Bioactive Lactones from the Mangrove-Derived Fungus Penicillium sp. TGM112
Abstract
:1. Introduction
2. Results and Discussion
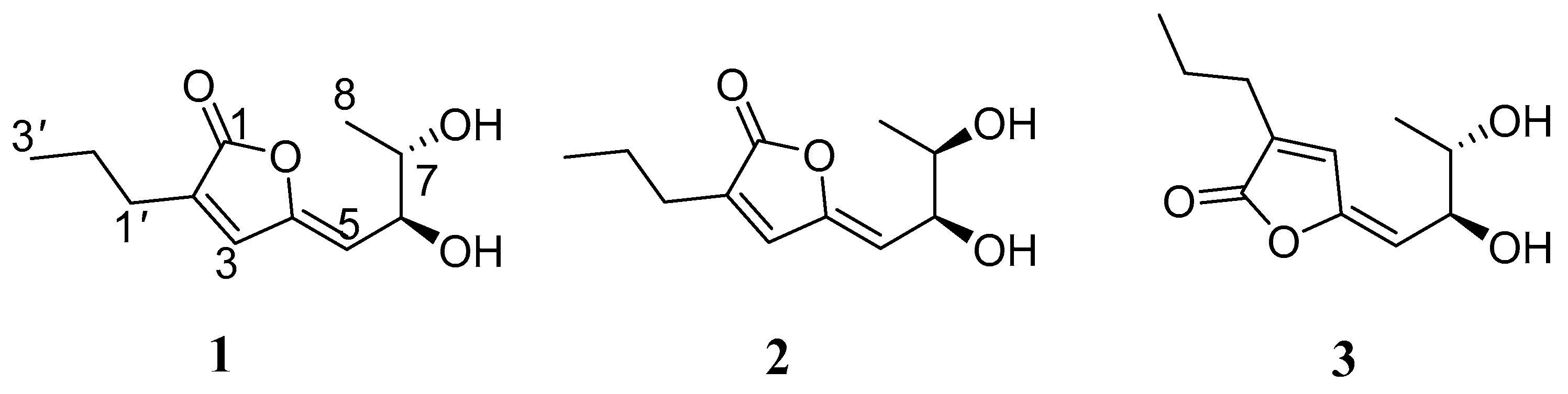
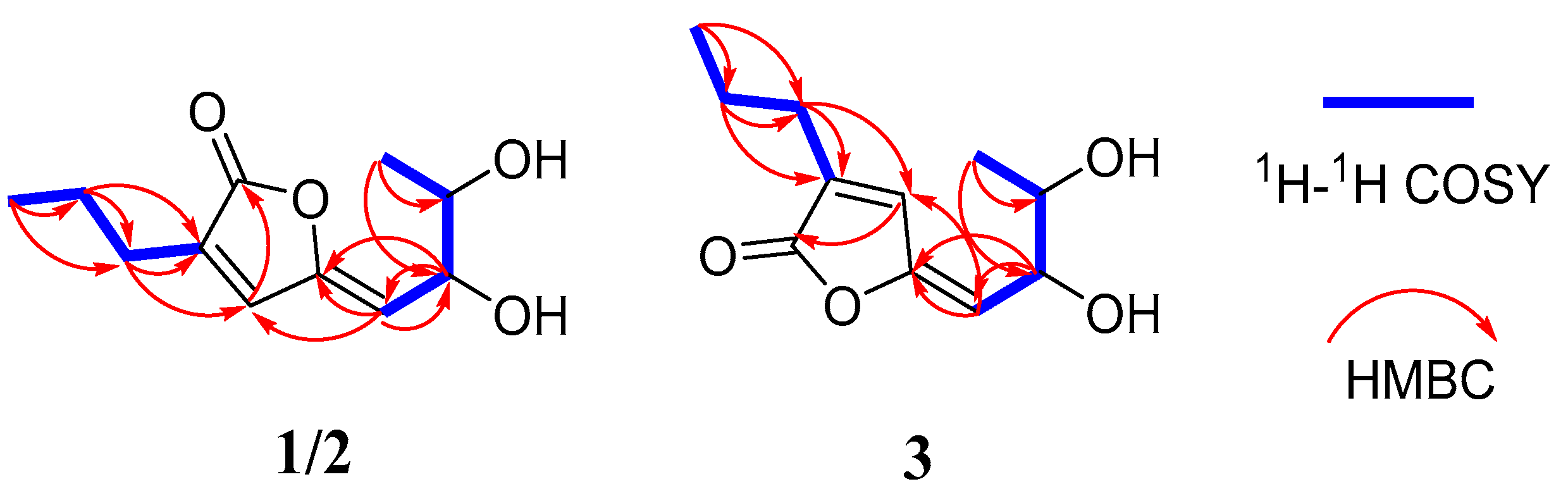
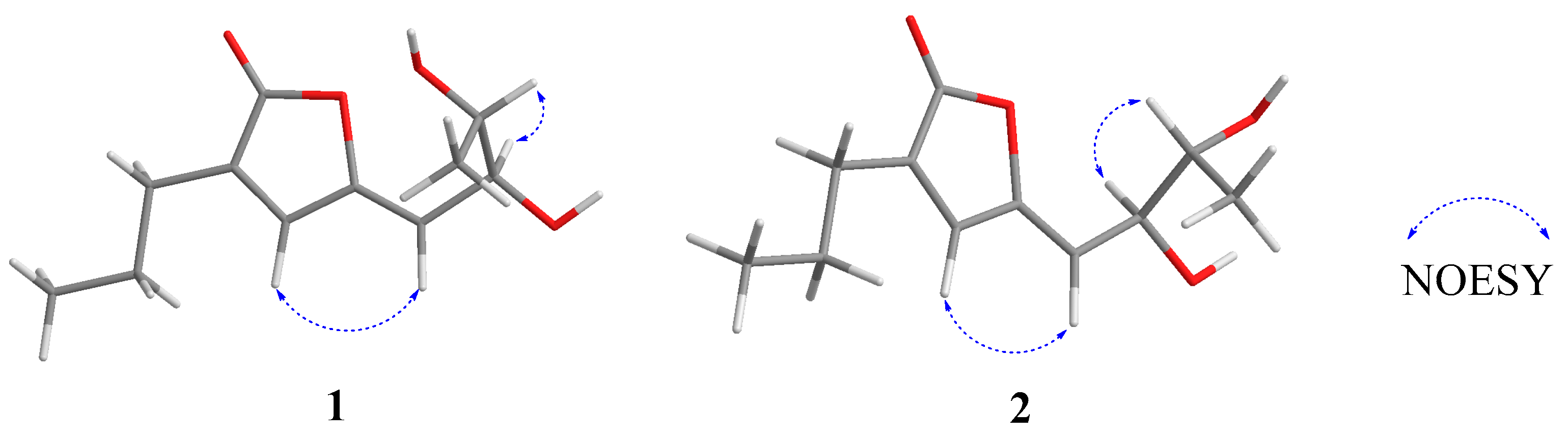
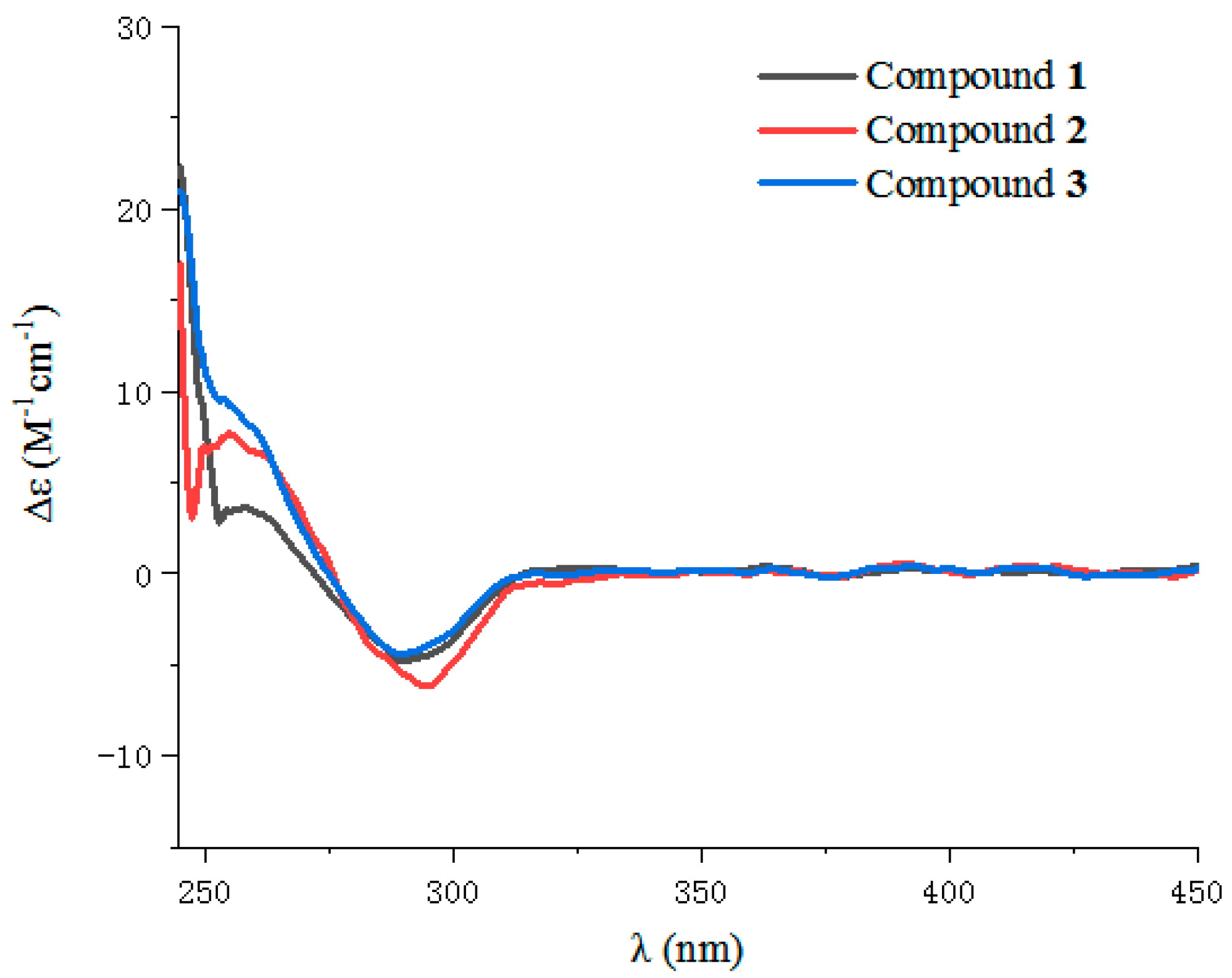
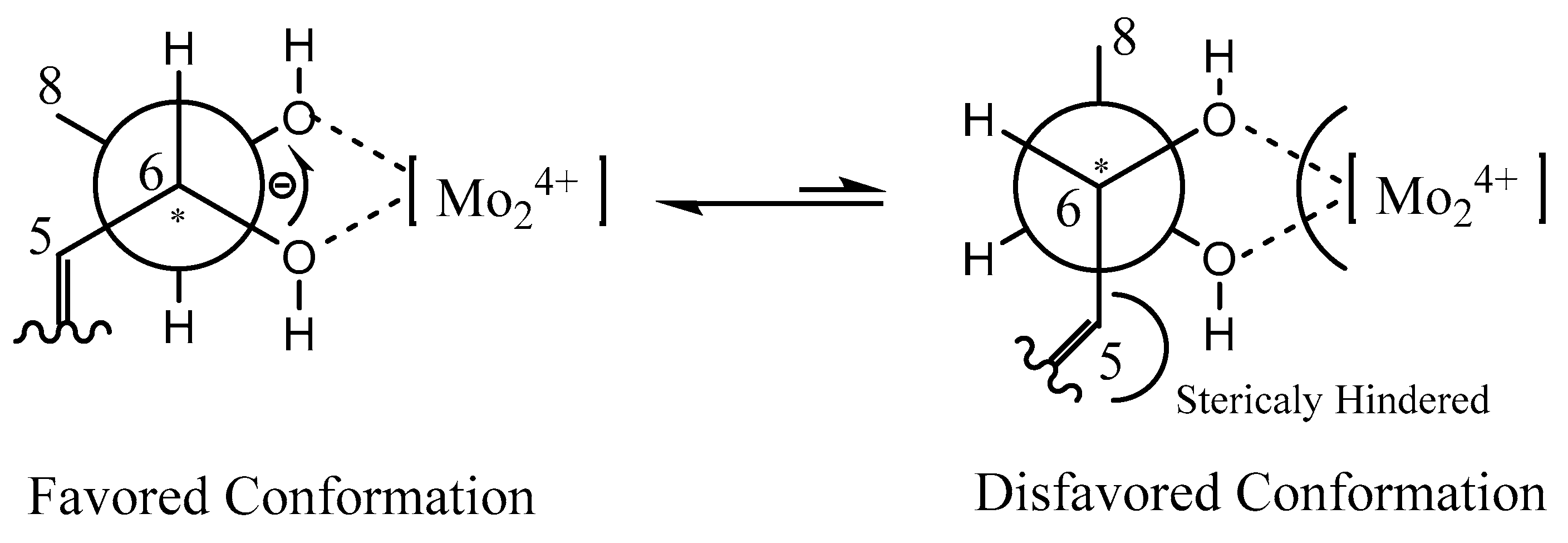
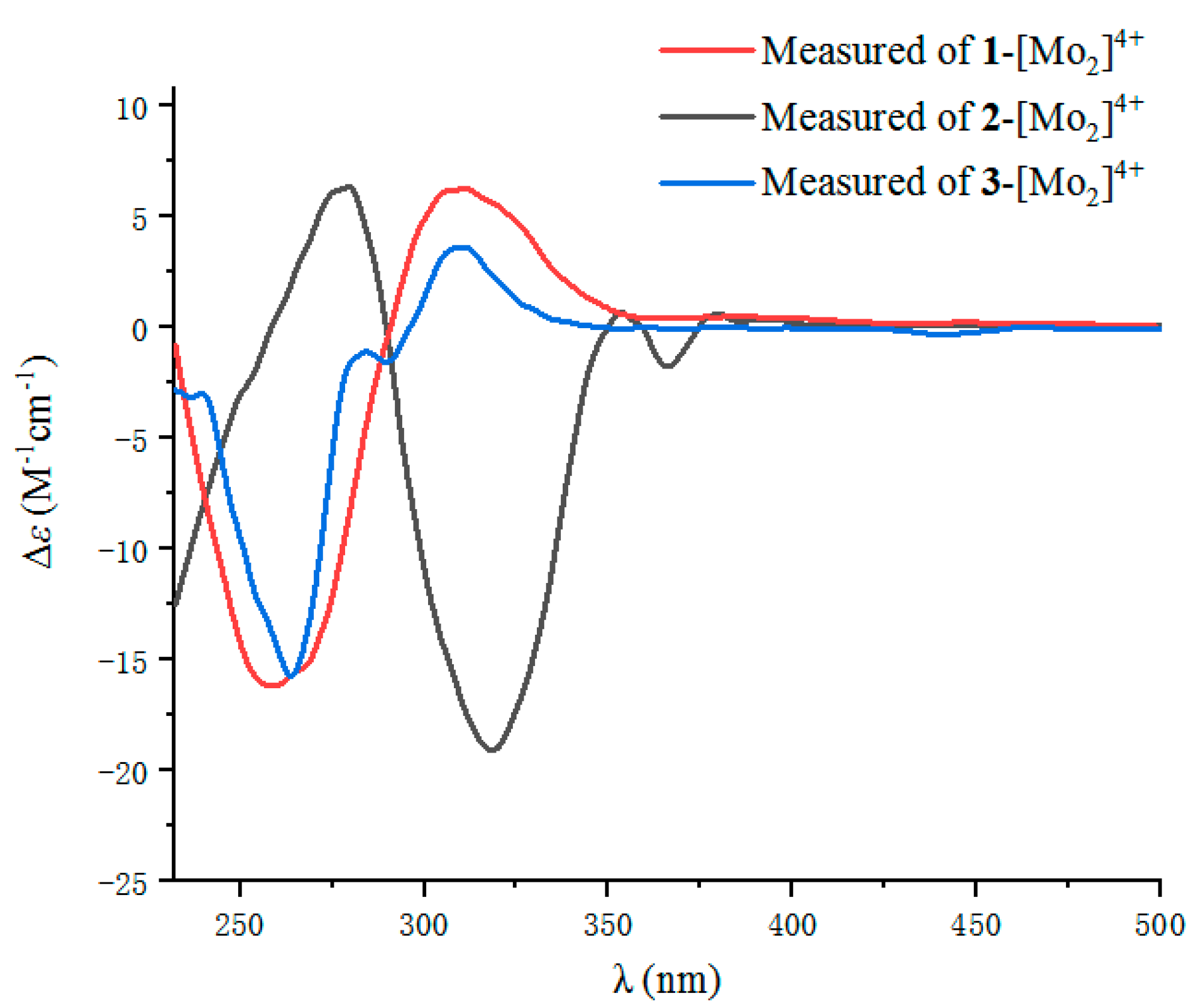
2.1. Structure Elucidation
2.2. Biological Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Materials
3.3. Extraction and Isolation
3.4. Computational Section
3.5. Antibacterial Activities
3.6. Insecticidal Activities against Newly Hatched Larvae of Culex Quinquefasciatus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yu, G.; Wang, Q.; Liu, S.; Zhang, X.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Methylsulfonylated Polyketides Produced by Neosartorya udagawae HDN13-313 via Exogenous Addition of Small Molecules. J. Nat. Prod. 2019, 82, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, L.-H.; Mándi, A.; Kurtán, T.; Li, X.-M.; Liu, Y.; Li, X.; Li, C.-S.; Wang, B.-G. Brocaeloids A-C, 4-Oxoquinoline and Indole Alkaloids with C-2 Reversed Prenylation from the Mangrove-Derived Endophytic Fungus Penicillium brocae. Eur. J. Org. Chem. 2014, 2014, 4029–4036. [Google Scholar] [CrossRef]
