Pyrogallol-Phloroglucinol-6,6’-Bieckol from Ecklonia cava Improved Blood Circulation in Diet-Induced Obese and Diet-Induced Hypertension Mouse Models
Abstract
1. Introduction
2. Results and Discussion
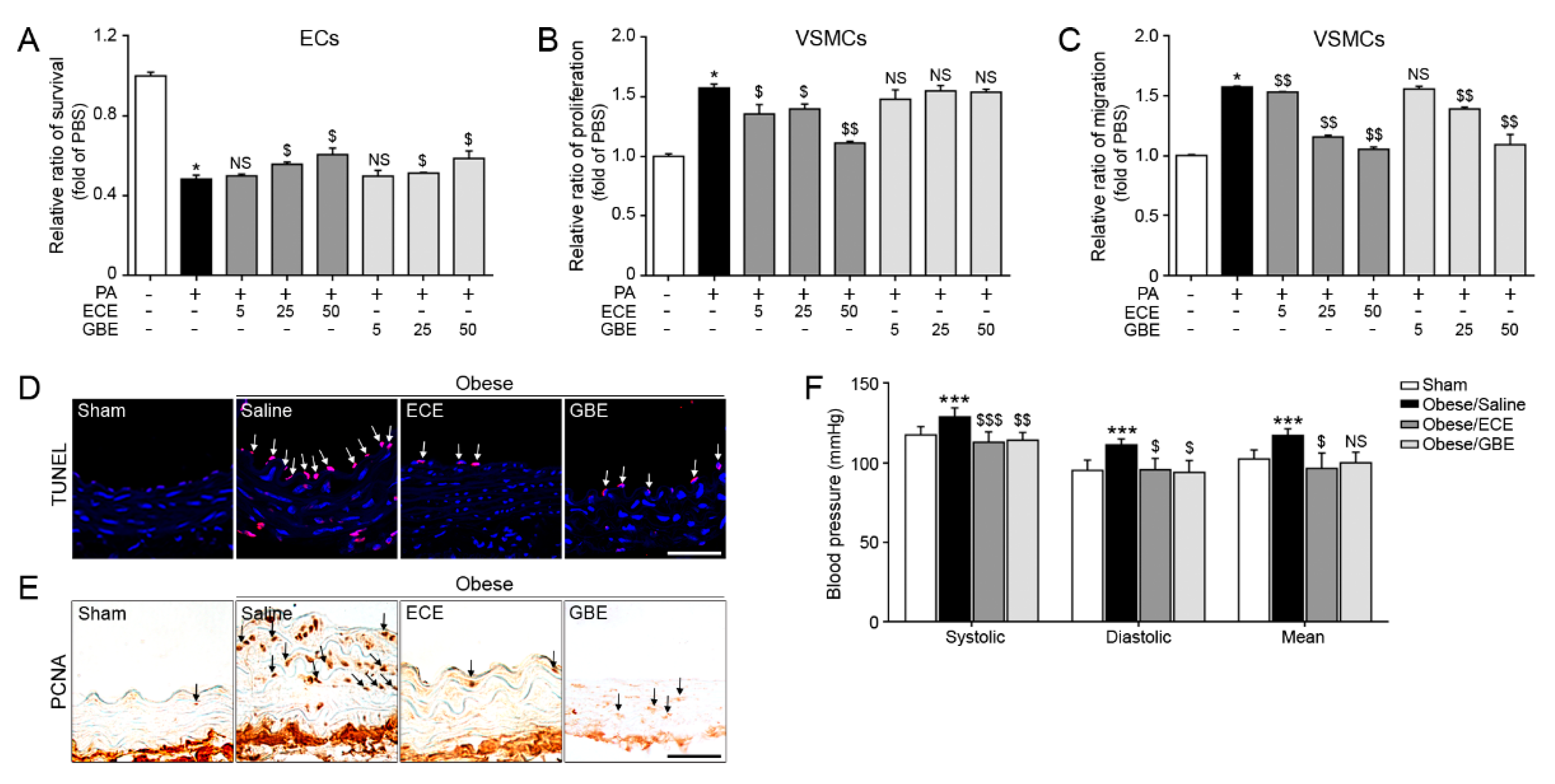
2.1. ECE and GBE Treatments Improved ECs and VSMCs Functions In Vitro and In Vivo
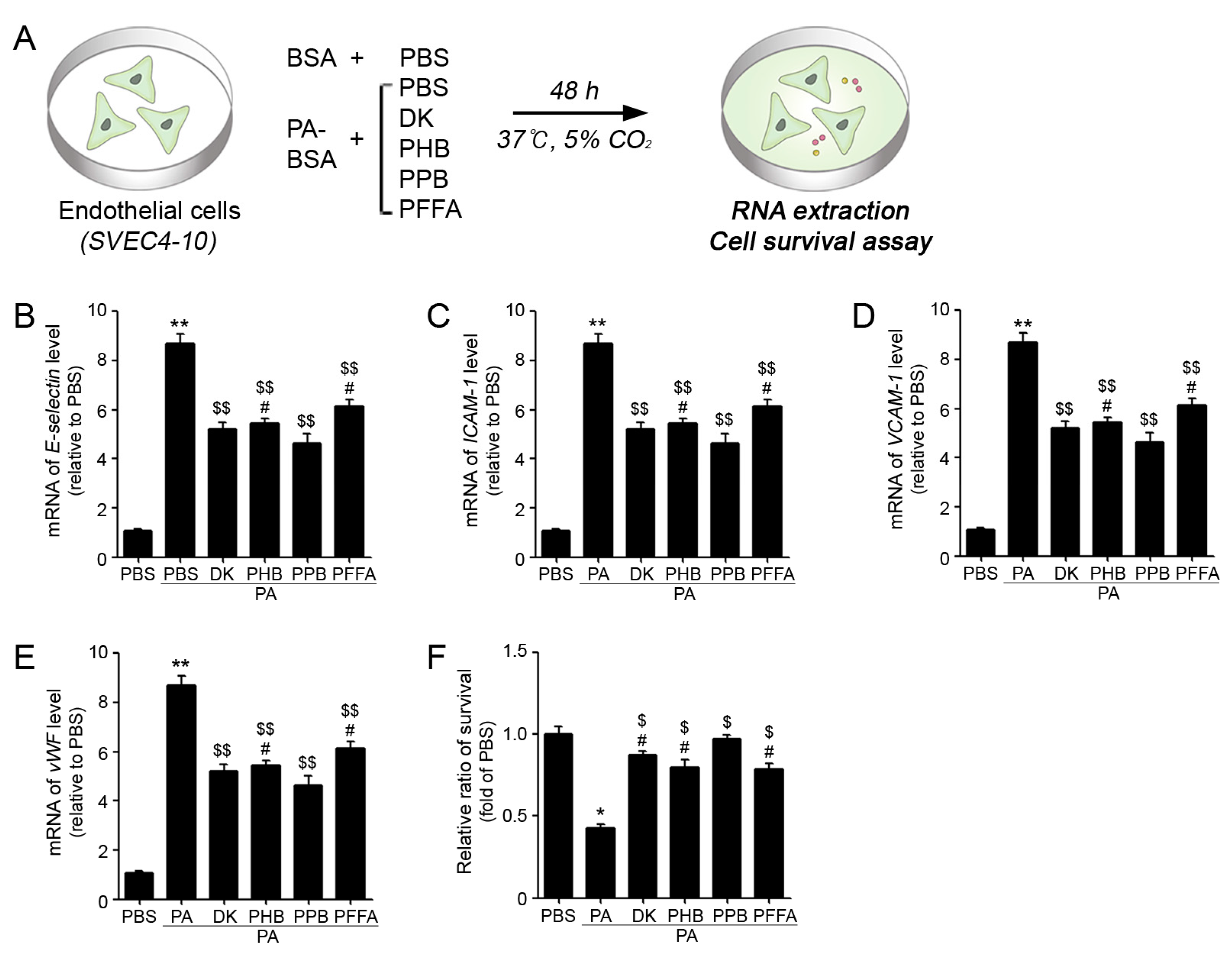
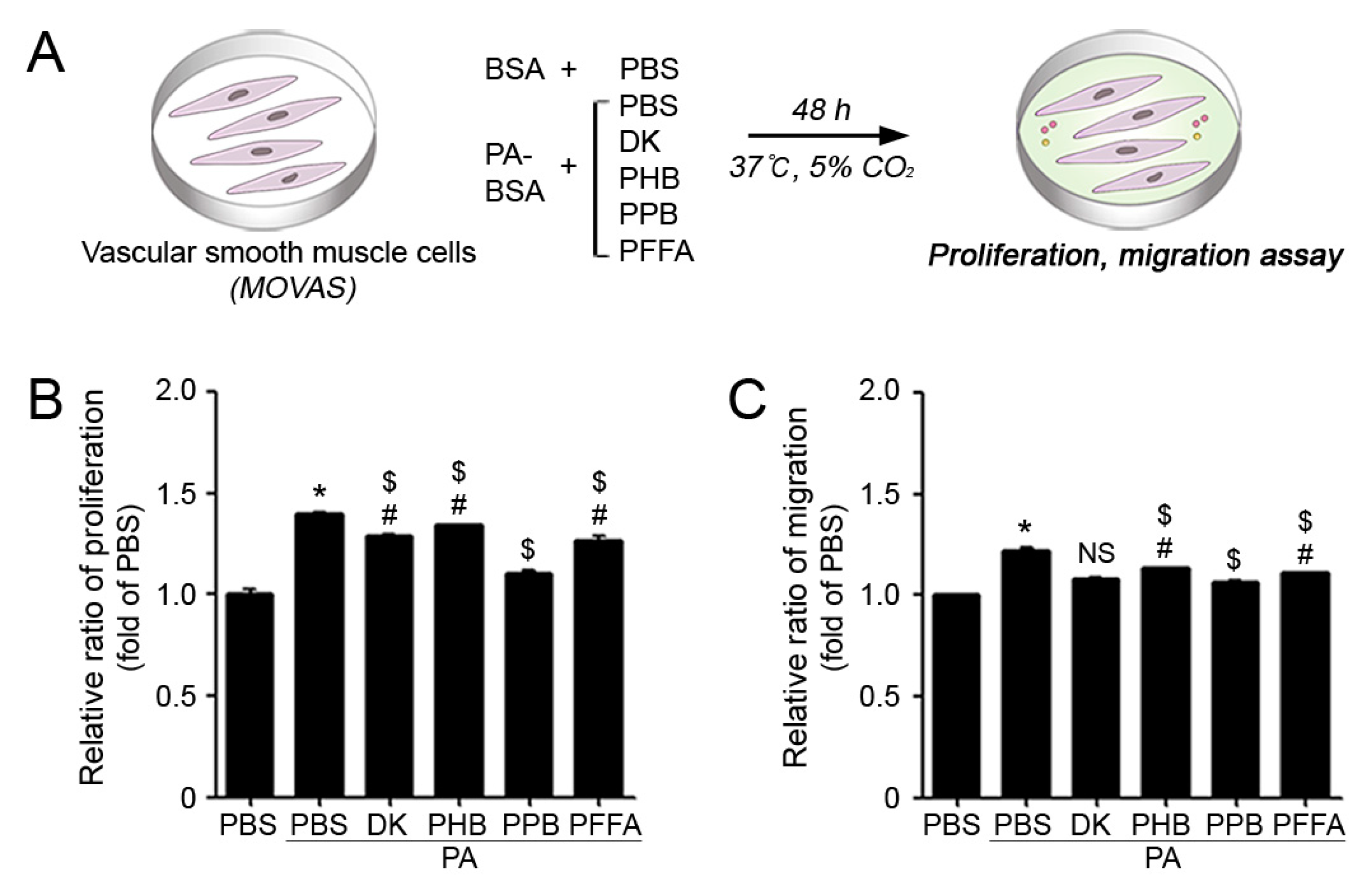
2.2. PPB Effectively Reduced ECs and VSMCs Dysfunction In Vitro
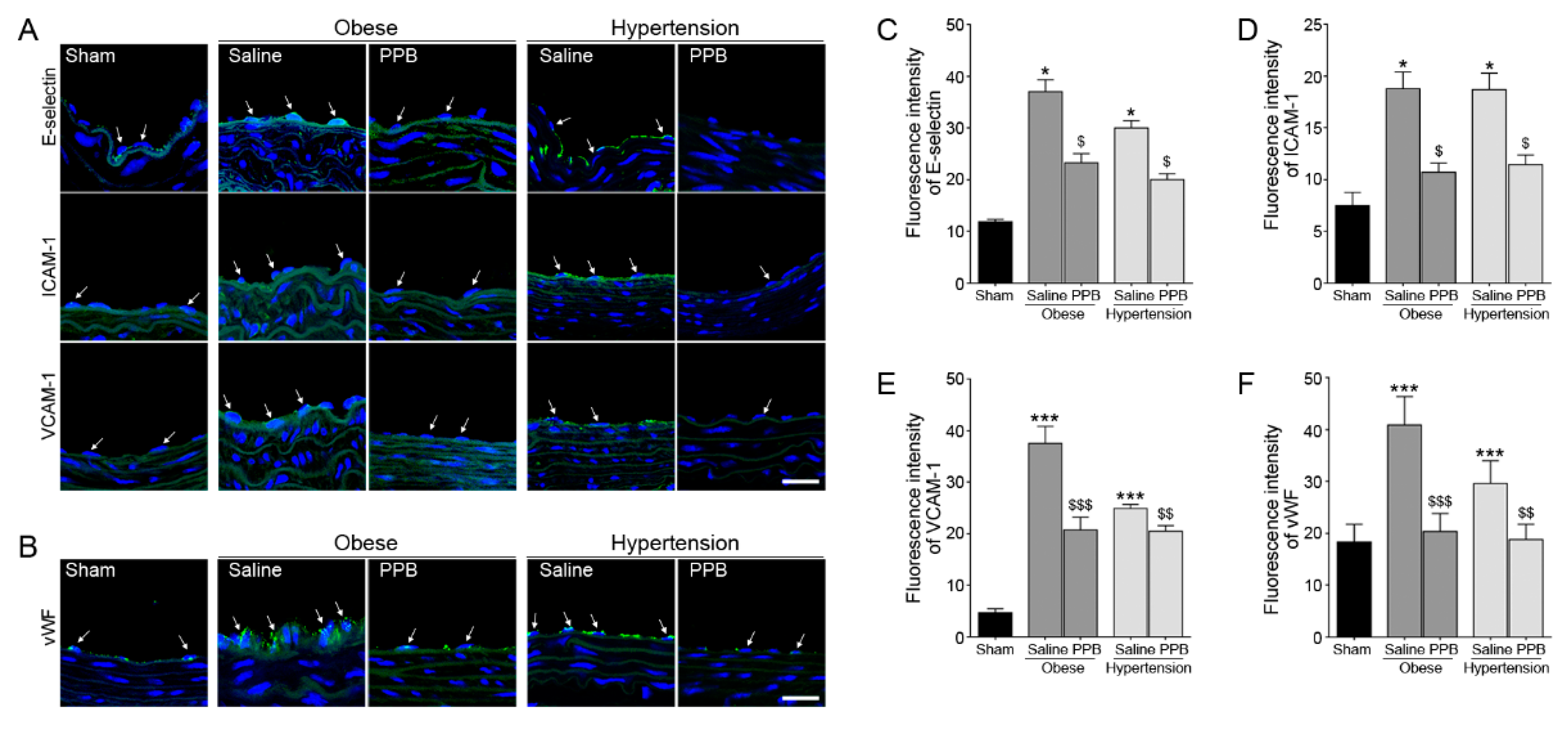
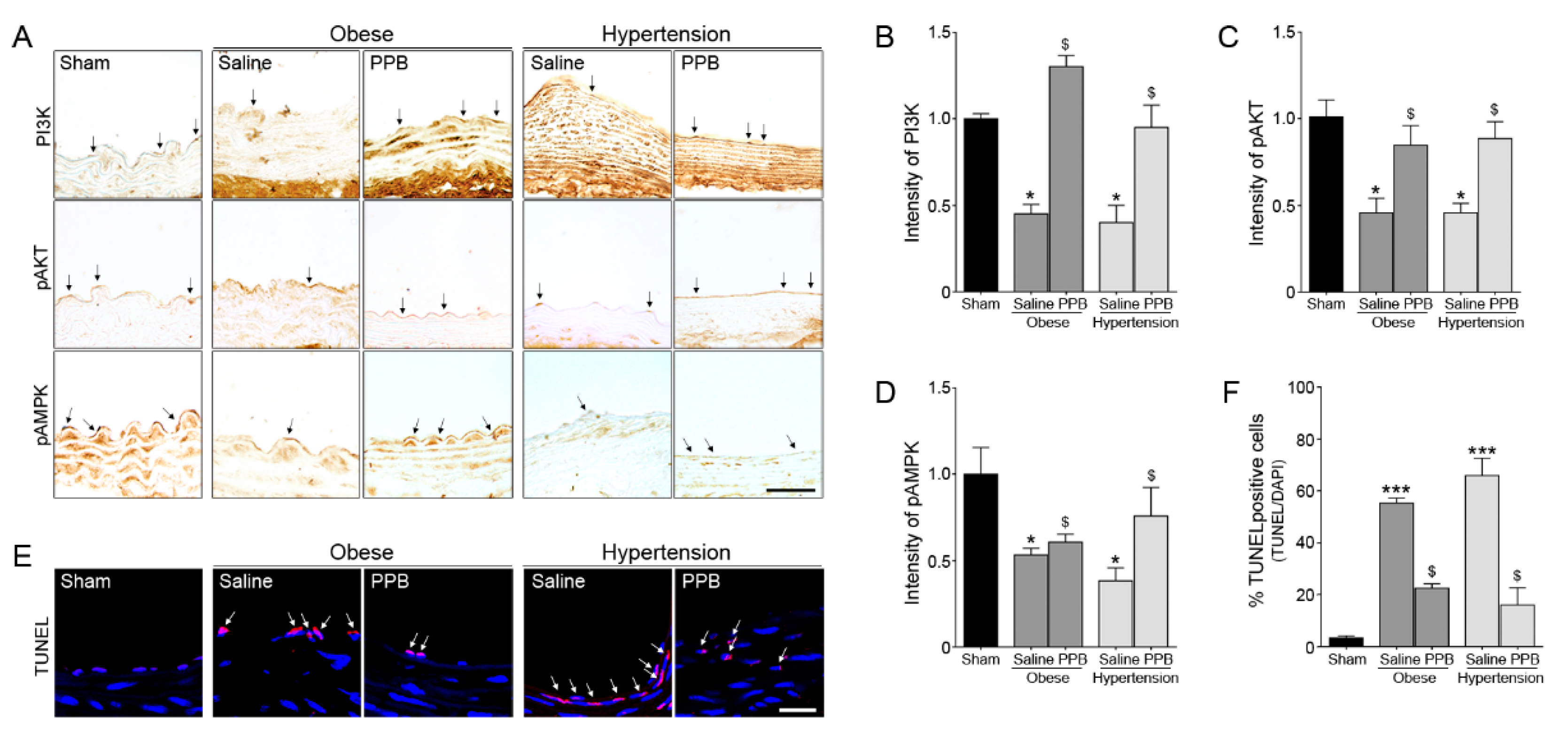
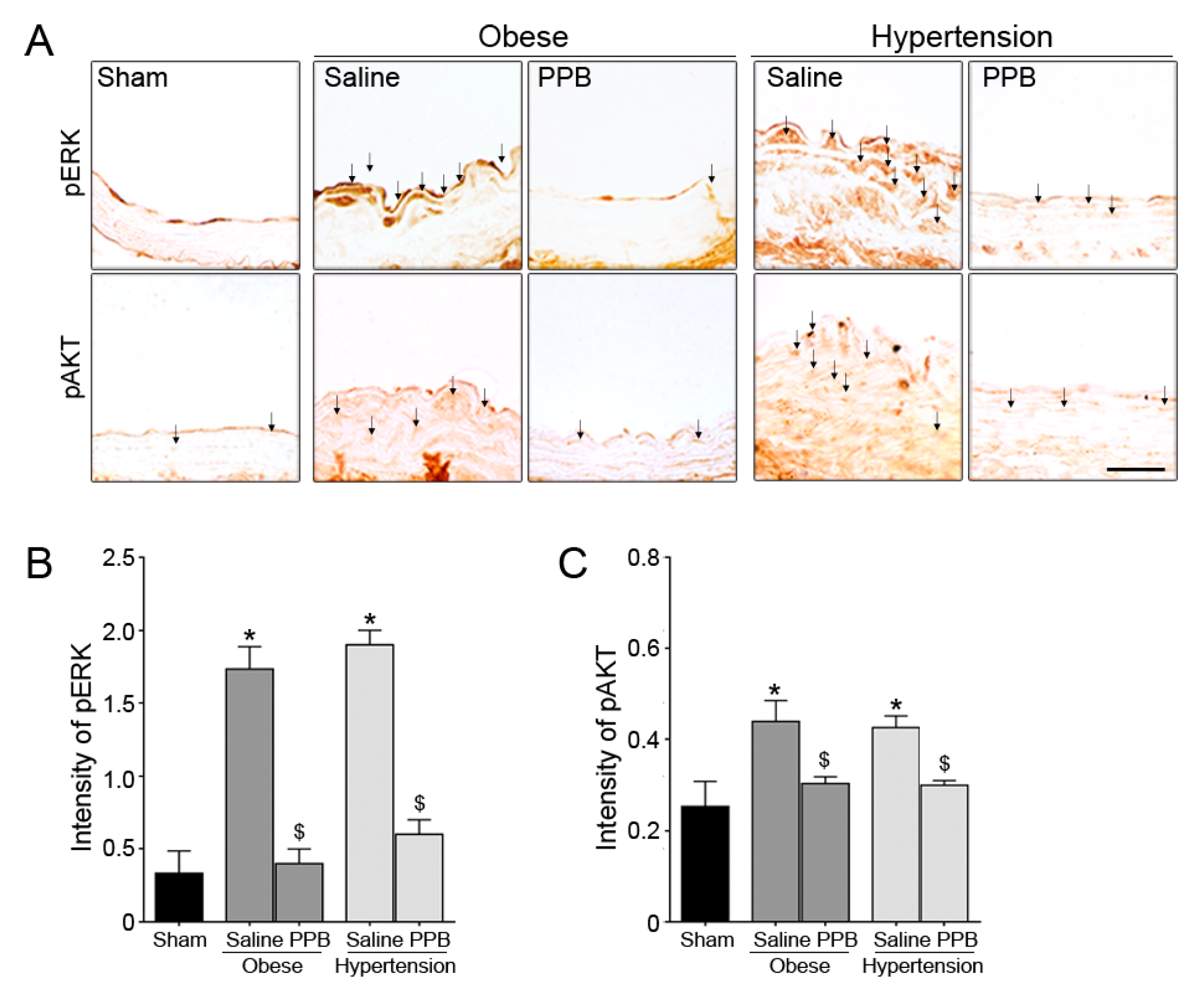
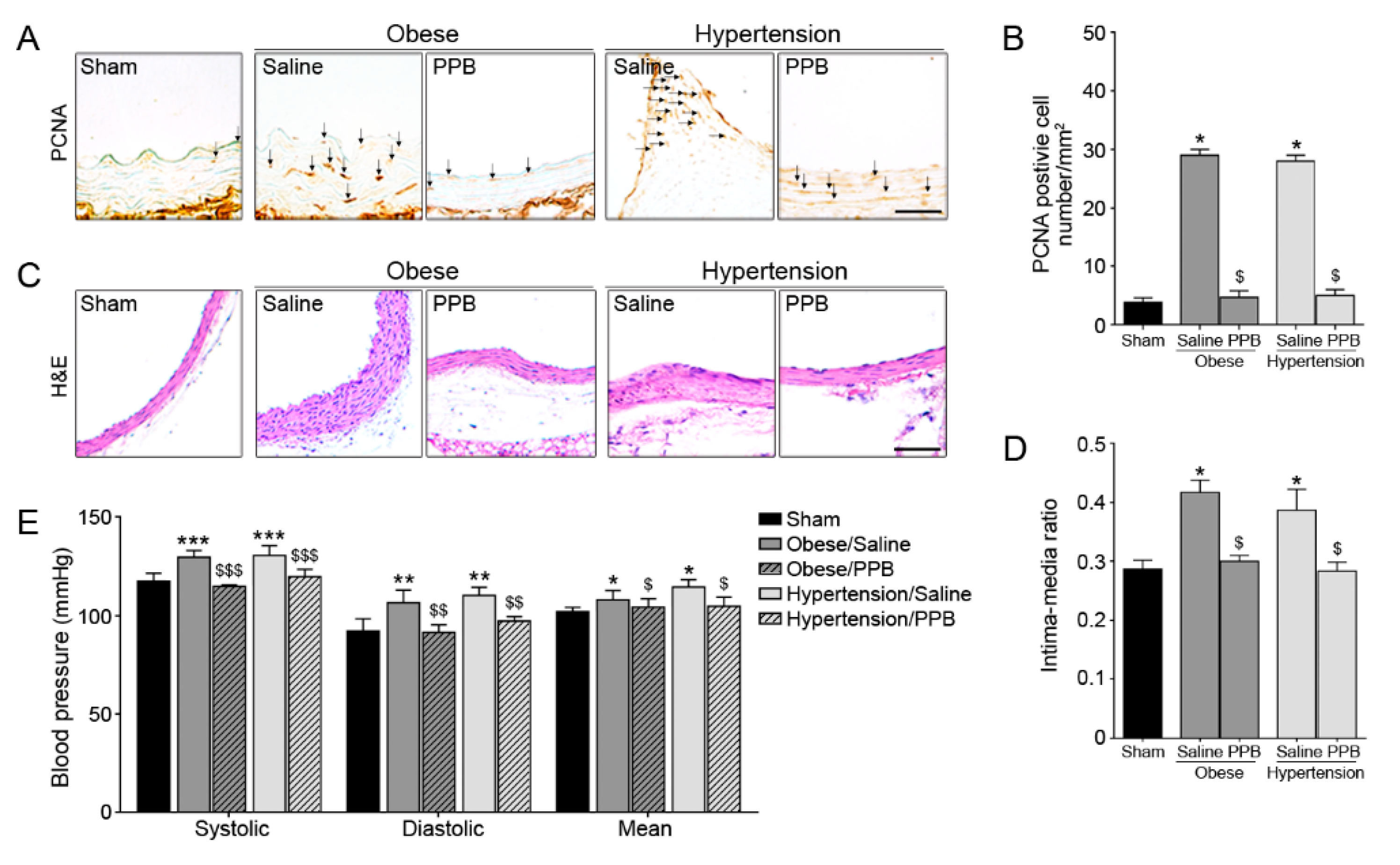
2.3. PPB Helps Rescue Vascular Dysfunction in the Diet-Induced Mouse Models
3. Materials and Methods
3.1. Preparations of ECE and GBE and Isolation of Four Phlorotannins
3.2. Endothelial Cell Survival Assay
3.3. VSMC Proliferation Assay
3.4. VSMC Trans-Well Migration Assay
3.5. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
3.6. Diet Induced Mice Model
3.7. DAB Immunohistochemistry
3.8. Immunofluorescence (IF)
3.9. Blood Pressure Measurements
3.10. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| PA | palmitate conjugation to BSA |
| DK | dieckol |
| PHB | 2,7-phloroglucinol-6,6-bieckol |
