella vulgaris: A double-blind placebo-controlled randomized clinical trial. Clin. Nutr. 2017, 36, 1001–1006. [Google Scholar] [CrossRef]
- Caroprese, M.; Albenzio, M.; Ciliberti, M.G.; Francavilla, M.; Sevi, A. A mixture of phytosterols from Dunaliella tertiolecta affects proliferation of peripheral blood mononuclear cells and cytokine production in sheep. Vet Immunol. Immunopathol. 2012, 150, 27–35. [Google Scholar] [CrossRef]
- Cutignano, A.; Nuzzo, G.; Ianora, A.; Luongo, E.; Romano, G.; Gallo, C.; Sansone, C.; Aprea, S.; Mancini, F.; D’Oro, U.; et al. Development and Application of a Novel SPE-Method for Bioassay-Guided Fractionation of Marine Extracts. Mar. Drugs 2015, 13, 5736–5749. [Google Scholar] [CrossRef]
- Guzman, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Paramylon, a Potent Immunomodulator from WZSL Mutant of Euglena gracilis. Molecules 2019, 24, 3114. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Barsanti, L.; Evangelista, V.; Frassanito, A.M.; Longo, V.; Pucci, L.; Penno, G.; Gualtieri, P. Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors. Food Sci. Nutr. 2017, 5, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.Y.; Yim, J.H.; Lee, H.K.; Pyo, S. Activation of murine peritoneal macrophages by sulfated exopolysaccharide from marine microalga Gyrodinium impudicum (strain KG03): Involvement of the NF-kappa B and JNK pathway. Int. Immunopharmacol. 2006, 6, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Park, G.T.; Go, R.E.; Lee, H.M.; Lee, G.A.; Kim, C.W.; Seo, J.W.; Hong, W.K.; Choi, K.C.; Hwang, K.A. Potential Anti-proliferative and Immunomodulatory Effects of Marine Microalgal Exopolysaccharide on Various Human Cancer Cells and Lymphocytes In Vitro. Mar. Biotechnol. 2017, 19, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Baek, S.H.; Woo, Y.; Han, J.K.; Kim, B.G.; Kim, O.Y.; Lee, J.H. Beneficial immunostimulatory effect of short-term Chlorella supplementation: Enhancement of natural killer cell activity and early inflammatory response (randomized, double-blinded, placebo-controlled trial). Nutr. J. 2012, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.C.; Ho, Y.C.; Liao, J.W.; Lu, F.J. Dunaliella salina Exhibits an Antileukemic Immunity in a Mouse Model of WEHI-3 Leukemia Cells. J. Agric. Food Chem. 2014, 62, 11479–11487. [Google Scholar] [CrossRef]
- Cerezuela, R.; Guardiola, F.A.; Meseguer, J.; Esteban, M.A. Enrichment of gilthead seabream (Sparus aurata L.) diet with microalgae: Effects on the immune system. Fish Physiol. Biochem. 2012, 38, 1729–1739. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.; De Morais, R.M. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Yim, J.H.; Kim, S.J.; Ahn, S.H.; Lee, H.K. Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresour. Technol. 2007, 98, 361–367. [Google Scholar] [CrossRef]
