Enantioselective Hydrolysis of Styrene Oxide and Benzyl Glycidyl Ether by a Variant of Epoxide Hydrolase from Agromyces mediolanus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of the Recombinant vEH-Am
2.2. Thermal Stability of vEH-Am
2.3. Hydrolysis of Racemic SO and BGE by vEH-Am
2.4. Kinetic Study of vEH-Am
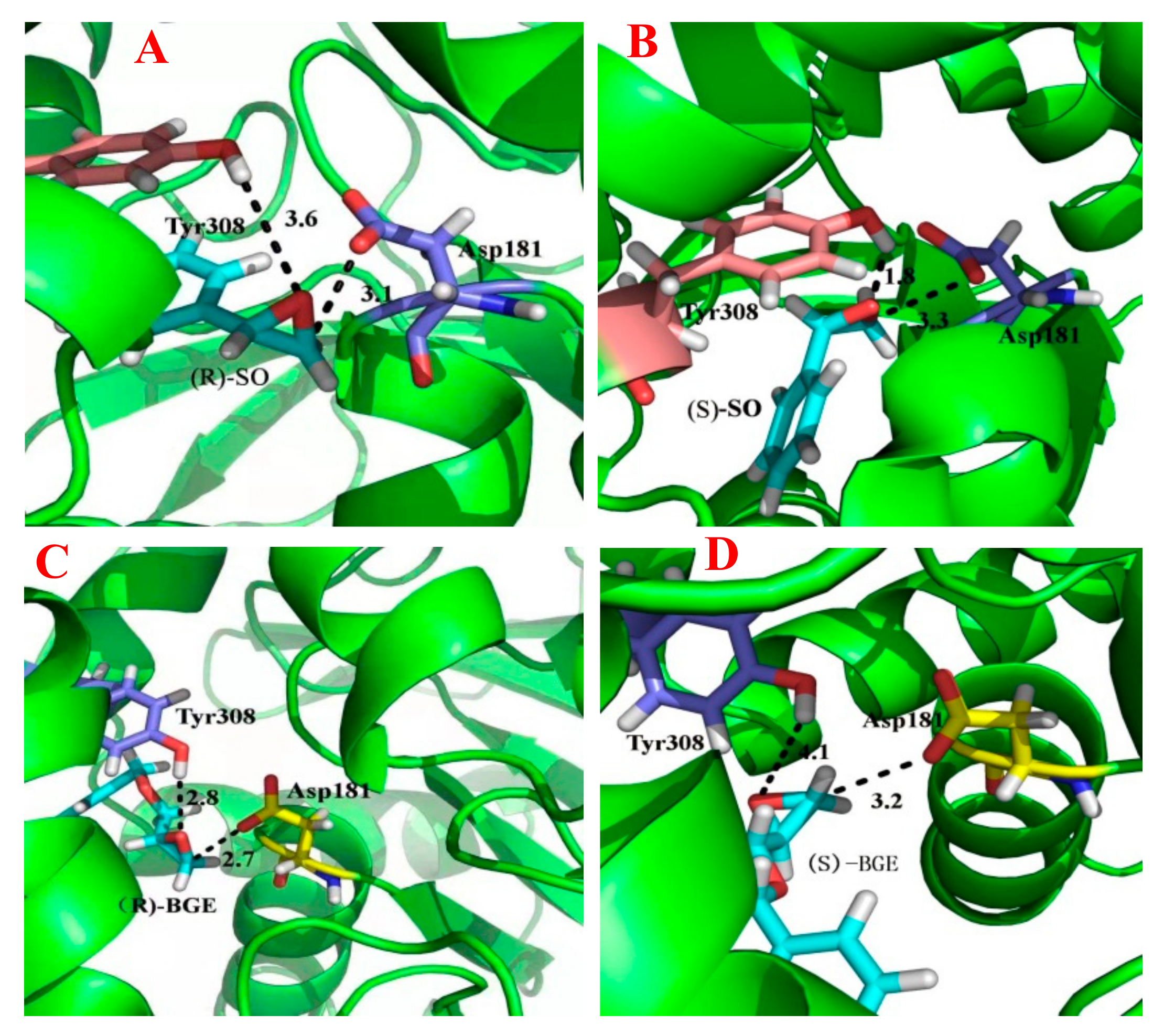
2.5. Homology Structual Modeling and Substrate Docking
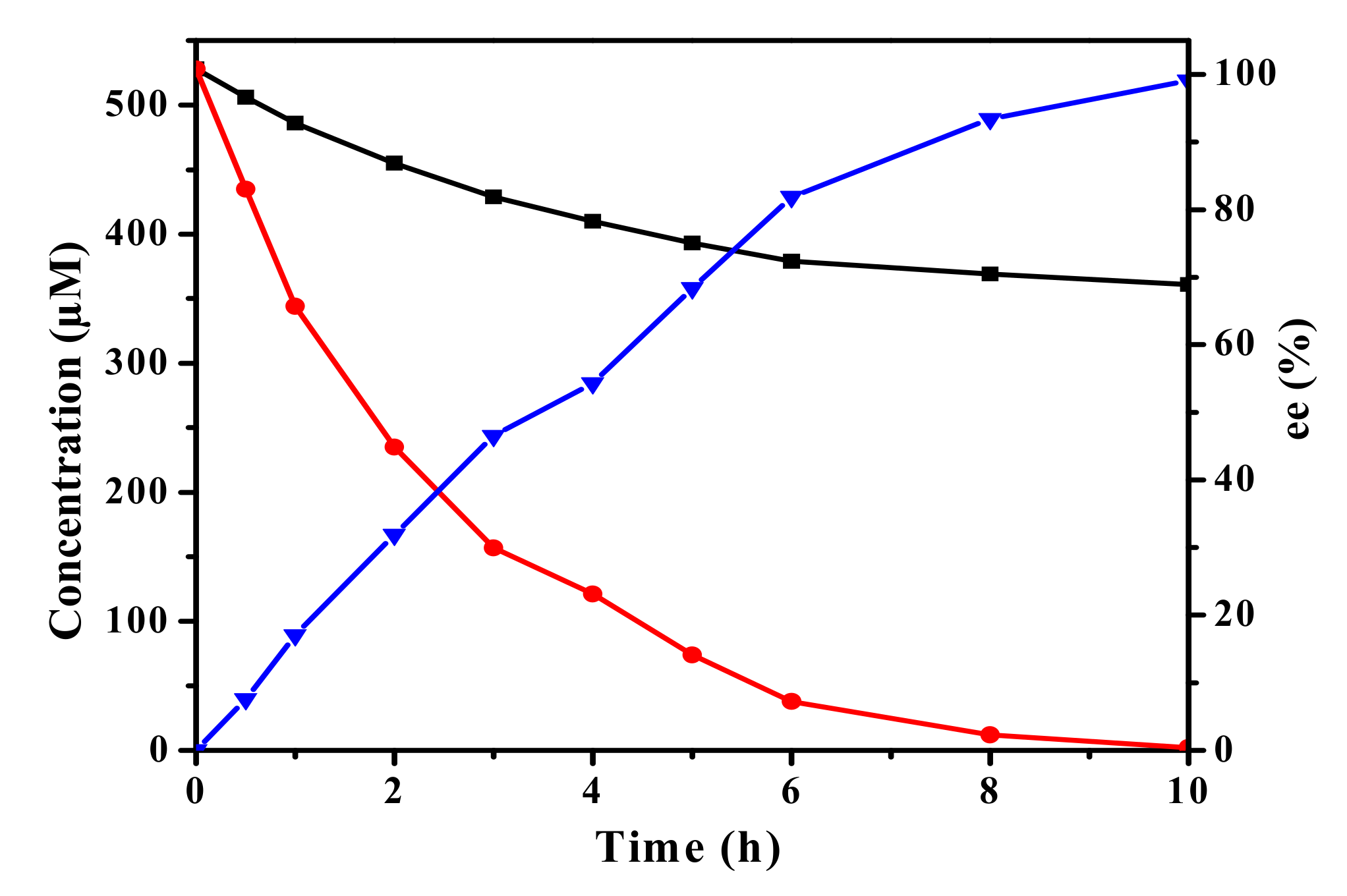
2.6. Biocatalytic Synthesis of (R)-SO and (S)-BGE
3. Materials and Methods
3.1. Materials
3.2. Cell Culture and Protein Expression
3.3. Purification of vEH-Am
3.4. Thermostability of vEH-Am
3.5. Activity Assay and Analytical Methods
3.6. Determination of Kinetic Properties
3.7. Homology Modeling and Docking
3.8. vEH-Am Hydrolysis of Racemic SO and BGE
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chang, D.; Wang, Z.; Heringa, M.F.; Wirthner, R.; Witholt, B.; Li, Z. Highly enantioselective hydrolysis of alicyclic meso-epoxides with a bacterial epoxide hydrolase from Sphingomonas sp. HXN-200: Simple syntheses of alicyclic vicinal trans-diols. Chem. Commun. 2003, 8, 960–961. [Google Scholar] [CrossRef]
- Wu, S.; Li, A.; Chin, Y.S.; Li, Z. Enantioselective Hydrolysis of Racemic and Meso-Epoxides with Recombinant Escherichia coli Expressing Epoxide Hydrolase from Sphingomonas sp. HXN-200: Preparation of Epoxides and Vicinal Diols in High ee and High Concentration. Acs Catal. 2013, 3, 752–759. [Google Scholar] [CrossRef]
- Hu, D.; Wang, R.; Shi, X.L.; Ye, H.H.; Wu, Q.; Wu, M.C.; Chu, J.J. Kinetic resolution of racemic styrene oxide at a high concentration by recombinant Aspergillus usamii epoxide hydrolase in an n-hexanol/buffer biphasic system. J. Biotechnol. 2016, 236, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Chen, Y.; Shen, H.; Wang, S.; Chen, L.; Zhu, Q. Biocatalytic resolution of benzyl glycidyl ether and its derivates by Talaromyces flavus: effect of phenyl ring substituents on enantioselectivity. Biotechnol. Lett. 2012, 34, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, G.; Gopal, P.; Reddy, C.N.; Yadav, J.S. First stereoselective synthesis of synargentolide A and revision of absolute stereochemistry. Tetrahedron Lett. 2009, 50, 6298–6302. [Google Scholar] [CrossRef]
