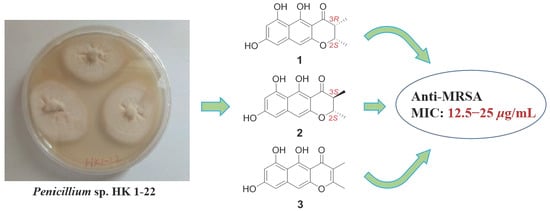
New Naphtho-γ-Pyrones Isolated from Marine-Derived Fungus Penicillium sp. HK1-22 and Their Antimicrobial Activities
Abstract
1. Introduction
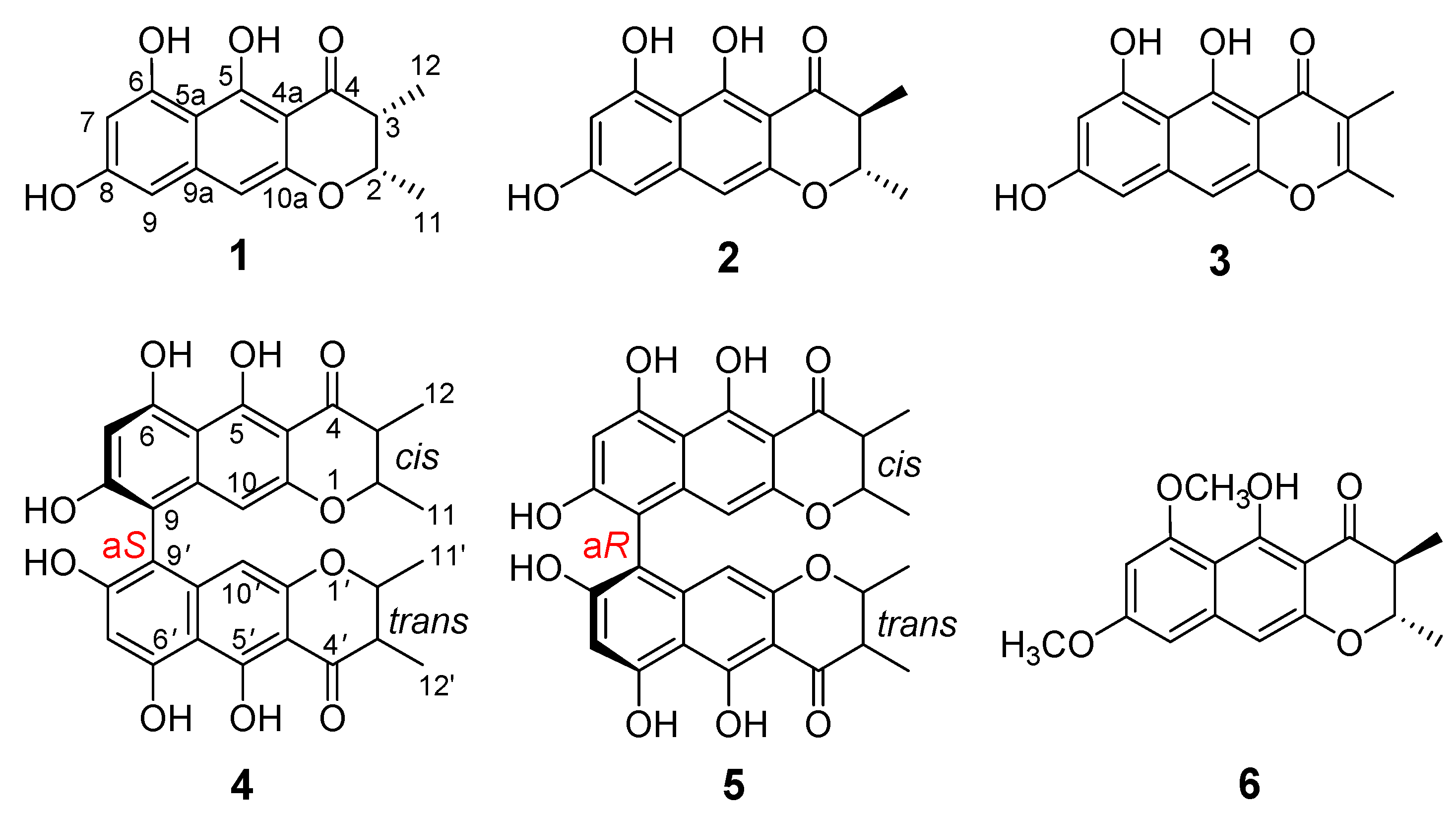
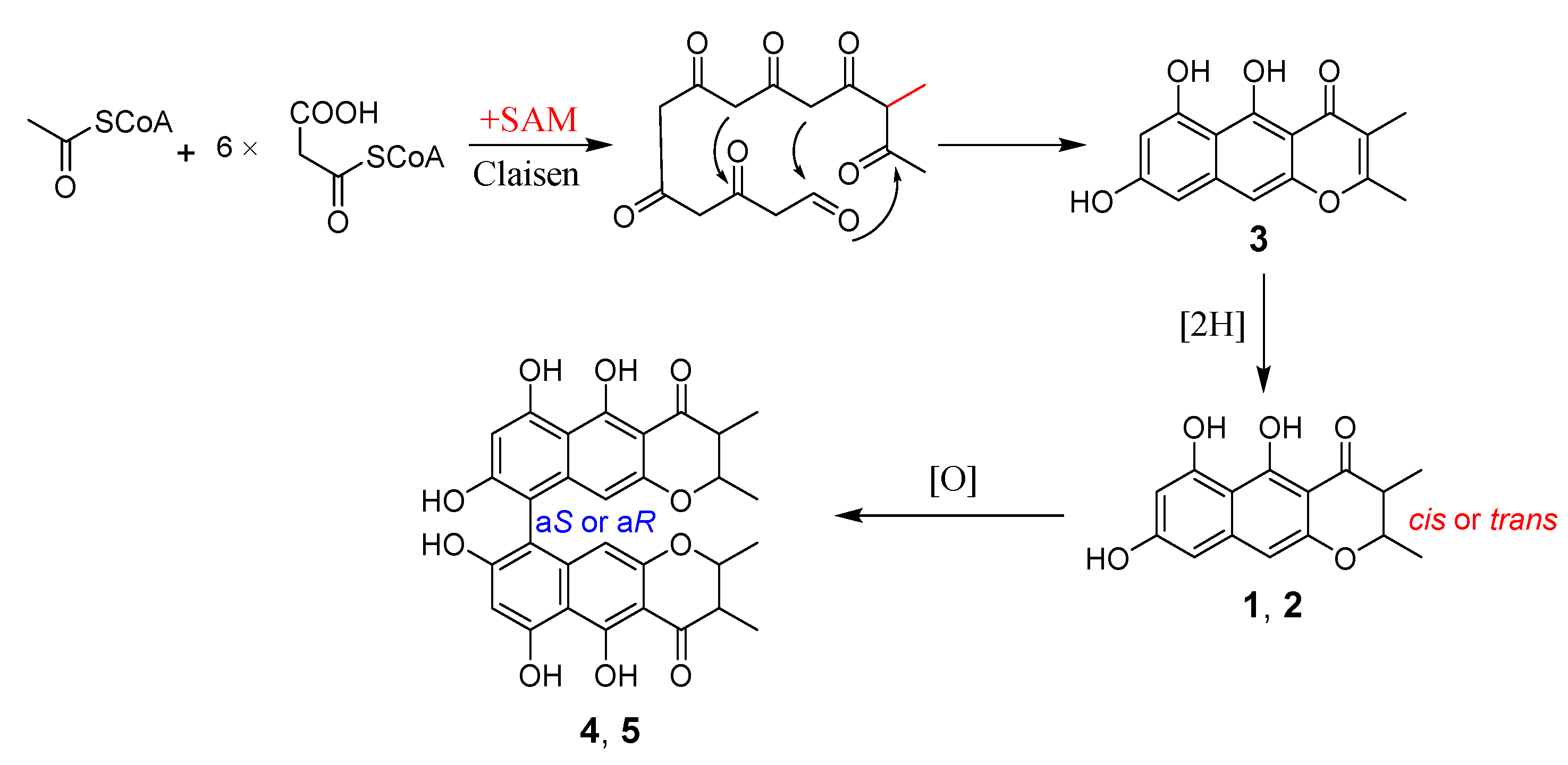
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
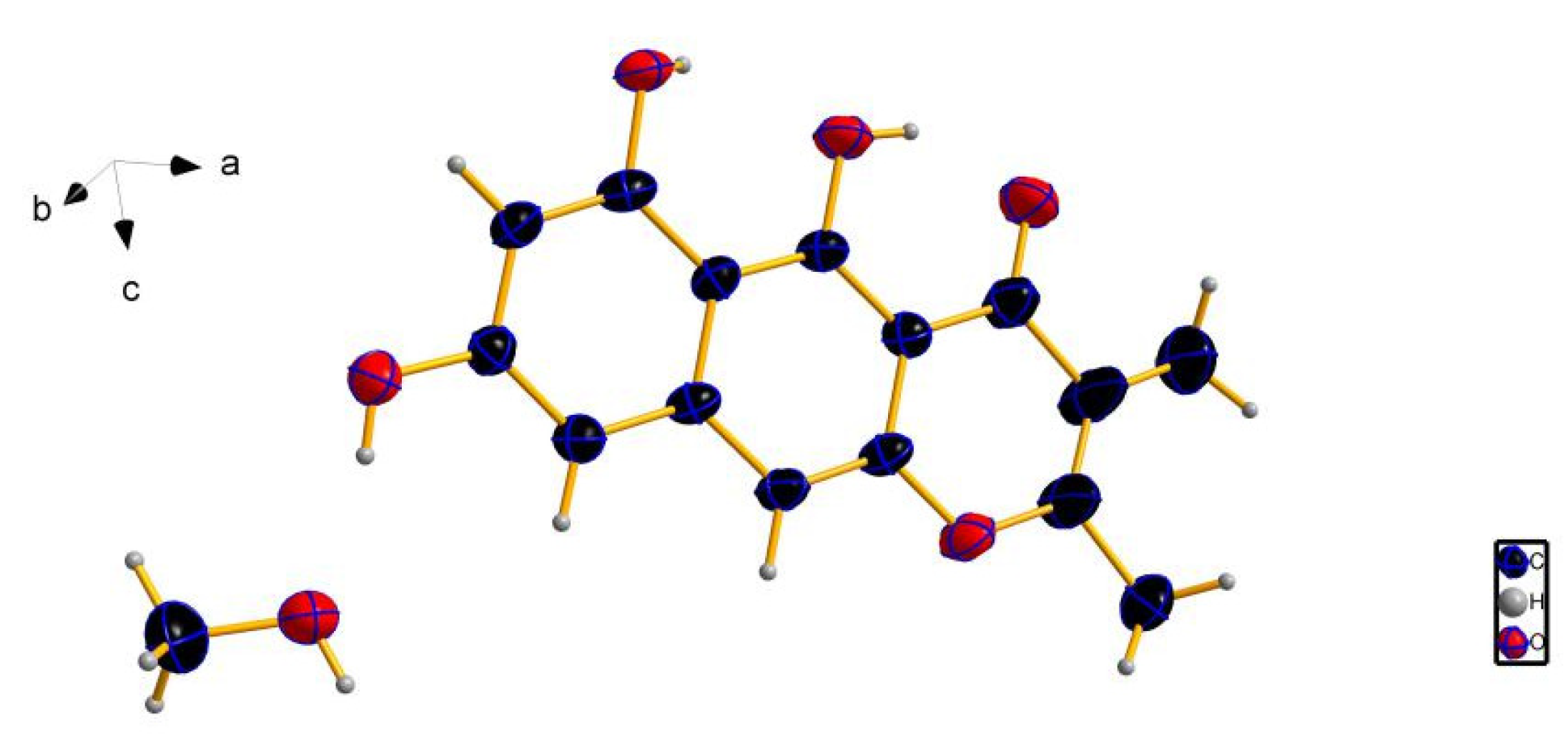
3.4. X-ray Crystallographic Analysis of Compound 3
3.5. Biological Assays
3.5.1. Antibacterial Assay
3.5.2. Anti-Phytopathogenic Assay
3.5.3. Cytotoxic Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef]
- Swathi, J.; Narendra, K.; Sowjanya, K.M.; Satya, A.K. Marine fungal metabolites as a rich source of bioactive compounds. Afr. J. Biochem. Res. 2013, 7, 184–196. [Google Scholar] [CrossRef]
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.Y.; Zhang, J.Y.; Liang, Y.J.; Chen, L.M.; Zhen, L.S.; Wang, F.; Mi, Y.J.; She, Z.G.; To, K.K.W.; Lin, Y.C.; et al. Anticancer effect and structure-activity analysis of marine products isolated from metabolites of mangrove fungi in the South China Sea. Mar. Drugs 2010, 8, 1094–1105. [Google Scholar] [CrossRef]
- Xu, J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Deng, C.M.; Liu, S.X.; Huang, C.H.; Pang, J.Y.; Lin, Y.C. Secondary metabolites of a mangrove endophytic fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Mar. Drugs 2013, 11, 2616–2624. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Koyama, K.; Aida, S.; Natori, S. Supplemental observations on atropisomerism of fungal bis(naphtho-γ-pyrone)s. Chem. Pharm. Bull. 1990, 38, 2259–2261. [Google Scholar] [CrossRef]
