Antioxidant Fucoidans Obtained from Tropical Seaweed Protect Pre-Osteoblastic Cells from Hydrogen Peroxide-Induced Damage
Abstract
1. Introduction
2. Results
2.1. Physicochemical Analyses of Fucoidans (FRFs)
2.2. Antioxidant Activity of FRFs
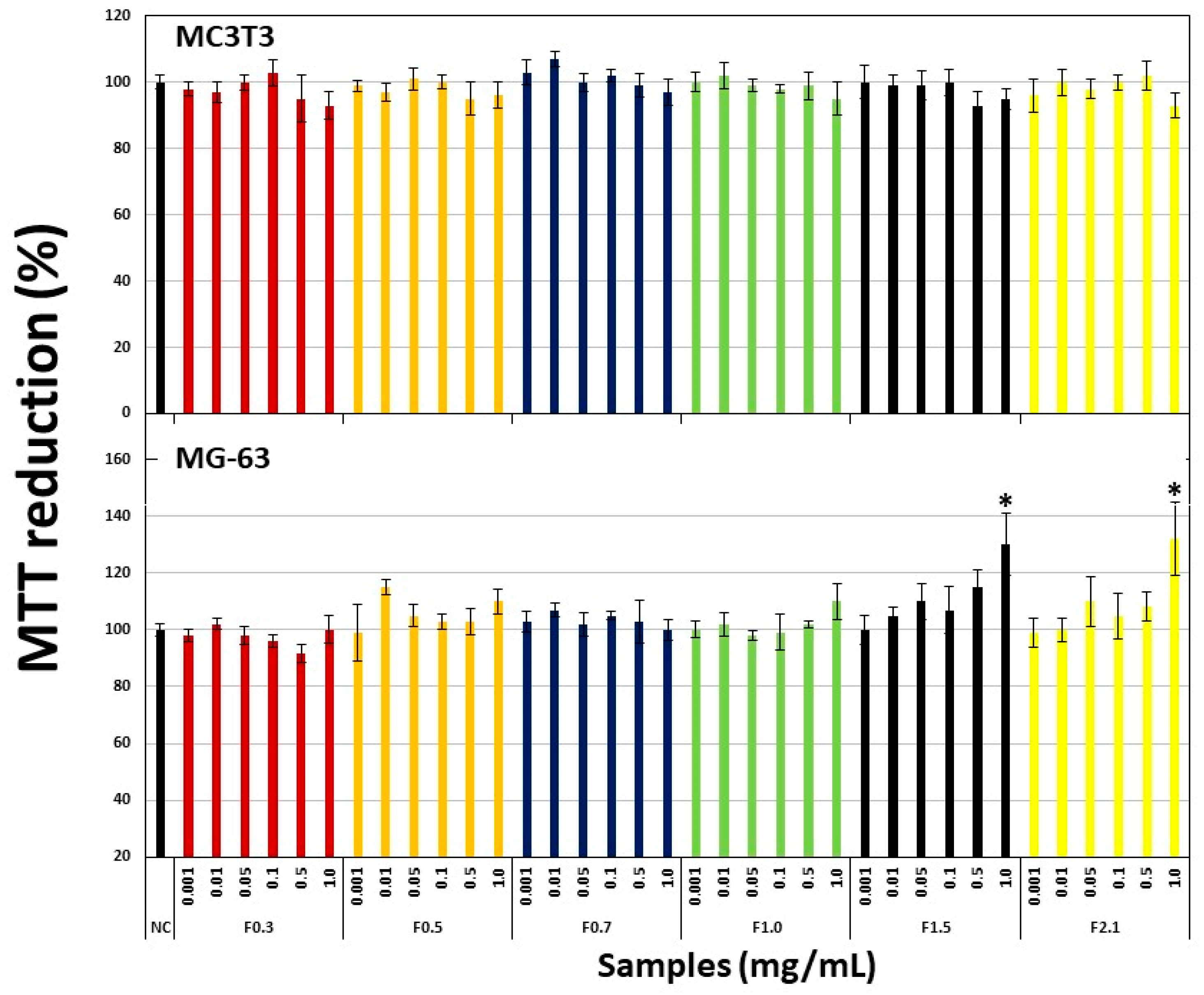
2.3. Effect of FRF on Viability of Pre-Osteoblastic Cells (MC3T3) and Osteosarcoma Cells (MG-63)
2.4. Effect of FRFs on Viability of Pre-Osteoblastic (MC3T3) Cells Exposed to Hydrogen Peroxide (H2O2).
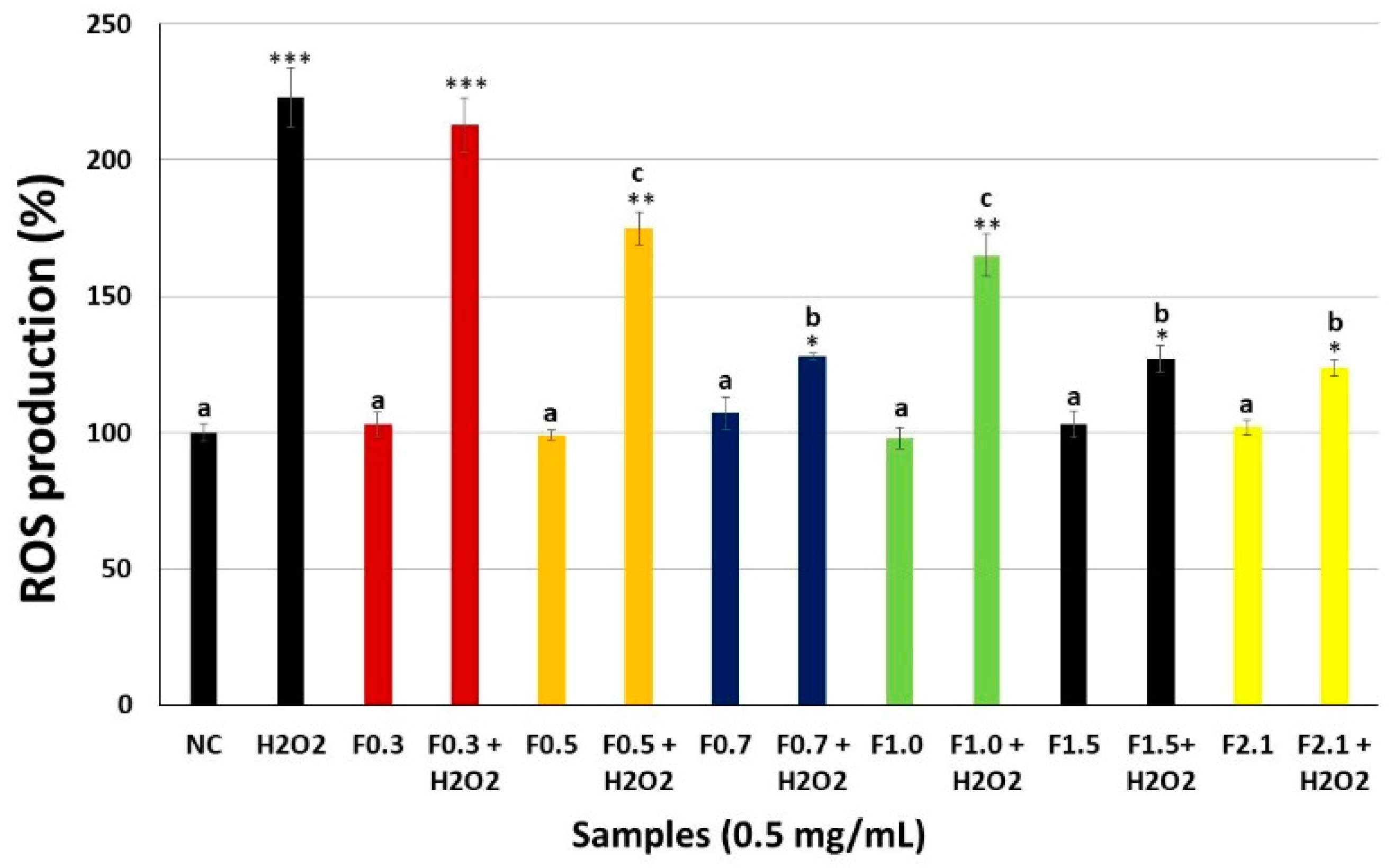
2.5. Intracellular Reactive Oxygen Species (ROS) Production
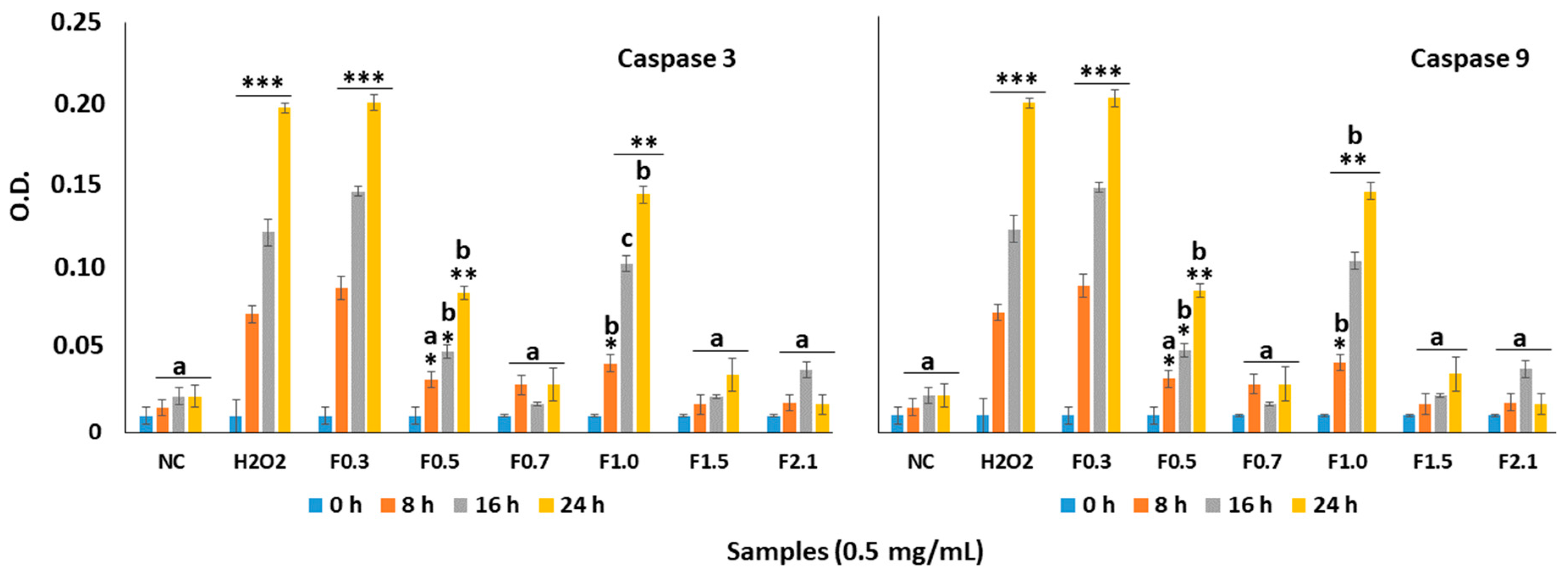
2.6. Effect of FRF on Caspase-3 and Caspase-9 in MC3T3 Cells
2.7. Assessment of the Cell Antioxidant Status
2.8. FRFs Attenuate H2O2-Mediated Inhibition of Alkaline Phosphatase Activity (ALP) Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction of Fucoidan (FRF)
4.3. Chemical Analysis and Monosaccharide Composition
4.4. 3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazolium Bromide (MTT) Test
4.5. Agarose Gel Electrophoresis in 1,3-Diamino Propane Acetate Buffer (PDA)
4.6. Antioxidant Activity
4.6.1. Determination of Total Antioxidant Capacity (TAC)
4.6.2. Hydroxyl Radical Scavenging Activity Assay
4.6.3. Ferrous Ion-Chelating Ability
4.6.4. Copper Chelating
4.6.5. Superoxide Radical Scavenging Activity Assay
4.6.6. Reducing Power
4.7. Induced Oxidative Stress Assay
4.8. Caspase-3 and -9 Activity Assays
4.9. Superoxide Dismutase Evaluation
4.10. Production of Intracellular ROS
4.11. Measurement of Alkaline Phosphatase Activity