| PPB | pyrogallol-phloroglucinol-6,6-bieckol |
| PFFA | phlorofucofuroeckol A |
| α-SMA | α-smooth muscle actin |
| PCNA | Proliferating cell nuclear antigen |
| H&E | Hematoxylin and eosin |
References
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; Rutherford, R.B. Inter-society consensus for the management of peripheral arterial disease (TASC II). J. Vasc. Surg. 2007, 45, 5–67. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W. Thirty years of saying NO: Sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circulation Res. 2016, 2, 375–396. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anaqnostopoulou, A.; Amer, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.; Feletou, M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular smooth muscle cells in atherosclerosis. Circulation Res. 2016, 4, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H. Inflammation and thrombosis: Roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemost. 2018, 2, 231–241. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Williamson, J.D.; Fitzpatrick, A.L.; Kronmal, R.A.; Ives, D.G.; Saxton, J.A.; Lopez, O.L.; Burke, G.; Carlson, M.C.; Fried, L.P.; et al. Ginkgo biloba for prevention of dementia: A randomized controlled trial. JAMA 2008, 19, 2253–2262. [Google Scholar] [CrossRef]
- Nash, K.M.; Shah, Z.A. Current perspectives on the beneficial role of Ginkgo biloba in neurological and cerebrovascular disorders. Integr. Med. Insights 2015, 10, 1–9. [Google Scholar] [CrossRef]
- Zuo, W.; Yan, F.; Zhang, B.; Li, J.; Mei, D. Advances in the studies of Ginkgo biloba leaves extract on aging-related diseases. Aging Dis. 2017, 6, 812. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Y.; Chen, K. Ginkgo biloba extract in vascular protection: Molecular mechanisms and clinical applications. Curr. Vasc. Pharmacol. 2017, 15, 532–548. [Google Scholar] [CrossRef] [PubMed]
- Fei, R.; Fei, Y.; Zheng, S.; Gao, Y.G.; Sun, H.X.; Zeng, X.L. Purified polysaccharide from Ginkgo biloba leaves inhibits P-selectin-mediated leucocyte adhesion and inflammation. Acta Pharmacol. Sin. 2008, 29, 499–506. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Li, Y.; Wang, J.; Sun, K.; Tao, W.; Wang, Z.; Xiao, W.; Pan, Y.; Zhang, S.; Wang, Y. Systematic Investigation of Ginkgo Biloba Leaves for Treating Cardio-cerebrovascular Diseases in an Animal Model. ACS Chem. Biol. 2017, 5, 1363–1372. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds. Mar. Drugs 2018, 7, 237. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K.; Shim, M. Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials 2016, 12, 235. [Google Scholar] [CrossRef]
