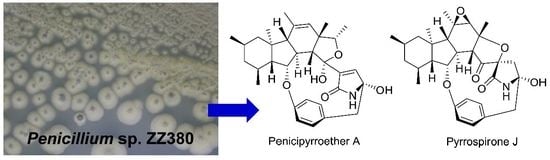
Novel Bioactive Penicipyrroether A and Pyrrospirone J from the Marine-Derived Penicillium sp. ZZ380
Abstract
1. Introduction
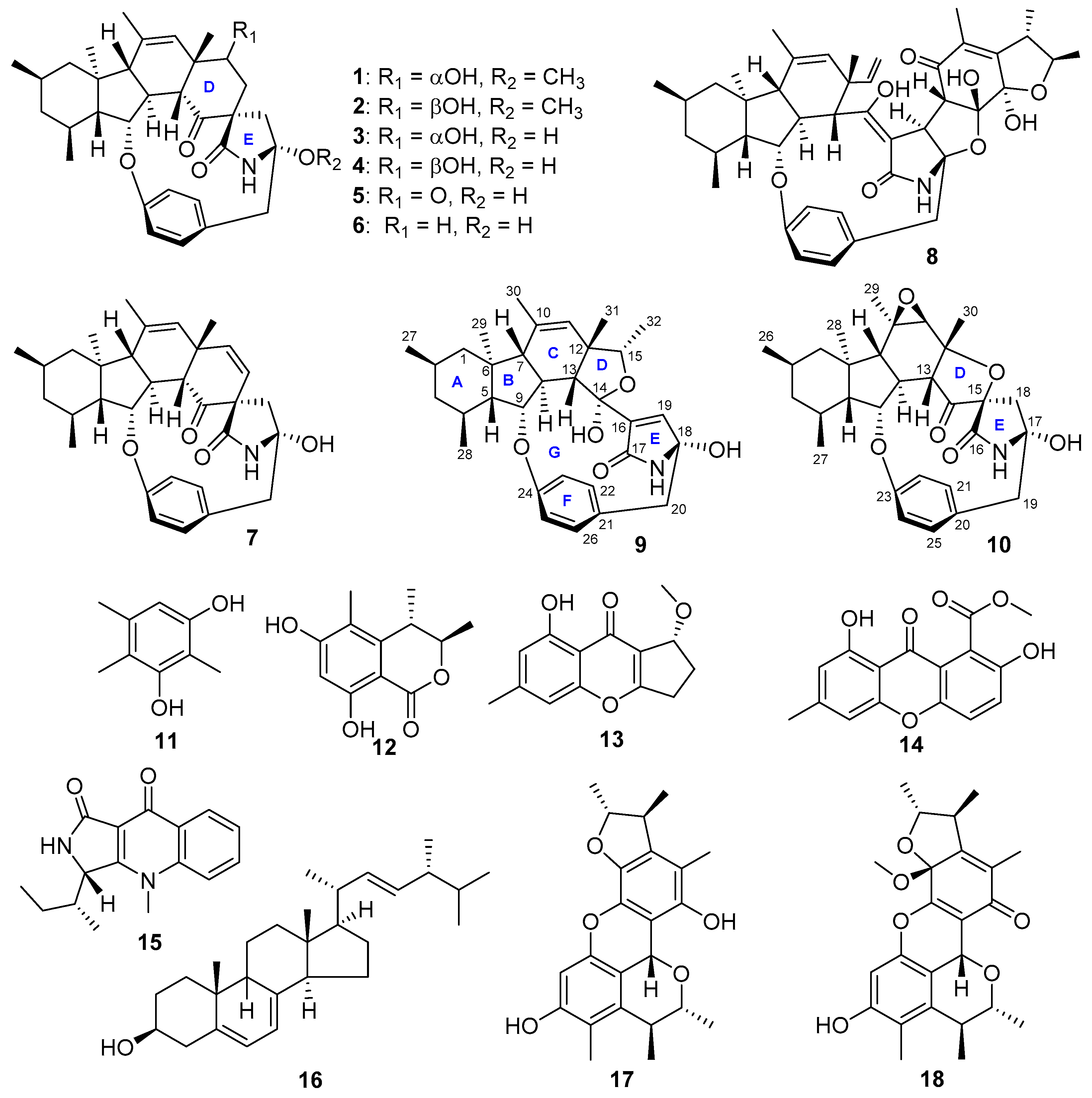
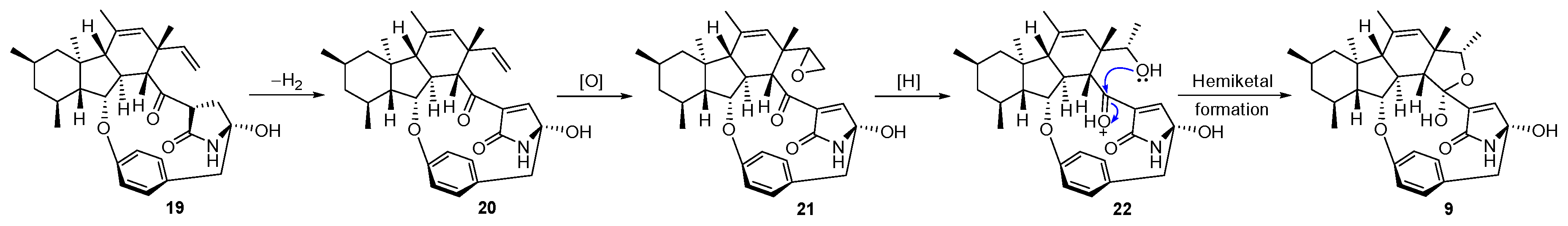
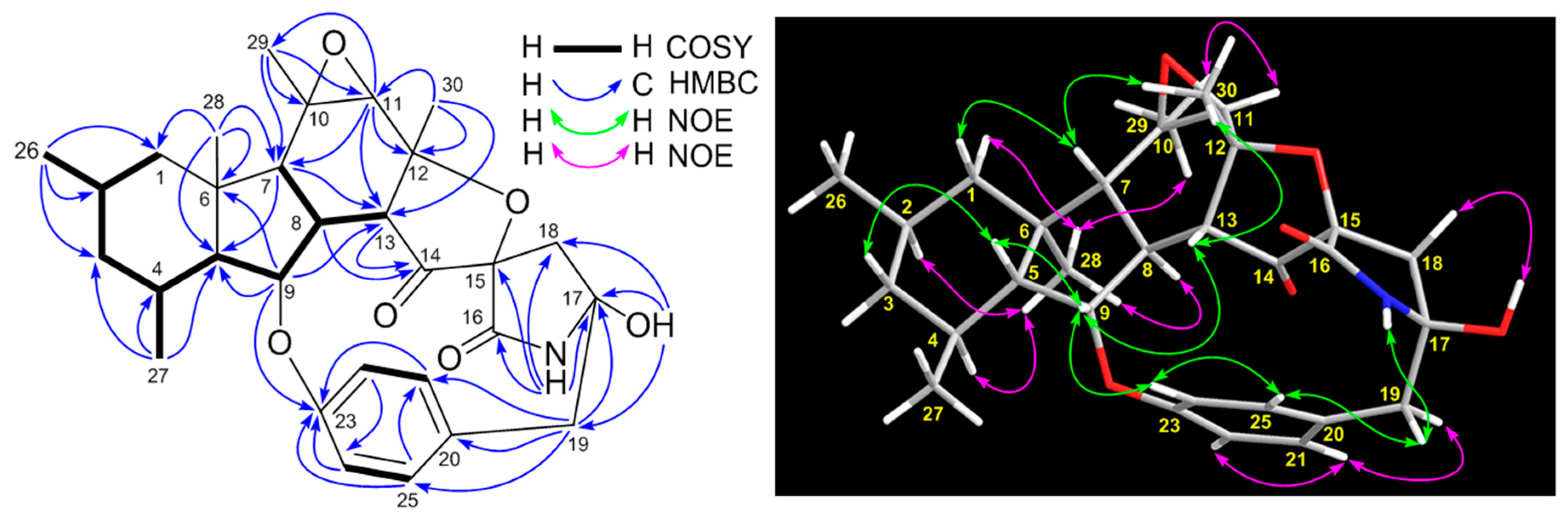
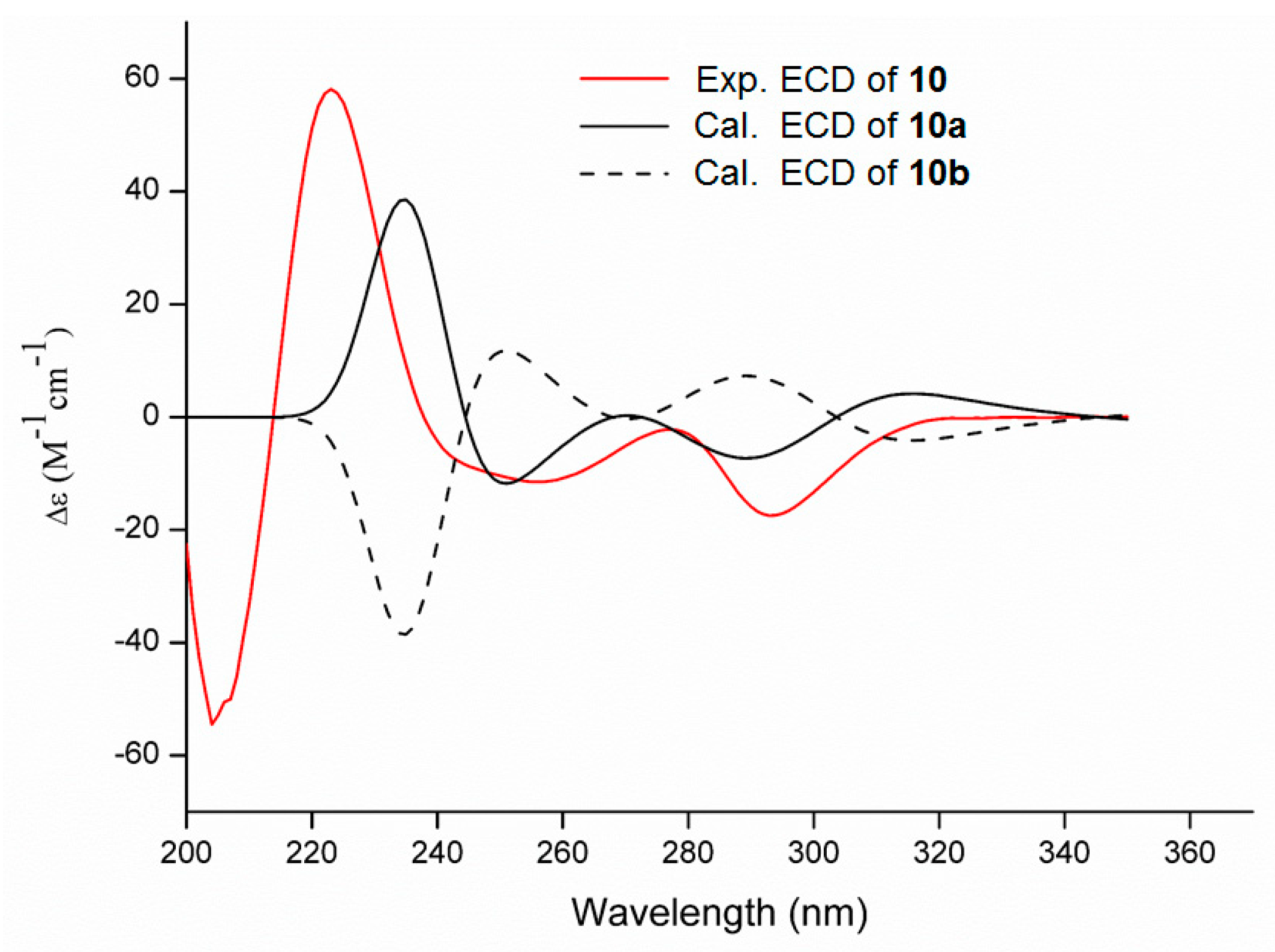
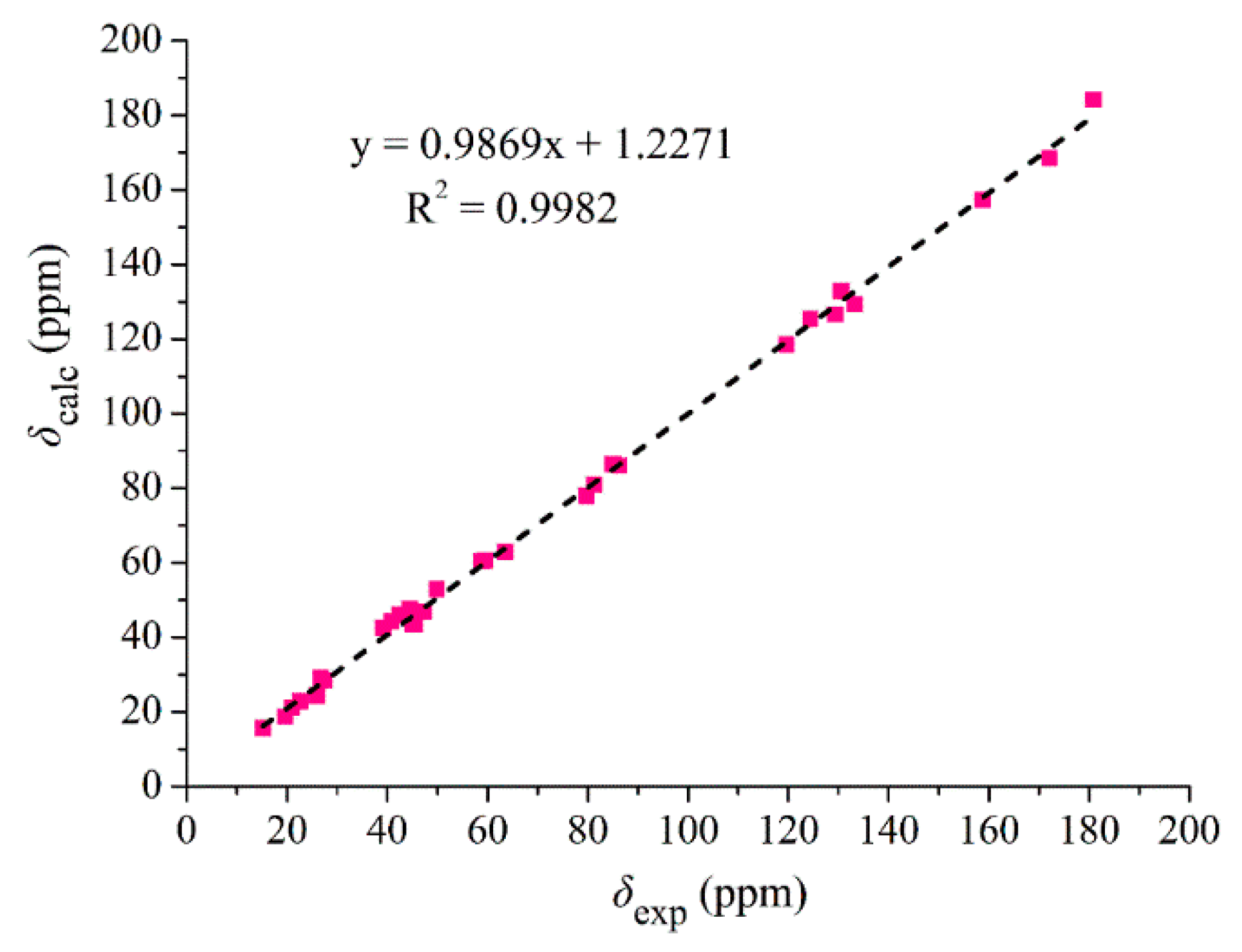
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Marine Strain ZZ380
3.3. Mass Culture of Strain ZZ380
3.4. Isolation of Compounds 9–18
3.5. ECD Calculation
3.6. 13C NMR Calculation
3.7. Sulforhodamine B (SRB) Assay
3.8. Antibacterial Activive Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.W.; Ear, A.; Nay, B. Hirsutellones and beyond: Figuring out the biological and synthetic logics toward chemical complexity in fungal PKS-NRPS compounds. Nat. Prod. Rep. 2013, 30, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Song, T.F.; Chen, M.X.; Chai, W.Y.; Zhang, Z.Z.; Lian, X.Y. New bioactive pyrrospirones C–I from a marine-derived fungus Penicillium sp. ZZ380. Tetrahedron 2018, 74, 884–891. [Google Scholar] [CrossRef]
- Song, T.; Chen, M.X.; Ge, Z.W.; Chai, W.Y.; Li, X.C.; Zhang, Z.Z.; Lian, X.Y. Bioactive penicipyrrodiether A, an adduct of GKK1032 analogue and phenol A derivative, from a marine-sourced fungus Penicillium sp. ZZ380. J. Org. Chem. 2018, 83, 13395–13401. [Google Scholar] [CrossRef]
