Marine Biosurfactants: Biosynthesis, Structural Diversity and Biotechnological Applications
Abstract
1. Introduction
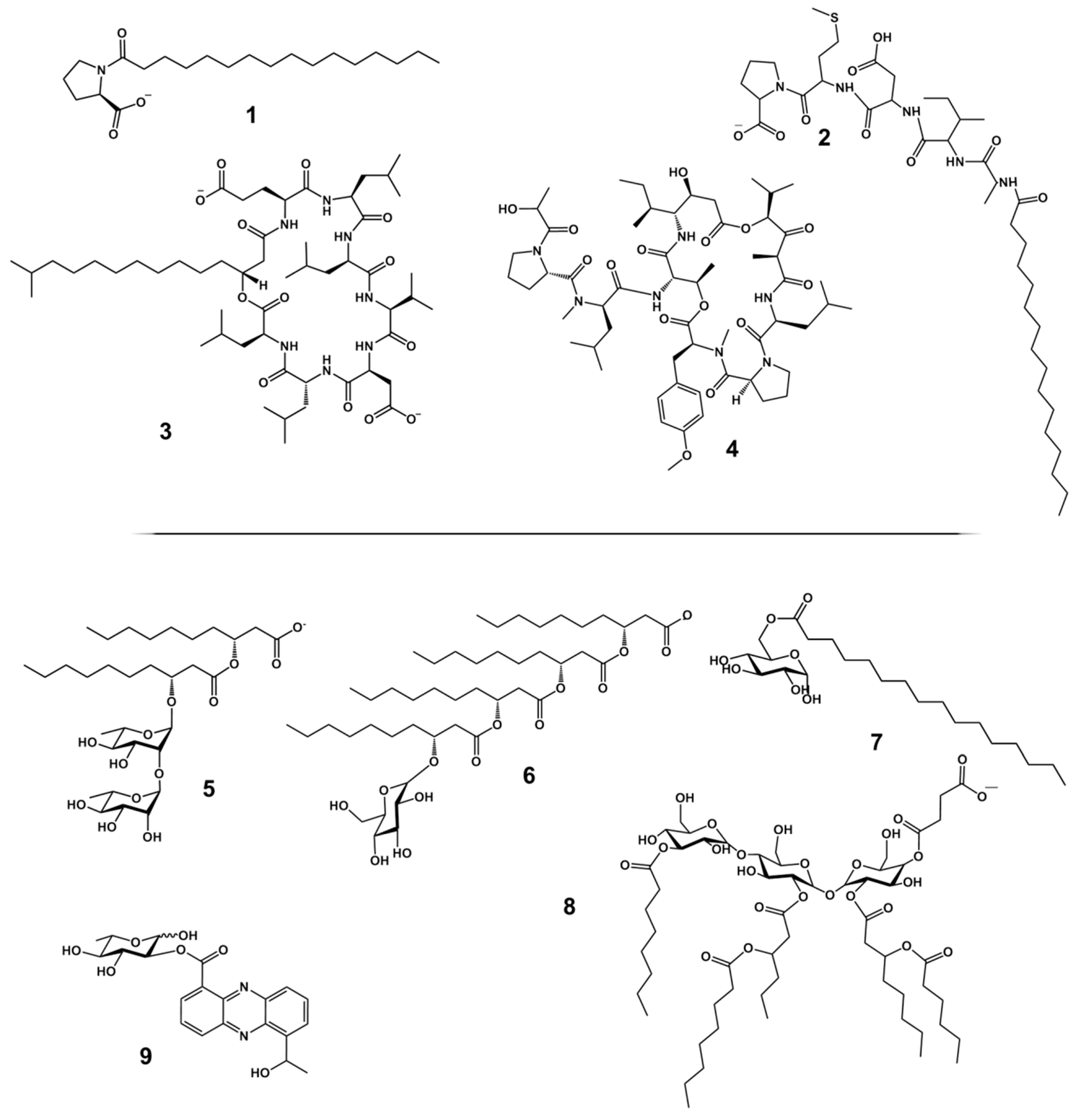
2. Structural Diversity of Biosurfactants
2.1. High Molecular Weight Polymeric Biosurfactants/Bioemulsifiers
2.2. Low Molecular Weight Biosurfactants
3. LMW Biosurfactants from Marine Sources
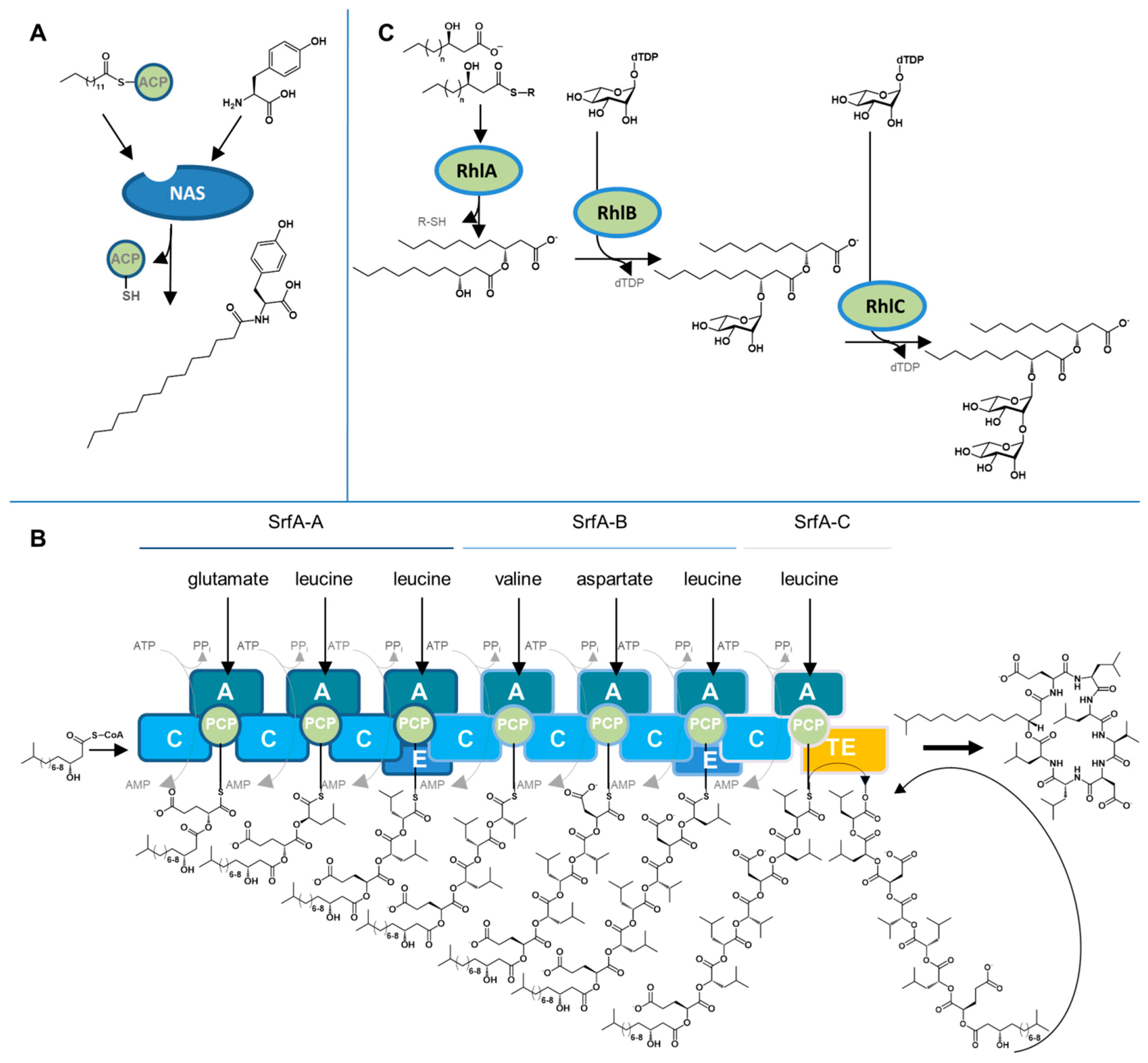
4. Biosynthetic Mechanisms
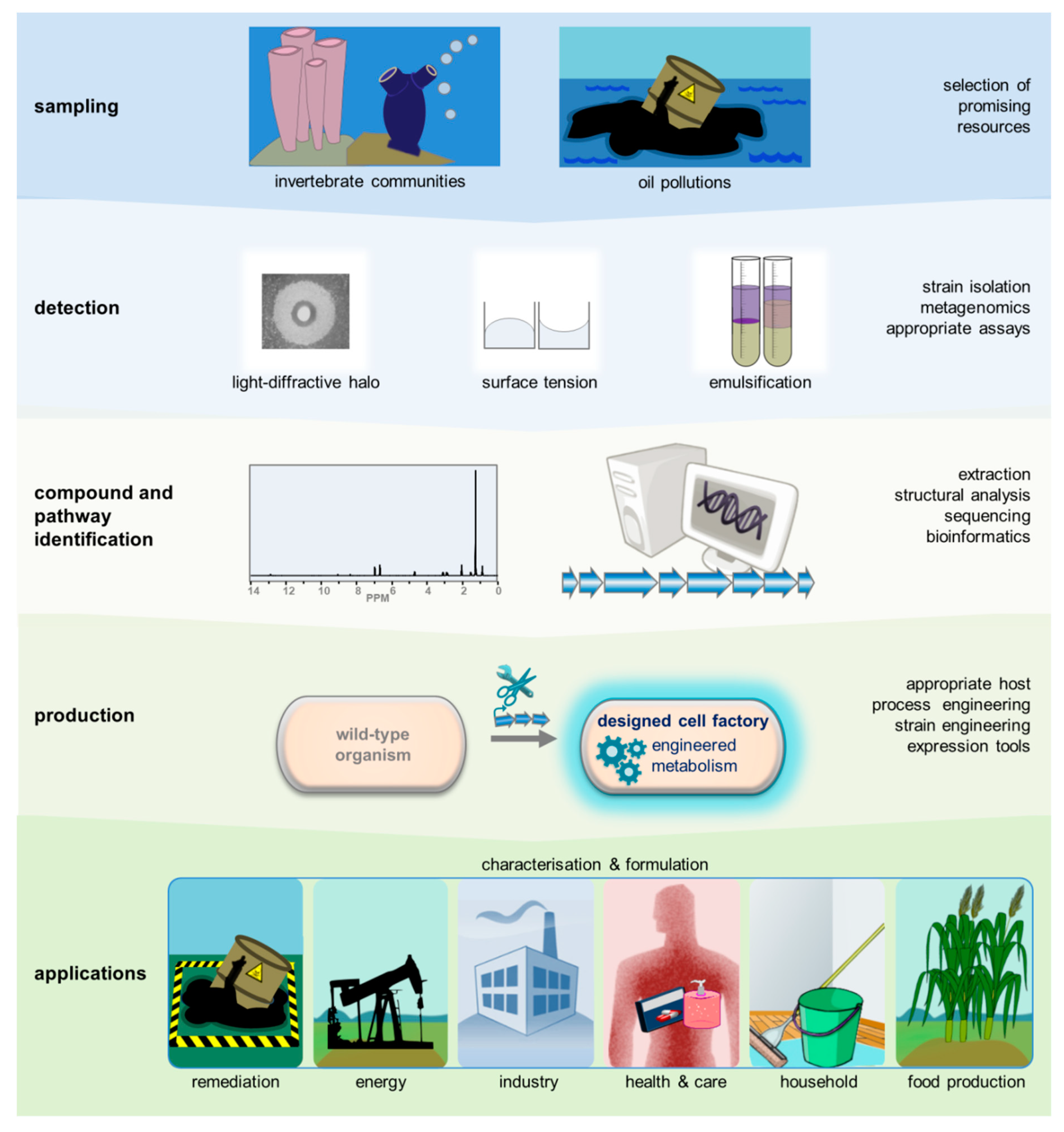
5. Prospecting for Novel Biosurfactants
6. Applications of Biosurfactants
6.1. Medical Applications
6.2. Food
6.3. Consumer Products
6.4. Bioremediation
6.5. Enhanced Recovery of Fossil Resources
6.6. Industrial Processes
7. Perspectives for the Biotechnological Exploitation of Marine Biosurfactants
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar] [PubMed]
- Ron, E.Z.; Rosenberg, E. Natural roles of biosurfactants. Environ. Microbiol. 2001, 3, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, R.; Syldatk, C. Types and classification of microbial surfactants. In Biosurfactants: Production and Utilization—Processes, Technologies, and Economics; Kosaric, N., Varder-Sukan, F., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 3–18. ISBN 978-1466596696. [Google Scholar]
- Bhadoriya, S.S.; Madoriya, N. Biosurfactants: A new pharmaceutical additive for solubility enhancement and pharmaceutical development. Biochem. Pharmacol. Open Access 2013, 2, 113. [Google Scholar] [CrossRef]
- Poremba, K.; Gunkel, W.; Lang, S.; Wagner, F. Toxicity testing of synthetic and biogenic surfactants on marine microorganisms. Environ. Toxicol. Water Qual. 1991, 6, 157–163. [Google Scholar] [CrossRef]
- Johann, S.; Seiler, T.-B.; Tiso, T.; Bluhm, K.; Blank, L.M.; Hollert, H. Mechanism-specific and whole-organism ecotoxicity of mono-rhamnolipids. Sci. Total Environ. 2016, 548–549, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Fenical, W. Strategies for the discovery of secondary metabolites from marine bacteria: Ecological Perspectives. Annu. Rev. Microbiol. 1994, 48, 559–584. [Google Scholar] [CrossRef]
- Kennedy, J.; O’Leary, N.D.; Kiran, G.S.; Morrissey, J.P.; O’Gara, F.; Selvin, J.; Dobson, A.D. Functional metagenomic strategies for the discovery of novel enzymes and biosurfactants with biotechnological applications from marine ecosystems. J. Appl. Microbiol. 2011, 111, 787–799. [Google Scholar] [CrossRef]
- Romanenko, L.A.; Uchino, M.; Tebo, B.M.; Tanaka, N.; Frolova, G.M.; Mikhailov, V.V. Pseudomonas marincola sp. nov., isolated from marine environments. Int. J. Syst. Evol. Microbiol. 2008, 58, 706–710. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Ron, E.Z. High- and low-molecular-mass microbial surfactants. Appl. Microbiol. Biotechnol. 1999, 52, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Soberón-Chávez, G.; Maier, R. Biosurfactants: A general overview. In Biosurfactants; Soberón-Chávez, G., Ed.; Microbiology Monographs; Springer: Berlin/Heidelberg, Germany, 2011; Volume 20, pp. 1–11. ISBN 978-3-642-14489-9. [Google Scholar]
- Uzoigwe, C.; Burgess, J.G.; Ennis, C.J.; Rahman, P.K.S.M. Bioemulsifiers are not biosurfactants and require different screening approaches. Front. Microbiol. 2015, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Ron, E.Z. Bioemulsans: Microbial polymeric emulsifiers. Curr. Opin. Biotechnol. 1997, 8, 313–316. [Google Scholar] [CrossRef]
- Sar, N.; Rosenberg, E. Emulsifier production by Acinetobacter calcoaceticus strains. Curr. Microbiol. 1983, 9, 309–313. [Google Scholar] [CrossRef]
- Zuckerberg, A.; Diver, A.; Peeri, Z.; Gutnick, D.L.; Rosenberg, E. Emulsifier of Arthrobacter RAG-1: Chemical and physical properties. Appl. Environ. Microbiol. 1979, 37, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Nakar, D.; Gutnick, D.L. Analysis of the wee gene cluster responsible for the biosynthesis of the polymeric bioemulsifier from the oil-degrading strain Acinetobacter lwoffii RAG-1. Microbiology 2001, 147, 1937–1946. [Google Scholar] [CrossRef]
- Cooper, A.; Kennedy, M.W. Biofoams and natural protein surfactants. Biophys. Chem. 2010, 151, 96–104. [Google Scholar] [CrossRef]
- Linder, M.B. Hydrophobins: Proteins that self assemble at interfaces. Curr. Opin. Colloid Interface Sci. 2009, 14, 356–363. [Google Scholar] [CrossRef]
- Cox, P.W.; Hooley, P. Hydrophobins: New prospects for biotechnology. Fungal Biol. Rev. 2009, 23, 40–47. [Google Scholar] [CrossRef]
- Fujii, T.; Yuasa, R.; Kawase, T.; Biodetergent, I.V. Monolayers of corynomycolic acids at the air-water interface. Colloid Polym. Sci. 1999, 277, 334–339. [Google Scholar] [CrossRef]
- Käppeli, O.; Finnerty, W.R. Characteristics of hexadecane partition by the growth medium of Acinetobacter sp. Biotechnol. Bioeng. 1980, 22, 495–503. [Google Scholar] [CrossRef]
- Kretschmer, A.; Bock, H.; Wagner, F. Chemical and physical characterization of interfacial-active lipids from Rhodococcus erythropolis grown on n-alkanes. Appl. Environ. Microbiol. 1982, 44, 864–870. [Google Scholar] [PubMed]
- Tahara, Y.; Kameda, M.; Yamada, Y.; Kondo, K. A new lipid; the ornithine and taurine-containing “cerilipin.”. Agric. Biol. Chem. 1976, 40, 243–244. [Google Scholar] [CrossRef]
- Kishimoto, N.; Adachi, K.; Tamura, S.; Nishihara, M.; Inagaki, K.; Sugio, T.; Tano, T. Lipoamino acids isolated from Acidiphilium organovorum. Syst. Appl. Microbiol. 1993, 16, 17–21. [Google Scholar] [CrossRef]
- Thies, S.; Rausch, S.C.; Kovacic, F.; Schmidt-Thaler, A.; Wilhelm, S.; Rosenau, F.; Daniel, R.; Streit, W.; Pietruszka, J.; Jaeger, K.-E. Metagenomic discovery of novel enzymes and biosurfactants in a slaughterhouse biofilm microbial community. Sci. Rep. 2016, 6, 27035. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.; Kunorozva, L.; Klaiber, I.; Henkel, M.; Pfannstiel, J.; Van Zyl, L.J.; Hausmann, R.; Burger, A.; Trindade, M. Novel metagenome-derived ornithine lipids identified by functional screening for biosurfactants. Appl. Microbiol. Biotechnol. 2019, 1–13. [Google Scholar] [CrossRef]
- Baltz, R.H.; Miao, V.; Wrigley, S.K. Natural products to drugs: Daptomycin and related lipopeptide antibiotics. Nat. Prod. Rep. 2005, 22, 717–741. [Google Scholar] [CrossRef]
- Inès, M.; Dhouha, G. Lipopeptide surfactants: Production, recovery and pore forming capacity. Peptides 2015, 71, 100–112. [Google Scholar] [CrossRef]
- Tally, F.P.; DeBruin, M.F. Development of daptomycin for Gram-positive infections. J. Antimicrob. Chemother. 2000, 46, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E.W. Polymyxin: Alternative mechanisms of action and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P. Surfactin and other lipopeptides from Bacillus spp. In Biosurfactants; Soberón-Chávez, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 57–92. ISBN 978-3-642-14489-9. [Google Scholar]
- Yeh, M.-S.; Wei, Y.-H.; Chang, J.-S. Enhanced production of surfactin from Bacillus subtilis by addition of solid carriers. Biotechnol. Prog. 2008, 21, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, A.; Ashby, R.; Foglia, T.A.; Solaiman, D.K.Y. Analysis and characterization of sophorolipids by liquid chromatography with atmospheric pressure chemical ionization. Chromatographia 2001, 53, 673–677. [Google Scholar] [CrossRef]
- Roelants, S.L.K.W.; Ciesielska, K.; De Maeseneire, S.L.; Moens, H.; Everaert, B.; Verweire, S.; Denon, Q.; Vanlerberghe, B.; Van Bogaert, I.N.A.; Van der Meeren, P.; et al. Towards the industrialization of new biosurfactants: Biotechnological opportunities for the lactone esterase gene from Starmerella bombicola. Biotechnol. Bioeng. 2016, 113, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Production of glycolipid biosurfactants by basidiomycetous yeasts. Biotechnol. Appl. Biochem. 2009, 53, 39–49. [Google Scholar] [CrossRef]
- Feldbrügge, M.; Kellner, R.; Schipper, K. The biotechnological use and potential of plant pathogenic smut fungi. Appl. Microbiol. Biotechnol. 2013, 97, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Christova, N.; Stoineva, I. Trehalose biosurfactants. In Biosurfactants—Recent Trends and Applications; Mulligan, C.N., Sharma, S.K., Mudhoo, A., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 197–216. ISBN 9781466518230. [Google Scholar]
- Kügler, J.H.; Le Roes-Hill, M.; Syldatk, C.; Hausmann, R. Surfactants tailored by the class Actinobacteria. Front. Microbiol. 2015, 6, 212. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef]
