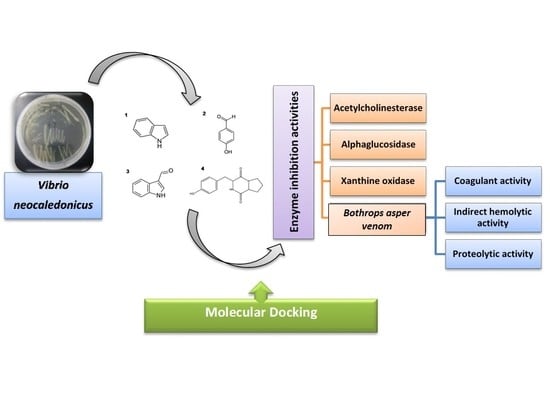
Bioactive Compounds Isolated from Marine Bacterium Vibrio neocaledonicus and Their Enzyme Inhibitory Activities
Abstract
1. Introduction
2. Results
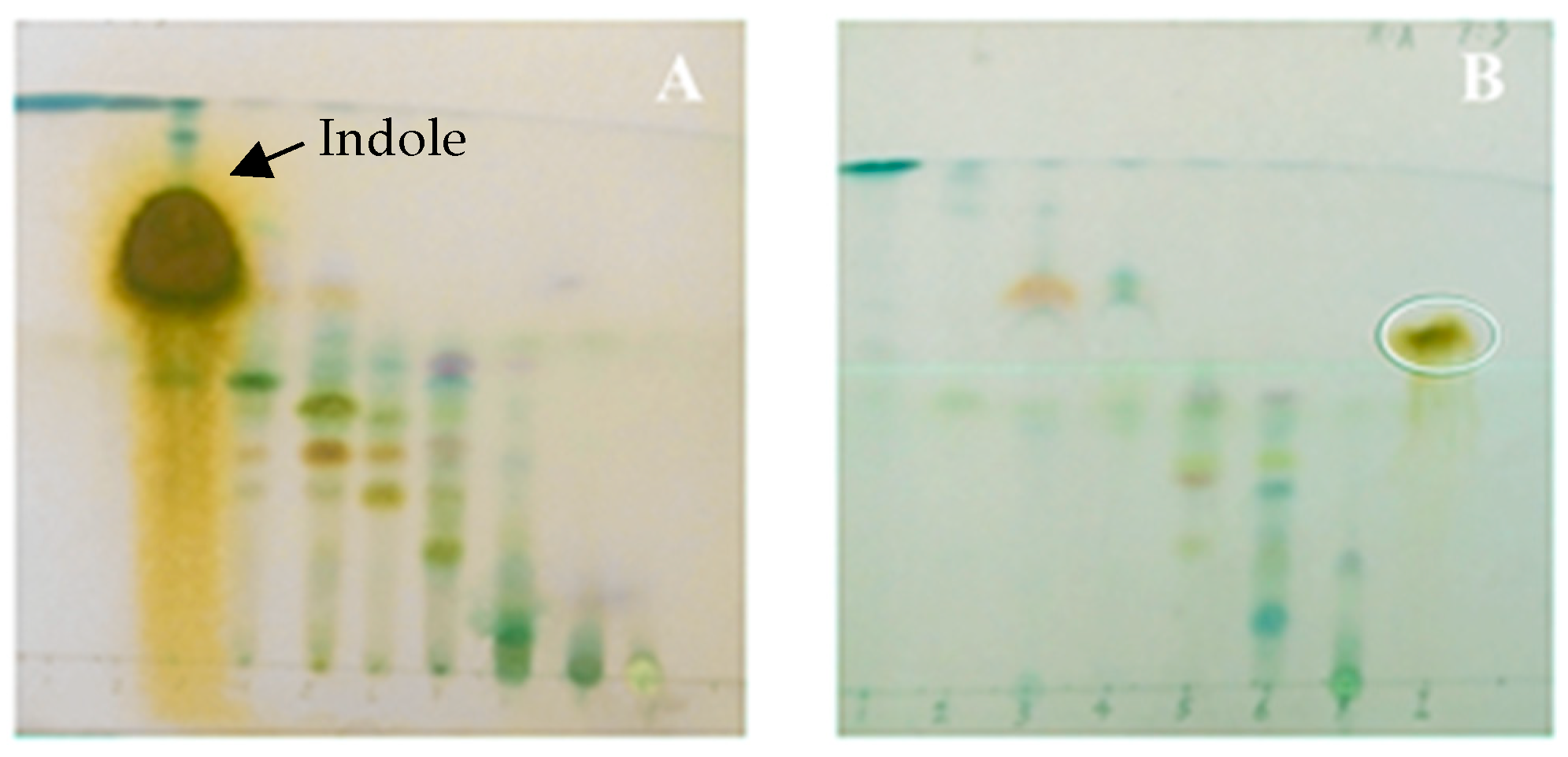
2.1. Culture Medium and Metabolite Production of Vibrio neocaledonicus
2.2. Structural Elucidation
2.3. In Vitro Acetylcholinesterase, Alphaglucosidase and Xanthine Oxidase Inhibition Assay
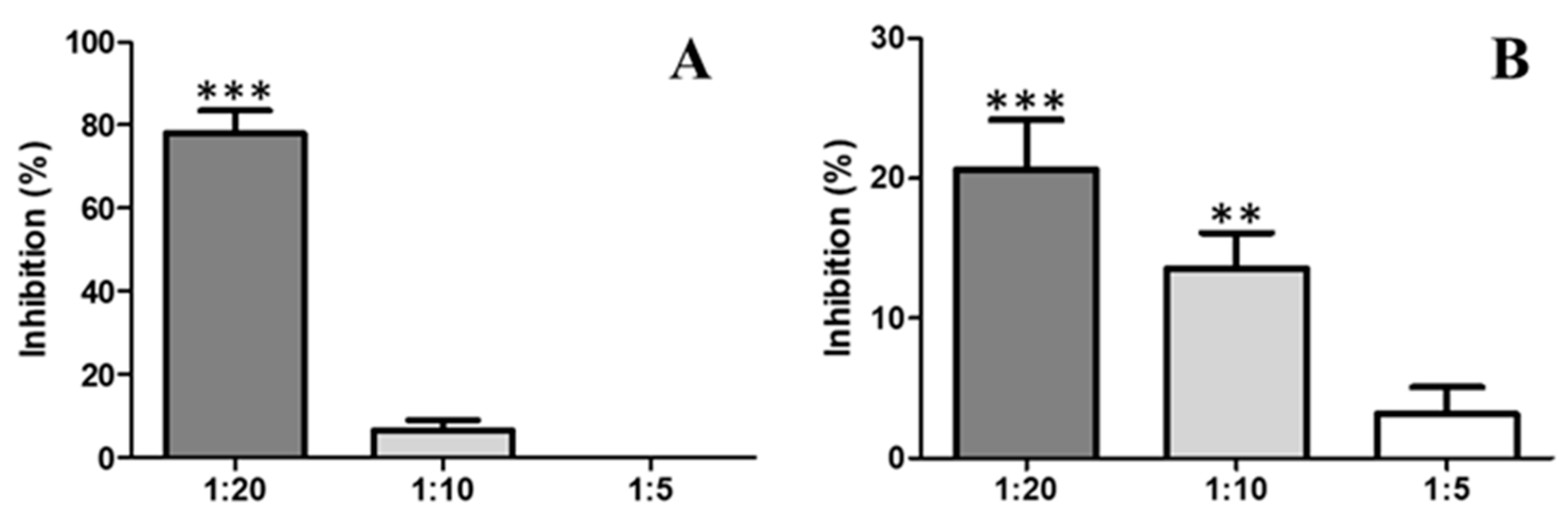
2.4. Antiophidic Activities
2.4.1. Inhibition of Proteolytic Activity by Indole
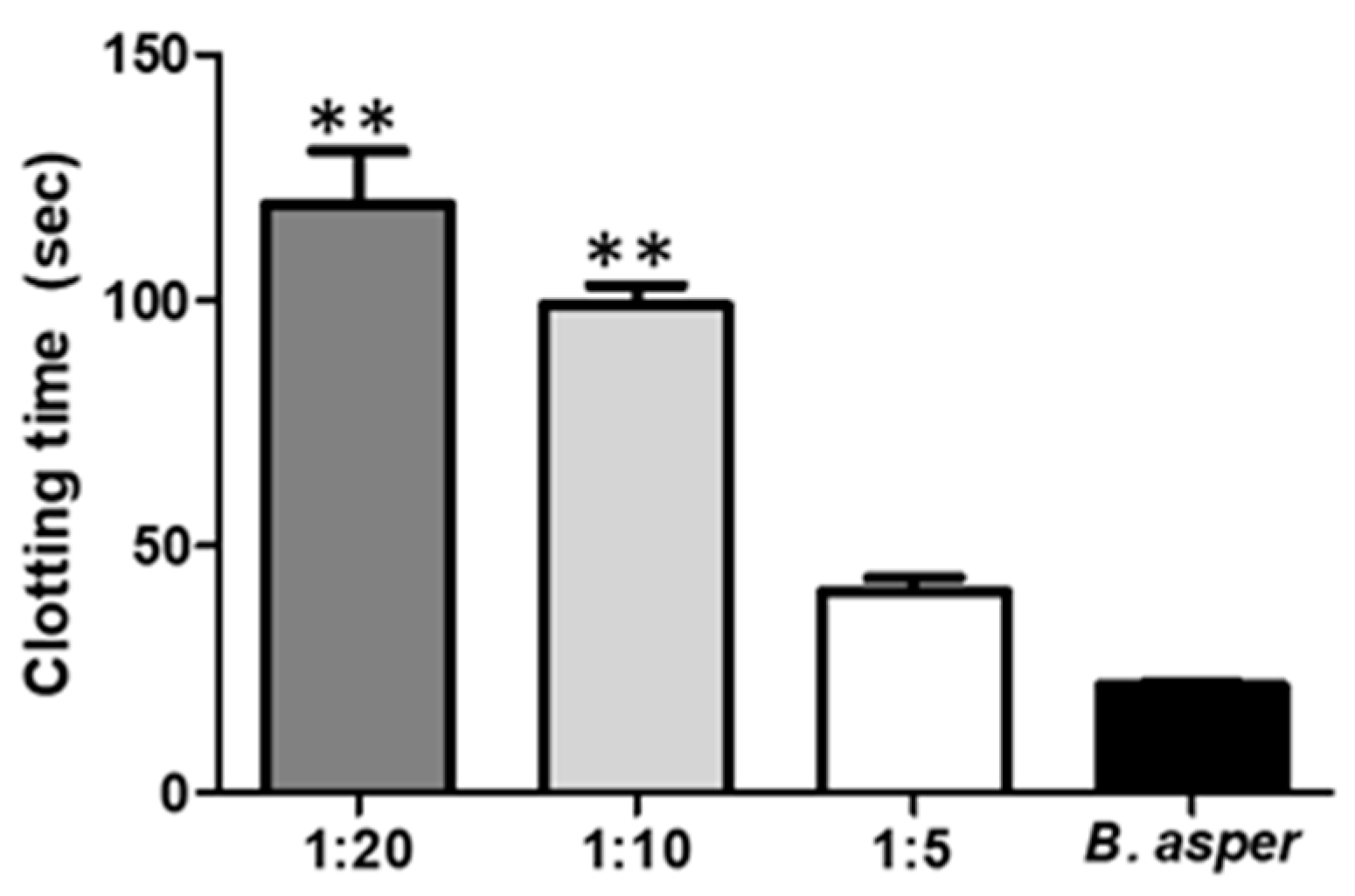
2.4.2. Inhibition of the Activity Coagulant by Indole
2.4.3. Inhibition of PLA2 Activity
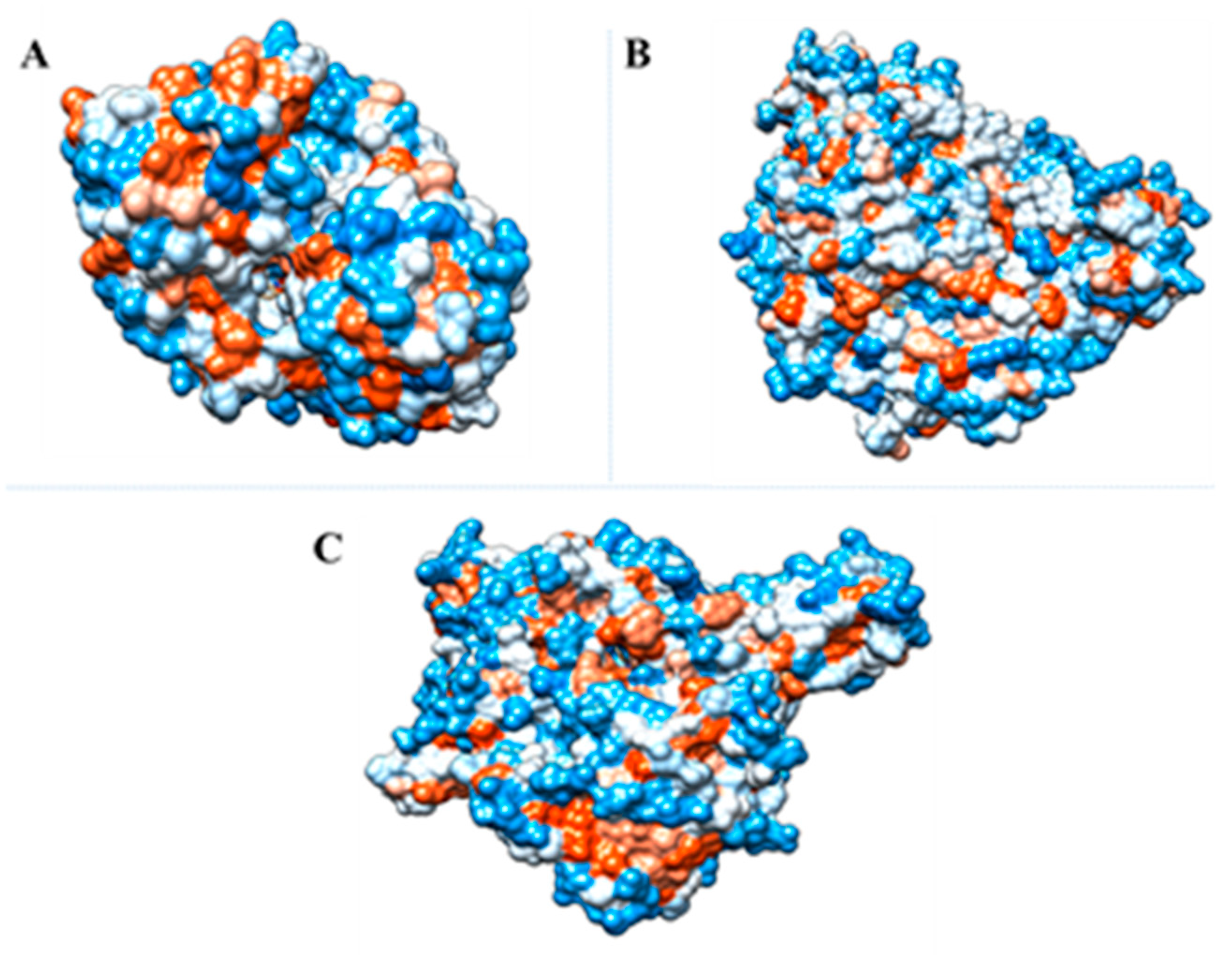
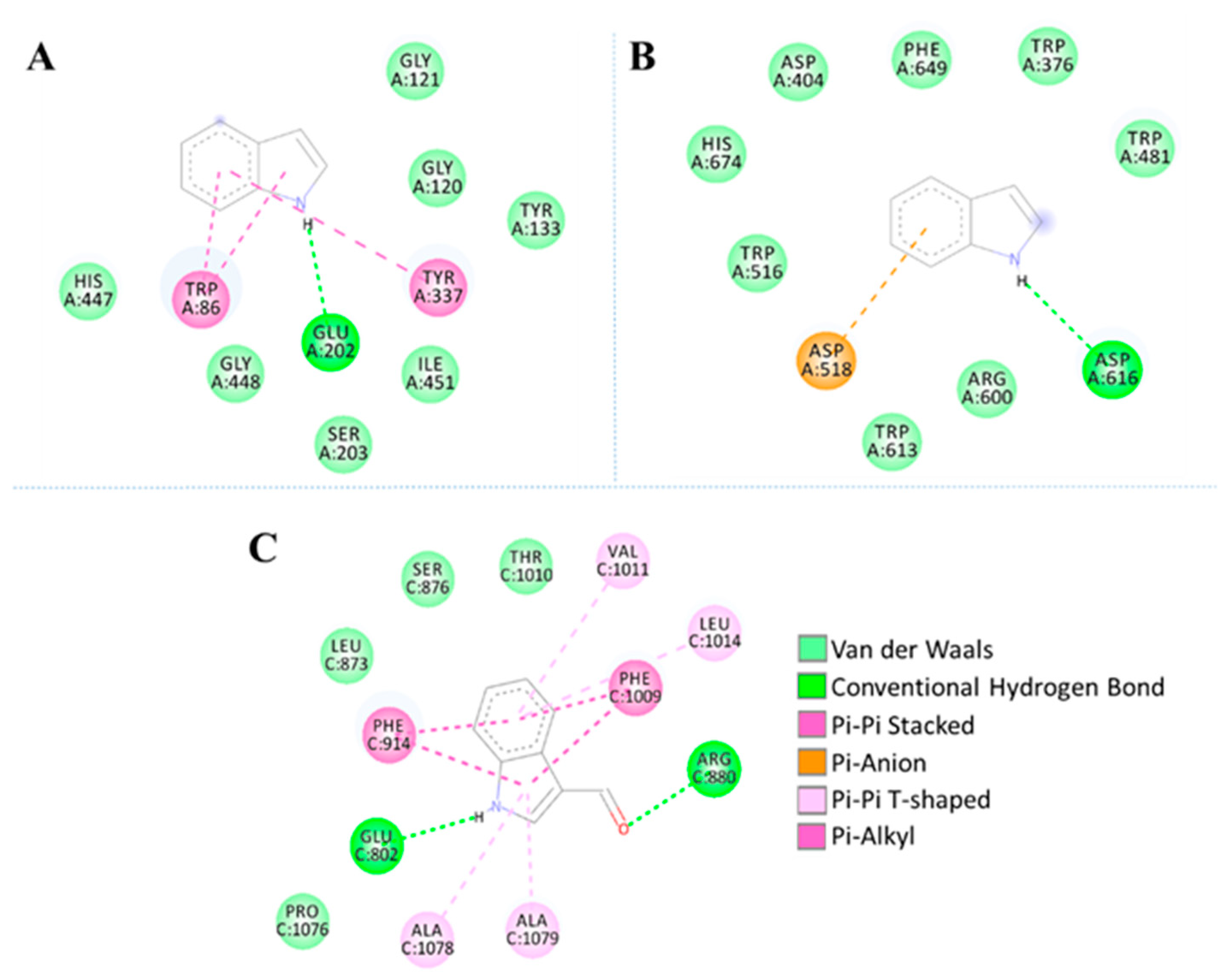
2.5. Molecular Docking Studies
3. Discussion
4. Materials and Methods
4.1. Sample and Reagents
4.2. Marine Bacterial Strain
4.3. Extraction Process
4.4. Isolation of Secondary Metabolites from Vibrio neocaledonicus
4.5. Acetylcholinesterase Inhibition Assay
4.6. Alphaglucosidase Inhibition Assay
4.7. Xanthine Oxidase Inhibition Assay
4.8. Antiophidic Activity
4.8.1. Purification of the Metalloproteinase (Batx-I) and Phospholipase A2 (Cdcum6)
4.8.2. Inhibition of PLA2 Activity
4.8.3. Inhibition of Coagulant Activity
4.8.4. Inhibition of Proteolytic Activity
4.9. Molecular Docking Studies