- Ebrahim, W.; Aly, A.H.; Wray, V.; Mándi, A.; Teiten, M.H.; Gaascht, F.; Orlikova, B.; Kassack, M.U.; Lin, W.; Diederich, M.; et al. Embellicines A and B: Absolute configuration and NF-κB transcriptional inhibitory activity. J. Med. Chem. 2013, 56, 2991–2999. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Guo, Y.H.; Wang, H.Y.; Ma, X.J.; Jiang, T.; Zhao, J.L.; Zou, Z.M.; Ding, G. Allelopathic polyketides from an endolichenic fungus Myxotrichum sp. by using OSMAC strategy. Sci. Rep. 2016, 6, 19350. [Google Scholar] [CrossRef]
- Wang, L.; Qin, D.; Zhang, K.; Huang, Q.; Liu, S.; Han, M.J.; Dong, J.Y. Metabolites from the co-culture of nigranoic acid and Umbelopsis dimorpha SWUKD 3.1410, an endophytic fungus from Kadsura angustifolia. Nat. Prod. Res. 2017, 31, 1414–1421. [Google Scholar]
- Xin, Z.H.; Li, T.; Zhu, T.J.; Wang, W.L.; Du, L.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Isocoumarin derivatives from the sea squirt-derived fungus Penicillium stoloniferum QY2-10 and the halotolerant fungus Penicillium notatum B-52. Arch. Pharm. Res. 2007, 30, 816–819. [Google Scholar] [CrossRef]
- Trisuwan, K.; Rukachaisirikul, V.; Borwornwiriyapan, K.; Phongpaichit, S.; Sakayaroj, J. Benzopyranone, benzophenone and xanthone derivatives from the soil fungus Penicillium citrinum PSU-RSPG95. Tetrahedron Lett. 2014, 55, 1336–1338. [Google Scholar] [CrossRef]