- Déziel, E.; Lépine, F.; Milot, S.; Villemur, R. Mass spectrometry monitoring of rhamnolipids from a growing culture of Pseudomonas aeruginosa strain 57RP. Biochim. Biophys. Acta 2000, 1485, 145–152. [Google Scholar] [CrossRef]
- Wittgens, A.; Santiago-Schuebel, B.; Henkel, M.; Tiso, T.; Blank, L.M.; Hausmann, R.; Hofmann, D.; Wilhelm, S.; Jaeger, K.-E.; Rosenau, F. Heterologous production of long-chain rhamnolipids from Burkholderia glumae in Pseudomonas putida-a step forward to tailor-made rhamnolipids. Appl. Microbiol. Biotechnol. 2018, 102, 1229–1239. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Stephanopoulos, G. Simple glycolipids of microbes: Chemistry, biological activity and metabolic engineering. Synth. Syst. Biotechnol. 2018, 3, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Cameotra, S.S.; Makkar, R.S.; Kaur, J.; Mehta, S.K. Synthesis of biosurfactants and their advantages to microorganisms and mankind. In Advances in Experimental Medicine and Biology; Sen, R., Ed.; Springer: New York, NY, USA, 2010; Volume 672, pp. 261–280. ISBN 978-1-4419-5978-2. [Google Scholar]
- Gautam, K.K.; Tyagi, V.K. Microbial surfactants: A review. J. Oleo Sci. 2006, 55, 155–166. [Google Scholar] [CrossRef]
- Satpute, S.K.; Bhuyan, S.S.; Pardesi, K.R.; Mujumdar, S.S.; Dhakephalkar, P.K.; Shete, A.M.; Chopade, B.A. Molecular genetics of biosurfactant synthesis in microorganisms. In Biosurfactants. Advances in Experimental Medicine and Biology; Sen, R., Ed.; Springer: New York, NY, USA, 2010; Volume 672, pp. 14–41, ISBN 0065-2598 (Print) 0065-2598 (Linking). [Google Scholar]
- Schulz, D.; Passeri, A.; Schmidt, M.; Lang, S.; Wagner, F.; Wray, V.; Gunkel, W. Marine biosurfactants, I. Screening for biosurfactants among crude oil degrading marine microorganisms from the North Sea. Z. Naturforsch. C 1991, 46, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Passeri, A.; Lang, S.; Wagner, F.; Wray, V. Marine biosurfactants, II. Production and characterization of an anionic trehalose tetraester from the marine bacterium Arthrobacter sp. EK 1. Z. Naturforsch. C 1991, 46, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Poremba, K.; Gunkel, W.; Lang, S.; Wagner, F. Marine biosurfactants, III. Toxicity testing with marine microorganisms and comparison with synthetic surfactants. Z. Naturforsch. C 1991, 46, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Passeri, A.; Schmidt, M.; Haffner, T.; Wray, V.; Lang, S.; Wagner, F. Marine biosurfactants. IV. Production, characterization and biosynthesis of an anionic glucose lipid from the marine bacterial strain MM1. Appl. Microbiol. Biotechnol. 1992, 37, 281–286. [Google Scholar] [CrossRef]
- Tripathi, L.; Irorere, V.U.; Marchant, R.; Banat, I.M. Marine derived biosurfactants: A vast potential future resource. Biotechnol. Lett. 2018, 40, 1441–1457. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactants produced by marine microorganisms with therapeutic applications. Mar. Drugs 2016, 14, 38. [Google Scholar] [CrossRef]
- Das, P.; Mukherjee, S.; Sivapathasekaran, C.; Sen, R. Microbial surfactants of marine origin: Potentials and prospects. In Biosurfactants. Advances in Experimental Medicine and Biology; Sen, R., Ed.; Springer: New York, NY, USA, 2010; Volume 672, pp. 88–101. ISBN 9781441959782. [Google Scholar]
- Dinamarca, M.; Ibacache-Quiroga, C.; Ojeda, J.; Troncoso, J. Marine microbial biosurfactants: Biological functions and physical properties as the basis for innovations to prevent and treat infectious diseases in aquaculture. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; Volume 2, pp. 1135–1144. ISBN 978-84-942134-0-3. [Google Scholar]
- Perfumo, A.; Banat, I.M.; Marchant, R. Going green and cold: Biosurfactants from low-temperature environments to biotechnology applications. Trends Biotechnol. 2018, 36, 277–289. [Google Scholar] [CrossRef]
- Sałek, K.; Gutierrez, T. Surface-active biopolymers from marine bacteria for potential biotechnological applications. AIMS Microbiol. 2016, 2, 92–107. [Google Scholar] [CrossRef]
- Luepongpattana, S.; Thaniyavarn, J.; Morikawa, M. Production of massoia lactone by Aureobasidium pullulans YTP6-14 isolated from the Gulf of Thailand and its fragrant biosurfactant properties. J. Appl. Microbiol. 2017, 123, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Ibacache-Quiroga, C.; Ojeda, J.; Espinoza-Vergara, G.; Olivero, P.; Cuellar, M.; Dinamarca, M.A. The hydrocarbon-degrading marine bacterium Cobetia sp. strain MM1IDA2H-1 produces a biosurfactant that interferes with quorum sensing of fish pathogens by signal hijacking. Microb. Biotechnol. 2013, 6, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Kaneda, K.; Ishizuka, I.; Toida, T.; Yano, I. Surface-active novel glycolipid and linked 3-hydroxy fatty acids produced by Serratia rubidaea. J. Bacteriol. 1990, 172, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Maneerat, S.; Bamba, T.; Harada, K.; Kobayashi, A.; Yamada, H.; Kawai, F. A novel crude oil emulsifier excreted in the culture supernatant of a marine bacterium, Myroides sp. strain SM1. Appl. Microbiol. Biotechnol. 2006, 70, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Shao, Z. Isolation and characterization of a novel biosurfactant produced by hydrocarbon-degrading bacterium Alcanivorax dieselolei B-5. J. Appl. Microbiol. 2010, 108, 1207–1216. [Google Scholar] [CrossRef]
- Unás, J.H.; de Alexandria Santos, D.; Azevedo, E.B.; Nitschke, M. Brevibacterium luteolum biosurfactant: Production and structural characterization. Biocatal. Agric. Biotechnol. 2018, 13, 160–167. [Google Scholar] [CrossRef]
- Seghal Kiran, G.; Anto Thomas, T.; Selvin, J.; Sabarathnam, B.; Lipton, A.P. Optimization and characterization of a new lipopeptide biosurfactant produced by marine Brevibacterium aureum MSA13 in solid state culture. Bioresour. Technol. 2010, 101, 2389–2396. [Google Scholar] [CrossRef]
- Peng, F.; Wang, Y.; Sun, F.; Liu, Z.; Lai, Q.; Shao, Z. A novel lipopeptide produced by a Pacific Ocean deep-sea bacterium, Rhodococcus sp. TW53. J. Appl. Microbiol. 2008, 105, 698–705. [Google Scholar] [CrossRef]
- Gandhimathi, R.; Seghal Kiran, G.; Hema, T.A.; Selvin, J.; Rajeetha Raviji, T.; Shanmughapriya, S. Production and characterization of lipopeptide biosurfactant by a sponge-associated marine actinomycetes Nocardiopsis alba MSA10. Bioprocess. Biosyst. Eng. 2009, 32, 825–835. [Google Scholar] [CrossRef]
- Selvin, J.; Sathiyanarayanan, G.; Lipton, A.N.; Al-Dhabi, N.A.; Valan Arasu, M.; Kiran, G.S. Ketide synthase (KS) domain prediction and analysis of iterative type II PKS gene in marine sponge-associated actinobacteria producing biosurfactants and antimicrobial agents. Front. Microbiol. 2016, 7, 63. [Google Scholar] [CrossRef]
- Saggese, A.; Culurciello, R.; Casillo, A.; Corsaro, M.; Ricca, E.; Baccigalupi, L. A marine isolate of Bacillus pumilus secretes a pumilacidin active against Staphylococcus aureus. Mar. Drugs 2018, 16, 180. [Google Scholar] [CrossRef] [PubMed]
- Khaneja, R.; Perez-Fons, L.; Fakhry, S.; Baccigalupi, L.; Steiger, S.; To, E.; Sandmann, G.; Dong, T.C.; Ricca, E.; Fraser, P.D.; et al. Carotenoids found in Bacillus. J. Appl. Microbiol. 2009, 108, 1889–1902. [Google Scholar] [CrossRef] [PubMed]
- Kalinovskaya, N.I.; Kuznetsova, T.A.; Rashkes, Y.V.; Mil’grom, Y.M.; Mil’grom, E.G.; Willis, R.H.; Wood, A.I.; Kurtz, H.A.; Carabedian, C.; Murphy, P.; et al. Surfactin-like structures of five cyclic depsipeptides from the marine isolate of Bacillus pumilus. Russ. Chem. Bull. 1995, 44, 951–955. [Google Scholar] [CrossRef]