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nichols, C.M.; Guezennec, J.; Bowman, J. Bacterial Exopolysaccharides from Extreme Marine Environments with Special Consideration of the Southern Ocean, Sea Ice, and Deep-Sea Hydrothermal Vents: A Review. Mar. Biotechnol. 2005, 7, 253–271. [Google Scholar] [CrossRef] [PubMed]
- Harder, T.; Lau, S.C.K.; Dobretsov, S.; Fang, T.K.; Qian, P.Y. A distinctive epibiotic bacterial community on the soft coral Dendronephthya sp. and antibacterial activity of coral tissue extracts suggest a chemical mechanism against bacterial epubiosis. FEMS Microbiol. Ecol. 2003, 43, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Nissimov, J.; Rosenberg, E.; Munn, C.B. Antimicrobial properties of resident coral mucus bacteria of Oculina patagonica. FEMS Microbiol. Lett. 2009, 292, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Xiao, T.; Song, Z. Investigation of corrosion inhibitory process of marine Vibrio neocaledonicus sp. bacterium for carbon steel. Corros. Sci. 2015, 100, 186–193. [Google Scholar] [CrossRef]
- Moradi, M.; Xiao, T.; Song, Z. Introducing a novel bacterium, Vibrio neocaledonicus sp., with the highest corrosion inhibition efficiency. Electrochem. Commun. 2015, 51, 64–68. [Google Scholar] [CrossRef]
- Maria, M.; Lone, G.; Thomas, L. Production of Bioactive Secondary Metabolites by Marine Vibrionaceae. Mar. Drugs 2011, 9, 1440–1468. [Google Scholar]