- Hirahashi, T.; Matsumoto, M.; Hazeki, K.; Saeki, Y.; Ui, M.; Seya, T. Activation of the human innate immune system by Spirulina: Augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int. Immunopharmacol. 2002, 2, 423–434. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klimova, B.; Wan, D.; Kuca, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.; De Morais, R.M.; Bernardo de Morais, A.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, N.; Sun, X.; Duan, M.; Luo, T.; Jiang, P.; Jiang, G.; Song, S.; Ai, C. Effect of intake pattern of sulfated polysaccharides on its biological activity in high fat diet-fed mice. Int. J. Biol. Macromol. 2019, 132, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, I.I.; Zulkifli, N.; Selvakumaran, S.A.; Lazim, N.A.M. Bioactive Algal-Derived Polysaccharides: Multi-Functionalization, Therapeutic Potential and Biomedical Applications. Curr. Pharm. Des. 2019, 25, 1147–1162. [Google Scholar] [CrossRef]
- Sun, C.; Liu, M.; Sun, P.; Yang, M.; Yates, E.A.; Guo, Z.; Fernig, D.G. Sulfated polysaccharides interact with fibroblast growth factors and protect from denaturation. FEBS Open Bio 2019, 9, 1477–1487. [Google Scholar] [CrossRef]
- Vishwakarma, J.; Vavilala, S.L. Evaluating the antibacterial and antibiofilm potential of sulfated polysaccharides extracted from green algae Chlamydomonas reinhardtii. J. Appl. Microbiol. 2019, 127, 1004–1017. [Google Scholar] [CrossRef]
- Abdala Diaz, R.T.; Casas Arrojo, V.; Arrojo Agudo, M.A.; Cardenas, C.; Dobretsov, S.; Figueroa, F.L. Immunomodulatory and Antioxidant Activities of Sulfated Polysaccharides from Laminaria ochroleuca, Porphyra umbilicalis, and Gelidium corneum. Mar. Biotechnol. 2019, 21, 577–587. [Google Scholar] [CrossRef]
- Bahramzadeh, S.; Tabarsa, M.; You, S.; Li, C.; Bita, S. Purification, structural analysis and mechanism of murine macrophage cell activation by sulfated polysaccharides from Cystoseira indica. Carbohydr. Polym. 2019, 205, 261–270. [Google Scholar] [CrossRef]
- Cui, J.F.; Ye, H.; Zhu, Y.J.; Li, Y.P.; Wang, J.F.; Wang, P. Characterization and Hypoglycemic Activity of a Rhamnan-Type Sulfated Polysaccharide Derivative. Mar. Drugs 2019, 17, 21. [Google Scholar] [CrossRef]
- Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Liu, Z.; Mao, W. Anticoagulant and Antithrombotic Properties in Vitro and in Vivo of a Novel Sulfated Polysaccharide from Marine Green Alga Monostroma nitidum. Mar. Drugs 2019, 17, 247. [Google Scholar] [CrossRef]
- Da Silva, F.R.P.; Moara, E.S.C.P.; de Carvalho Franca, L.F.; Alves, E.H.P.; Dos Santos Carvalho, J.; Di Lenardo, D.; Brito, T.V.; Medeiros, J.R.; de Oliveira, J.S.; Freitas, A.L.P.; et al. Sulfated polysaccharides from the marine algae Gracilaria caudata prevent tissue damage caused by ligature-induced periodontitis. Int. J. Biol. Macromol. 2019, 132, 1–8. [Google Scholar] [PubMed]
- Frentzen, M. Phosphatidylglycerol and sulfoquinovosyldiacylglycerol: Anionic membrane lipids and phosphate regulation. Curr. Opin. Plant Biol. 2004, 7, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K. Role of membrane glycerolipids in photosynthesis, thylakoid biogenesis and chloroplast development. J. Plant Res. 2016, 129, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.B.; Hewlins, M.J.; Ellis, A.J.; Harwood, J.L.; White, G.F. Glycolytic breakdown of sulfoquinovose in bacteria: A missing link in the sulfur cycle. Appl. Environ. Microbiol. 2003, 69, 6434–6441. [Google Scholar] [CrossRef]
- Speciale, G.; Jin, Y.; Davies, G.J.; Williams, S.J.; Goddard-Borger, E.D. YihQ is a sulfoquinovosidase that cleaves sulfoquinovosyl diacylglyceride sulfolipids. Nat. Chem. Biol. 2016, 12, 215–217. [Google Scholar] [CrossRef]