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Drissi, H.; Sanjay, A. The multifaceted osteoclast; far and beyond bone resorption. J. Cell. Biochem. 2016, 117, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Osteoporosis: Fracture epidemiology update 2016. Curr. Opin. Rheumatol. 2017, 29, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Investig. 2003, 112, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, S.; Vandromme, J.; Antoine, C. Postmenopausal hormone therapy: Risks and benefits. Nat. Rev. Endocrinol. 2013, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Where are we 10 years after the Women’s Health Initiative? J. Clin. Endocrinol. Metab. 2013, 98, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Downey, P.A.; Siegel, M.I. Bone biology and the clinical implications for osteoporosis. Phys. Ther. 2006, 86, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.O.R.; Gomes, D.L.; Dantas, L.A.; Viana, R.L.S.; Chiquetti, S.C.; Almeida-Lima, J.; Costa, L.S.; Rocha, H.A.O. Fucan-coated silver nanoparticles synthesized by a green method induce human renal adenocarcinoma cell death. Int. J. Biol. Macromol. 2016, 93, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Yuguchi, Y.; Bui, L.M.; Takebe, S.; Suzuki, S.; Nakajima, N.; Kitamura, S.; Thanh, T.T.T. Primary structure, conformation in aqueous solution, and intestinal immunomodulating activity of fucoidan from two brown seaweed species Sargassum crassifolium and Padina australis. Carbohydr. Polym. 2016, 147, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Yang, S.S.; You, H.K.; Shin, H.I.; Lee, J. Fucoidan-induced osteogenic differentiation promotes angiogenesis by inducing vascular endothelial growth factor secretion and accelerates bone repair. J. Tissue Eng. Regen. Med. 2018, 12, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Changotade, S.I.T.; Korb, G.; Bassil, J.; Barroukh, B.; Willig, C.; Colliec-Jouault, S.; Durand, P.; Gondeau, G.; Senni, K. Potential effects of a low-molecular-weight fucoidan extracted from