- Saini, P.; Kumar, N.; Wani, S.I.; Sharma, S.; Chimni, S.S.; Sareen, D. Bioresolution of racemic phenyl glycidyl ether by a putative recombinant epoxide hydrolase from Streptomyces griseus NBRC 13350. World J. Microbiol. Biotechnol. 2017, 33, 82. [Google Scholar] [CrossRef]
- Woo, J.H.; Lee, E.Y. Enantioselective hydrolysis of racemic styrene oxide and its substituted derivatives using newly-isolated Sphingopyxis sp. exhibiting a novel epoxide hydrolase activity. Biotechnol. Lett. 2014, 36, 357–362. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, H.S.; Kim, S.J.; Park, S.; Kim, B.J.; Shuler, M.L.; Lee, E.Y. Cloning, expression and enantioselective hydrolytic catalysis of a microsomal epoxide hydrolase from a marine fish, Mugil cephalus. Biotechnol. Lett. 2007, 29, 237–246. [Google Scholar] [CrossRef]
- Hu, D.; Tang, C.; Li, C.; Kan, T.; Shi, X.; Feng, L.; Wu, M. Stereoselective Hydrolysis of Epoxides by reVrEH3, a Novel Vigna radiata Epoxide Hydrolase with High Enantioselectivity or High and Complementary Regioselectivity. J. Agric. Food Chem. 2017, 65, 9861–9870. [Google Scholar] [CrossRef]
- Woo, J.H.; Kwon, T.H.; Kim, J.T.; Kim, C.G.; Lee, E.Y. Identification and characterization of epoxide hydrolase activity of polycyclic aromatic hydrocarbon-degrading bacteria for biocatalytic resolution of racemic styrene oxide and styrene oxide derivatives. Biotechnol. Lett. 2013, 35, 599–606. [Google Scholar] [CrossRef]
- Bala, N.; Kaur, K.; Chimni, S.S.; Saini, H.S.; Kanwar, S.S. Bioresolution of benzyl glycidyl ether using whole cells of Bacillus alcalophilus. J. Basic Microbial. 2012, 52, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Kotik, M.; Stepanek, V.; Kyslik, P.; Maresova, H. Cloning of an epoxide hydrolase-encoding gene from Aspergillus niger M200, overexpression in E. coli, and modification of activity and enantioselectivity of the enzyme by protein engineering. J. Biotechnol. 2007, 132, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Hwang, Y.O.; Kang, S.G.; Lee, H.S.; Cho, J.C.; Kim, S.J. Cloning and characterization of three epoxide hydrolases from a marine bacterium, Erythrobacter litoralis HTCC2594. Appl. Microbial. Biotechnol. 