- Anburajan, L.; Meena, B.; Raghavan, R.V.; Shridhar, D.; Joseph, T.C.; Vinithkumar, N.V.; Dharani, G.; Dheenan, P.S.; Kirubagaran, R. Heterologous expression, purification, and phylogenetic analysis of oil-degrading biosurfactant biosynthesis genes from the marine sponge-associated Bacillus licheniformis NIOT-06. Bioprocess. Biosyst. Eng. 2015, 38, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Hentati, D.; Chebbi, A.; Hadrich, F.; Frikha, I.; Rabanal, F.; Sayadi, S.; Manresa, A.; Chamkha, M. Production, characterization and biotechnological potential of lipopeptide biosurfactants from a novel marine Bacillus stratosphericus strain FLU5. Ecotoxicol. Environ. Saf. 2019, 167, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, G.; Zhou, S.; Sha, Z.; Sun, C.; Wu, S.; Liu, G.; Zhou, S.; Sha, Z.; Sun, C. Characterization of antifungal lipopeptide biosurfactants produced by marine bacterium Bacillus sp. CS30. Mar. Drugs 2019, 17, 199. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.-H.; Ye, Z.-W.; Zheng, Q.-W.; Wei, T.; Lin, J.-F.; Guo, L.-Q. Isolation and characterization of cyclic lipopeptides with broad-spectrum antimicrobial activity from Bacillus siamensis JFL15. 3 Biotech. 2018, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Timmis, K.N.; Wray, V.; Fredrickson, H.L. Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BAS50. Appl. Environ. Microbiol. 1995, 61, 1706–1713. [Google Scholar] [PubMed]
- Sivapathasekaran, C.; Mukherjee, S.; Samanta, R.; Sen, R. High-performance liquid chromatography purification of biosurfactant isoforms produced by a marine bacterium. Anal. Bioanal. Chem. 2009, 395, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Dey, G.; Bharti, R.; Dhanarajan, G.; Das, S.; Dey, K.K.; Kumar, B.N.P.; Sen, R.; Mandal, M. Marine lipopeptideiturin A inhibits Akt mediated GSK3β and FoxO3a signaling and triggers apoptosis in breast cancer. Sci. Rep. 2015, 5, 10316. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Ko, S.-K.; Jang, M.; Kim, J.; Kim, G.; Lee, J.; Jeon, E.; Futamura, Y.; Ryoo, I.-J.; Lee, J.-S.; et al. New cyclic lipopeptides of the iturin class produced by saltern-derived Bacillus sp. KCB14S006. Mar. Drugs 2016, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hu, J. Plipastatin A1 produced by a marine sediment-derived Bacillus amyloliquefaciens SH-B74 contributes to the control of gray mold disease in tomato. 3 Biotech. 2018, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Quinn, G.A.; Maloy, A.P.; McClean, S.; Carney, B.; Slater, J.W. Lipopeptide biosurfactants from Paenibacillus polymyxa inhibit single and mixed species biofilms. Biofouling 2012, 28, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Desjardine, K.; Pereira, A.; Wright, H.; Matainaho, T.; Kelly, M.; Andersen, R.J. Tauramamide, a lipopeptide antibiotic produced in culture by Brevibacillus laterosporus isolated from a marine habitat: Structure elucidation and synthesis. J. Nat. Prod. 2007, 70, 1850–1853. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.S.; Kumar, C.G.; Jayalakshmi, S. Aneurinifactin, a new lipopeptide biosurfactant produced by a marine Aneurinibacillus aneurinilyticus SBP-11 isolated from Gulf of Mannar: Purification, characterization and its biological evaluation. Microbiol. Res. 2017, 194, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barakat, K.M.; Hassan, S.W.M.; Darwesh, O.M. Biosurfactant production by haloalkaliphilic Bacillus strains isolated from Red Sea, Egypt. Egypt. J. Aquat. Res. 2017, 43, 205–211. [Google Scholar] [CrossRef]
- Xu, Y.; Kersten, R.D.; Nam, S.-J.; Lu, L.; Al-Suwailem, A.M.; Zheng, H.; Fenical, W.; Dorrestein, P.C.; Moore, B.S.; Qian, P.-Y. Bacterial biosynthesis and maturation of the didemnin anti-cancer Agents. J. Am. Chem. Soc. 2012, 134, 8625–8632. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.C.; Li, J.; Hong, Y.H.; Xu, X.M.; Chen, W.X.; Yuan, J.P.; Peng, J.; Yi, M.; Wang, J.H. Characterization of a novel biosurfactant produced by marine hydrocarbon-degrading bacterium Achromobacter sp. HZ01. J. Appl. Microbiol. 2016, 120, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.; Lloyd, R.; Barsby, T.; Haden, P.; Kelly, M.T.; Andersen, R.J. Massetolides A−H, antimycobacterial cyclic depsipeptides produced by two pseudomonads isolated from marine habitats. J. Nat. Prod. 1997, 60, 223–229. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, I.; de Kock, M.J.; de Waard, P.; van Beek, T.A.; Raaijmakers, J.M. Massetolide A biosynthesis in Pseudomonas fluorescens. J. Bacteriol. 2008, 190, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.S.; Kumar, C.G.; Jayalakshmi, S. Pontifactin, a new lipopeptide biosurfactant produced by a marine Pontibacter korlensis strain SBK-47: Purification, characterization and its biological evaluation. Process. Biochem. 2016, 51, 2198–2207. [Google Scholar] [CrossRef]
- Cheng, T.; Liang, J.; He, J.; Hu, X.; Ge, Z.; Liu, J. A novel rhamnolipid-producing Pseudomonas aeruginosa ZS1 isolate derived from petroleum sludge suitable for bioremediation. AMB Express 2017, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Das, S. Characterization of the metabolic pathway and catabolic gene expression in biphenyl degrading marine bacterium Pseudomonas aeruginosa JP-11. Chemosphere 2016, 144, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, A.; Zhang, X.; Si, X.; Cao, J. Comparative analysis of rhamnolipid congener synthesis in neotype Pseudomonas aeruginosa ATCC 10145 and two marine isolates. Bioresour. Technol. 2019, 286, 121380. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, P.; Maida, I.; Palma Esposito, F.; Tortorella, E.; Subko, K.; Ezeofor, C.; Zhang, Y.; Tabudravu, J.; Jaspars, M.; Fani, R.; et al. Antimicrobial activity of monoramnholipids produced by bacterial strains isolated from the Ross Sea (Antarctica). Mar. Drugs 2016, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.S.; Tripathi, L.; Zompra, A.; Salek, K.; Irorere, V.U.; Gutierrez, T.; Spyroulias, G.A.; Marchant, R.; Banat, I.M. Identification and characterisation of short chain rhamnolipid production in a previously uninvestigated, non-pathogenic marine pseudomonad. Appl. Microbiol. Biotechnol. 2018, 102, 8537–8549. [Google Scholar] [CrossRef] [PubMed]
- Marzban, A.; Ebrahimipour, G.; Danesh, A. Bioactivity of a novel glycolipid produced by a halophilic Buttiauxella sp. and improving submerged fermentation using a response surface method. Molecules 2016, 21, 1256. [Google Scholar] [CrossRef]
- Dusane, D.H.; Pawar, V.S.; Nancharaiah, Y.V.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling 2011, 27, 645–654. [Google Scholar] [CrossRef]
- Abraham, W.R.; Meyer, H.; Yakimov, M. Novel glycine containing glucolipids from the alkane using bacterium Alcanivorax borkumensis. Biochim. Biophys. Acta 1998, 1393, 57–62. [Google Scholar] [CrossRef]
- Yakimov, M.M.; Golyshin, P.N.; Lang, S.; Moore, E.R.; Abraham, W.R.; Lünsdorf, H.; Timmis, K.N. Alcanivorax borkumensis gen. nov., sp. nov., a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int. J. Syst. Bacteriol. 1998, 48, 339–348. [Google Scholar] [CrossRef]
- Antoniou, E.; Fodelianakis, S.; Korkakaki, E.; Kalogerakis, N. Biosurfactant production from marine hydrocarbon-degrading consortia and pure bacterial strains using crude oil as carbon source. Front. Microbiol. 2015, 6, 274. [Google Scholar] [CrossRef] [PubMed]
- White, D.A.; Hird, L.C.; Ali, S.T. Production and characterization of a trehalolipid biosurfactant produced by the novel marine bacterium Rhodococcus sp. strain PML026. J. Appl. Microbiol. 2013, 115, 744–755. [Google Scholar] [CrossRef]
- Konishi, M.; Nishi, S.; Fukuoka, T.; Kitamoto, D.; Watsuji, T.; Nagano, Y.; Yabuki, A.; Nakagawa, S.; Hatada, Y.; Horiuchi, J. Deep-sea Rhodococcus sp. BS-15, lacking the phytopathogenic fas genes, produces a novel glucotriose lipid biosurfactant. Mar. Biotechnol. 2014, 16, 484–493. [Google Scholar] [CrossRef]
- Choi, B.-K.; Lee, H.-S.; Kang, J.S.; Shin, H.J.; Choi, B.-K.; Lee, H.-S.; Kang, J.S.; Shin, H.J. Dokdolipids A−C, hydroxylated rhamnolipids from the marine-derived Actinomycete Actinoalloteichus hymeniacidonis. Mar. Drugs 2019, 17, 237. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Sims, J.; Wang, B.; Hamann, M.T. Marine actinomycete Streptomyces sp. ISP2-49E, a new source of rhamnolipid. Biochem. Syst. Ecol. 2014, 55, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, C.; Jensen, P.R.; Dwight, R.; Fenical, W. Rare phenazine l-quinovose esters from a marine actinomycete. J. Org. Chem. 1992, 57, 740–742. [Google Scholar] [CrossRef]
- Hamza, F.; Satpute, S.; Banpurkar, A.; Kumar, A.R.; Zinjarde, S. Biosurfactant from a marine bacterium disrupts biofilms of pathogenic bacteria in a tropical aquaculture system. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [PubMed]
- Senthil Balan, S.; Ganesh Kumar, C.; Jayalakshmi, S. Physicochemical, structural and biological evaluation of Cybersan (trigalactomargarate), a new glycolipid biosurfactant produced by a marine yeast, Cyberlindnera saturnus strain SBPN-27. Process. Biochem. 2019, 80, 171–180. [Google Scholar] [CrossRef]