- Tan, L.; Chen, C.; Guo, S.; Ma, F.; Gómez-Betancur, I.C. In vitro inhibition of acetylcholinesterase, alphaglucosidase and xanthine oxidase by bacterial from coral reef in Hainan, South China Sea. J. Mar. Sci. Eng. 2018, 6, 33. [Google Scholar] [CrossRef]
- Khan, M.T.H. Molecular interactions of cholinesterases inhibitors using in silico methods: Current status and future prospects. New Biotechnol. 2009, 25, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Ren, Y.; Howes, M.-J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181. [Google Scholar] [CrossRef]
- Tabet, N. Acetylcholinesterase inhibitors for Alzheimer’s disease: Anti-inflammatories in acetylcholine clothing. Age Ageing 2006, 35, 336–338. [Google Scholar] [CrossRef]
- Williams, P.; Sorribasab, A.; Howesc, M.J.R. Natural products as a source of Alzheimer’s drug leads. Nat. Prod. Rep. 2011, 28, 48–77. [Google Scholar] [CrossRef]
- Sony, P.; Ayinampudi, S.; Dipti, P.; Chityal, G.K.; Sudha, K.; Lipsa, C.; Soumya, S.D. A marine sponge associated strain of Bacillus subtilis and other marine bacteria can produce anticholinesterase compounds. Microb. Cell Factories 2014, 13, 24. [Google Scholar]