2007, 76, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.O.; Kang, S.G.; Woo, J.H.; Kwon, K.K.; Sato, T.; Lee, E.Y.; Han, M.S.; Kim, S.J. Screening enantioselective epoxide hydrolase activities from marine microorganisms: detection of activities in Erythrobacter spp. Mar. Biotechnol. 2008, 10, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.P.; Mouad, A.M.; Boschini, L.; Regali Seleghim, M.H.; Sette, L.D.; Meleiro Porto, A.L. Marine fungi Aspergillus sydowii and Trichoderma sp. catalyze the hydrolysis of benzyl glycidyl ether. Mar. Biotechnol. 2011, 13, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Kang, J.H.; Hwang, Y.O.; Cho, J.C.; Kim, S.J.; Kang, S.G. Biocatalytic resolution of glycidyl phenyl ether using a novel epoxide hydrolase from a marine bacterium, Maritimibacter alkaliphilus KCCM 42376. J. Biosci. Bioeng. 2010, 109, 539–544. [Google Scholar] [CrossRef]
- Xue, F.; Liu, Z.Q.; Zou, S.P.; Wan, N.W.; Zhu, W.Y.; Zhu, Q.; Zheng, Y.G. A novel enantioselective epoxide hydrolase from Agromyces mediolanus ZJB120203: Cloning, characterization and application. Process Biochem. 2014, 49, 409–417. [Google Scholar] [CrossRef]
- Xue, F.; Liu, Z.Q.; Wan, N.W.; Zhu, H.Q.; Zheng, Y.G. Engineering the epoxide hydrolase from Agromyces mediolanus for enhanced enantioselectivity and activity in the kinetic resolution of racemic epichlorohydrin. RSC Adv. 2015, 5, 31525–31532. [Google Scholar] [CrossRef]
- Yoo, S.S.; Park, S.; Lee, E.Y. Enantioselective resolution of racemic styrene oxide at high concentration using recombinant Pichia pastoris expressing epoxide hydrolase of Rhodotorula glutinis in the presence of surfactant and glycerol. Biotechnol. Lett. 2008, 30, 1807–1810. [Google Scholar] [CrossRef]
- Weijers, C.A.G.M. Enantioselective hydrolysis of aryl, alicyclic and aliphatic epoxides by Rhodotorula glutinis. Tetrahedron Asymm. 1997, 8, 639–647. [Google Scholar] [CrossRef]
- Bendigiri, C.; Harini, K.; Yenkar, S.; Zinjarde, S.; Sowdhamini, R.; RaviKumar, A. Evaluating Ylehd, a recombinant epoxide hydrolase from Yarrowia lipolytica as a potential biocatalyst for the resolution of benzyl glycidyl ether. RSC Adv. 2018, 8, 12918–12926. [Google Scholar] [CrossRef]