- Hielscher-Michael, S.; Griehl, C.; Buchholz, M.; Demuth, H.U.; Arnold, N.; Wessjohann, L.A. Natural Products from Microalgae with Potential against Alzheimer’s Disease: Sulfolipids Are Potent Glutaminyl Cyclase Inhibitors. Mar. Drugs 2016, 14, 203. [Google Scholar] [CrossRef]
- Manzo, E.; Gallo, C.; Fioretto, L.; Nuzzo, G.; Barra, G.; Pagano, D.; Krauss, I.R.; Paduano, L.; Ziaco, M.; DellaGreca, M.; et al. Diasteroselective Colloidal Self-Assembly Affects the Immunological Response of the Molecular Adjuvant Sulfavant. ACS Omega 2019, 4, 7807–7814. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Leu, S.; Boussiba, S. Microalgae as a source for VLC-PUFA production. Subcell Biochem. 2016, 86, 471–510. [Google Scholar]
- Colombo, S.M.; Wacker, A.; Parrish, C.C.; Kainz, M.J.; Arts, M.T. A fundamental dichotomy in long-chain polyunsaturated fatty acid abundance between and within marine and terrestrial ecosystems. Environ. Rev. 2017, 25, 163–174. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Maroneze, M.M.; Depra, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Cui, Y.; Thomas-Hall, S.R.; Schenk, P.M. Phaeodactylum tricornutum microalgae as a rich source of omega-3 oil: Progress in lipid induction techniques towards industry adoption. Food Chem. 2019, 297, 124937. [Google Scholar] [CrossRef] [PubMed]
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Le, P.N.T.; Desbois, A.P. Antibacterial Effect of Eicosapentaenoic Acid against Bacillus cereus and Staphylococcus aureus: Killing Kinetics, Selection for Resistance, and Potential Cellular Target. Mar. Drugs 2017, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Albracht-Schulte, K.; Gonzalez, S.; Jackson, A.; Wilson, S.; Ramalingam, L.; Kalupahana, N.S.; Moustaid-Moussa, N. Eicosapentaenoic Acid Improves Hepatic Metabolism and Reduces Inflammation Independent of Obesity in High-Fat-Fed Mice and in HepG2 Cells. Nutrients 2019, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.; Erkan, N.; Yasmin, A. Antioxidant protection of eicosapentaenoic acid and fish oil oxidation by polyphenolic-enriched apple skin extract. J. Agric. Food Chem. 2010, 58, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Garcia de Acilu, M.; Leal, S.; Caralt, B.; Roca, O.; Sabater, J.; Masclans, J.R. The Role of Omega-3 Polyunsaturated Fatty Acids in the Treatment of Patients with Acute Respiratory Distress Syndrome: A Clinical Review. BioMed Res. Int. 2015, 2015, 653750. [Google Scholar] [CrossRef][Green Version]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef]
- Nappo, M.; Berkov, S.; Massucco, C.; Di Maria, V.; Bastida, J.; Codina, C.; Avila, C.; Messina, P.; Zupo, V.; Zupo, S. Apoptotic activity of the marine diatom Cocconeis scutellum and eicosapentaenoic acid in BT20 cells. Pharm. Biol. 2012, 50, 529–535. [Google Scholar] [CrossRef]
- Yamada, H.; Umemoto, T.; Kakei, M.; Momomura, S.I.; Kawakami, M.; Ishikawa, S.E.; Hara, K. Eicosapentaenoic acid shows anti-inflammatory effect via GPR120 in 3T3-L1 adipocytes and attenuates adipose tissue inflammation in diet-induced obese mice. Nutr. Metab. 2017, 14, 33. [Google Scholar] [CrossRef]
- Onodera, T.; Fukuhara, A.; Shin, J.; Hayakawa, T.; Otsuki, M.; Shimomura, I. Eicosapentaenoic acid and 5-HEPE enhance macrophage-mediated Treg induction in mice. Sci. Rep. 2017, 7, 4560. [Google Scholar] [CrossRef]