- Inkster, M.E.; Cotter, M.A.; Cameron, N.E. Treatment with the xanthine oxidase inhibitor, allopurinol, improves nerve and vascular function in diabetic rats. Eur. J. Pharmacol. 2007, 561, 63–71. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Otero, R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon 2009, 54, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venom: Structural characterization and mechanism of catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.A.; Moraes, F.R.; DeUllah, A.; Masood, R.; Santana, V.S.; Mariutti, R.; Arni, R.K. Three-Dimensional Structures and Mechanisms of Snake Venom Serine Proteinases, Metalloproteinases, and Phospholipase A2s. In Venom Genomics and Proteomics; Gopalakrishnakone, P., Calvete, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Bon, C. Venoms, antivenoms and immunotherapy. Toxicon 1996, 34, 142–143. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; León, G.; Lomonte, B.; Angulo, Y. Antivenoms for snakebite envenomings. Inflamm. Allergy Drug Targets 2011, 10, 369–380. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; León, G.; Rojas, G.; Lomonte, B.; Rucavado, A.; Chaves, F.; Gutiérrez, J.M. Neutralization of local tissue damage induced by Bothrops asper (terciopelo) snake venom. Toxicon 1998, 36, 1529–1538. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B.; León, G.; Rucavado, A.; Chaves, F.; Angulo, Y. Trends in Snakebite Envenomation Therapy: Scientific, Technological and Public Health Considerations. Curr. Pharm. Des. 2007, 13, 2935–2950. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Tan, K.; Yisha, B.; Fan, M.; Sun, X. Methods for Preparing Indole-3-Formaldehyde and Its Application in the Preparation of Drug for the Treatment of Hyperucemia and/or Gut. CN 108624633, 9 October 2018. [Google Scholar]
- Tan, L.; Yisha, B.; Sun, X.; Fan, M.; Ma, F. Natural Indole, Its Preparation Method and Application for Preparing Drugs for Treating Alzheimer’s Disease or Diabetes. CN 108299274, 20 July 2018. [Google Scholar]
- Jason, S.; Ian, R.; Baxendale, V.L.; Steven, V. Microwave assisted Leimgruber–Batcho reaction for the preparation of indoles, azaindoles and pyrroylquinolines. Org. Biomol. Chem. 2004, 2, 160–167. [Google Scholar]
- Ingrit, C.D.; Eduardo, G.; Massayoshi, Y. Constituyentes químicos del túbero de Dracontium spruceanum (Schott) G.Zhu ex Dracontium loretense Krause (Araceae). Rev. Soc. Química Perú 2011, 77, 117–126. [Google Scholar]
- Hiort, J. Marburg an der Lahn. Neue Naturstoffe aus Schwamm-Assoziierten Pilzen des Mittelmeeres-Isolierung Strukturaufklärung und Biologischen Aktivität. Ph.D. Thesis, Heinrich-Heine Universität, Düsseldorf, Gernany, 2002. [Google Scholar]
- SDBSWeb. Available online: http://sdbs.riodb.aist.go.jp National Institute of Advanced Industrial Science and Technology (accessed on 28 February 2018).
