Cyclonerane Derivatives from the Algicolous Endophytic Fungus Trichoderma asperellum A-YMD-9-2
Abstract
:1. Introduction
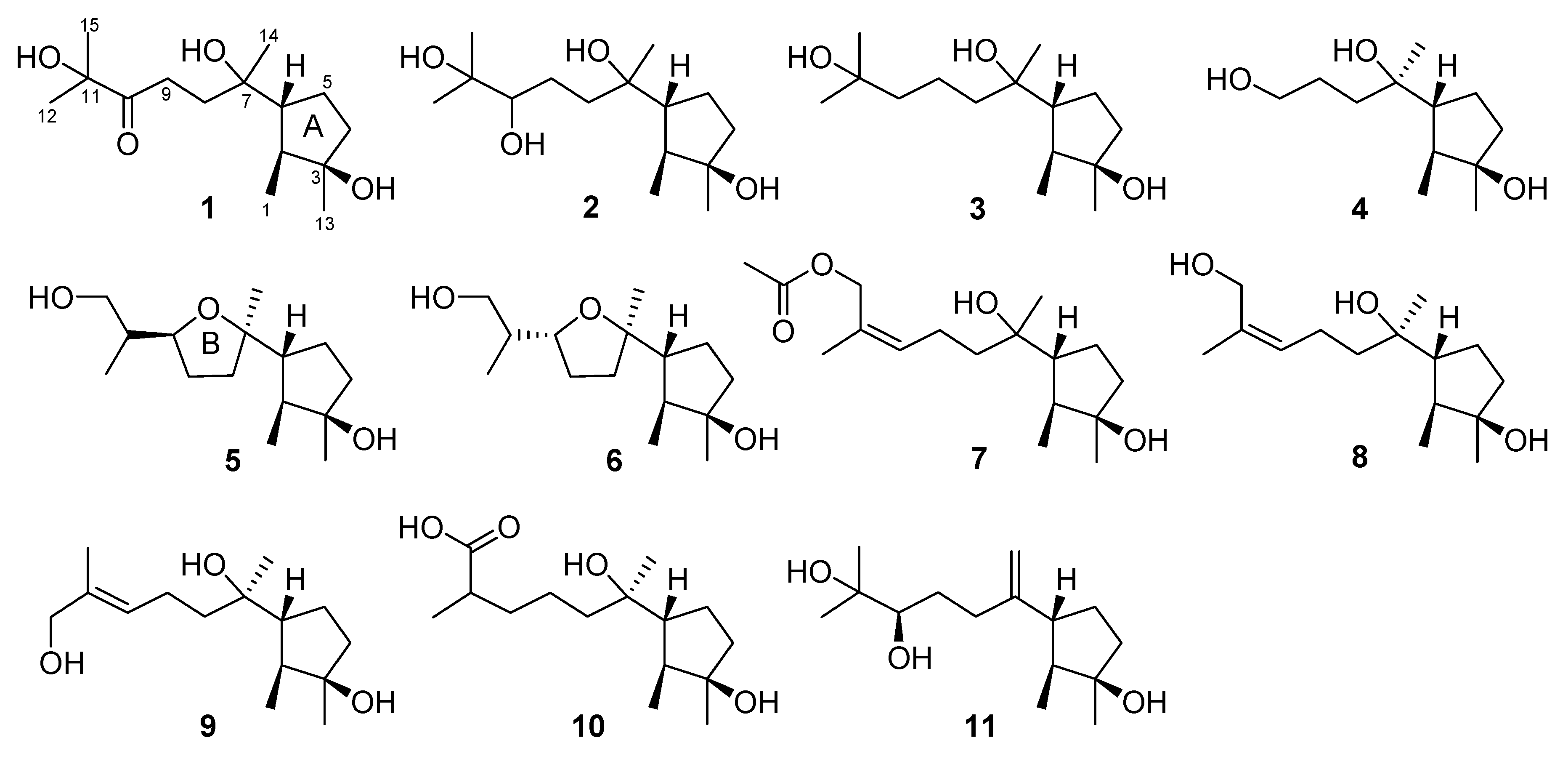
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. Extraction and Isolation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Su, D.; Ding, L.; He, S. Marine-derived Trichoderma species as a promising source of bioactive secondary metabolites. Mini-Rev. Med. Chem. 2018, 18, 1702–1713. [Google Scholar] [CrossRef]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Trichocarotins A–H and trichocadinin A, nine sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma virens. Bioorg. Chem. 2018, 81, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.P.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Diterpenes and sesquiterpenes from the marine algicolous fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018, 81, 2553–2559. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.P.; Liu, X.H.; Shi, Z.Z.; Miao, F.P.; Fang, S.T.; Ji, N.Y. Bisabolane, cyclonerane, and harziane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Phytochemistry 2018, 152, 45–52. [Google Scholar] [CrossRef]
- Liang, X.R.; Miao, F.P.; Song, Y.P.; Liu, X.H.; Ji, N.Y. Citrinovirin with a new norditerpene skeleton from the marine algicolous fungus Trichoderma citrinoviride. Bioorg. Med. Chem. Lett. 2016, 26, 5029–5031. [Google Scholar] [CrossRef]
- Miao, F.P.; Liang, X.R.; Yin, X.L.; Wang, G.; Ji, N.Y. Absolute configurations of unique harziane diterpenes from Trichoderma species. Org. Lett. 2012, 14, 3815–3817. [Google Scholar] [CrossRef]
- Yamamoto, T.; Izumi, N.; Ui, H.; Sueki, A.; Masuma, R.; Nonaka, K.; Hirose, T.; Sunazuka, T.; Nagai, T.; Yamada, H.; et al. Wickerols A and B: novel anti-influenza virus diterpenes produced by Trichoderma atroviride FKI-3849. Tetrahedron 2012, 68, 9267–9271. [Google Scholar] [CrossRef]
- Fujita, T.; Takaishi, Y.; Takeda, Y.; Fujiyama, T.; Nishi, T. Fungal metabolites. II. Structural elucidation of minor metabolites, valinotricin, cyclonerodiol oxide, and epicyclonerodiol oxide, from Trichoderma polysporum. Chem. Pharm. Bull. 1984, 32, 4419–4425. [Google Scholar] [CrossRef]