- Macías, M.; Ulloa, M.; Gamboa, A.; Mata, R. Phytotoxic compounds from the new coprophilous fungus Guanomyces polythrix. J. Nat. Prod. 2000, 63, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Biswas, K.; Chakrabarti, D.K. Toxic naphtho-γ-pyrones from Aspergillus niger. J. Agric. Food Chem. 1979, 27, 1347–1351. [Google Scholar] [CrossRef]
- Lu, S.Q.; Sun, W.B.; Meng, J.J.; Wang, A.L.; Wang, X.H.; Tian, J.; Fu, X.X.; Dai, J.G.; Liu, Y.; Lai, D.W.; et al. Bioactive bis-naphtho-γ-pyrones from rice false smut pathogen Ustilaginoidea virens. J. Agric. Food Chem. 2015, 63, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.B.; Wang, A.L.; Xu, D.; Wang, W.X.; Meng, J.J.; Dai, J.G.; Liu, Y.; Lai, D.W.; Zhou, L.G. New ustilaginoidins from rice false smut balls caused by Villosiclava virens and their phytotoxic and cytotoxic activities. J. Agric. Food Chem. 2017, 65, 5151–5160. [Google Scholar] [CrossRef] [PubMed]
- Ugaki, N.; Matsuda, D.; Yamazaki, H.; Nonaka, K.; Masuma, R.; Ōmura, S.; Tomoda, H. New isochaetochromin, an inhibitor of triacylglycerol synthesis in mammalian cells, produced by Penicillium sp. FKI-4942: I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 2012, 65, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Donner, C.D. Naphthopyranones–isolation, bioactivity, biosynthesis and synthesis. Nat. Prod. Rep. 2015, 32, 578–604. [Google Scholar] [CrossRef] [PubMed]