- Cui, X.Q.; Zhu, G.L.; Liu, S.H.; Jiang, G.L.; Wang, Y.; Zhu, W.M. Diversity and function of the antarctic krill microorganisms from Euphausia superba. Sci. Rep. 2016, 6, 36496. [Google Scholar] [CrossRef]
- Kim, W.G.; Song, N.K.; Yoo, I.D. Quinolactacins A1 and A2, new acetylcholinesterase inhibitors from Penicillium citrinum. J. Antibiot. 2001, 54, 831–835. [Google Scholar] [CrossRef]
- Shirane, N.; Takenaka, H.; Ueda, K.; Hashimoto, Y.; Katoh, K.; Ishii, H. Sterol analysis of DMI-resistant and sensitive strains of Venturia inaequalis. Phytochemistry 1996, 41, 1301–1308. [Google Scholar] [CrossRef]
- Wakana, D.; Hosoe, T.; Itabashi, T.; Okada, K.; de Campos Takaki, G.; Yaguchi, T.; Fukushima, K.; Kawai, K. New citrinin derivatives isolated from Penicillium citrinum. J. Nat. Med. 2006, 60, 279–284. [Google Scholar] [CrossRef]
- Lu, Z.Y.; Lin, Z.J.; Wang, W.L.; Du, L.; Zhu, T.J.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Citrinin dimers from the halotolerant fungus Penicillium citrinum B-57. J. Nat. Prod. 2008, 71, 543–546. [Google Scholar] [CrossRef]
- Shiono, Y.; Shimanuki, K.; Hiramatsu, F.; Koseki, T.; Tetsuya, M.; Fujisawa, N.; Kimura, K. Pyrrospirones A and B, apoptosis inducers in HL-60 cells, from an endophytic fungus, Neonectria ramulariae Wollenw KS-246. Bioorg. Med. Chem. Lett. 2008, 18, 6050–6053. [Google Scholar] [CrossRef]