- Chun, H.G.; Davies, B.; Hoth, D.; Suffness, M.; Plowman, J.; Flora, K.; Grieshaber, C.; Leyland-Jones, B.; Didemnin, B. The first marine compound entering clinical trials as an antineoplastic agent. Invest. New Drugs 1986, 4, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.-C.; Li, J.; Liang, F.-R.; Yi, M.; Xu, X.-M.; Yuan, J.-P.; Peng, J.; Wu, C.-F.; Wang, J.-H. Isolation and characterization of a novel hydrocarbon-degrading bacterium Achromobacter sp. HZ01 from the crude oil-contaminated seawater at the Daya Bay, southern China. Mar. Pollut. Bull. 2014, 83, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Acharya, D.; Adholeya, A.; Barrow, C.J.; Deshmukh, S.K. Nonribosomal peptides from marine microbes and their antimicrobial and anticancer potential. Front. Pharmacol. 2017, 8, 828. [Google Scholar] [CrossRef] [PubMed]
- Tiso, T.; Thies, S.; Müller, M.; Tsvetanova, L.; Carraresi, L.; Bröring, S.; Jaeger, K.-E.; Blank, L.M. Rhamnolipids: Production, performance, and application. In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals; Lee, S.Y., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–37. ISBN 978-3-319-31421-1. [Google Scholar]
- Kiran, G.S.; Ninawe, A.S.; Lipton, A.N.; Pandian, V.; Selvin, J. Rhamnolipid biosurfactants: Evolutionary implications, applications and future prospects from untapped marine resource. Crit. Rev. Biotechnol. 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Laursen, J.B.; Nielsen, J. Phenazine natural products: Biosynthesis, synthetic analogues, and biological activity. Chem. Rev. 2004, 104, 1663–1685. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.M.; Trauger, J.W.; Schwarzer, D.; Marahiel, M.A.; Walsh, C.T. Generality of peptide cyclization catalyzed by isolated thioesterase domains of nonribosomal peptide synthetases. Biochemistry 2001, 40, 7099–7108. [Google Scholar] [CrossRef] [PubMed]
- Tanovic, A.; Samel, S.A.; Essen, L.-O.; Marahiel, M.A. Crystal structure of the termination module of a nonribosomal peptide synthetase. Science 2008, 321, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, S.N. Structural and molecular characteristics of lichenysin and its relationship with surface activity. In Advances in Experimental Medicine and Biology; Sen, R., Ed.; Springer: New York, NY, USA, 2010; Volume 672, pp. 304–315. ISBN 978-1-4419-5978-2. [Google Scholar]
- Yakimov, M.M.; Kröger, A.; Slepak, T.N.; Giuliano, L.; Timmis, K.N.; Golyshin, P.N. A putative lichenysin A synthetase operon in Bacillus licheniformis: Initial characterization. Biochim. Biophys. Acta—Gene Struct. Expr. 1998, 1399, 141–153. [Google Scholar] [CrossRef]
- Steller, S.; Vollenbroich, D.; Leenders, F.; Stein, T.; Conrad, B.; Hofemeister, J.; Jacques, P.; Thonart, P.; Vater, J. Structural and functional organization of the fengycin synthetase multienzyme system from Bacillus subtilis b213 and A1/3. Chem. Biol. 1999, 6, 31–41. [Google Scholar] [CrossRef]
- Tsuge, K.; Akiyama, T.; Shoda, M. Cloning, sequencing, and characterization of the iturin A operon. J. Bacteriol. 2001, 183, 6265–6273. [Google Scholar] [CrossRef]
- Tsuge, K.; Ano, T.; Hirai, M.; Nakamura, Y.; Shoda, M. The genes degQ, pps, and lpa-8 (sfp) are responsible for conversion of Bacillus subtilis 168 to plipastatin production. Antimicrob. Agents Chemother. 1999, 43, 2183–2192. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Park, S.-Y.; Choi, S.-K.; Park, S.-H. Biosynthesis of polymyxins B, E, and P using genetically engineered polymyxin synthetases in the surrogate host Bacillus subtilis. J. Microbiol. Biotechnol. 2015, 25, 1015–1025. [Google Scholar] [CrossRef]
- Choi, S.-K.; Park, S.-Y.; Kim, R.; Lee, C.-H.; Kim, J.F.; Park, S.-H. Identification and functional analysis of the fusaricidin biosynthetic gene of Paenibacillus polymyxa E681. Biochem. Biophys. Res. Commun. 2008, 365, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.F.; Chao, C.J.; Clardy, J. Long-chain N-acyltyrosine synthases from environmental DNA. Appl. Environ. Microbiol. 2004, 70, 6865–6870. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.F.; Clardy, J. N-acyl derivatives of arginine and tryptophan isolated from environmental DNA expressed in Escherichia coli. Org. Lett. 2005, 7, 3613–3616. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, R.M.; Clardy, J. FeeM, an N-acyl amino acid synthase from an uncultured soil microbe: Structure, mechanism, and acyl carrier protein binding. Structure 2006, 14, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Samel, S.A.; Wagner, B.; Marahiel, M.A.; Essen, L.-O. The thioesterase domain of the fengycin biosynthesis cluster: A structural base for the macrocyclization of a non-ribosomal lipopeptide. J. Mol. Biol. 2006, 359, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Marahiel, M.A. Working outside the protein-synthesis rules: Insights into non-ribosomal peptide synthesis. J. Pept. Sci. 2009, 15, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jensen, S.E. Nonribosomal biosynthesis of fusaricidins by Paenibacillus polymyxa PKB1 involves direct activation of a D-amino acid. Chem. Biol. 2008, 15, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Wittgens, A.; Kovacic, F.; Müller, M.M.; Gerlitzki, M.; Santiago-Schübel, B.; Hofmann, D.; Tiso, T.; Blank, L.M.; Henkel, M.; Hausmann, R.; et al. Novel insights into biosynthesis and uptake of rhamnolipids and their precursors. Appl. Microbiol. Biotechnol. 2017, 101, 2865–2878. [Google Scholar] [CrossRef] [PubMed]
- Tiso, T.; Zauter, R.; Tulke, H.; Leuchtle, B.; Li, W.-J.; Behrens, B.; Wittgens, A.; Rosenau, F.; Hayen, H.; Blank, L.M. Designer rhamnolipids by reduction of congener diversity: Production and characterization. Microb. Cell Fact. 2017, 16, 225. [Google Scholar] [CrossRef]
- Das, P.; Mukherjee, S.; Sen, R. Genetic Regulations of the biosynthesis of microbial surfactants: An overview. Biotechnol. Genet. Eng. Rev. 2008, 25, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Roongsawang, N.; Washio, K.; Morikawa, M. Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants. Int. J. Mol. Sci. 2010, 12, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Schneiker, S.; Martins dos Santos, V.A.P.; Bartels, D.; Bekel, T.; Brecht, M.; Buhrmester, J.; Chernikova, T.N.; Denaro, R.; Ferrer, M.; Gertler, C.; et al. Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis. Nat. Biotechnol. 2006, 24, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Sambles, C.M.; White, D.A. Genome sequence of Rhodococcus sp. strain PML026, a trehalolipid biosurfactant producer and biodegrader of oil and alkanes. Genome Announc. 2015, 3, e00433-15. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Margesin, R. Psychrophilic lifestyles: Mechanisms of adaptation and biotechnological tools. Appl. Microbiol. Biotechnol. 2019, 103, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Hochmuth, T.; Piel, J. Polyketide synthases of bacterial symbionts in sponges—Evolution-based applications in natural products research. Phytochemistry 2009, 70, 1841–1849. [Google Scholar] [CrossRef]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine sponge natural products with anticancer potential: An updated review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef]
- Negi, B.; Kumar, D.; Rawat, D.S. Marine peptides as anticancer agents: A remedy to mankind by nature. Curr. Protein Pept. Sci. 2017, 18, 885–904. [Google Scholar] [CrossRef]
- Perfumo, A.; Rudden, M.; Marchant, R.; Banat, I.M. Biodiversity of biosurfactants and roles in enhancing the (bio)availability of hydrophobic substrates. In Cellular Ecophysiology of Microbe; Krell, T., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–29. [Google Scholar]
- Head, I.M.; Jones, D.M.; Röling, W.F.M. Marine microorganisms make a meal of oil. Nat. Rev. Microbiol. 2006, 4, 173–182. [Google Scholar] [CrossRef]
- Rojo, F. Degradation of alkanes by bacteria. Environ. Microbiol. 2009, 11, 2477–2490. [Google Scholar] [CrossRef]
- Coscolín, C.; Bargiela, R.; Martínez-Martínez, M.; Alonso, S.; Bollinger, A.; Thies, S.; Chernikova, T.N.; Hai, T.; Golyshina, O.V.; Jaeger, K.-E.; et al. Hydrocarbon-degrading microbes as sources of new biocatalysts. In Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes; McGenity, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–21. [Google Scholar]