- Macías, F.F.; Varela, R.M.; Simonet, A.M.; Cutler, H.G.; Cutler, S.J.; Eden, M.A.; Hill, R.A. Bioactive carotanes from Trichoderma virens. J. Nat. Prod. 2000, 63, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Sun, P.X.; Jin, G.L.; Qin, L.P. Sesquiterpenoids from Trichoderma atroviride, an endophytic fungus in Cephalotaxus fortunei. Fitoterapia 2011, 82, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Zhao, L.X.; Chen, Y.W.; Huang, R.; Miao, C.P.; Wang, J. Sesquiterpenoids from the endophytic fungus Trichoderma sp. PR-35 of Paeonia delavayi. Chem. Biodivers. 2011, 8, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, J.L.; Liu, J.M.; Chen, R.D.; Xie, K.B.; Chen, D.W.; Feng, K.P.; Zhang, D.; Dai, J.G. Neural anti-inflammatory sesquiterpenoids from the endophytic fungus Trichoderma sp. Xy24. J. Asian Nat. Prod. Res. 2017, 19, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lin, X.; Tian, Y.; Liang, R.; Wang, J.; Yang, B.; Zhou, X.; Kaliyaperumal, K.; Luo, X.; Tu, Z.; Liu, Y. Three new polyketides from the marine sponge-derived fungus Trichoderma sp. SCSIO41004. Nat. Prod. Res. 2018, 32, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.Y.; Wang, B.G. Mycochemistry of marine algicolous fungi. Fungal Divers. 2016, 80, 301–342. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Kim, Y.H.; Jung, J.H.; Kang, J.S.; Kim, D.K.; Choi, H.D.; Son, B.W. Microbial transformation of the bioactive sesquiterpene, cyclonerodiol, by the ascomycete Penicillium sp. and the actinomycete Streptomyces sp. Enzyme Microb. Technol. 2007, 40, 1188–1192. [Google Scholar] [CrossRef]
- Hanson, J.R.; Hitchcock, P.B.; Nyfeler, R. Cyclonerotriol [6-(3-hydroxy-2,3-dimethylcyclopentyl)-2- methylhept-2-ene-1,6-diol], a new sesquiterpenoid metabolite of Fusariurn culmorum. J. Chem. Soc. Perkin Trans. 1 1975, 1586–1590. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wang, Y.L.; Tan, J.L.; Zhu, C.Y.; Li, D.X.; Huang, R.; Zhang, K.Q.; Niu, X.M. Regulation of the growth of cotton bollworms by metabolites from an entomopathogenic fungus Paecilomyces cateniobliquus. J. Agric. Food Chem. 2012, 60, 5604–5608. [Google Scholar] [CrossRef] [PubMed]
- Koshino, H.; Togiya, S.; Terada, S.; Yoshihara, T.; Sakamura, S.; Shimanuki, T.; Sato, T.; Tajimi, A. New fungitoxic sesquiterpenoids, chokols A-G, from stromata of Epichloe typhina and the absolute configuration of chokol E. Agric. Biol. Chem. 1989, 53, 789–796. [Google Scholar] [CrossRef]
- Laurent, D.; Goasdoue, N.; Kohler, F.; Pellegrin, F.; Platzer, N. Characterization of cyclonerodiol isolated from corn infested by Fusarium Moniliforme sheld.: one- and two-dimensional 1H and 13C NMR study. Magn. Reson. Chem. 1990, 28, 662–664. [Google Scholar] [CrossRef]