- Kotik, M.; Kyslik, P. Purification and characterisation of a novel enantioselective epoxide hydrolase from Aspergillus niger M200. Biochim. Biophys. Acta 2006, 1760, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Reetz, M.T. Manipulating the stereoselectivity of limonene epoxide hydrolase by directed evolution based on iterative saturation mutagenesis. J. Am. Chem. Soc. 2010, 132, 15744–15751. [Google Scholar] [CrossRef] [PubMed]
- Saenz-Mendez, P.; Katz, A.; Perez-Kempner, M.L.; Ventura, O.N.; Vazquez, M. Structural insights into human microsomal epoxide hydrolase by combined homology modeling, molecular dynamics simulations, and molecular docking calculations. Proteins 2017, 85, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T.; Bocola, M.; Wang, L.W.; Sanchis, J.; Cronin, A.; Arand, M.; Zou, J.; Archelas, A.; Bottalla, A.L.; Naworyta, A.; et al. Directed evolution of an enantioselective epoxide hydrolase: Uncovering the source of enantioselectivity at each evolutionary stage. J. Am. Chem. Soc. 2009, 131, 7334–7343. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hu, D.; Zong, X.; Li, J.; Ding, L.; Wu, M.; Li, J. Enantioconvergent hydrolysis of racemic styrene oxide at high concentration by a pair of novel epoxide hydrolases into (R)-phenyl-1,2-ethanediol. Biotechnol. Lett. 2017, 39, 1917–1923. [Google Scholar] [CrossRef]
- Zou, S.P.; Zheng, Y.G.; Wu, Q.; Wang, Z.C.; Xue, Y.P.; Liu, Z.Q. Enhanced catalytic efficiency and enantioselectivity of epoxide hydrolase from Agrobacterium radiobacter AD1 by iterative saturation mutagenesis for (R)-epichlorohydrin synthesis. Appl. Microbial. Biotechnol. 2018, 102, 733–742. [Google Scholar] [CrossRef]
- Jin, H.X.; Liu, Z.Q.; Hu, Z.C.; Zheng, Y.G. Biosynthesis of (R)-epichlorohydrin at high substrate concentration by kinetic resolution of racemic epichlorohydrin with a recombinant epoxide hydrolase. Eng. Life Sci. 2013, 13, 385–392. [Google Scholar] [CrossRef]