- Oikawa, H. Biosynthesis of structurally unique fungal metabolite GKK1032A2: indication of novel carbocyclic formation mechanism in polyketide biosynthesis. J. Org. Chem. 2003, 68, 3552–3557. [Google Scholar] [CrossRef]
- Yang, X.W.; Yang, J.; Xu, G. Skeleton reassignment of type C polycyclic polyprenylated acylphloroglucinols. J. Nat. Prod. 2017, 80, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.B.; Wu, W.; Jiao, F.R.; Jiao, W.H.; Li, L.; Sun, F.; Wang, S.P.; Yang, F.; Lin, H.W. Asperflotone, an 8(14→15)-abeo-ergostane from the sponge-derived fungus Aspergillus flocculosus 16D-1. J. Org. Chem. 2019, 84, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Y.; Li, J.; Huang, X.; Yan, T.; Cao, W.; Liu, H.; Long, Y.; She, Z. Diaporindenes A-D: Four unusual 2,3-dihydro-1H-indene analogues with anti-inflammatory activities from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. J. Org. Chem. 2018, 83, 11804–11813. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.X.; Ye, X.W.; Yu, S.R.; Lian, X.Y.; Zhang, Z.Z. New capoamycin-type antibiotics and polyene acids from marine Streptomyces fradiae PTZ0025. Mar. Drugs 2012, 10, 2388–2402. [Google Scholar] [CrossRef]