- Su, B.N.; Park, E.J.; Mbwambo, Z.H.; Santarsiero, B.D.; Mesecar, A.D.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. New chemical constituents of euphorbia quinquecostata and absolute configuration assignment by a convenient Mosher ester procedure carried out in NMR tubes. J. Nat. Prod. 2002, 65, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Z.; Miao, F.P.; Fang, S.T.; Yin, X.L.; Ji, N.Y. Sulfurated diketopiperazines from an algicolous isolate of Trichoderma virens. Phytochem. Lett. 2018, 27, 101–104. [Google Scholar] [CrossRef]


| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1 (β) | 1.03, d (6.8) | 1.03, d (6.8) | 1.03, d (6.8) | 1.05, d (6.8) | 1.02, d (6.8) | 1.02, d (6.8) | 1.04, d (6.8) |
| 2 (α) | 1.56, m | 1.55, m | 1.59, m | 1.60, m | 1.50, m | 1.54, m | 1.60, m |
| 4a | 1.67, m | 1.69, m | 1.67, m | 1.69, m | 1.68, m | 1.68, m | 1.68, m |
| 4b | 1.56, m | 1.56, m | 1.55, m | 1.56, m | 1.59, m | 1.58, m | 1.55, m |
| 5a | 1.88, m | 1.88, m | 1.85, m | 1.88, m | 1.89, m | 1.89, m | 1.85, m |
| 5b | 1.63, m | 1.56, m | 1.54, m | 1.55, m | 1.49, m | 1.40, m | 1.55, m |
| 6 (β) | 1.97, m | 1.89, m | 1.85, m | 1.87, m | 1.95, m | 2.00, m | 1.84, m |
| 8a | 2.12, m | 1.73, m | 1.44, m | 1.57, m | 1.75, m | 1.86, m | 1.50, t (8.3) |
| 8b | 1.89, m | 1.59, m | 1.64, m | 1.69, m | |||
| 9a | 2.51, m | 1.61, m | 1.44, m | 1.68, m | 1.92, m | 1.83, m | 2.16, m |
| 9b | 1.40, m | 1.71, m | 1.79, m | ||||
| 10 | 3.38, br d (10.3) a | 1.45, m | 3.68, m | 4.07, ddd (7.6, 6.9, 4.1) | 4.04, ddd (9.8, 5.6, 4.3) | 5.41, t (7.3) | |
| 11 | 1.93, m | 2.00, m | |||||
| 12 | 1.30, s | 1.16, s | 1.22, s | 0.93, d (7.0) | 0.90, d (7.1) | 1.74, br s | |
| 13 (α) | 1.25, s | 1.25, s | 1.25, s | 1.26, s | 1.24, s | 1.25, s | 1.25, s |
| 14 | 1.19, s | 1.15, s | 1.16, s | 1.17, s | 1.14, s | 1.17, s | 1.16, s |
| 15a | 1.29, s | 1.21, s | 1.22, s | 3.65, d (10.8, 6.1) | 3.69, dd (10.8, 6.9) | 4.62, d (11.9) | |
| 15b | 3.61, d (10.8, 4.1) | 3.58, dd (10.8, 3.7) | 4.57, d (11.9) | ||||
| CH3CO | 2.06, s |
| Position | δC, Type | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1 | 14.4, CH3 | 14.5, CH3 | 14.7, CH3 | 14.6, CH3 | 14.0, CH3 | 13.8, CH3 | 14.7, CH3 |
| 2 | 44.9, CH | 44.7, CH | 44.4, CH | 44.5, CH | 45.4, CH | 45.3, CH | 44.4, CH |
| 3 | 81.4, C | 81.5, C | 81.5, C | 81.4, C | 81.3, C | 81.4, C | 81.4, C |
| 4 | 40.4, CH2 | 40.4, CH2 | 40.5, CH2 | 40.5, CH2 | 40.5, CH2 | 40.4, CH2 | 40.5, CH2 |
