The Immune Activity of PT-Peptide Derived from Anti-Lipopolysaccharide Factor of the Swimming Crab Portunus trituberculatus Is Enhanced when Encapsulated in Milk-Derived Extracellular Vesicles
Abstract
1. Introduction
2. Results
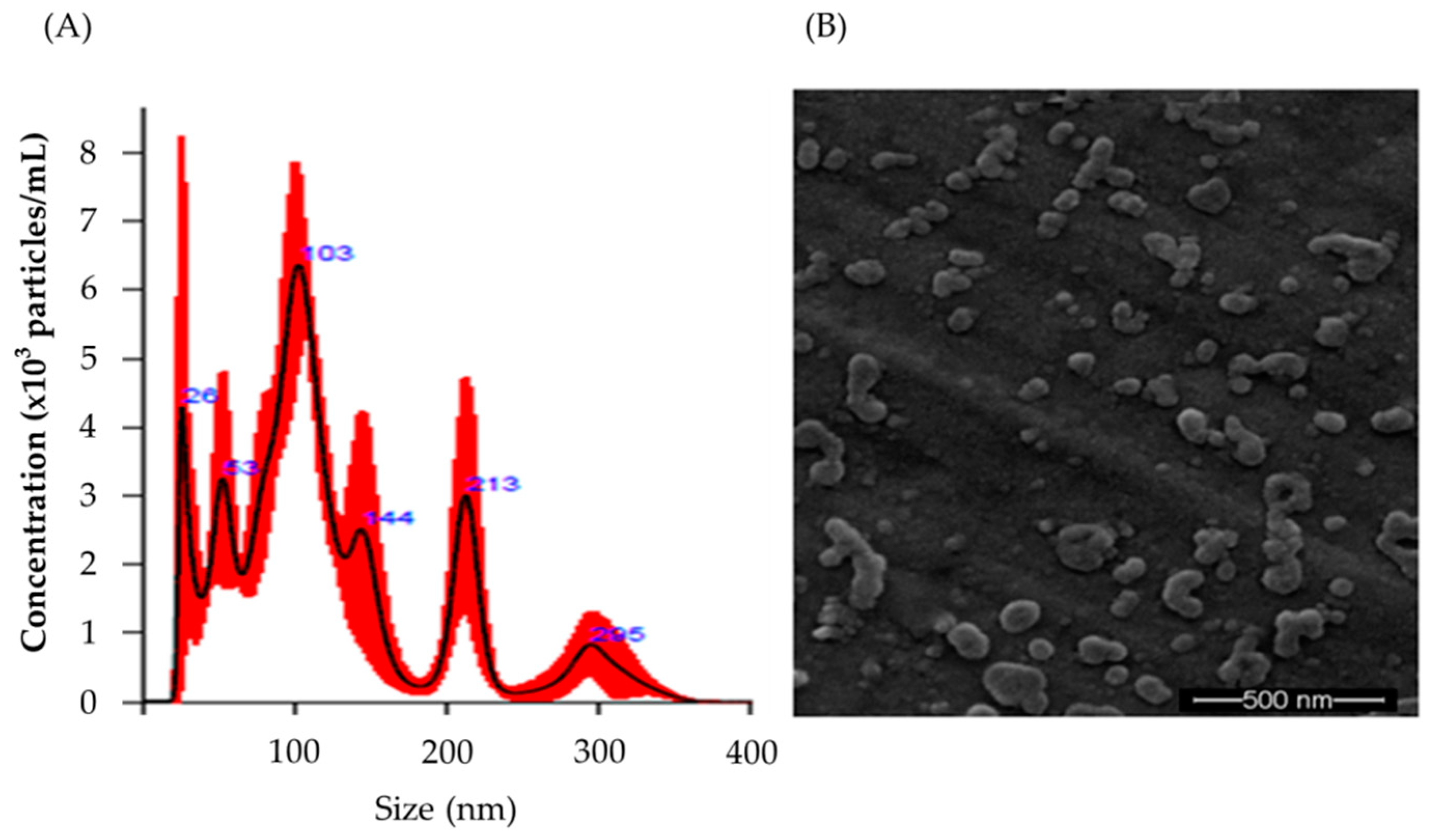
2.1. Characterization of Raw Milk-Derived EVs Encapsulating PT-Peptide