- Fang, X.-P.; Anderson, J.E.; Chang, C.-J.; McLaughlin, J.L. Three new bioactive styryllactones from Goniothalamus giganteus (Annonaceae). Tetrahedron 1991, 47, 9751–9758. [Google Scholar] [CrossRef]
- Findlay, J.A.; Miller, J.D.; Womiloju, T.O.; Li, G. Insect toxins from spruce endophytes. Can. J. Chem. 2003, 81, 284–292. [Google Scholar] [CrossRef]
- Zhuang, Y.; Teng, X.; Wang, Y.; Liu, P.; Wang, H.; Li, J.; Li, G.; Zhu, W. Cyclopeptides and polyketides from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Tetrahedron 2011, 67, 7085–7089. [Google Scholar] [CrossRef]
- Wang, L.J.; Luo, G.D.; Ma, Z.J.; Liu, X.M. Chemical constituents with antionxident activities from litchi (Litchi chinensis sonn.) seeds. Food Chem. 2011, 126, 1081–1087. [Google Scholar] [CrossRef]
- Wang, K.; Bao, L.; Ma, K.; Qi, W.; Song, F.H.; Yao, Y.J.; Yin, W.B.; Zhang, L.X.; Huang, Y.; Han, J.J.; et al. Bioactive spirobisnaphthalenes and lactones from a cup fungus Plectania sp. collected in the Tibet Platesu Region. Eur. J. Org. Chem. 2016, 25, 4338–4346. [Google Scholar] [CrossRef]