- Andrea, C.S.; John, H.C.; Gary, A.S. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternate. Proc. Natl. Acad. Sci. USA 1998, 85, 8008–8011. [Google Scholar]
- Knight, V.; Sanglier, J.-J.; DiTullio, D.; Braccili, S.; Bonner, P.; Waters, J.; Hughes, D.; Zhang, L. Diversifying microbial natural products for drug discovery. Appl. Microbiol. Biotechnol. 2003, 62, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Bull, A.T. The renaissance of continuous culture in the post-genomics age. J. Ind. Microbiol. Biotechnol. 2010, 37, 993–1021. [Google Scholar] [CrossRef]
- Shimada, N.; Hasegawa, S.; Harada, T.; Tomisawa, T.; Fujii, A.; Takita, T. Oxetanocin, a novel nucleoside from bacteria. J. Antibiot. 1986, 39, 1623–1625. [Google Scholar] [CrossRef]
- Aharonowitz, Y. Nitrogen Metabolite Regulation of Antibiotic Biosynthesis. Annu. Rev. Microbiol. 1980, 34, 209–233. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef]
- Veale, C.G.L.; Davies-Coleman, M.T. Chapter One—Marine Bi-, Bis-, and Trisindole Alkaloids. Alkaloids Chem. Biol. 2014, 73, 1–64. [Google Scholar] [PubMed]
- Mariyana, A.; Georgi, S.; Irena, P.; Dimitrina, Z.; Nikola, Y.; Irini, D. Galantamine derivatives with indole moiety: Docking, design, synthesis and acetylcholinesterase inhibitory activity. Bioorg. Med. Chem. 2015, 23, 5382–5389. [Google Scholar]
- Kim, K.; Nam, K.; Kurihara, H.; Kim, S. Potent α-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry 2008, 69, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.R.; Kokil, G.R. Development and Discovery Avenues in Bioactive Natural Products for Glycemic Novel Therapeutics; Elsevier: Amsterdam, The Netherlands, 2013; Volume 39, pp. 431–466. [Google Scholar]
- Liu, T.; Yip, Y.M.; Song, L.; Feng, S.; Liu, Y.; Lai, F.; Zhang, D.; Huang, D. Inhibiting enzymatic starch digestion by the phenolic compound diboside A: A mechanistic and in silico study. Food Res. Int. 2013, 54, 595–600. [Google Scholar] [CrossRef]
- Ha, J.-H.; Lee, D.-U.; Lee, J.-T.; Kim, J.-S.; Yong, C.-S.; Kim, J.-A.; Ha, J.-S.; Huh, K. 4-Hydroxybenzaldehyde from Gastrodia elata B1. is active in the antioxidation and GABAergic neuromodulation of the rat brain. J. Ethnopharmacol. 2000, 73, 329–333. [Google Scholar] [CrossRef]
- Tao, Y.-H.; Yuan, Z.; Tang, X.-Q.; Xu, H.-B.; Yang, X.-L. Inhibition of GABA shunt enzymes’ activity by 4-hydroxybenzaldehyde derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Heda, L.C.C.; Sharma, R.; Pareek, C.; Chaudhari, P.B. Synthesis and Antimicrobial Activity of Some Derivatives of 5-Substituted Indole Dihydropyrimidines. E-J. Chem. 2009, 6, 770–774. [Google Scholar] [CrossRef]
- Kakul, H.; Mohammad, A.; Amir, A. Synthesis, characterization and antiamoebic activity of new indole-3-carboxaldehyde thiosemicarbazones and their Pd(II) complexes. Eur. J. Med. Chem. 2007, 42, 1300–1308. [Google Scholar]
- Bell, R.; Carmeli, S.; Sar, N.; Vibrindole, A. A Metabolite of the Marine Bacterium, Vibrio parahaemolyticus, Isolated from the Toxic Mucus of the Boxfish Ostracion cubicus. J. Nat. Prod. 1994, 57, 1587–1590. [Google Scholar] [CrossRef]
- Unson, M.D.; Faulkner, D.J. Cyanobacterial symbiont biosynthesis of chlorinated metabolites from Dysidea herbacea (Porifera). Cell. Mol. Life Sci. 1993, 49, 349–353. [Google Scholar] [CrossRef]
- Laatsch, H. In Frontier in Marine Biotechnology; Proksch, P., Ed.; Horizon Bioscience: Norfolk, UK, 2006; pp. 225–288. [Google Scholar]