- Yakimov, M.M.; Timmis, K.N.; Golyshin, P.N. Obligate oil-degrading marine bacteria. Curr. Opin. Biotechnol. 2007, 18, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; Golyshin, P.N.; Crisafi, F.; Denaro, R.; Giuliano, L. Marine, Aerobic hydrocarbon-degrading gammaproteobacteria: The family Alcanivoracaceae. In Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–13. [Google Scholar] [CrossRef]
- Korzhenkov, A.A.; Toshchakov, S.V.; Golyshina, O.V.; Ferrer, M.; Chernikova, T.N.; Jaeger, K.-E.; Yakimov, M.M.; Golyshin, P.N. Aerobic hydrocarbon-degrading Gammaproteobacteria: Oleiphilaceae. In Taxonomy, Genomics and Ecophysiology of Hydrocarbon-Degrading Microbes; McGenity, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2019; in press. [Google Scholar]
- Wu, Y.; Lai, Q.; Zhou, Z.; Qiao, N.; Liu, C.; Shao, Z. Alcanivorax hongdengensis sp. nov., an alkane-degrading bacterium isolated from surface seawater of the straits of Malacca and Singapore, producing a lipopeptide as its biosurfactant. Int. J. Syst. Evol. Microbiol. 2009, 59, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Eberlein, C.; Baumgarten, T.; Starke, S.; Heipieper, H.J. Immediate response mechanisms of Gram-negative solvent-tolerant bacteria to cope with environmental stress: Cis-trans isomerization of unsaturated fatty acids and outer membrane vesicle secretion. Appl. Microbiol. Biotechnol. 2018, 102, 2583–2593. [Google Scholar] [CrossRef] [PubMed]
- Mahjoubi, M.; Cappello, S.; Souissi, Y.; Jaouani, A.; Cherif, A. Microbial bioremediation of petroleum hydrocarbon– contaminated marine environments. In Recent Insights in Petroleum Science and Engineering; Zoveidavianpoor, M., Ed.; InTech: London, UK, 2018; pp. 325–350. [Google Scholar]
- Cafaro, V.; Izzo, V.; Notomista, E.; Di Donato, A. Marine hydrocarbonoclastic bacteria. In Marine Enzymes for Biocatalysis; Trincone, A., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; Volume 48, pp. 373–402. ISBN 9781907568800. [Google Scholar]
- Souza, E.C.; Vessoni-Penna, T.C.; de Souza Oliveira, R.P. Biosurfactant-enhanced hydrocarbon bioremediation: An overview. Int. Biodeterior. Biodegradation 2014, 89, 88–94. [Google Scholar] [CrossRef]
- Godfrin, M.P.; Sihlabela, M.; Bose, A.; Tripathi, A. Behavior of marine bacteria in clean environment and oil spill conditions. Langmuir 2018, 34, 9047–9053. [Google Scholar] [CrossRef]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z.; Cameotra, S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef]
- Chrzanowski, Ł.; Ławniczak, Ł.; Czaczyk, K. Why do microorganisms produce rhamnolipids? World J. Microbiol. Biotechnol. 2012, 28, 401–419. [Google Scholar] [CrossRef]
- Martins dos Santos, V.; Sabirova, J.; Timmis, K.N.; Yakimov, M.M.; Golyshin, P.N. Alcanivorax borkumensis. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1265–1288. ISBN 978-3-540-77584-3. [Google Scholar]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Paulino, B.N.; Pessôa, M.G.; Mano, M.C.R.; Molina, G.; Neri-Numa, I.A.; Pastore, G.M. Current status in biotechnological production and applications of glycolipid biosurfactants. Appl. Microbiol. Biotechnol. 2016, 100, 10265–10293. [Google Scholar] [CrossRef]
- Olasanmi, I.; Thring, R.; Olasanmi, I.O.; Thring, R.W. The role of biosurfactants in the continued drive for environmental sustainability. Sustainability 2018, 10, 4817. [Google Scholar] [CrossRef]
- Hames, E.; Vardar-Sukan, F.; Kosaric, N. Patents on biosurfactants and future trends. In Biosurfactants: Production and Utilization—Processes, Technologies, and Economics; Kosaric, N., Varder-Sukan, F., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 165–244. ISBN 978-1466596696. [Google Scholar]
- Khopade, A.; Ren, B.; Liu, X.Y.; Mahadik, K.; Zhang, L.; Kokare, C. Production and characterization of biosurfactant from marine Streptomyces species B3. J. Colloid Interface Sci 2012, 367, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.K.; Pradhan, N.; Mall, G.; Panda, H.T.; Sukla, L.B.; Panda, P.K.; Mishra, B.K. Application of lipopeptide biosurfactant isolated from a halophile: Bacillus tequilensis CH for inhibition of biofilm. Appl. Biochem. Biotechnol. 2013, 171, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Sivasankar, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Optimization, production and characterization of glycolipid biosurfactant from the marine actinobacterium, Streptomyces sp. MAB36. Bioprocess. Biosyst Eng 2014, 37, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, S.A.; Lohans, C.T.; van Belkum, M.J.; Bels, M.A.; Vederas, J.C. Studies on tridecaptin B(1), a lipopeptide with activity against multidrug resistant Gram-negative bacteria. Org. Biomol. Chem. 2015, 13, 6073–6081. [Google Scholar] [CrossRef] [PubMed]
- Díaz De Rienzo, M.A.; Banat, I.M.; Dolman, B.; Winterburn, J.; Martin, P.J. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. N. Biotechnol. 2015, 32, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Díaz De Rienzo, M.A.; Stevenson, P.; Marchant, R.; Banat, I.M. Antibacterial properties of biosurfactants against selected Gram-positive and -negative bacteria. FEMS Microbiol. Lett. 2016, 363, fnv224. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Díaz De Rienzo, M.A.; Stevenson, P.S.; Marchant, R.; Banat, I.M. Effect of biosurfactants on Pseudomonas aeruginosa and Staphylococcus aureus biofilms in a BioFlux channel. Appl. Microbiol. Biotechnol. 2016, 100, 5773–5779. [Google Scholar] [CrossRef]
- Sotirova, A.; Avramova, T.; Stoitsova, S.; Lazarkevich, I.; Lubenets, V.; Karpenko, E.; Galabova, D. The importance of rhamnolipid-biosurfactant-induced changes in bacterial membrane lipids of Bacillus subtilis for the antimicrobial activity of thiosulfonates. Curr. Microbiol. 2012, 65, 534–541. [Google Scholar] [CrossRef]
- Yin, X. Formulations Combining Ramoplanin and Rhamnolipids for Combating Bacterial Infection. U.S. Patent 20,140,294,925, 10 February 2014. [Google Scholar]
- Hage-Hülsmann, J.; Grünberger, A.; Thies, S.; Santiago-Schübel, B.; Klein, A.S.; Pietruszka, J.; Binder, D.; Hilgers, F.; Domröse, A.; Drepper, T.; et al. Natural biocide cocktails: Combinatorial antibiotic effects of prodigiosin and biosurfactants. PLoS ONE 2018, 13, e0200940. [Google Scholar] [CrossRef]
- Joshi-Navare, K.; Prabhune, A. A biosurfactant-sophorolipid acts in synergy with antibiotics to enhance their efficiency. Biomed. Res. Int. 2013, 2013, 512495. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; De Rienzo, M.A.D.; Quinn, G.A. Microbial biofilms: Biosurfactants as antibiofilm agents. Appl. Microbiol. Biotechnol. 2014, 98, 9915–9929. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Klein, D.J. Polymyxin B hemoperfusion: A mechanistic perspective. Crit. Care 2014, 18, 309. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.; Lydon, H.; Roelants, S.L.K.W.; Van Bogaert, I.N.A.; Marchant, R.; Banat, I.M.; Mitchell, C.A. Lactonic sophorolipids increase tumor burden in Apcmin +/− mice. PLoS ONE 2016, 11, e0156845. [Google Scholar] [CrossRef] [PubMed]
- Jerala, R. Synthetic lipopeptides: A novel class of anti-infectives. Expert Opin. Investig. Drugs 2007, 16, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gao, L.; Bie, X.; Lu, Z.; Liu, H.; Zhang, C.; Lu, F.; Zhao, H. Identification of novel surfactin derivatives from NRPS modification of Bacillus subtilis and its antifungal activity against Fusarium moniliforme. BMC Microbiol. 2016, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H. Synthetic biology, genome mining, and combinatorial biosynthesis of NRPS-derived antibiotics: A perspective. J. Ind. Microbiol. Biotechnol. 2018, 45, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Stipcevic, T.; Piljac, A.; Piljac, G. Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns 2006, 32, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Renfro, T.D.; Xie, W.; Yang, G.; Chen, G. Rhamnolipid surface thermodynamic properties and transport in agricultural soil. Colloids Surf. B Biointerfaces 2014, 115, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Vatsa, P.; Sanchez, L.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. Int. J. Mol. Sci. 2010, 11, 5095–5108. [Google Scholar] [CrossRef]