- Ye, X.W.; Anjum, K.; Song, T.F.; Wang, W.L.; Yu, S.R.; Huang, H.C.; Lian, X.Y.; Zhang, Z.Z. A new curvularin glycoside and its cytotoxic and antibacterial analogues from marine actinomycete Pseudonocardia sp. HS7. Nat. Prod. Res. 2016, 30, 1156–1161. [Google Scholar] [CrossRef]
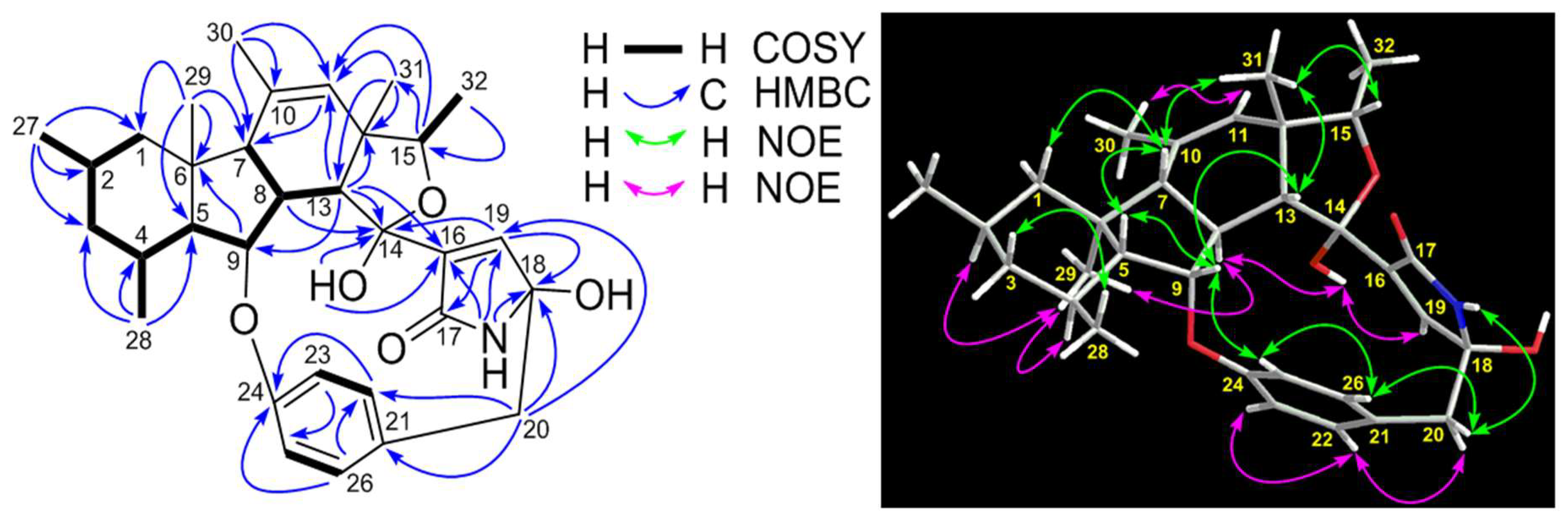
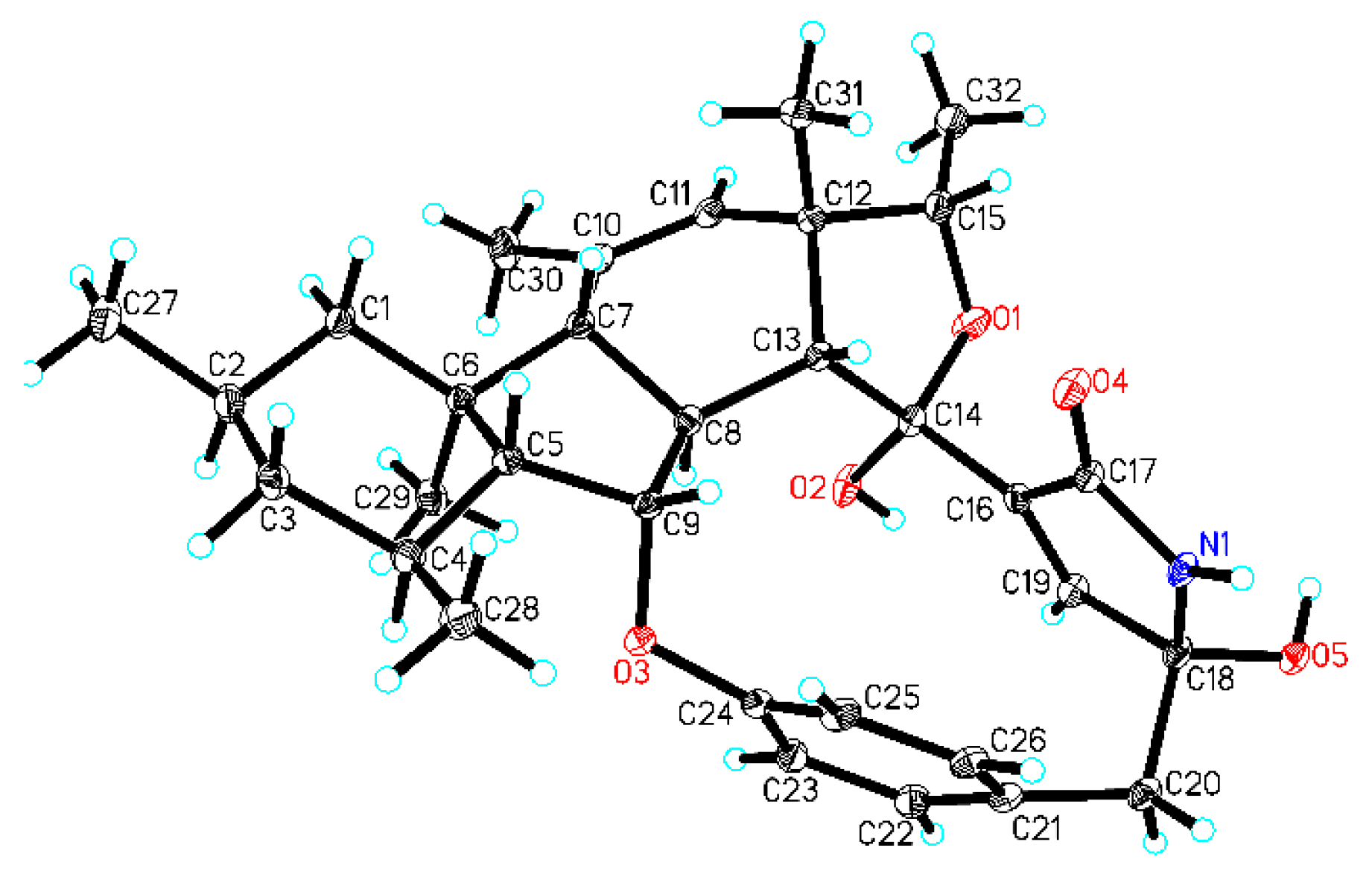

| No. | δC, Type | δH (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 49.2, CH2 | βH: 0.76, t (12.0); αH: 1.88, dd (12.2, 3.3) | H-2, αH-1; H-2, βH-1 | C-27, C-29 |
| 2 | 28.5, CH | 1.76, m | H-1, H-3, H3-27 | |
| 3 | 46.1, CH2 | βH: 0.57, q (12.0); αH: 1.69, m | H-2, αH-3, H-4; H-2, βH-3, H-4 | C-27, C-28 |
| 4 | 28.0, CH | 1.97, m | H-3, H-5, H3-28 | |
| 5 | 62.0, CH | 1.22, dd (11.3, 7.6) | H-4, H-9 | C-4, C-6, C-29 |
| 6 | 41.3, C | – | ||
| 7 | 54.4, CH | 2.36, d (13.2) | H-8 | C-10 |
| 8 | 48.9, CH | 3.12, m | H-7, H-9, H-13 | C-7, C-9, C-13, C-14 |
| 9 | 87.5, CH | 5.01, dd (7.6, 4.8) | H-5, H-8 | C-6 |
| 10 | 139.9, C | – | ||
| 11 | 126.8, CH | 5.62, s | C-7, C-30 | |
| 12 | 48.6, C | – | ||
| 13 | 56.3, CH | 3.78, d (5.7) | H-8 | C-9, C-11, C-12, C-14, C-16, C-31 |
| 14 | 102.1, C | – | ||
| 15 | 79.4, CH | 4.35, q (6.4) | H3-32 | C-11, C-31 |
| 16 | 139.2, C | – | ||
| 17 | 172.7, C | – | ||
| 18 | 88.1, C | – | ||
| 19 | 147.3, CH | 6.95, d (1.8) | C-14, C-17, C-18 | |