- Zapata-Gonzalez, F.; Rueda, F.; Petriz, J.; Domingo, P.; Villarroya, F.; Diaz-Delfin, J.; de Madariaga, M.A.; Domingo, J.C. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR(gamma):RXR heterodimers: Comparison with other polyunsaturated fatty acids. J. Leukoc. Biol. 2008, 84, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Liddle, D.M.; Cohen, D.J.; Tsang, D.H.; Hillyer, L.M.; Abdelmagid, S.A.; Nakamura, M.T.; Power, K.A.; Ma, D.W.; Robinson, L.E. The delta 6 desaturase knock out mouse reveals that immunomodulatory effects of essential n-6 and n-3 polyunsaturated fatty acids are both independent of and dependent upon conversion. J. Nutr. Biochem. 2016, 32, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Song, S.; Niu, Y.; Meng, M.; Wang, C. Eicosapentaenoic Acid (EPA) Induced Macrophages Activation through GPR120-Mediated Raf-ERK1/2-IKKbeta-NF-kappaB p65 Signaling Pathways. Nutrients 2017, 9, 397. [Google Scholar]
- Bigogno, C.; Khozin-Goldberg, I.; Boussiba, S.; Vonshak, A.; Cohen, Z. Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 2002, 60, 497–503. [Google Scholar] [CrossRef]
- Dunstan, G.A.; Volkman, J.K.; Barrett, S.M.; Leroi, J.M.; Jeffrey, S.W. Essential Polyunsaturated Fatty-Acids from 14 Species of Diatom (Bacillariophyceae). Phytochemistry 1994, 35, 155–161. [Google Scholar] [CrossRef]
- Martins, T.G.; Odebrecht, C.; Jensen, L.V.; D’Oca, M.G.M.; Wasielesky, W. The contribution of diatoms to bioflocs lipid content and the performance of juvenile Litopenaeus vannamei (Boone, 1931) in a BFT culture system. Aquac. Res. 2016, 47, 1315–1326. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Cuine, S.; Beyly-Adriano, A.; Legeret, B.; Billon, E.; Auroy, P.; Beisson, F.; Peltier, G.; Li-Beisson, Y. The green microalga Chlamydomonas reinhardtii has a single omega-3 fatty acid desaturase that localizes to the chloroplast and impacts both plastidic and extraplastidic membrane lipids. Plant Physiol. 2013, 163, 914–928. [Google Scholar] [CrossRef]
- Haigh, W.G.; Yoder, T.F.; Ericson, L.; Pratum, T.; Winget, R.R. The characterisation and cyclic production of a highly unsaturated homoserine lipid in Chlorella minutissima. Biochim. Biophys. Acta Lipids Lipid Metab. 1996, 1299, 183–190. [Google Scholar] [CrossRef]
- Nappo, M.; Berkov, S.; Codina, C.; Avila, C.; Messina, P.; Zupo, V.; Bastida, J. Metabolite profiling of the benthic diatom Cocconeis scutellum by GC-MS. J. Appl. Phycol. 2009, 21, 295–306. [Google Scholar] [CrossRef]
- Renaud, S.M.; Thinh, L.V.; Lambrinidis, G.; Parry, D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Gouveia, L.; Coutinho, C.; Mendonca, E.; Batista, A.P.; Sousa, I.; Bandarra, N.M.; Raymundo, A. Functional biscuits with PUFA-omega 3 from Isochrysis galbana. J. Sci. Food Agric. 2008, 88, 891–896. [Google Scholar] [CrossRef]
- Adolf, J.E.; Place, A.R.; Stoecker, D.K.; Harding, L.W. Modulation of polyunsaturated fatty acids in mixotrophic Karlodinium veneficum (Dinophyceae) and its prey, Storeatula major (Cryptophyceae). J. Phycol. 2007, 43, 1259–1270. [Google Scholar] [CrossRef]