- Sachdev, D.P.; Cameotra, S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013, 97, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shao, B.; Long, X.; Yao, Y.; Meng, Q. Foliar penetration enhanced by biosurfactant rhamnolipid. Colloids Surf. B Biointerfaces 2016, 145, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Priyadharsini, G.B.; Poulose, N.; Selvin, J. Production of lipopeptide biosurfactant by a marine Nesterenkonia sp. and its application in food industry. Front. Microbiol 2017, 8, 1138. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, L.V.; Freire, D.M.G.; Nitschke, M. Perspectives on using biosurfactants in food industry. In Biosurfactants: Production and Utilization—Processes, Technologies, and Economics; Kosaric, N., Varder-Sukan, F., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 295–312. ISBN 978-1466596696. [Google Scholar]
- Mnif, I.; Ghribi, D. Glycolipid biosurfactants: Main properties and potential applications in agriculture and food industry. J. Sci Food Agric 2016, 96, 4310–4320. [Google Scholar] [CrossRef] [PubMed]
- Haba, E.; Bouhdid, S.; Torrego-Solana, N.; Marques, A.M.; Espuny, M.J.; Garcia-Celma, M.J.; Manresa, A. Rhamnolipids as emulsifying agents for essential oil formulations: Antimicrobial effect against Candida albicans and methicillin-resistant Staphylococcus aureus. Int J. Pharm 2014, 476, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Varvaresou, A.; Iakovou, K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015, 61, 214–223. [Google Scholar] [CrossRef]
- Rincón-Fontán, M.; Rodríguez-López, L.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Study of the synergic effect between mica and biosurfactant to stabilize Pickering emulsions containing Vitamin E using a triangular design. J. Colloid Interface Sci. 2019, 537, 34–42. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Rincón-Fontán, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Preservative and irritant capacity of biosurfactants from different sources: A comparative study. J. Pharm. Sci. 2019, 108, 2296–2304. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Natural surfactants used in cosmetics: Glycolipids. Int. J. Cosmet. Sci. 2009, 31, 255–261. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Lourith, N. Lipopeptides in cosmetics. Int. J. Cosmet. Sci. 2010, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in cosmetic formulations: Trends and challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Das, K. Microbial surfactants and their potential applications: An overview. In Biosurfactants. Advances in Experimental Medicine and Biology; Sen, R., Ed.; Springer: New York, NY, USA, 2010; Volume 672, pp. 54–64. ISBN 978-1-4419-5978-2. [Google Scholar]
- Van Renterghem, L.; Roelants, S.L.K.W.; Baccile, N.; Uyttersprot, K.; Taelman, M.C.; Everaert, B.; Mincke, S.; Ledegen, S.; Debrouwer, S.; Scholtens, K.; et al. From lab to market: An integrated bioprocess design approach for new-to-nature biosurfactants produced by Starmerella bombicola. Biotechnol. Bioeng. 2018, 115, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Sekhon Randhawa, K.K.; Rahman, P.K.S.M. Rhamnolipid biosurfactants—Past, present, and future scenario of global market. Front. Microbiol. 2014, 5, 454. [Google Scholar] [CrossRef] [PubMed]
- Vecino Bello, X.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Study of the synergistic effects of salinity, pH, and temperature on the surface-active properties of biosurfactants produced by Lactobacillus pentosus. J. Agric. Food Chem. 2012, 60, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Michaud, L.; Syldatk, C.; Hausmann, R.; De Domenico, E.; Lo Giudice, A. Influence of salinity and temperature on the activity of biosurfactants by polychaete-associated isolates. Environ. Sci. Pollut. Res. Int. 2014, 21, 2988–3004. [Google Scholar] [CrossRef] [PubMed]
- Ivshina, I.; Kostina, L.; Krivoruchko, A.; Kuyukina, M.; Peshkur, T.; Anderson, P.; Cunningham, C. Removal of polycyclic aromatic hydrocarbons in soil spiked with model mixtures of petroleum hydrocarbons and heterocycles using biosurfactants from Rhodococcus ruber IEGM 231. J. Hazard. Mater. 2016, 312, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bezza, F.A.; Chirwa, E.M.N. Pyrene biodegradation enhancement potential of lipopeptide biosurfactant produced by Paenibacillus dendritiformis CN5 strain. J. Hazard. Mater. 2017, 321, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Van Hamme, J.; Urban, J. Biosurfactants in bioremediation. In Advances in Applied Bioremediation; Singh, A., Kuhad, R.C., Ward, O.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-89620-3. [Google Scholar]
- Vecino, X.; Rodríguez-López, L.; Cruz, J.M.; Moldes, A.B. Sewage sludge polycyclic aromatic hydrocarbon (PAH) decontamination technique based on the utilization of a lipopeptide biosurfactant extracted from corn steep liquor. J. Agric. Food Chem. 2015, 63, 7143–7150. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, A.R.; Wick, L.Y.; Harms, H. Principles of microbial PAH-degradation in soil. Environ. Pollut. 2005, 133, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Shahzad, S.; Munir, A.; Nadagouda, M.N.; Khan, G.S.; Shams, D.F.; Dionysiou, D.D.; Rana, U.A. Micelles as soil and water decontamination agents. Chem Rev. 2016, 116, 6042–6074. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Borgohain, S.; Kumar, M.; Rangarajan, V.; Somasundaran, P.; Sen, R. Recent developments in microbial enhanced oil recovery. Renew. Sustain. Energy Rev. 2015, 52, 1539–1558. [Google Scholar] [CrossRef]
- Sen, R. Biotechnology in petroleum recovery: The microbial EOR. Prog. Energy Combust. Sci. 2008, 34, 714–724. [Google Scholar] [CrossRef]
- Khire, J.M. Bacterial biosurfactants, and their role in microbial enhanced oil recovery (MEOR). In Biosurfactants: Advances in Experimental Medicine and Biology; Sen, R., Ed.; Springer: New York, NY, USA, 2010; Volume 672, pp. 146–157. ISBN 9781441959782. [Google Scholar]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.-S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Zhang, G.; Rogers, R.E.; French, W.T.; Lao, W. Investigation of microbial influences on seafloor gas-hydrate formations. Mar. Chem. 2007, 103, 359–369. [Google Scholar] [CrossRef]
- Arora, A.; Singh Cameotra, S. Effects of biosurfactants on gas hydrates. J. Pet. Environ. Biotechnol. 2014, 5, 170. [Google Scholar] [CrossRef]
- Kitamoto, D.; Yanagishita, H.; Endo, A.; Nakaiwa, M.; Nakane, T.; Akiya, T. Remarkable antiagglomeration effect of a yeast biosurfactant, diacylmannosylerythritol, on ice-water slurry for cold thermal storage. Biotechnol. Prog. 2001, 17, 362–365. [Google Scholar] [CrossRef]
- Madihalli, C.; Sudhakar, H.; Doble, M. Mannosylerythritol lipid-A as a pour point depressant for enhancing the low-temperature fluidity of biodiesel and hydrocarbon fuels. Energy Fuels 2016, 30, 4118–4125. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, N.; Yang, J.; Yang, Y.; Wang, R.; Liu, L.; Yuan, H. Lipopeptide produced from Bacillus sp. W112 improves the hydrolysis of lignocellulose by specifically reducing non-productive binding of cellulases with and without CBMs. Biotechnol Biofuels 2017, 10, 301. [Google Scholar] [CrossRef]
- Sharma, P.; Melkania, U. Biosurfactant-enhanced hydrogen production from organic fraction of municipal solid waste using co-culture of Escherichia coli and Enterobacter aerogenes. Bioresour Technol 2017, 243, 566–572. [Google Scholar] [CrossRef]
- Gomes, N.G.M.; Dasari, R.; Chandra, S.; Kiss, R.; Kornienko, A. marine invertebrate metabolites with anticancer activities: Solutions to the “supply problem”. Mar. Drugs 2016, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Geys, R.; Soetaert, W.; Van Bogaert, I. Biotechnological opportunities in biosurfactant production. Curr. Opin. Biotechnol. 2014, 30, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sekhon Randhawa, K.K. Biosurfactants produced by genetically maniplated microorganisms—Challenges and opportunities. In Biosurfactants: Production and Utilization—Processes, Technologies, and Economics; Kosaric, N., Vardar-Sukan, F., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 49–72. ISBN 9781466596696. [Google Scholar]
- Bages, S.; White, D.A.; Winterburn, J.B.; Webb, C.; Martin, P.J. Production and separation of a trehalolipid biosurfactant. Biochem. Eng. J. 2018. [Google Scholar] [CrossRef]