- Oh, S.; Son, M.; Lee, H.S.; Kim, H.S.; Jeon, Y.J.; Byun, K. Protective Effect of Pyrogallol-Phloroglucinol-6,6-Bieckol from Ecklonia cava on Monocyte-Associated Vascular Dysfunction. Mar. Drugs 2018, 16, 441. [Google Scholar] [CrossRef]
- Lee, J.H.; Ko, J.Y.; Oh, J.Y.; Kim, C.Y.; Lee, H.J.; Kim, J.; Jeon, Y.J. Preparative isolation and purification of phlorotannins from Ecklonia cava using centrifugal partition chromatography by one-step. Food Chem. 2014, 158, 433–437. [Google Scholar] [CrossRef]
- Kim, T.H.; Bae, J.S. Ecklonia cava extracts inhibit lipopolysaccharide induced inflammatory responses in human endothelial cells. Food Chem. Toxicol. 2010, 48, 1682–1687. [Google Scholar] [CrossRef]
- Takahashi, M.; Satake, N.; Yamashita, H.; Tamura, A.; Sasaki, M.; Matsui-Yuasa, I.; Tabuchi, M.; Akahoshi, Y.; Terada, M.; Kojima-Yuasa, A. Ecklonia cava polyphenol protects the liver against ethanol-induced injury in rats. Biochim. Biophys. Acta 2012, 1820, 978–988. [Google Scholar] [CrossRef]