- Molino, A.; Martino, M.; Larocca, V.; Di Sanzo, G.; Spagnoletta, A.; Marino, T.; Karatza, D.; Iovine, A.; Mehariya, S.; Musmarra, D. Eicosapentaenoic Acid Extraction from Nannochloropsis gaditana using Carbon Dioxide at Supercritical Conditions. Mar. Drugs 2019, 17, 132. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.N.; Chen, T.P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Y.; Chen, F. Production potential of eicosapentaenoic acid by the diatom Nitzschia laevis. Biotechnol. Lett. 2000, 22, 727–733. [Google Scholar] [CrossRef]
- Zhang, D.M.; Wen, S.M.; Wu, X.; Cong, W. Effect of culture condition on the growth, biochemical composition and EPA production of alkaliphilic Nitzschia plea isolated in the Southeast of China. Bioprocess Biosyst. Eng. 2018, 41, 831–839. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Pontes, I.; Gaspar, H.; Malcata, F.X. Metabolic relationships between macro- and micronutrients, and the eicosapentaenoic acid and docosahexaenoic acid contents of Pavlova lutheri. Enzym. Microb. Technol. 2006, 38, 358–366. [Google Scholar] [CrossRef]
- Mayer, C.; Come, M.; Ulmann, L.; Zittelli, G.C.; Faraloni, C.; Nazih, H.; Ouguerram, K.; Chenais, B.; Mimouni, V. Preventive Effects of the Marine Microalga Phaeodactylum tricornutum, Used as a Food Supplement, on Risk Factors Associated with Metabolic Syndrome in Wistar Rats. Nutrients 2019, 11, 1069. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Borowitzka, M.A. The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J. Appl. Phycol. 2006, 18, 703–712. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Wu, H.; Jiang, P.; Chen, Z.; Xiang, W. Growth and Biochemical Composition of Porphyridium purpureum SCS-02 under Different Nitrogen Concentrations. Mar. Drugs 2019, 17, 124. [Google Scholar] [CrossRef]
- Pezzolesi, L.; Pichierri, S.; Samori, C.; Totti, C.; Pistocchi, R. PUFAs and PUAs production in three benthic diatoms from the northern Adriatic Sea. Phytochemistry 2017, 142, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Blanchemain, A.; Grizeau, D. Increased production of eicosapentaenoic acid by Skeletonema costatum cells after decantation at low temperature. Biotechnol. Tech. 1999, 13, 497–501. [Google Scholar] [CrossRef]
- Vidoudez, C.; Pohnert, G. Comparative metabolomics of the diatom Skeletonema marinoi in different growth phases. Metabolomics 2012, 8, 654–669. [Google Scholar] [CrossRef]
- Fabregas, J.; Otero, A.; Dominguez, A.; Patino, M. Growth Rate of the microalga Tetraselmis suecica changes the biochemical composition of Artemia species. Mar. Biotechnol. 2001, 3, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; De Lutiis, M.A.; Grilli, A.; Felaco, M. Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokines secretion in U937: SHP-1 as a novel biological target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef]
- Hussein, G.; Nakamura, M.; Zhao, Q.; Iguchi, T.; Goto, H.; Sankawa, U.; Watanabe, H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol. Pharm. Bull. 2005, 28, 47–52. [Google Scholar] [CrossRef]
- Brendler, T.; Williamson, E.M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 240. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Grimmig, B.; Morganti, J.; Nash, K.; Bickford, P.C. Immunomodulators as therapeutic agents in mitigating the progression of Parkinson’s disease. Brain Sci. 2016, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Lin, K.C.; Lu, W.J.; Thomas, P.A.; Jayakumar, T.; Sheu, J.R. Astaxanthin, a carotenoid, stimulates immune responses by enhancing IFN-γ and Il-2 secretion in primary cultured lymphocytes in vitro and ex vivo. Int. J. Mol. Sci. 2015, 17, 44. [Google Scholar] [CrossRef]