- Jackson, S.A.; Borchert, E.; O’Gara, F.; Dobson, A.D. Metagenomics for the discovery of novel biosurfactants of environmental interest from marine ecosystems. Curr. Opin. Biotechnol. 2015, 33, 176–182. [Google Scholar] [CrossRef]
- Trindade, M.; van Zyl, L.J.; Navarro-Fernández, J.; Abd Elrazak, A. Targeted metagenomics as a tool to tap into marine natural product diversity for the discovery and production of drug candidates. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Baker, K.V.; Takano, E.; Breitling, R. The “three Cs” of novel antibiotic discovery and production through synthetic biology: Biosynthetic gene clusters, heterologous chassis, and synthetic microbial consortia. Adv. Biosyst. 2018, 2, 1800064. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wang, Y.; Ang, E.L.; Zhao, H. Engineering microbial hosts for production of bacterial natural products. Nat. Prod. Rep. 2016, 33, 963–987. [Google Scholar] [CrossRef]
- Kim, E.; Moore, B.S.; Yoon, Y.J. Reinvigorating natural product combinatorial biosynthesis with synthetic biology. Nat. Chem. Biol. 2015, 11, 649–659. [Google Scholar] [CrossRef]
- Nishizaki, T.; Tsuge, K.; Itaya, M.; Doi, N.; Yanagawa, H. Metabolic engineering of carotenoid biosynthesis in Escherichia coli by ordered gene assembly in Bacillus subtilis. Appl. Environ. Microbiol. 2007, 73, 1355–1361. [Google Scholar] [CrossRef]
- Andreou, A.I.; Nakayama, N. Mobius Assembly: A versatile Golden-Gate framework towards universal DNA assembly. PLoS ONE 2018, 13, e0189892. [Google Scholar] [CrossRef]
- Gibson, D.G. Enzymatic assembly of overlapping DNA fragments. Methods Enzymol. 2011, 498, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Cobb, R.E.; Ning, J.C.; Zhao, H. DNA assembly techniques for next-generation combinatorial biosynthesis of natural products. J. Ind. Microbiol. Biotechnol. 2014, 41, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Perlova, O.; Fu, J.; Kuhlmann, S.; Krug, D.; Stewart, A.F.; Zhang, Y.; Müller, R. Reconstitution of the myxothiazol biosynthetic gene cluster by Red/ET recombination and heterologous expression in Myxococcus xanthus. Appl. Environ. Microbiol. 2006, 72, 7485–7494. [Google Scholar] [CrossRef] [PubMed]
- Weihmann, R.; Domröse, A.; Drepper, T.; Jaeger, K.; Loeschcke, A. Protocols for yTREX/Tn5-based gene cluster expression in Pseudomonas putida. Microb. Biotechnol. 2019, 1751–7915.13402. [Google Scholar] [CrossRef] [PubMed]
- Nah, H.-J.; Pyeon, H.-R.; Kang, S.-H.; Choi, S.-S.; Kim, E.-S. Cloning and heterologous expression of a large-sized natural product biosynthetic gene cluster in Streptomyces species. Front. Microbiol. 2017, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Yamanaka, K.; Xu, Y.; Zhang, W.; Vlamakis, H.; Kolter, R.; Moore, B.S.; Qian, P.-Y. Directed natural product biosynthesis gene cluster capture and expression in the model bacterium Bacillus subtilis. Sci. Rep. 2015, 5, 9383. [Google Scholar] [CrossRef] [PubMed]
- Brigulla, M.; Wackernagel, W. Molecular aspects of gene transfer and foreign DNA acquisition in prokaryotes with regard to safety issues. Appl. Microbiol. Biotechnol. 2010, 86, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Troeschel, S.C.; Drepper, T.; Leggewie, C.; Streit, W.R.; Jaeger, K.-E. Novel tools for the functional expression of metagenomic DNA. Methods Mol. Biol. 2010, 668, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Vojcic, L.; Despotovic, D.; Martinez, R.; Maurer, K.; Schwaneberg, U. An efficient transformation method for Bacillus subtilis DB104. Appl. Microbiol. Biotechnol. 2012, 94, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Yin, J.; Fu, J.; Herrmann, J.; Li, Y.; Yin, Y.; Stewart, A.F.; Müller, R.; Zhang, Y. Room temperature electrocompetent bacterial cells improve DNA transformation and recombineering efficiency. Sci. Rep. 2016, 6, 24648. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Huang, F.; Stewart, F.A.; Xia, L.; Zhang, Y.; Müller, R. Direct cloning, genetic engineering, and heterologous expression of the syringolin biosynthetic gene cluster in E. coli through Red/ET recombineering. Chembiochem 2012, 13, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Loeschcke, A.; Thies, S. Pseudomonas putida-a versatile host for the production of natural products. Appl. Microbiol. Biotechnol. 2015, 99, 6197–6214. [Google Scholar] [CrossRef] [PubMed]
- Thies, S.; Santiago-Schübel, B.; Kovačić, F.; Rosenau, F.; Hausmann, R.; Jaeger, K.-E. Heterologous production of the lipopeptide biosurfactant serrawettin W1 in Escherichia coli. J. Biotechnol. 2014, 181, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.R.; Cho, J.S.; Cho, I.J.; Park, D.; Lee, S.Y. Markerless gene knockout and integration to express heterologous biosynthetic gene clusters in Pseudomonas putida. Metab. Eng. 2018, 47, 463–474. [Google Scholar] [CrossRef]
- Zhang, H.; Boghigian, B.A.; Armando, J.; Pfeifer, B.A. Methods and options for the heterologous production of complex natural products. Nat. Prod. Rep. 2011, 28, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Sydow, A.; Schempp, F.; Becher, D.; Schewe, H.; Schrader, J.; Buchhaupt, M. Investigation of plasmid-induced growth defect in Pseudomonas putida. J. Biotechnol. 2016, 231, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Guell, M.; Church, G.M.; Pfeifer, B.A. Heterologous erythromycin production across strain and plasmid construction. Biotechnol. Prog. 2018, 34, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fang, X.; Bai, B.; Liang, X.; Shuler, P.J.; Goddard, W.A.; Tang, Y. Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnol. Bioeng. 2007, 98, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Zobel, S.; Benedetti, I.; Eisenbach, L.; de Lorenzo, V.; Wierckx, N.; Blank, L.M. Tn7-based device for calibrated heterologous gene expression in Pseudomonas putida. ACS Synth. Biol. 2015, 4, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Kryachko, Y.; Nathoo, S.; Lai, P.; Voordouw, J.; Prenner, E.J.; Voordouw, G. Prospects for using native and recombinant rhamnolipid producers for microbially enhanced oil recovery. Int. Biodeterior. Biodegrad. 2013, 81, 133–140. [Google Scholar] [CrossRef]
- Domröse, A.; Weihmann, R.; Thies, S.; Jaeger, K.-E.; Drepper, T.; Loeschcke, A. Rapid generation of recombinant Pseudomonas putida secondary metabolite producers using yTREX. Synth. Syst. Biotechnol. 2017, 2, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Domröse, A.; Hage-Hülsmann, J.; Thies, S.; Weihmann, R.; Kruse, L.; Otto, M.; Wierckx, N.; Jaeger, K.-E.; Drepper, T.; Loeschcke, A. Pseudomonas putida rDNA is a favored site for the expression of biosynthetic genes. Sci. Rep. 2019, 9, 7028. [Google Scholar] [CrossRef] [PubMed]
- Wittgens, A.; Rosenau, F. On the road towards tailor-made rhamnolipids: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 8175–8185. [Google Scholar] [CrossRef] [PubMed]
- Henkel, M.; Syldatk, C.; Hausmann, R. The prospects for the production of rhamnolipids on renewable resources: Evaluation of novel feedstocks and perspectives of strain engineering. In Biosurfactants: Production and Utilization; Kosaric, N., Varder-Sukan, F., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 83–100. ISBN 978-1-4665-9669-6. [Google Scholar]
- Cabrera-Valladares, N.; Richardson, A.-P.; Olvera, C.; Treviño, L.G.; Déziel, E.; Lépine, F.; Soberón-Chávez, G. Monorhamnolipids and 3-(3-hydroxyalkanoyloxy) alkanoic acids (HAAs) production using Escherichia coli as a heterologous host. Appl. Microbiol. Biotechnol. 2006, 73, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wittgens, A.; Tiso, T.; Arndt, T.T.; Wenk, P.; Hemmerich, J.; Müller, C.; Wichmann, R.; Küpper, B.; Zwick, M.; Wilhelm, S.; et al. Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440. Microb. Cell Fact. 2011, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.; Li, X.; Whiting, A.; Latif, M.; Gibson, T.; Silva, C.J.; Brian, P.; Davies, J.; Miao, V.; Wrigley, S.K.; et al. Heterologous production of daptomycin in Streptomyces lividans. J. Ind. Microbiol. Biotechnol. 2006, 33, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.P.; Roongsawang, N.; Washio, K.; Morikawa, M. Flexible exportation mechanisms of arthrofactin in Pseudomonas sp. MIS38. J. Appl. Microbiol. 2009, 107, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, H.; Zhang, D.; Li, X.; Yu, H.; Shen, Z. Overexpression of specific proton motive force-dependent transporters facilitate the export of surfactin in Bacillus subtilis. J. Ind. Microbiol. Biotechnol. 2015, 42, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Wu, Q.; Xu, Y. Genome and transcriptome analysis of surfactin biosynthesis in Bacillus amyloliquefaciens MT45. Sci. Rep. 2017, 7, 40976. [Google Scholar] [CrossRef]