- Xu, D.Q.; Sharpless, K.B. Synthesis and stereochemical assignments for Goniobutenolodes A and B. Tetrahedron Lett. 1994, 35, 4685–4688. [Google Scholar] [CrossRef]
- Wang, L.P.; Zhu, W.M. Versicolactones A and B: Total synthesis and structure revision. Tetrahedron Lett. 2013, 54, 6729–6731. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Bai, M.; Zhou, X.-M.; Huang, G.-L.; Shao, T.-M.; Luo, Y.-P.; Niu, Z.-G.; Niu, Y.-Y.; Chen, G.-Y.; Han, C.-R. Penicilindoles A–C, Cytotoxic Indole Diterpenes from the Mangrove-Derived Fungus Eupenicillium sp. HJ002. J. Nat. Prod. 2018, 81, 1045–1049. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Li, G.-N.; Zheng, C.-J.; Chen, G.-Y.; Song, X.-P.; Han, C.-R.; Fu, Y.-H.; Chen, W.-H.; Niu, Z.-G. Bioactive Anthraquinone Derivatives from the Mangrove-Derived Fungus Stemphylium sp. 33231. J. Nat. Prod. 2014, 77, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-L.; Zhou, X.-M.; Bai, M.; Liu, Y.-X.; Zhao, Y.-L.; Luo, Y.-P.; Niu, Y.-Y.; Zheng, C.-J.; Chen, G.