brown algae on bone biomaterial osteoconductive properties. J. Biomed. Mater. Res. 2008, 87, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Hung, Y.L.; Phan, N.N.; Chang, P.M.; Li, K.L.; Lin, Y.C. The in vitro and in vivo effects of the low molecular weight fucoidan on the bone osteogenic differentiation properties. Cytotechnology 2016, 68, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, K.; Lim, D.; Lee, S. The Sulfated Polysaccharide Fucoidan Stimulates Osteogenic Differentiation of Human Adipose-Derived Stem Cells. Stem Cells Dev. 2012, 21, 2204–2211. [Google Scholar] [CrossRef]
- Kim, B.S.; Kang, H.J.; Park, J.Y.; Lee, J. Fucoidan promotes osteoblast differentiation via JNK-and ERK-dependent BMP2–Smad 1/5/8 signaling in human mesenchymal stem cells. Exp. Mol. Med. 2015, 47, 128. [Google Scholar] [CrossRef]
- Menezes, M.M.; Nobre, L.T.D.B.; Rossi, G.R.; Almeida-Lima, J.; Melo-Silveira, R.F.; Franco, C.R.C.; Trindade, E.S.; Nader, H.B.; Rocha, H.A.O. A low-molecular-weight galactofucan from the seaweed, Spatoglossum schröederi, binds fibronectin and inhibits capillary-like tube formation in vitro. Int. J. Biol. Macromol. 2018, 111, 1067–1075. [Google Scholar] [CrossRef]
- Castro, L.S.E.P.W.; de Sousa Pinheiro, T.; Castro, A.J.G.; Santos, M.D.S.N.; Soriano, E.M.; Leite, E.L. Potential anti-angiogenic, antiproliferative, antioxidant, and anticoagulant activity of anionic polysaccharides, fucans, extracted from brown algae Lobophora variegata. J. Appl. Phycol. 2015, 27, 1315–1325. [Google Scholar] [CrossRef]
- Melo, K.R.T.; Câmara, R.B.G.; Queiroz, M.F.; Vidal, A.A.J.; Lima, C.R.M.; Melo-Silveira, R.F.; Almeida-Lima, J.; Rocha, H.A.O. Evaluation of sulfated polysaccharides from the brown seaweed Dictyopteris justii as antioxidant agents and as inhibitors of the formation of calcium oxalate crystals. Molecules 2013, 18, 14543–14563. [Google Scholar] [CrossRef]