- Jezierska, S.; Claus, S.; Van Bogaert, I. Yeast glycolipid biosurfactants. FEBS Lett. 2017, 140, 874–888. [Google Scholar] [CrossRef]
- Lv, H.; Li, J.; Wu, Y.; Garyali, S.; Wang, Y. Transporter and its engineering for secondary metabolites. Appl. Microbiol. Biotechnol. 2016, 100, 6119–6130. [Google Scholar] [CrossRef] [PubMed]
- Willenbacher, J.; Zwick, M.; Mohr, T.; Schmid, F.; Syldatk, C.; Hausmann, R. Evaluation of different Bacillus strains in respect of their ability to produce surfactin in a model fermentation process with integrated foam fractionation. Appl. Microbiol. Biotechnol. 2014, 98, 9623–9632. [Google Scholar] [CrossRef] [PubMed]
- Beuker, J.; Steier, A.; Wittgens, A.; Rosenau, F.; Henkel, M.; Hausmann, R. Integrated foam fractionation for heterologous rhamnolipid production with recombinant Pseudomonas putida in a bioreactor. AMB Express 2016, 6, 11. [Google Scholar] [CrossRef] [PubMed]
| Producing Species 1 | Compound 2 | Alkane-Dependent 3 | Sampling Site 4 | Reference | |
|---|---|---|---|---|---|
| Isolation | Production | ||||
| fatty acids | |||||
| Aureobasidium pullulans | massoia lactone | coastal seawater, Koh Sichang, Gulf of Thailand | [59] | ||
| Cobetia sp. strain MM1IDA2H-1 | 3-hydroxy fatty acids | x | eulitoral pond, Montemar, Chile | [60] | |
| Serratia rubidaea | rubiwettin R1 | n.a. | n.a. | [61] | |
| lipoamino acids | |||||
| Myroides sp. SM1 | ornithine lipid | x | x | seawater, Thailand | [62] |
| Alcanivorax dieselolei | proline lipid | x | x | surface water, Yellow River delta, Bohai Sea, China | [63] |
| Brevibacterium luteolum | proline lipid | x | tunicate, north coast of São Paulo, Brazil | [64] | |
| lipopeptides | |||||
| Brevibacterium luteolum | Thr-Pro- Pro-Leu/Ile-Leu/Ile- Ala- Phe | x | tunicate, north coast of São Paulo, Brazil | [64] | |
| Brevibacterium aureum | Gly-Gly-Leu-Pro | x | sponge, southwest coast of India | [65] | |
| Rhodococcus sp. TW53 | rhodofactin(miao)Ala-Ile-Asp-Met-Pro | x | x | deep sea sediment, Pacific Ocean | [66] |
| Nocardiopsis alba | phenyl alanine dipeptide | x | sponge, southwest coast of India | [67,68] | |
| Bacillus pumilus | pumilacidin | seawater | [69,70] | ||
| Bacillus pumilus | surfactin-like | sponge, Hautman Reef, Australia | [71] | ||
| Bacillus licheniformis NIOT-06 | surfactin | sponge, North Bay of Port Blair, South Andaman | [72] | ||
| Bacillus stratophericus | Surfactin(miao)pumilacidin | x | harbour, Sfax, Tunisia | [73] | |
| Bacillus sp. CS30 | surfactin | deep sea sediment, Formosa ridge, South China Sea | [74] | ||
| Bacillus siamensis | surfactin(miao)bacillomycin F | fish intestine, Guangzhou, China | [75] | ||
| Bacillus licheniformis | lichenysin | x | deep oil well, Northern Germany | [76] | |
| Bacillus circulans | fengycins | marine samples, Andaman Nicobar Islands, India | [77] | ||
| Bacillus megaterium | iturin | seawater, Andaman Nicobar Islands, India | [78] | ||
| Bacillus sp. KCB14S006 | iturins | saltern, Incheon, South Korea | [79] | ||
| Bacillus amyloliquefaciens SH-B74 | plipastatin A1 | deep sea, South China Sea | [80] | ||
| Paenibacillus polymyxa | polymyxin B | red algae | [81] | ||
| fusaricidin B | |||||
| Brevibacillus laterosporus | tauramamide | tube worm, Loloata Island, Papua New Guinea | [82] | ||
| Aneurinibacillus aneurinilyticus | aneurinifactin | x | sea sediment, Gulf of Mannar, India | [83] | |
| Bacillus amyloliquefaciens | didemnin B | x | oil-contaminated water, Red Sea, Egypt | [84] | |
| Tistrella mobilis | didemnin B | seawater/tunicates, Red Sea, Pacific Ocean, and marine sediments in Japan | [85] | ||
| Achromobacter sp. HZ01. | Gly-Gly-Leu-Met-Leu-Leu | x | oil-contaminated water, Mabianzhou Island, southern China | [86] | |
| Pseudomonas sp. | massetolide | red algae, tubeworm, Moira Island and Masset Inlet, Canada | [87,88] | ||
| Pontibacter korlensis | pontifactin(miao)Ser-Asp-Val-Ser-Ser | x | contaminated seawater and sediment, coastal sites of Karaikal, India | [89] | |
| glycolipids | |||||
| Pseudomonas aeruginosa | rhamnolipid | x | x | contaminated seawater, Zhoushan Islands, China | [90] |
| Pseudomonas aeruginosa | rhamnolipid | x | coastal sediment, Odisha, India | [91] | |
| Pseudomonas aeruginosa | rhamnolipid | offshore sediment, Xiamen, China | [92] | ||
| Pseudomonas sp. BTN-1 | rhamnolipid | sediments, Baia Terranova, Antarctica | [93] | ||
| Pseudomonas sp. MCTG214(3b1) | rhamnolipid | x | x | coastal seawater, Sarasota Bay, Florida, US | [94] |
| Buttiauxella sp. | glucosyl ester lipid | mangrove forest, Qeshm Island, Iran | [95] | ||
| Serratia marcescens | glucosyl ester lipid | coral, Mandapam, India | [96] | ||
| Serratia rubidea | rubiwettin RG1 | n.a. | n.a. | [61] | |
| Alcanivorax borkumensis | glucose lipid | x | x | sediments, isle of Borkum, North Sea, Germany | [52,97,98] |
| Alcanivorax | rhamnolipid | x | x | chronically polluted harbour water, Elefsina Bay, Aegean Sea, Greece | [99] |
| Paracoccus | sophorolipid | x | x | chronically polluted harbour sediment, Elefsina Bay, Aegean Sea, Greece | |
| Arthrobacter sp. EK 1 | trehalose lipid tetraester | x | x | seawater, North Sea, Germany | [49,50] |
| Arthrobacter sp. SI 1 | trehalose lipid diester | x | x | seawater, North Sea, Germany | |
| Rhodococcus sp. strain PML026 | trehalose lipid | x | seawater, Plymouth, UK | [100] | |
| Rhodococcus sp. BS-15 | tri-glucose lipid tetraester, | x | deep sea sediment, Okinawa Trough | [101] | |
| Actinoalloteichus hymeniacidonis | Doktolipids(miao)(rhamnose lipids) | coastal sediment, Dokdo island, South Korea | [102] | ||
| Streptomyces sp. IA49E | di-rhamnolipid | x | coast with petrochemical facilities, Galveston Bay, Texas, USA | [103] | |
| Streptomyces sp.CNB-253 | phenazine-l quinovose ester | shallow sediments, Bodega Bay, CA, USA | [104] | ||
| Staphylococcus lentus | threose diester | snail, Mandapam, Tamil Nadu, India | [105] | ||
| Cyberlindnera saturnus | cybersan (galactose lipid) | x | polluted coastal sediment, Tamil Nadu, India | [106] | |
| Surface-Active Compound | Chemical Classification | Producing Bacterium | Marine Isolate 1 | Reference |
|---|---|---|---|---|
| surfactin | lipopeptide | Bacillus subtilis | - | [33,113,114] |
| lichenysin | lipopeptide | Bacillus licheniformis | + | [115,116] |
| fengycins | lipopeptide | Bacillus subtilis | - | [117] |
| iturin | lipopeptide | Bacillus subtilis | - | [118] |
| plipastatin A1 | lipopeptide | Bacillus subtilis | - | [119] |
| polymyxin B | lipopeptide | Paenibacillus polymyxa | - | [120] |
| fusaricidin B | lipopeptide | Paenibacillus polymyxa | - | [121] |
| didemnin B | lipopeptide | Tistrella mobilis | + | [85] |
| massetolides | lipopeptide | Pseudomonas fluorescens | - | [88] |
| rhamnolipids | glycolipid | Pseudomonas aeruginosa | - | [42,110] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubicki, S.; Bollinger, A.; Katzke, N.; Jaeger, K.-E.; Loeschcke, A.; Thies, S. Marine Biosurfactants: Biosynthesis, Structural Diversity and Biotechnological Applications. Mar. Drugs 2019, 17, 408. https://doi.org/10.3390/md17070408
Kubicki S, Bollinger A, Katzke N, Jaeger K-E, Loeschcke A, Thies S. Marine Biosurfactants: Biosynthesis, Structural Diversity and Biotechnological Applications. Marine Drugs. 2019; 17(7):408. https://doi.org/10.3390/md17070408
Chicago/Turabian StyleKubicki, Sonja, Alexander Bollinger, Nadine Katzke, Karl-Erich Jaeger, Anita Loeschcke, and Stephan Thies. 2019. "Marine Biosurfactants: Biosynthesis, Structural Diversity and Biotechnological Applications" Marine Drugs 17, no. 7: 408. https://doi.org/10.3390/md17070408
APA StyleKubicki, S., Bollinger, A., Katzke, N., Jaeger, K.-E., Loeschcke, A., & Thies, S. (2019). Marine Biosurfactants: Biosynthesis, Structural Diversity and Biotechnological Applications. Marine Drugs, 17(7), 408. https://doi.org/10.3390/md17070408