-Y. Dihydroisocoumarins from the Mangrove-Derived Fungus Penicillium citrinum. Mar. Drugs 2016, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Huang, G.; Wang, B.; Bai, M.; Luo, Y.; Chen, G.; Zheng, C. Two New Isocoumarins Isolated from a Mangrove-Derived Fungus Penicillium citrinum HL-5126. Chin. J. Org. Chem. 2019, 39, 1479–1482. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.-J.; Huang, G.-L.; Mei, R.-Q.; Wang, B.; Luo, Y.-P.; Zheng, C.; Niu, Z.-G.; Chen, G.-Y. Bioactive Meroterpenoids and Isocoumarins from the Mangrove-Derived Fungus Penicillium sp. TGM112. J. Nat. Prod. 2019, 82, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Snatzke, G.; Wagner, U.; Wolff, H.P. Cirulardichroism-LXXV 1: Cottonogenic derivatives of chiral bidentate ligands with the comples [Mo2(OAc)4]. Tetrahedron 1981, 37, 349–361. [Google Scholar] [CrossRef]
- Di Bari, L.; Pescitelli, G.; Pratelli, C.; Pini, D.; Salvadori, P. Determination of absolute configuration of acyclic 1,2-diols with Mo2(OAc)4. 1. Snatzke′s method revisited. J. Org. Chem. 2001, 66, 4819–4825. [Google Scholar] [CrossRef]