- Aliança, A.S.S.; Anjos, K.F.L.; Reis, T.N.V.; Higino, T.M.M.; Brelaz-de-Castro, M.C.A.; Bianco, E.M.; Figueiredo, R.C.B.Q. The in vitro biological activity of the Brazilian brown seaweed Dictyota mertensii against Leishmania amazonensis. Molecules 2014, 19, 14052–14065. [Google Scholar] [CrossRef]
- Fernandes-Negreiros, M.M.; Machado, R.I.A.; Bezerra, F.L.; Melo, M.C.N.; Alves, M.G.C.F.; Filgueira, L.G.A.; Morgano, M.A.; Trindade, E.S.; Costa, L.S.; Rocha, H.A.O. Antibacterial, antiproliferative, and immunomodulatory activity of silver nanoparticles synthesized with fucans from the alga Dictyota mertensii. Nanomaterials 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.D.A.; Câmara, R.B.G.; Nobre, L.T.D.B.; Costa, M.S.S.P.; Almeida-Lima, J.; Farias, E.H.C.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Nobre, L.T.D.B.; Vidal, A.A.J.; Almeida-Lima, J.; Oliveira, R.M.; Paredes-Gamero, E.J.; Medeiros, V.P.; Trindade, E.S.; Franco, C.R.C.; Nader, H.B.; Rocha, H.A.O. Fucan effect on CHO cell proliferation and migration. Carbohydr. Polym. 2013, 98, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Rocha, H.A.O.; Bezerra, L.C.M.; Albuquqerque, I.R.L.; Costa, L.S.; Guerra, C.M.P.; Abreu, L.R.D.; Nader, H.B.; Leite, E.L. A xylogalactofucan from the brown seaweed Spatoglossum schröederi stimulates the synthesis of an antithrombotic heparan sulfate from endothelial cells. Planta Med. 2005, 71, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Wynne, M.J.; Ballatine, D.L. The genus Hypoglossum Kützing (Delesseriaceae, Rhodophyta) in the tropical western Atlantic, including H. anomalum sp. nov. J. Phycol. 1986, 22, 185–193. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Spector, T. Refinement of the Coomassie blue method of protein quantification. Anal. Biochem. 1978, 86, 142–146. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar]