- Macías, M.; Gamboa, A.; Ulloa, M.; Toscano, R.A.; Mata, R. Phytotoxic naphthopyranone derivatives from the coprophilous fungus Guanomyces polythrix. Phytochemistry 2001, 58, 751–758. [Google Scholar] [CrossRef]
- Kong, X.L.; Ma, X.H.; Xie, Y.Y.; Cai, S.X.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Aromatic polyketides from a sponge-derived fungus Metarhizium anisopliae mxh-99 and their antitubercular activities. Arch. Pharm. Res. 2013, 36, 739–744. [Google Scholar] [CrossRef]
- Koyama, K.; Ominato, K.; Natori, S.; Tashiro, T.; Tsuruo, T. Cytotoxicity and antitumor activities of fungal bis (naphtho-γ-pyrone) derivatives. J. Pharmacobio-Dyn. 1998, 11, 630–635. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Bills, G.F.; Teran, A.; Silverman, K.C.; Lingham, R.B.; Felock, P.; Hazuda, D.J. Four novel bis-(naphtho-γ-pyrones) isolated from Fusarium species as inhibitors of HIV-1 integrase. Bioorg. Med. Chem. Lett. 2003, 13, 713–717. [Google Scholar] [CrossRef]
- Xu, G.B.; Yang, T.; Bao, J.K.; Fang, D.M.; Li, G.Y. Isochaetomium A2, a new bis(naphthodihydropyran-4-one) with antimicrobial and immunological activities from fungus Chaetomium microcephalum. Arch. Pharm. Res. 2014, 37, 575–579. [Google Scholar] [CrossRef]