- Dong, S.-H.; Zhang, C.-R.; Dong, L.; Wu, Y.; Yue, J.-M. Onoceranoid-Type Triterpenoids fromLansium domesticum. J. Nat. Prod. 2011, 74, 1042–1048. [Google Scholar] [CrossRef]
- Zhang, L.-B.; Ji, J.; Lei, C.; Wang, H.-Y.; Zhao, Q.-S.; Hou, A.-J. Isoprenylated Flavonoid and Adipogenesis-Promoting Constituents of Dodonaea viscosa. J. Nat. Prod. 2012, 75, 699–706. [Google Scholar] [CrossRef]
- Gorecki, M.; Kaminska, A.; Ruskowska, P.; Suszczynska, A.; Frelek, J. Dimolybdenum method for determination of the absolute configuration of vic-diols-foundations and developments. Pol. J. Chem. 2006, 80, 523–534. [Google Scholar]
- Elnaggar, M.S.; Ebrahim, W.; Mándi, A.; Kurtán, T.; Müller, W.E.G.; Kalscheuer, R.; Singab, A.; Lin, W.; Liu, Z.; Proksch, P. Hydroquinone derivatives from the marine-derived fungus Gliomastix sp. RSC Adv. 2017, 7, 30640–30649. [Google Scholar] [CrossRef]
- Ren, J.; Ding, S.-S.; Zhu, A.; Cao, F.; Zhu, H.-J. Bioactive Azaphilone Derivatives from the Fungus Talaromyces aculeatus. J. Nat. Prod. 2017, 80, 2199–2203. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Crickmore, N.; George, Z.; Xie, L.; He, Y.-Q.; Li, Y.; Tang, J.-L.; Tian, L.; Wang, X.; Fang, X. Characterization of a new highly mosquitocidal isolate of Bacillus thuringiensis—An alternative to Bti? J. Invertebr. Pathol. 2012, 109, 217–222. [Google Scholar] [CrossRef] [PubMed]