- Fernández, M.; Ortiz, W.; Pereañez, J.A.; Martínez, D. Evaluación de las propiedades antiofídicas del extracto etanólico y fracciones obtenidas de Renealmia alpinia (Rottb) mass (Zingiberaceae) cultivada in vitro. Vitae 2011, 17, 75–82. [Google Scholar]
- Otero, R.; Núñez, V.; Barona, J.; Fonnegra, R.; Jimenez, S.L.; Osorio, R.; Saldarriaga, M.A. Snakebites and ethnobotany in the North West region of Colombia. Part III: Neutralization of the haemorrhagic effect of Bothrops atrox venom. J. Ethnopharmacol. 2000, 73, 233–241. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Zhang, Y. Catalytic reaction mechanism of acetylcholinesterase determined by Born-Oppenheimer ab initio QM/MM Molecular Dynamics Simulations. J. Phys. Chem. B 2010, 114, 8817–8825. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Hongnan, C.; Russ, H. Substrate Orientation and Catalysis at the Molybdenum Site in Xanthine Oxidase. J. Biol. Chem. 2009, 284, 8760–8767. [Google Scholar]
- Véronique, R.Z.; Beatrice, C.P.; Roberta, I.; Maria, C.F.; Stanley, G.; Yves, B.; Giancarlo, P.; Marco, M.; Gerlind, S. Structure of human lysosomal acid α-glucosidase–A guide for the treatment of Pompe disease. Nat. Commun. 2017, 8, 1111. [Google Scholar]
- Atsushi, K.; Erina, H.; Saori, M.; Isao, A.; Tatsushi, I.; Yoshihiro, N.; Yuichi, Y.; Robert, N.; Hideyuki, S.; Izumi, N.; et al. α-1-C-Butyl-1,4-dideoxy-1,4-imino-l-arabinitol as a Second-Generation Iminosugar-Based Oral α-Glucosidase Inhibitor for Improving Postprandial Hyperglycemia. J. Med. Chem. 2012, 55, 10347–10362. [Google Scholar]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Pistia-Brueggeman, G.; Hollingsworth, R.I. A preparation and screening strategy for glycosidase inhibitors. Tetrahedron 2001, 57, 8773–8778. [Google Scholar] [CrossRef]
- Valentao, P.; Frenandes, E.; Carvalho, F.; Andrade, B.P.; Seabra, R.M.; Bastos, M.L. Studies on the antioxidant activity of Lippia citfiodora infusion: Scavenging effect on superoxide radical, hydroxyl radical and hypochlorous acid. Biol. Pharm. Bull. 2002, 25, 1324–1327. [Google Scholar] [CrossRef]
- López-Cruz, R.I.; Zenteno-Savín, T.; Galván-Magaña, F. Superoxide production, oxidative damage and enzymatic antioxidant defenses in shark skeletal muscle. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 50–56. [Google Scholar] [CrossRef]
- Patiño, A.C.; Pereañez, J.A.; Núñez, V.; Benjumea, D.M.; Fernández, M.; Rucavado, A.; Sanz, L.; Calvete, J.J. Isolation and biological characterization of Batx-I, a weak hemorrhagic and fibrinogenolytic PI metalloproteinase from Colombian Bothrops atrox venom. Toxicon 2010, 56, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Pereañez, J.A.; Núñez, V.; Huancahuire-Vega, S.; Marangoni, S.; Ponce-Soto, L.A. Biochemical and biological characterization of a PLA2 from crotoxin complex of Crotalus durissus cumanensis. Toxicon 2009, 53, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Mackessy, S.P. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon 1996, 34, 1149–1155. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Williams, D.; Fan, H.W.; Warrell, D.A. Snakebite envenoming from a global perspective: Towards an integrated approach. Toxicon 2010, 56, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Theakston, R.D.G.; Reid, H.A. Development of simple standard assay procedures for the characterization of snake venoms. Bull. World Health. Organ. 1983, 61, 949–956. [Google Scholar] [PubMed]
- Wang, W.-J.; Shih, C.-H.; Huang, T.-F. A novel P-I class metalloproteinase with broad substrate-cleaving activity, agkislysin, from Agkistrodon acutus venom. Biochem. Biophys. Res. Commun. 2004, 324, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Vriend, G. WHAT IF: A molecular modeling and drug design program. J. Mol. Graph. 1990, 8, 52–56. [Google Scholar] [CrossRef]