- Jang, J.; Ye, B.R.; Heo, S.J.; Oh, C.; Kang, D.H.; Kim, J.H.; Affan, A.; Yoon, K.T.; Choi, Y.U.; Park, S.C.; et al. Photo-oxidative stress by ultraviolet-B radiation and antioxidative defense of eckstolonol in human keratinocytes. Environ. Toxicol. Pharmacol. 2012, 34, 926–934. [Google Scholar] [CrossRef]
- Lee, S.H.; Heo, S.J.; Hwang, J.Y.; Han, J.S.; Jeon, Y.J. Protective effects of enzymatic digest from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. J. Sci. Food Agric. 2010, 90, 349–356. [Google Scholar] [CrossRef]
- Beck, S.M.; Ruge, H.; Schindler, C.; Burkart, M.; Miller, R.; Kirschbaum, C.; Goschke, T. Effects of Ginkgo biloba extract EGb 761® on cognitive control functions, mental activity of the prefrontal cortex and stress reactivity in elderly adults with subjective memory impairment–a randomized double-blind placebo-controlled trial. Hum. Psychopharmacol. Clin. Exp. 2016, 3, 227–242. [Google Scholar] [CrossRef]
- Mansour, S.M.; Bahgat, A.K.; El-Khatib, A.S.; Khayyal, M.T. Ginkgo biloba extract (EGb 761) normalizes hypertension in 2K, 1C hypertensive rats: Role of antioxidant mechanisms, ACE inhibiting activity and improvement of endothelial dysfunction. Phytomedicine 2011, 18, 641–647. [Google Scholar] [CrossRef]