| 20 | 45.7, CH2 | βH: 3.56, d (12.2); αH: 3.51, d (12.2) | αH-20; βH-20 | C-18, C-19, C-21, C-22, C-26 |
| 21 | 130.8, C | – | ||
| 22 | 132.7, CH | 7.31, dd (8.1, 1.9) | H-23 | C-20, C-24, C-26 |
| 23 | 122.4, CH | 7.13, dd (8.1, 2.4) | H-22 | C-21, C-25 |
| 24 | 159.6, C | – | ||
| 25 | 118.4, CH | 7.22 a | H-26 | C-21, C-23 |
| 26 | 130.2, CH | 7.38, dd (8.4, 1.9) | H-25 | C-22, C-24 |
| 27 | 23.4, CH3 | 0.91, d (6.4) | H-2 | C-1, C-2, C-3 |
| 28 | 20.2, CH3 | 1.18, d (6.3) | H-4 | C-3, C-4, C-5 |
| 29 | 16.8, CH3 | 1.31, s | C-1, C-5, C-6, C-7 | |
| 30 | 20.8, CH3 | 1.81, s | C-7, C-10, C-11 | |
| 31 | 22.0, CH3 | 1.35, s | C-11, C-12, C-13, C-15 | |
| 32 | 14.8, CH3 | 1.26, d (6.4) | H-15 | C-12, C-15 |
| OH-14 | – | 6.25, s | C-14, C-16 | |
| OH-18 | – | 8.35, s | ||
| NH-17 | – | 9.43, s | C-16, C-18, C-19 |
| No. | δC, Type | δH (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|
| 1 | 47.3, CH2 | βH: 0.81, t (12.3); αH: 1.80, m | H-2, αH-2; H-2, βH-2 | C-26, C-28 |
| 2 | 27.4, CH | 1.82, m | H-1, H-3, H3-26 | C-6 |
| 3 | 45.5, CH2 | βH: 0.51, q (12.1); αH: 1.75, m | H-2, αH, H-4 H-2, βH, H-4 | C-2, C-27 |
| 4 | 26.8, CH | 1.78, m | H-3, H-5, H3-27 | C-2 |
| 5 | 59.5, CH | 1.22, dd (11.6, 8.8) | H-4, H-9 | C-4, C-6, C-28 |
| 6 | 42.5, C | – | ||
| 7 | 49.9, CH | 1.55, d (14.3) | H-8 | C-1, C-5, C-6, C-8, C-13, C-28 |