- Chen, W.J.; Lou, W.Y.; Yu, C.Y.; Wu, H.; Zong, M.H.; Smith, T.J. Use of hydrophilic ionic liquids in a two-phase system to improve Mung bean epoxide hydrolases-mediated asymmetric hydrolysis of styrene oxide. J. Biotechnol. 2012, 162, 183–190. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zhang, L.; Sun, L.H.; Li, X.J.; Wan, N.W.; Zheng, Y.G. Enzymatic production of 5’-inosinic acid by a newly synthesised acid phosphatase/phosphotransferase. Food Chem. 2012, 134, 948–956. [Google Scholar] [CrossRef]






| Temperature (°C) | Inactivation Constant (h−1) | Half-Life (h) |
|---|---|---|
| 30 37 50 | 0.014 0.087 0.15 | 51.1 8.0 4.7 |
| EH Source | Absolute Configuration | ee (%) | E-Values | Reference |
|---|---|---|---|---|
| Talaromyces flavus | R | 96 | 13 | [4] |
| Rhodotorula glutinis | R | 98 | 6.5 | [20] |
| Yarrowia lipolytica | R | 95 | 10.4 | [21] |
| Aspergillus niger | R | <60 | 3 | [22] |
| Aspergillus sydowii | R | <46 | / | [15] |
| Streptomyces griseus | R | 7 | 1.5 | [6] |
| Trichoderma sp. | S | <60 | / | [15] |
| Bacillus alcalophilus | S | 30 | / | [11] |
| Agromyces mediolanus (W182F/S207V/N240D) | S | >99 | 14.5 | This study |
| Kinetic Parameters | (R)-SO | (S)-SO | (R)-BGE | (S)-BGE |
|---|---|---|---|---|
| Vm (μmol·min−1·mg−1) Km (mM) | 15.9 5.2 | 3.4 0.9 | 32.1 9.3 | 63.3 36.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Li, Y.; Zhang, Q.; Lin, S.; Yang, Z.; Ding, G. Enantioselective Hydrolysis of Styrene Oxide and Benzyl Glycidyl Ether by a Variant of Epoxide Hydrolase from Agromyces mediolanus. Mar. Drugs 2019, 17, 367. https://doi.org/10.3390/md17060367
Jin H, Li Y, Zhang Q, Lin S, Yang Z, Ding G. Enantioselective Hydrolysis of Styrene Oxide and Benzyl Glycidyl Ether by a Variant of Epoxide Hydrolase from Agromyces mediolanus. Marine Drugs. 2019; 17(6):367. https://doi.org/10.3390/md17060367
Chicago/Turabian StyleJin, Huoxi, Yan Li, Qianwei Zhang, Saijun Lin, Zuisu Yang, and Guofang Ding. 2019. "Enantioselective Hydrolysis of Styrene Oxide and Benzyl Glycidyl Ether by a Variant of Epoxide Hydrolase from Agromyces mediolanus" Marine Drugs 17, no. 6: 367. https://doi.org/10.3390/md17060367
APA StyleJin, H., Li, Y., Zhang, Q., Lin, S., Yang, Z., & Ding, G. (2019). Enantioselective Hydrolysis of Styrene Oxide and Benzyl Glycidyl Ether by a Variant of Epoxide Hydrolase from Agromyces mediolanus. Marine Drugs, 17(6), 367. https://doi.org/10.3390/md17060367