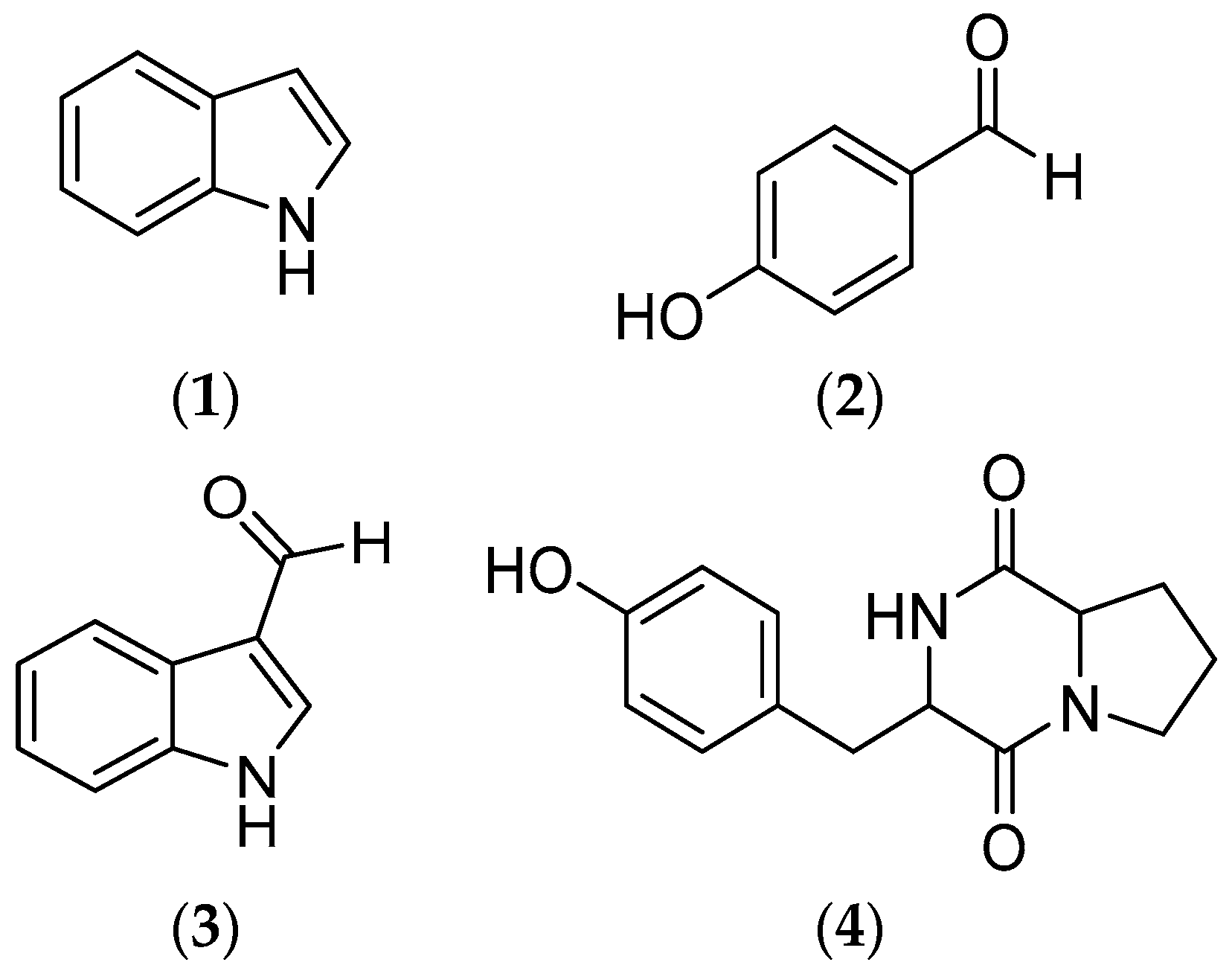

| Group | AchE (IC50 µM) | AG (IC50 µM) | XO (IC50 µM) |
|---|---|---|---|
| Huperzine (positive control) | 0.445 | - | - |
| Acarbose (positive control) | - | 387.21 ± 12.54 | - |
| Allopurinol (positive control) | - | - | 7.82 ± 0.12 |
| V. neocaledonicus extract | 15.27 ± 0.07 µg/mL | 53.52 ± 1.4 µg/mL | 341 ± 2.53 µg/mL |
| Indole | 334.81 ± 13.12 | 18.65 ± 1.1 | 842.58 ± 16.73 |
| 4-hydroxybenzaldehyde | 6892.25 ± 117.58 | - | 506.23 ± 16.01 |
| 1H-indole-3-carboxaldehyde | - | - | 13.36 ± 0.39 |
| Group | Dose (µg/mL) | AChEI% | AGI% | XOI% |
|---|---|---|---|---|
| V. neocaledonicus extract | 50 | 98.34 ± 0.52 *** | 47.28 ± 0.18 | 26.11 ± 0.03 |
| 25 | 69.67 ± 0.6 ** | 33.33 ± 0.95 | 25.54 ± 0.13 | |
| 12.5 | 22.34 ± 0.56 | 31.77 ± 0.45 | 24.62 ± 0.2 | |
| 6.25 | 15.1 ± 0.11 | 29.06 ± 0.26 | 22.49 ± 0.03 | |
| 3.125 | 6.23 ± 0.1 | 8.24 ± 0.03 | 7.62 ± 0.17 | |
| Indole | 50 | 51.3 ± 1.67 * | 100 *** | 44.30 ± 1.14 |
| 25 | 37.54 ± 1.26 | 94.4 ± 1.01 *** | 25.17 ± 0.65 | |
| 12.5 | 36.63 ± 1.04 | 85.49 ± 0.83 *** | 22.07 ± 0.55 | |
| 6.25 | 9.42 ± 0.07 | 81.49 ± 1.17 *** | 17.77 ± 0.52 | |
| 3.125 | 0 | 51.43 ± 1.06 * | 15.01 ± 0.31 | |
| 4-hydroxybenzaldehyde | 50 | 25.7 ± 0.56 | 0 | 50.75 ± 0.50 |
| 25 | 14.26 ± 0.011 | 0 | 31.67 ± 0.52 | |
| 12.5 | 6.55 ± 0.02 | 0 | 27.28 ± 0.27 | |
| 6.25 | 4.21 ± 0.01 | 0 | 25.27 ± 0.19 | |
| 3.125 | 0 | 0 | 17.39 ± 0.49 | |
| 1H-indole-3-carboxaldehyde | 50 | 0 | 0 | 100 *** |
| 25 | 0 | 0 | 86.49 ± 0.89 *** | |
| 12.5 | 0 | 0 | 71.29 ± 0.99 *** | |
| 6.25 | 0 | 0 | 67.69 ± 0.34 ** | |
| 3.125 | 0 | 0 | 61.14 ± 0.14 ** |
| Enzyme | Ligand | Interactions | Affinity (Kcal/mol) | |||||
|---|---|---|---|---|---|---|---|---|
| Hydrogen bonds | Van der Waals | π − π stacked | π-anion | π − π T-shaped | π-alkyl | |||
| AChE (PDB code 4PQE) | Indole | Glu202 | Gly120 Gly121 Tyr133 Ser203 His447 Ile451 Gly448 | Trp86 Tyr337 | - | - | - | −6.3 |
| AG (PDB code 5NN8) | Indole | Asp616 | Trp376 Asp404 Trp481 Trp516 Trp613 Arg600 His647 Phe649 | - | Asp518 | - | - | −5.5 |
| XO (PDB code 3NVY) | 1H-indole-3- carboxaldehyde | Arg880 Glu802 | Leu873 Ser876 Thr1010 Pro1076 | - | - | Phe914 Phe1009 | Val1011 Leu1014 Ala1078 Ala1079 | −7.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Betancur, I.; Zhao, J.; Tan, L.; Chen, C.; Yu, G.; Rey-Suárez, P.; Preciado, L. Bioactive Compounds Isolated from Marine Bacterium Vibrio neocaledonicus and Their Enzyme Inhibitory Activities. Mar. Drugs 2019, 17, 401. https://doi.org/10.3390/md17070401
Gómez-Betancur I, Zhao J, Tan L, Chen C, Yu G, Rey-Suárez P, Preciado L. Bioactive Compounds Isolated from Marine Bacterium Vibrio neocaledonicus and Their Enzyme Inhibitory Activities. Marine Drugs. 2019; 17(7):401. https://doi.org/10.3390/md17070401
Chicago/Turabian StyleGómez-Betancur, Isabel, Jianping Zhao, Lin Tan, Chang Chen, Ge Yu, Paola Rey-Suárez, and Lina Preciado. 2019. "Bioactive Compounds Isolated from Marine Bacterium Vibrio neocaledonicus and Their Enzyme Inhibitory Activities" Marine Drugs 17, no. 7: 401. https://doi.org/10.3390/md17070401
APA StyleGómez-Betancur, I., Zhao, J., Tan, L., Chen, C., Yu, G., Rey-Suárez, P., & Preciado, L. (2019). Bioactive Compounds Isolated from Marine Bacterium Vibrio neocaledonicus and Their Enzyme Inhibitory Activities. Marine Drugs, 17(7), 401. https://doi.org/10.3390/md17070401