| 8 | 39.2, CH | 2.79, m | H-7, H-9, H-13 | C-7, C-9, C-10, C-13, C-14 |
| 9 | 85.0, CH | 4.82, dd (8.6, 7.0) | H-5, H-8 | C-5, C-6, C-13, C-23 |
| 10 | 58.8, C | – | ||
| 11 | 63.5, CH | 2.46, s | C-10, C-12, C-13, C-29, C-30 | |
| 12 | 81.3, C | – | ||
| 13 | 44.5, CH | 3.21, d (8.0) | C-8, C-9, C-11, C-12, C-14, C-30 | |
| 14 | 180.9, C | – | ||
| 15 | 79.7, C | – | ||
| 16 | 172.1, C | – | ||
| 17 | 86.2, C | – | ||
| 18 | 40.9, CH2 | βH: 1.99, d (12.1); αH: 2.45, d (12.1) | αH-18; βH-18 | C-14, C-15, C-16, C-17, C-19 |
| 19 | 45.0, CH2 | βH: 2.99, d (14.5); αH: 2.69, d (14.5) | αH-19; βH-19 | C-17, C-18, C-20, C-21, C-25 |
| 20 | 129.4, C | – | ||
| 21 | 133.3, CH | 6.92, dd (8.3, 2.1) | H-22 | C-19, C-23, C-25 |
| 22 | 124.4, CH | 6.82, dd (8.3, 2.7) | H-21 | C-20, C-23, C-24 |
| 23 | 158.7, C | – | ||
| 24 | 119.7, CH | 6.94, dd (8.8, 2.7) | H-25 | C-20, C-22, C-23 |
| 25 | 130.6, CH | 6.78, dd (8.8, 2.1) | H-24 | C-19, C-21, C-23 |
| 26 | 22.7, CH3 | 0.86, d (6.1) | H-2 | C-1, C-2, C-3 |
| 27 | 19.7, CH3 | 1.02, d (6.2) | H-4 | C-3, C-4, C-5 |
| 28 | 15.2, CH3 | 1.05, s | C-1, C-5, C-6, C-7 | |
| 29 | 21.0, CH3 | 1.20, s | C-7, C-10, C-11 | |
| 30 | 26.0, CH3 | 1.59, s | C-11, C-12, C-13 | |
| OH-17 | – | 6.24, s | C-17, C-18, C-19 | |
| NH-16 | – | 8.76, s | C-15, C-16, C-17, C-18 |