- Davinelli, S.; Melvang, H.M.; Andersen, L.P.; Scapagnini, G.; Nielsen, M.E. Astaxanthin from shrimp cephalothorax stimulates the immune response by enhancing IFN-γ, IL-10, and IL-2 secretion in splenocytes of Helicobacter pylori-infected mice. Mar. Drugs 2019, 17, 44. [Google Scholar] [CrossRef]
- Barzkar, N.; Tamadoni Jahromi, S.; Poorsaheli, H.B.; Vianello, F. Metabolites from Marine Microorganisms, Micro, and Macroalgae: Immense Scope for Pharmacology. Mar. Drugs 2019, 17, 464. [Google Scholar] [CrossRef]
- Yanai, H.; Masui, Y.; Katsuyama, H.; Adachi, H.; Kawaguchi, A.; Hakoshima, M.; Waragai, Y.; Harigae, T.; Sako, A. An Improvement of Cardiovascular Risk Factors by Omega-3 Polyunsaturated Fatty Acids. J. Clin. Med. Res. 2018, 10, 281–289. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Gille, A.; Louis, S.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Bioavailability and safety of nutrients from the microalgae Chlorella vulgaris, Nannochloropsis oceanica and Phaeodactylum tricornutum in C57BL/6 mice. Nutrients 2018, 10, 965. [Google Scholar] [CrossRef]
- Vinayak, V.; Manoylov, K.M.; Gateau, H.; Blanckaert, V.; Hérault, J.; Pencréac’H, G.; Marchand, J.; Gordon, R.; Schoefs, B. Diatom milking? A review and new approaches. Mar. Drugs 2015, 13, 2629–2665. [Google Scholar] [CrossRef]
- Vaz, B.D.S.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Bule, M.H.; Ahmed, I.; Maqbool, F.; Bilal, M.; Iqbal, H.M.N. Microalgae as a source of high-value bioactive compounds. Front. Biosci. Sch. 2018, 10, 197–216. [Google Scholar]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Schadendorf, D.; Ascierto, P.A.; Haanen, J.; Espinosa, E.; Demidov, L.; Garbe, C.; Guida, M.; Lorigan, P.; Chiarion-Sileni, V.; Gogas, H.; et al. Safety and efficacy of nivolumab in challenging subgroups with advanced melanoma who progressed on or after ipilimumab treatment: A single-arm, open-label, phase II study (CheckMate 172). Eur. J. Cancer 2019, 121, 144–153. [Google Scholar] [CrossRef]
- Wang, J.; Shen, T.; Wang, Q.; Zhang, T.; Li, L.; Wang, Y.; Fang, Y. The long-term efficacy of cytokine-induced killer cellular therapy for hepatocellular carcinoma: A meta-analysis. Immunotherapy 2019, 11, 1325–1335. [Google Scholar] [CrossRef]
- Maus, M.V.; Grupp, S.A.; Porter, D.L.; June, C.H. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014, 123, 2625–2635. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Thalhammer, T.; Tzakos, A.G.; Stojanovska, L. Targeting antigens to dendritic cell receptors for vaccine development. J. Drug Deliv. 2013, 2013, 869718. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef]
- Mellman, I. Dendritic cells: Master regulators of the immune response. Cancer Immunol. Res. 2013, 1, 145–149. [Google Scholar] [CrossRef]