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) | |
| 1 | 171.9, C | 172.0, C | 172.1, C | |||
| 2 | 135.9, C | 135.7, C | 136.0, C | |||
| 3 | 139.0, CH | 7.31, s | 139.0, CH | 7.32, s | 136.4, CH | 7.66, s |
| 4 | 150.6, C | 150.6, C | 152.0, C | |||
| 5 | 113.9, CH | 5.30, d (9.2) | 113.6, CH | 5.33, d (9.2) | 113.7, CH | 5.68, d (8.8) |
| 6 | 71.7, CH | 4.45, dd (6.0, 9.2) | 71.5, CH | 4.51, dd (4.4, 9.2) | 72.7, CH | 4.27, dd (5.2, 8.8) |
| 7 | 71.5, CH | 3.69, m | 71.4, CH | 3.80, m | 71.6, CH | 3.75, m |
| 8 | 18.8, CH3 | 1.13, d (6.8) | 18.8, CH3 | 1.15, d (6.4) | 19.1, CH3 | 1.17, d (6.4) |
| 1′ | 28.0, CH2 | 2.31, t (6.8) | 28.0, CH2 | 2.32, t (6.8) | 28.2, CH2 | 2.33, t (6.8) |
| 2′ | 21.9, CH2 | 1.60, m | 21.9, CH2 | 1.62, m | 21.9, CH2 | 1.62, m |
| 3′ | 13.9, CH3 | 0.98, t (7.2) | 13.9, CH3 | 0.98, t (7.2) | 13.9, CH3 | 0.98, t (7.2) |
| Compounds | MIC (µg/mL) | LC50 (µg/mL) |
|---|---|---|
| S. aureus | C. quinquefasciatus | |
| 1 | 6.25 | >80 |