- Dietrich, C.P.; McDuffie, N.M.; Sampaio, L.O. Identification of acidic mucopolysaccharides by agarose gel electrophoresis. J. Chromatogr. A 1977, 130, 299–304. [Google Scholar] [CrossRef]
- Galinari, É.; Almeida-Lima, J.; Macedo, G.R.; Mantovani, H.C.; Rocha, H.A.O. Antioxidant, antiproliferative, and immunostimulatory effects of cell wall α-D-mannan fractions from Kluyveromyces marxianus. Int. J. Biol. Macromol. 2018, 109, 837–846. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Anton, A. Colorimetric estimation of aluminum with pyrocatechol violet. Anal. Chem. 1960, 32, 725–726. [Google Scholar] [CrossRef]
- Chaves Filho, G.P.; de Sousa, A.F.G.; Câmara, R.B.G.; Rocha, H.A.O.; de Medeiros, S.R.B.; Moreira, S.M.G. Genotoxicity and osteogenic potential of sulfated polysaccharides from Caulerpa prolifera seaweed. Int. J. Biol. Macromol. 2018, 114, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Ulber, R. Fucoidan production: Approval key challenges and opportunities. Carbohydr. Polym. 2019, 211, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Barroso, E.M.A.; Costa, L.S.; Cordeiro, S.L.; Costa, M.S.S.P.; Franco, C.R.C.; Nader, H.B.; Leite, E.L.; Rocha, H.A.O. A non anticoagulant heterofucan has antithrombotic activity in vivo. Planta Med. 2008, 74, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, K.D.; Costa, L.S.; Fidelis, G.P.; Oliveira, R.M.; Nobre, L.T.D.B.; Dantas-Santos, N.; Câmara, R.B.G.; Albuquerque, I.R.L.; Cordeiro, S.L.; Sabry, D.A.; et al. Anticoagulant, antioxidant and antitumor activities of heterofucans from the seaweed Dictyopteris delicatula. Int. J. Mol. Sci. 2011, 12, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, I.R.L.; Cordeiro, S.L.; Gomes, D.L.; Dreyfuss, J.L.; Filgueira, L.G.A.; Leite, E.L.; Nader, H.B.; Rocha, H.A.O. Evaluation of anti-nociceptive and anti-inflammatory activities of a heterofucan from Dictyota menstrualis. Mar. Drugs 2013, 11, 2722–2740. [Google Scholar] [CrossRef]
- Câmara, R.B.G.; Costa, L.S.; Fidelis, G.P.; Nobre, L.T.D.B.; Dantas-Santos, N.; Cordeiro, S.L.; Costa, M.S.S.P.; Alves, L.G.; Rocha, H.A.O. Heterofucans from the Brown Seaweed Canistrocarpus cervicornis with Anticoagulant and Antioxidant Activities. Mar. Drugs 2011, 9, 124–138. [Google Scholar] [CrossRef]
- Athukorala, Y.; Kim, K.N.; Jeon, Y.J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Moure, A.; Falqué, E.; Domínguez, H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013, 132, 1764–1785. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Yin, Z.; Qu, G.; Wang, C. Fucoidan and Its Health Benefits. In Bioactive Seaweeds for Food Applications; Academic Press: Cambridge, MA, USA, 2018; pp. 223–238. [Google Scholar]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.C.R.; Marques, C.T.; Dore, C.M.G.; Silva, F.R.F.; Rocha, H.A.O. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ozgocmen, S.; Kaya, H.; Fadillioglu, E.; Aydogan, R.; Yilmaz, Z. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol. Cell. Biochem. 2007, 295, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Terruzzi, I.; Montesano, A.; Senesi, P.; Villa, I.; Ferraretto, A.; Bottani, M.; Vacante, F.; Spinello, A.; Bolamperti, S.; Luzi, L.; et al. L-Carnitine reduces oxidative stress and promotes cells differentiation and bone matrix proteins expression in human osteoblast-like cells. BioMed Res. Int. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Chae, H.J.; Park, S.Y.; Kim, J.H. Porcine placenta hydrolysates enhance osteoblast differentiation through their antioxidant activity and effects on ER stress. BMC Complement. Altern. Med. 2016, 16, 291. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Ochi, M.; Ono, M.; Aoyama, E.; Ogino, T.; Kondo, Y.; Ohuchi, H. Glutathione accelerates osteoclast differentiation and inflammatory bone destruction. Free Radic. Res. 2019, 53, 226–236. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Alves, M.G.D.C.F.; Will, L.S.E.P.; Costa, T.G.; Sabry, D.A.; de Souza Rêgo, L.A.R.; Accardo, C.M.; Rocha, H.A.O.; Filgueira, L.G.A.; Leite, E.L. A sulfated polysaccharide, fucans, isolated from brown algae Sargassum vulgare with anticoagulant, antithrombotic, antioxidant and anti-inflammatory effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209. [Google Scholar] [CrossRef]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
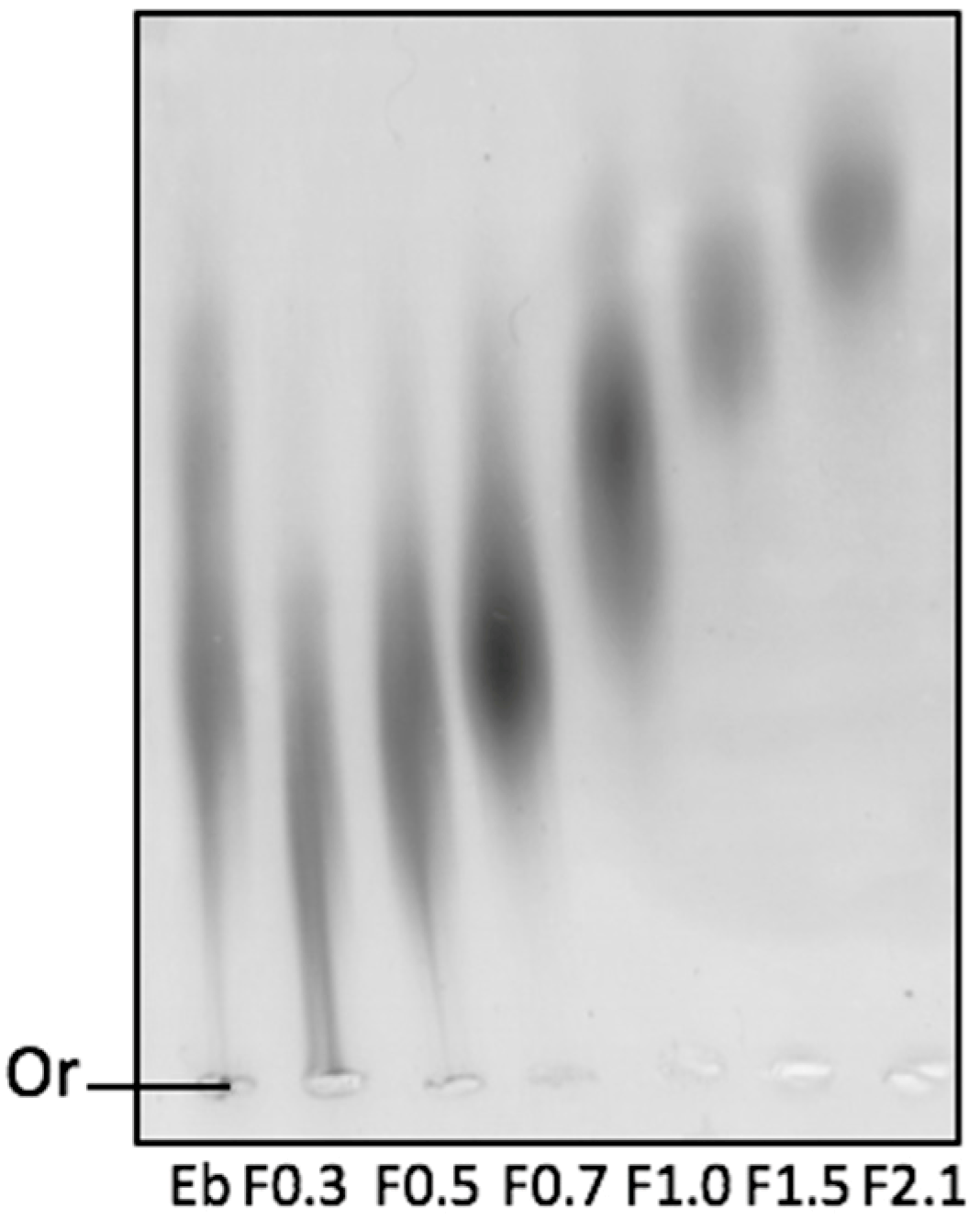



| FRF | Yield (%) | Proteins (%) | Sulfate Content (%) | Molar Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| Fuc | Glu | Man | Gal | Xyl | ||||
| F0.3 | 19.1 | nd | 1.50 | 1.00 | 0.70 | 0.30 | 1.46 | 0.22 |
| F0.5 | 24.1 | 0.06 | 4.70 | 1.00 | 2.02 | 1.02 | 1.79 | 0.49 |
| F0.7 | 18.2 | 0.04 | 7.80 | 1.00 | 0.24 | 0.26 | 1.78 | 0.68 |
| F1.0 | 8.9 | 0.02 | 6.30 | 1.00 | 1.23 | 1.01 | 5.01 | 1.80 |
| F1.5 | 21.1 | 0.00 | 6.40 | 1.00 | 2.11 | 0.68 | 4.10 | 0.71 |
| F2.1 | 8.8 | 0.00 | 1.20 | 1.00 | 1.56 | 0.00 | 7.26 | 0.52 |
| Concentration (mg/mL) | Scavenging % | ||
|---|---|---|---|
| OH. | O2 | ||
| F0.3 | 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| F0.5 | 0.5 | 1.2 ± 1.7 | 0.5 ± 1.1 |
| F0.7 | 0.5 | 9.0 ± 1.4 * | 10.7 ± 1.7 * |
| F1.0 | 0.5 | 0.2 ± 0.9 | 1.0 ± 1.7 |
| F1.5 | 0.5 | 0.9 ± 2.3 | 1.3 ± 2.0 |