- Chen, M.; Shen, N.X.; Chen, Z.Q.; Zhang, F.M.; Chen, Y. Penicilones A–D, anti-MRSA azaphilones from the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2017, 80, 1081–1086. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, Y.Y.; Chen, Z.Q.; Shen, N.X.; Shen, L.; Zhang, F.M.; Zhou, X.J.; Wang, C.Y. NaBr-induced production of brominated azaphilones and related tricyclic polyketides by the marine-derived fungus Penicillium janthinellum HK1-6. J. Nat. Prod. 2019, 82, 368–374. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Shen, N.X.; Liang, Z.Y.; Shen, L.; Chen, M.; Wang, C.Y. Paraherquamide J, a new prenylated indole alkaloid from the marine-derived fungus Penicillium janthinellum HK1-6. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Gaffield, W. Circular dichroism, optical rotatory dispersion and absolute configuration of flavanones, 3-hydroxyflavanones and their glycosides: Determination of aglycone chirality in flavanone glycosides. Tetrahedron 1970, 26, 4093–4108. [Google Scholar] [CrossRef]
- Ugaki, N.; Yamazaki, H.; Uchida, R.; Tomoda, H. New isochaetochromin, an inhibitor of triacylglycerol synthesis in mammalian cells, produced by Penicillium sp. FKI-4942: II. structure elucidation. J. Antibiot. 2012, 65, 21–24. [Google Scholar] [CrossRef]
- Koyama, K.; Natori, S. Further characterization of seven bis(naphtho-γ-pyrones) congeners of ustilaginoidins, coloring matters of Claviceps virens (Ustilaginoidea virens). Chem. Pharm. Bull. 1998, 36, 146–152. [Google Scholar] [CrossRef]
- Strange, R.N.; Scott, P.R. A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef]