- Rossi, M.; Young, J.W. Human dendritic cells: Potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 2005, 175, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Hirano, N.; Butler, M.O.; Xia, Z.; Ansen, S.; von Bergwelt-Baildon, M.S.; Neuberg, D.; Freeman, G.J.; Nadler, L.M. Engagement of CD83 ligand induces prolonged expansion of CD8(+) T cells and preferential enrichment for antigen specificity. Blood 2006, 107, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.T.; Brauer, M.; Kee, R.; Batt, K.; Karaca-Mandic, P.; Zhang, J.; Goldman, D.P. The Potential Impact of CAR T-Cell Treatment Delays on Society. Am. J. Manag. Care 2019, 25, 379–386. [Google Scholar]
- Lauritano, C.; Ianora, A. Grand Challenges in Marine Biotechnology: Overview of Recent EU-Funded Projects. In Grand Challenges in Marine Biotechnology; Springer: Cham, Switzerland, 2018; pp. 425–449. [Google Scholar]
- Rubin, B.A. A note on the development of the bifurcated needle for smallpox vaccination. WHO Chronic 1980, 34, 180–181. [Google Scholar]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Cameron, F.; Whiteside, G.; Perry, C. Ipilimumab: First global approval. Drugs 2011, 71, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Seimetz, D.; Heller, K.; Richter, J. Approval of First CAR-Ts: Have we Solved all Hurdles for ATMPs? Cell Med. 2019, 11, 2155179018822781. [Google Scholar] [CrossRef]
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From Marine Origin to Therapeutics: The Antitumor Potential of Marine Algae-Derived Compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef]
- De Coêlho, D.F.; Tundisi, L.L.; Cerqueira, K.S.; da Silva Rodrigues, J.R.; Mazzola, P.G.; Tambourgi, E.B.; de Souza, R.R. Microalgae: Cultivation Aspects and Bioactive Compounds. Braz. Arch. Biol. Technol. 2019, 62, e19180343. [Google Scholar] [CrossRef]
- Serif, M.; Dubois, G.; Finoux, A.L.; Teste, M.A.; Jallet, D.; Daboussi, F. One-step generation of multiple gene knock-outs in the diatom Phaeodactylum tricornutum by DNA-free genome editing. Nat. Commun. 2018, 9, 3924. [Google Scholar] [CrossRef] [PubMed]
- Nymark, M.; Sharma, A.K.; Sparstad, T.; Bones, A.M.; Winge, P. A CRISPR/Cas9 system adapted for gene editing in marine algae. Sci. Rep. 2016, 6, 24951. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Nakagawa, T.; Kitamura, H.; Atsumi, T.; Kamon, H.; Sawa, S.; Kamimura, D.; Ueda, N.; Iwakura, Y.; Ishihara, K.; et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J. Immunol. 2004, 173, 3844–3854. [Google Scholar] [CrossRef] [PubMed]
| Microalgae | Extract/Fraction/Compound | Active Concentration | Mechanism/ Organism and Target Cells (or Model) | Reference |
|---|---|---|---|---|
| Alexandrium tamarense | Total Extract/Fractions | NA | Activation of IL-6/Human PBMC | [49] |
| Chaetoceros calcitrans | Fractions | NA | Activation of IL-6/Human PBMC | [49] |
| Chaetoceros socialis | Total extract | NA | Activation of IL-6/Human PBMC | [49] |
| Chlorella stigmatophora | Crude polysaccharide extracts | 5 or 10 mg/kg | Activation of phagocytic activity - SRBC/Mouse | [50] |
| Chlorella vulgaris | Diet supplementation/ commercially available pills | 5 g/day | Improve of NK activity and serum level of INF-γ, IL-1β and IL-12/Human trials | [55] |
| Dunaliella salina | Diet supplementation of commercially available spray-dried preparations | 369, and 922.5 mg/kg | MiceNK and Macrophage activation/In vivo mice model | [56] [49] |
| Dunaliella salina | Fractions | NA | Activation of IL-6/Human PBMC | [49] |