| 2 | >20.0 | 78.5 (±0.58) |
| 3 | >20.0 | >80 |
| Ciprofloxacin a | 0.39 | |
| Azadirachtin b | 9.8 (±0.58) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, M.; Huang, G.-L.; Mei, R.-Q.; Wang, B.; Luo, Y.-P.; Nong, X.-H.; Chen, G.-Y.; Zheng, C.-J. Bioactive Lactones from the Mangrove-Derived Fungus Penicillium sp. TGM112. Mar. Drugs 2019, 17, 433. https://doi.org/10.3390/md17080433
Bai M, Huang G-L, Mei R-Q, Wang B, Luo Y-P, Nong X-H, Chen G-Y, Zheng C-J. Bioactive Lactones from the Mangrove-Derived Fungus Penicillium sp. TGM112. Marine Drugs. 2019; 17(8):433. https://doi.org/10.3390/md17080433
Chicago/Turabian StyleBai, Meng, Guo-Lei Huang, Rong-Qing Mei, Bin Wang, You-Ping Luo, Xu-Hua Nong, Guang-Ying Chen, and Cai-Juan Zheng. 2019. "Bioactive Lactones from the Mangrove-Derived Fungus Penicillium sp. TGM112" Marine Drugs 17, no. 8: 433. https://doi.org/10.3390/md17080433
APA StyleBai, M., Huang, G.-L., Mei, R.-Q., Wang, B., Luo, Y.-P., Nong, X.-H., Chen, G.-Y., & Zheng, C.-J. (2019). Bioactive Lactones from the Mangrove-Derived Fungus Penicillium sp. TGM112. Marine Drugs, 17(8), 433. https://doi.org/10.3390/md17080433