- Boukaew, S.; Prasertsan, P. Suppression of rice sheath blight disease using a heat stable culture filtrate from Streptomyces philanthi RM-1-138. Crop Prot. 2014, 61, 1–10. [Google Scholar] [CrossRef]
- Suprapta, D.N. Potential of microbial antagonists as biocontrol agents against plant fungal pathogens. J. ISSAAS 2012, 18, 1–8. [Google Scholar]
- Zheng, C.J.; Shao, C.L.; Guo, Z.Y.; Chen, J.F.; Deng, D.S.; Yang, K.L.; Chen, Y.Y.; Fu, X.M.; She, Z.G.; Lin, Y.C.; et al. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Yang, R.; Gao, Z.F.; Zhao, J.Y.; Li, W.B.; Zhou, L.; Miao, F. New class of 2-aryl-6-chloro-3,4-dihydroisoquinolinium salts as potential antifungal agents for plant protection: Synthesis, bioactivity and structure-activity relationships. J. Agric. Food Chem. 2015, 63, 1906–1914. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
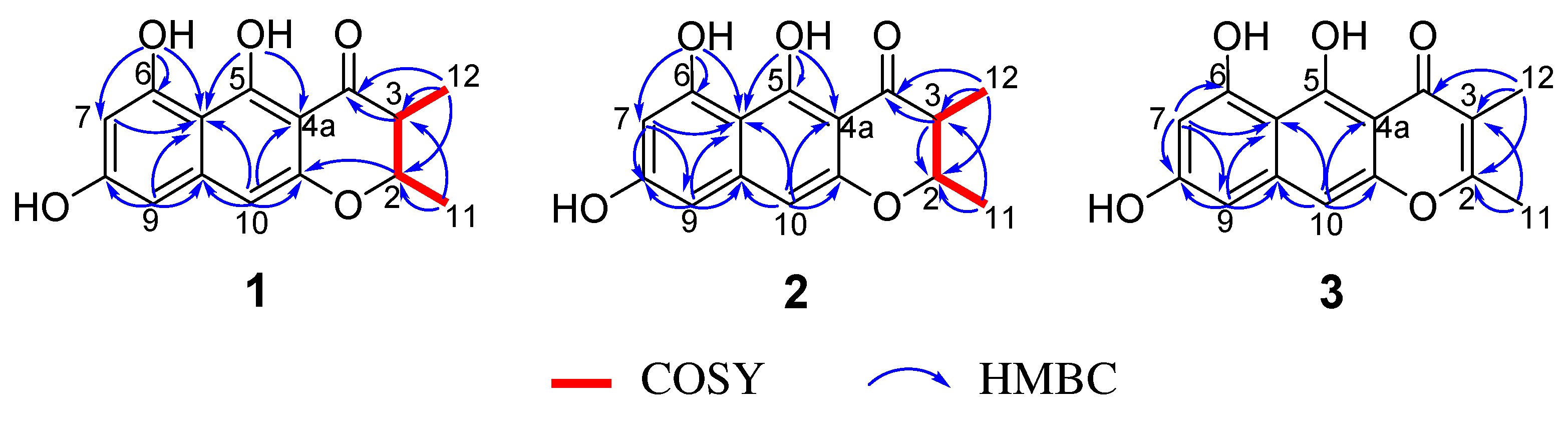
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC Type | δH (Mult. J in Hz) | δC Type | δH (Mult. J in Hz) | δC Type | δH (Mult. J in Hz) | |
| 2 | 76.4, CH | 4.67 (dq, 3.0, 6.6) | 79.1, CH | 4.29 (dq, 10.8, 6.0) | 165.2, C | |
| 3 | 44.9, CH | 2.82 (dq, 3.0, 6.6) | 46.7, CH | 2.80 (dq, 10.8, 6.6) | 111.8, C | |
| 4 | 203.2, C | 201.6, C | 182.6, C | |||
| 4a | 101.8, C | 102.3, C | 101.3, C | |||
| 5 | 166.3, C | 165.6, C | 162.0, C | |||
| 5a | 105.0, C | 105.0, C | 105.2, C | |||
| 6 | 160.8, C | 160.8, C | 158.3, C | |||
| 7 | 100.7, CH | 6.28 (brs) | 100.7, CH | 6.27 (brs) | 100.3, CH | 6.31 (d, 1.8) |