- Ahn, G.; Park, E.; Lee, W.W.; Hyun, J.W.; Lee, K.W.; Shin, T.; Jeon, Y.J.; Jee, Y. Enzymatic extract from Ecklonia cava induces the activation of lymphocytes by IL-2 production through the classical NF-κB pathway. Mar. Biotechnol. 2011, 13, 66–73. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, M.Y.; Shim, B.J.; Youn, H.J.; Hwang, H.J.; Shin, H.C.; Jeon, H.K. Effects of Ecklonia cava polyphenol in individuals with hypercholesterolemia: A pilot study. J. Med. Food 2012, 15, 1038–1044. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Ryu, B.; Lee, S.H.; Kim, M.M.; Kim, S.K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorg. Med. Chem. 2009, 17, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Lee, S.H.; Heo, S.J.; Kim, K.N.; Jeon, Y.J. Evaluation of antioxidant properties of a new compound, pyrogallol-phloroglucinol-6,6’-bieckol isolated from brown algae, Ecklonia cava. Nutr. Res. Pract. 2011, 5, 495–502. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.K. Insulinotrophic and hypolipidemic effects of Ecklonia cava in streptozotocin–induced diabetic mice. Asian Pac. J. Trop. Med. 2012, 5, 374–379. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, J.A.; Ahn, B.N.; Vo, T.S.; Yoon, N.T.; Kim, S.K. 1-(3′, 5′-dihydroxyphenoxy)-7-(2″, 4″, 6-trihydroxyphenoxy)-2, 4, 9-trihydroxydibenzo-1, 4-dioxin Inhibits Adipocyte Differentiation of 3T3-L1 Fibroblasts. Mar. Biotechnol. 2010, 12, 299–307. [Google Scholar] [CrossRef]