| F2.1 | 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Effect of FRF (0.5 mg/mL) on Total Superoxide Dismutase Levels (% of Control) of MC3T3 Cells Exposed to Oxidative Damage | |||
|---|---|---|---|
| . | . | FRF | FRF + H2O2 |
| NC | 100 ± 1.1% | ||
| H2O2 | 52.2 ± 1.1% *** | ||
| F0.3 | 101.8 ± 2.1% | 53.9 ± 0.5% *** | |
| F0.5 | 101.9 ± 1.0% | 74.7 ± 1.1% ** | |
| F0.7 | 104.2 ± 2.4% | 88.8 ± 2.5% * | |
| F1.0 | 105.8 ± 3.7% | 52.9 ± 2.8% *** | |
| F1.5 | 99.7 ± 0.7% | 86.3 ± 2.8% * | |
| F2.1 | 95.0 ± 3.7% | 87.0 ± 0.8% * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidelis, G.P.; Silva, C.H.F.; Nobre, L.T.D.B.; Medeiros, V.P.; Rocha, H.A.O.; Costa, L.S. Antioxidant Fucoidans Obtained from Tropical Seaweed Protect Pre-Osteoblastic Cells from Hydrogen Peroxide-Induced Damage. Mar. Drugs 2019, 17, 506. https://doi.org/10.3390/md17090506
Fidelis GP, Silva CHF, Nobre LTDB, Medeiros VP, Rocha HAO, Costa LS. Antioxidant Fucoidans Obtained from Tropical Seaweed Protect Pre-Osteoblastic Cells from Hydrogen Peroxide-Induced Damage. Marine Drugs. 2019; 17(9):506. https://doi.org/10.3390/md17090506
Chicago/Turabian StyleFidelis, Gabriel Pereira, Cynthia Haynara Ferreira Silva, Leonardo Thiago Duarte Barreto Nobre, Valquíria Pereira Medeiros, Hugo Alexandre Oliveira Rocha, and Leandro Silva Costa. 2019. "Antioxidant Fucoidans Obtained from Tropical Seaweed Protect Pre-Osteoblastic Cells from Hydrogen Peroxide-Induced Damage" Marine Drugs 17, no. 9: 506. https://doi.org/10.3390/md17090506
APA StyleFidelis, G. P., Silva, C. H. F., Nobre, L. T. D. B., Medeiros, V. P., Rocha, H. A. O., & Costa, L. S. (2019). Antioxidant Fucoidans Obtained from Tropical Seaweed Protect Pre-Osteoblastic Cells from Hydrogen Peroxide-Induced Damage. Marine Drugs, 17(9), 506. https://doi.org/10.3390/md17090506