| 5 | 24.8, CH2 | 24.5, CH2 | 24.4, CH2 | 24.6, CH2 | 25.1, CH2 | 25.4, CH2 | 24.5, CH2 |
| 6 | 55.1, CH | 54.5, CH | 54.3, CH | 54.9, CH | 54.2, CH | 54.1, CH | 54.6, CH |
| 7 | 76.0, C | 75.1, C | 75.0, C | 74.8, C | 85.7, C | 86.0, C | 74.8, C |
| 8 | 31.5, CH2 | 37.0, CH2 | 41.1, CH2 | 36.8, CH2 | 35.7, CH2 | 34.8, CH2 | 40.5, CH2 |
| 9 | 32.8, CH2 | 25.8, CH2 | 18.7, CH2 | 27.2, CH2 | 28.1, CH2 | 27.4, CH2 | 22.5, CH2 |
| 10 | 215.1, C | 79.0, CH | 44.5, CH2 | 63.6, CH2 | 80.3, CH | 84.2, CH | 131.1, CH |
| 11 | 79.4, C | 73.4, C | 71.2, C | 38.2, CH | 37.4, CH | 130.0, C | |
| 12 | 27.8, CH3 | 23.5, CH3 | 29.4, CH3 | 11.8, CH3 | 12.0, CH3 | 21.6, CH3 | |
| 13 | 26.3, CH3 | 26.2, CH3 | 26.2, CH3 | 26.2, CH3 | 26.2, CH3 | 26.2, CH3 | 26.2, CH3 |
| 14 | 23.5, CH3 | 25.0, CH3 | 25.2, CH3 | 25.2, CH3 | 23.1, CH3 | 26.4, CH3 | 25.0, CH3 |
| 15 | 28.0, CH3 | 26.7, CH3 | 29.5, CH3 | 66.9, CH2 | 66.7, CH2 | 63.3, CH2 | |
| CH3CO | 171.3, C | ||||||
| CH3CO | 21.1, CH3 | ||||||
| Compound | IC50 (μg/mL) | |||
|---|---|---|---|---|
| Chattonella marina | Heterosigma akashiwo | Karlodinium veneficum | Prorocentrum donghaiense | |
| 1 | 5.2 | 8.0 | 10 | 9.9 |
| 2 | 8.8 | 21 | 76 | 6.5 |
| 3 | 61 | 73 | 71 | 40 |
| 4 | 13 | 73 | 6.3 | 34 |
| 5 | 2.4 | 26 | 3.9 | 20 |
| 6 | 5.8 | 37 | 5.5 | 15 |
| 7 | 59 | 14 | 35 | 7.3 |
| 8 | 42 | 50 | 12 | 3.4 |
| 9 | 15 | 53 | 43 | 1.1 |
| 10 | 1.6 | 1.8 | 1.6 | 2.0 |
| 11 | 62 | 9.4 | 13 | 6.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-P.; Miao, F.-P.; Liu, X.-H.; Yin, X.-L.; Ji, N.-Y. Cyclonerane Derivatives from the Algicolous Endophytic Fungus Trichoderma asperellum A-YMD-9-2. Mar. Drugs 2019, 17, 252. https://doi.org/10.3390/md17050252
Song Y-P, Miao F-P, Liu X-H, Yin X-L, Ji N-Y. Cyclonerane Derivatives from the Algicolous Endophytic Fungus Trichoderma asperellum A-YMD-9-2. Marine Drugs. 2019; 17(5):252. https://doi.org/10.3390/md17050252
Chicago/Turabian StyleSong, Yin-Ping, Feng-Ping Miao, Xiang-Hong Liu, Xiu-Li Yin, and Nai-Yun Ji. 2019. "Cyclonerane Derivatives from the Algicolous Endophytic Fungus Trichoderma asperellum A-YMD-9-2" Marine Drugs 17, no. 5: 252. https://doi.org/10.3390/md17050252
APA StyleSong, Y.-P., Miao, F.-P., Liu, X.-H., Yin, X.-L., & Ji, N.-Y. (2019). Cyclonerane Derivatives from the Algicolous Endophytic Fungus Trichoderma asperellum A-YMD-9-2. Marine Drugs, 17(5), 252. https://doi.org/10.3390/md17050252