- Shin, H.C.; Kim, S.H.; Park, Y.; Lee, B.H.; Hwang, H.J. Effects of 12-week oral supplementation of Ecklonia cava polyphenols on anthropometric and blood lipid parameters in overweight Korean individuals: A double-blind randomized clinical trial. Phytother. Res. 2012, 26, 363–368. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Cai, Y.; Knight, W.E.; Guo, S.; Li, J.D.; Knight, P.A.; Yan, C. Vinpocetine suppresses pathological vascular remodeling by inhibiting vascular smooth muscle cell proliferation and migration. J. Pharmacol. Exp. Ther. 2012, 343, 479–488. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, Z.J.; Li, Y.; Kim, M.M.; Lee, S.H.; Kim, S.K. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
- Banin, R.M.; de Andrade, I.S.; Cerutti, S.M.; Oyama, L.M.; Telles, M.M.; Ribeiro, E.B. Ginkgo biloba Extract (GbE) Stimulates the Hypothalamic Serotonergic System and Attenuates Obesity in Ovariectomized Rats. Front. Pharmacol. 2017, 8, 605. [Google Scholar] [CrossRef]
- Wang, Y.; Thatcher, S.E.; Cassis, L.A. Measuring blood pressure using a noninvasive tail cuff method in mice. Methods Mol. Biol. 2017, 1614, 69–72. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, M.; Oh, S.; Lee, H.S.; Ryu, B.; Jiang, Y.; Jang, J.T.; Jeon, Y.-J.; Byun, K. Pyrogallol-Phloroglucinol-6,6’-Bieckol from Ecklonia cava Improved Blood Circulation in Diet-Induced Obese and Diet-Induced Hypertension Mouse Models. Mar. Drugs 2019, 17, 272. https://doi.org/10.3390/md17050272
Son M, Oh S, Lee HS, Ryu B, Jiang Y, Jang JT, Jeon Y-J, Byun K. Pyrogallol-Phloroglucinol-6,6’-Bieckol from Ecklonia cava Improved Blood Circulation in Diet-Induced Obese and Diet-Induced Hypertension Mouse Models. Marine Drugs. 2019; 17(5):272. https://doi.org/10.3390/md17050272
Chicago/Turabian StyleSon, Myeongjoo, Seyeon Oh, Hye Sun Lee, BoMi Ryu, Yunfei Jiang, Ji Tae Jang, You-Jin Jeon, and Kyunghee Byun. 2019. "Pyrogallol-Phloroglucinol-6,6’-Bieckol from Ecklonia cava Improved Blood Circulation in Diet-Induced Obese and Diet-Induced Hypertension Mouse Models" Marine Drugs 17, no. 5: 272. https://doi.org/10.3390/md17050272
APA StyleSon, M., Oh, S., Lee, H. S., Ryu, B., Jiang, Y., Jang, J. T., Jeon, Y.-J., & Byun, K. (2019). Pyrogallol-Phloroglucinol-6,6’-Bieckol from Ecklonia cava Improved Blood Circulation in Diet-Induced Obese and Diet-Induced Hypertension Mouse Models. Marine Drugs, 17(5), 272. https://doi.org/10.3390/md17050272