| 8 | 162.9, C | 162.9, C | 160.6, C | |||
| 9 | 102.3, CH | 6.52 (brs) | 102.3, CH | 6.51 (brs) | 100.4, CH | 6.56 (d, 1.8) |
| 9a | 143.6, C | 143.6, C | 140.3, C | |||
| 10 | 101.5, CH | 6.46 (s) | 101.4, CH | 6.45 (s) | 99.2, CH | 6.95 (s) |
| 10a | 155.8, C | 156.1, C | 151.4, C | |||
| 11 | 16.6, CH3 | 1.38 (d, 6.6) | 19.9, CH3 | 1.50 (d, 6.0) | 18.6, CH3 | 2.38 (s) |
| 12 | 9.8, CH3 | 1.20 (d, 6.6) | 10.3, CH3 | 1.25 (d, 6.6) | 8.6, CH3 | 1.92 (s) |
| OH-5 | 15.59 (s) | 15.62 (s) | 16.05 (s) | |||
| OH-6 | 9.48 (s) | 9.46 (s) | 10.18 (s) | |||
| OH-8 | 9.15 (s) | 9.13 (s) | 9.82 (s) | |||
| Pathogenic Bacteria | Antibiotic Resistant (–)/Susceptible (+) | MIC (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Ob | Vc | Cd | ||
| Staphylococcus aureus ATCC 43300 | methicillin (–) | 12.5 | 12.5 | 12.5 | >50 | >50 | >50 | 1.56 | – |
| Staphylococcus aureus ATCC 33591 | methicillin (–) | 25 | 25 | 25 | >50 | >50 | >50 | 0.39 | – |
| Staphylococcus aureus ATCC 25923 | methicillin (+) | 50 | 50 | 50 | >50 | >50 | 0.39 | 1.56 | – |
| Staphylococcus aureus ATCC 29213 | methicillin (+) | 25 | 25 | 25 | >50 | >50 | 0.39 | 0.78 | – |
| Enterococcus faecalis ATCC 51299 | vancomycin (–) | >50 | >50 | >50 | >50 | >50 | >50 | >50 | – |
| Enterococcus faecium ATCC 35667 | vancomycin (+) | >50 | >50 | >50 | >50 | >50 | >50 | 0.78 | – |
| Escherichia coli ATCC 25922 | – | >50 | >50 | >50 | >50 | >50 | >50 | >50 | 1.56 |