| Euglena gracilis | β-Glucans | 150 μg/mL | Activation of NK cells and improve in inflammatory mediator/Human PBMC | [51,52] |
| Gyrodinium impudicum | Sulfated exopolysaccharides | 0.1–10 μg/mL | Macrophage activation/Murine | [53,59] |
| Skeletonema costatum | Total Extract/Fractions | NA | Activation of IL-6/Human PBMC | [49] |
| Skeletonema dohrnii | Total Extract/Fractions | NA | Activation of IL-6/Human PBMC | [49] |
| Skeletonema marinoi | Total Extract | NA | Activation of IL-6/Human PBMC | [49] |
| Spirulina sp. | Food supplement of condensed water-soluble extract of commercially available spray dried Spirulina | NA | Augmentation of interferon production and NK cytotoxicity /Human trials | [60,61] |
| Tetraselmis chuii | Orally administration of lyophilized microalgae | 50 or 100 g/Kg | Increase in hemolytic complement activity, phagocytic capacity and expression levels of β-defensin, major histocompatibility complex IIα and colony-stimulating factor receptor-1/Gilthead sea bream | [57] |
| Thalassiosira weissflogii | Fractions | Activation of IL-6/Human PBMC | [49] | |
| Thraustochytriidae sp. | Exopolysaccharides | 10−3 to 10−9 w/v | B-cells proliferation/Human | [54] |
| Tribonema sp. | Sulfated polysaccharides | 12.5–200 μg/mL | Macrophage proliferation and improved expression of cytokines/Mouse | [2] |
| Microalgae | PUFA | Ref |
|---|---|---|
| Amphidinium carterae | ARA/EPA | [94] |
| Amphiprora hyaline | EPA | [95] |
| Amphora coffeaeformis | EPA/DHA | [96] |
| Chlamydomonas reinhardtii | ω-3 | [97] |
| Chlorella minutissima | EPA | [98] |
| Cocconeis scutellum | ω-3/ω-6 | [99] |
| Conticribra weissflogii | EPA/DHA | [96] |
| Cryptomonas sp. | EPA/DHA | [100] |
| Cylindrotheca fusiformis | EPA | [95] |
| Fragilaria pinnata | EPA | [95] |
| Isochrysis galbana | EPA/DHA | [101] |
| Karlodinium veneficum | ω-3 | [102] |
| Nannochloropsis gaditana | EPA | [103] |
| Nannochloropsis sp. | EPA | [104] |
| Nitzschia closterium | EPA | [95] |
| Nitzschia laevis | ARA/EPA | [105] |
| Nitzschia plea | EPA | [106] |
| Parietochloris incisa | ARA/EPA | [94] |
| Pavlova lutheri | EPA/DHA | [107] |
| Phaeodactylum tricornutum | EPA | [108] |
| Pleurochrysis carterae | ω-3 | [109] |
| Porphyridium purpureum | ARA/EPA | [110] |
| Proschkinia complanatoides | EPA | [111] |
| Rhizosolenia setigera | EPA | [95] |
| Rhodomonas sp. | EPA/DHA | [100] |
| Skeletonema costatum | EPA | [112] |
| Skeletonema marinoi | EPA | [113] |
| Storeatula major | ω-3 | [102] |
| Tetraselmis suecica | EPA | [114] |
| Thalassionema nitzschioides | EPA | [95] |
| Thalassiosira pseudonana | ARA/EPA | [94] |
| Thalassiosira stellaris | EPA | [95] |
| Thalassiothrix heteromorpha | EPA | [95] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2020, 18, 2. https://doi.org/10.3390/md18010002
Riccio G, Lauritano C. Microalgae with Immunomodulatory Activities. Marine Drugs. 2020; 18(1):2. https://doi.org/10.3390/md18010002
Chicago/Turabian StyleRiccio, Gennaro, and Chiara Lauritano. 2020. "Microalgae with Immunomodulatory Activities" Marine Drugs 18, no. 1: 2. https://doi.org/10.3390/md18010002
APA StyleRiccio, G., & Lauritano, C. (2020). Microalgae with Immunomodulatory Activities. Marine Drugs, 18(1), 2. https://doi.org/10.3390/md18010002