| Compounds | Glioma Cells (μM) | Bacteria (μg/mL) | ||
|---|---|---|---|---|
| U87MG | U251 | MRSA | E. coli | |
| 9 | 1.64 ± 0.05 | 5.50 ± 0.12 | 1.7 | 3.0 |
| 10 | 10.52 ± 0.62 | 17.92 ± 0.93 | >50 | >50 |
| DOX | 1.20 ± 0.06 | 8.03 ± 1.20 | NT | NT |
| Gentamicin | NT | NT | 0.36 | 1.44 |
| Vancomycin | NT | NT | 0.20 | NT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Tang, M.; Ge, H.; Chen, M.; Lian, X.; Zhang, Z. Novel Bioactive Penicipyrroether A and Pyrrospirone J from the Marine-Derived Penicillium sp. ZZ380. Mar. Drugs 2019, 17, 292. https://doi.org/10.3390/md17050292
Song T, Tang M, Ge H, Chen M, Lian X, Zhang Z. Novel Bioactive Penicipyrroether A and Pyrrospirone J from the Marine-Derived Penicillium sp. ZZ380. Marine Drugs. 2019; 17(5):292. https://doi.org/10.3390/md17050292
Chicago/Turabian StyleSong, Tengfei, Mingmin Tang, Hengju Ge, Mengxuan Chen, Xiaoyuan Lian, and Zhizhen Zhang. 2019. "Novel Bioactive Penicipyrroether A and Pyrrospirone J from the Marine-Derived Penicillium sp. ZZ380" Marine Drugs 17, no. 5: 292. https://doi.org/10.3390/md17050292
APA StyleSong, T., Tang, M., Ge, H., Chen, M., Lian, X., & Zhang, Z. (2019). Novel Bioactive Penicipyrroether A and Pyrrospirone J from the Marine-Derived Penicillium sp. ZZ380. Marine Drugs, 17(5), 292. https://doi.org/10.3390/md17050292