| Pathogenic Fungus | Average Inhibition Rate (%) | ||
|---|---|---|---|
| 50 μg/mL | 10 μg/mL | 2 μg/mL | |
| Rhizoctonia solani | 100 | 50.2 | 12 |
| Rhizoctonia cerealis | 100 | 30.4 | 0 |
| Gaeumannomyces graminis | 98.3 | 23.1 | 0 |
| Alternaria alternata | 80.3 | 25.9 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.-Y.; Liang, Z.-Y.; Shen, N.-X.; Liu, W.-L.; Zhou, X.-J.; Fu, X.-M.; Chen, M.; Wang, C.-Y. New Naphtho-γ-Pyrones Isolated from Marine-Derived Fungus Penicillium sp. HK1-22 and Their Antimicrobial Activities. Mar. Drugs 2019, 17, 322. https://doi.org/10.3390/md17060322
Zheng Y-Y, Liang Z-Y, Shen N-X, Liu W-L, Zhou X-J, Fu X-M, Chen M, Wang C-Y. New Naphtho-γ-Pyrones Isolated from Marine-Derived Fungus Penicillium sp. HK1-22 and Their Antimicrobial Activities. Marine Drugs. 2019; 17(6):322. https://doi.org/10.3390/md17060322
Chicago/Turabian StyleZheng, Yao-Yao, Zhao-Yang Liang, Nan-Xing Shen, Wen-Long Liu, Xiao-Jian Zhou, Xiu-Mei Fu, Min Chen, and Chang-Yun Wang. 2019. "New Naphtho-γ-Pyrones Isolated from Marine-Derived Fungus Penicillium sp. HK1-22 and Their Antimicrobial Activities" Marine Drugs 17, no. 6: 322. https://doi.org/10.3390/md17060322
APA StyleZheng, Y.-Y., Liang, Z.-Y., Shen, N.-X., Liu, W.-L., Zhou, X.-J., Fu, X.-M., Chen, M., & Wang, C.-Y. (2019). New Naphtho-γ-Pyrones Isolated from Marine-Derived Fungus Penicillium sp. HK1-22 and Their Antimicrobial Activities. Marine Drugs, 17(6), 322. https://doi.org/10.3390/md17060322