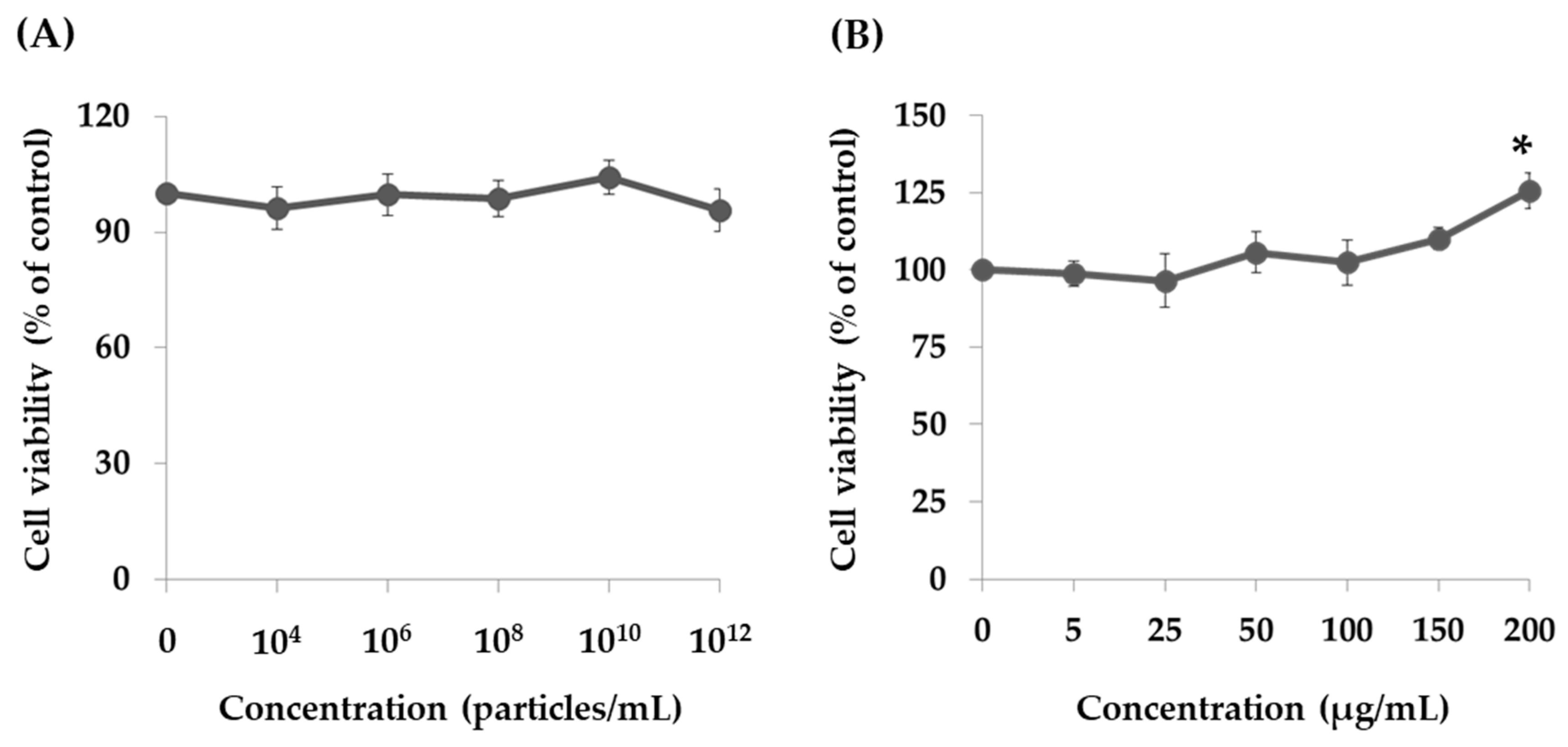
2.2. Effect of PT-Peptide and Raw Milk-Derived EVs in THP-1 Cells
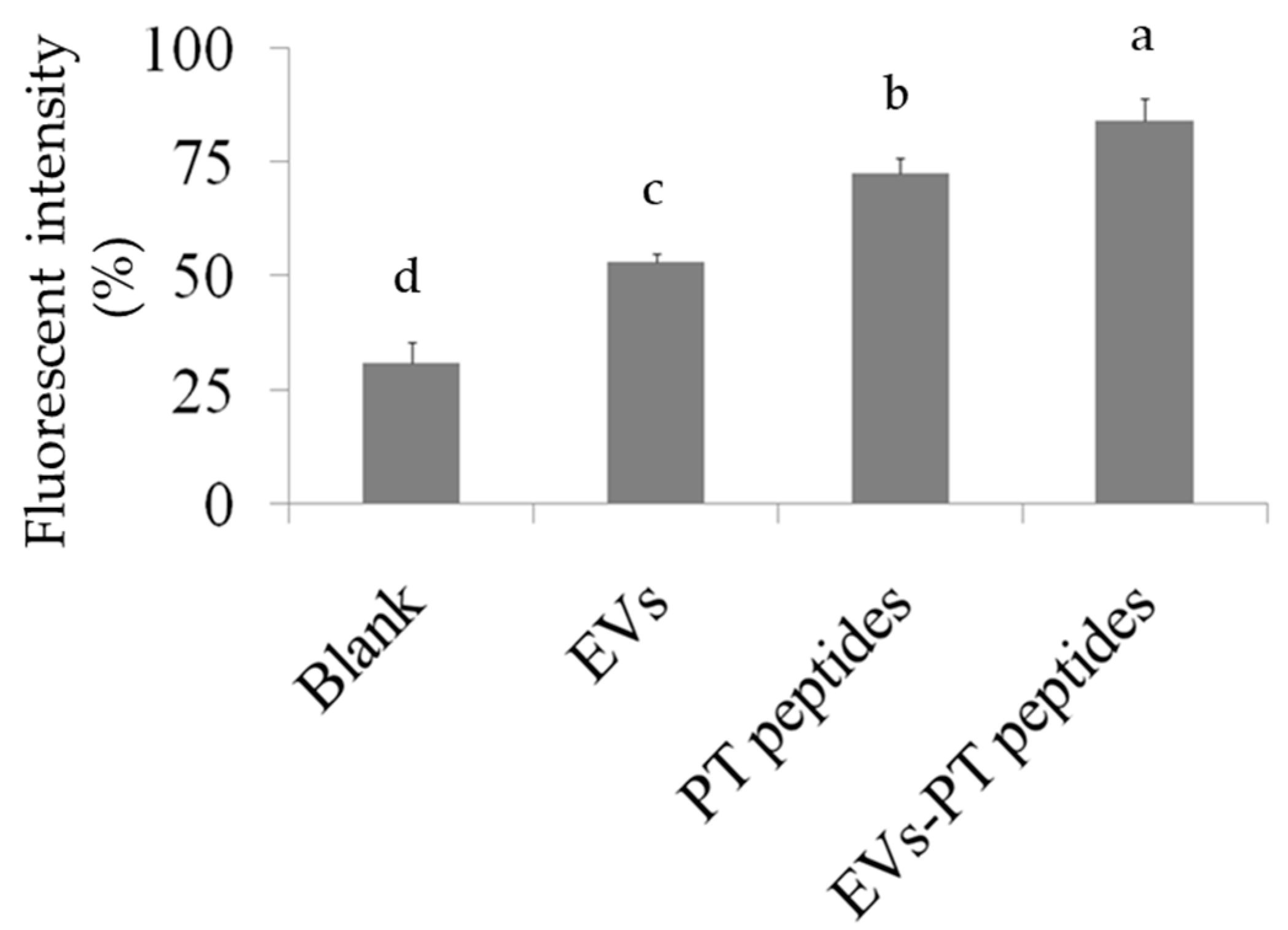
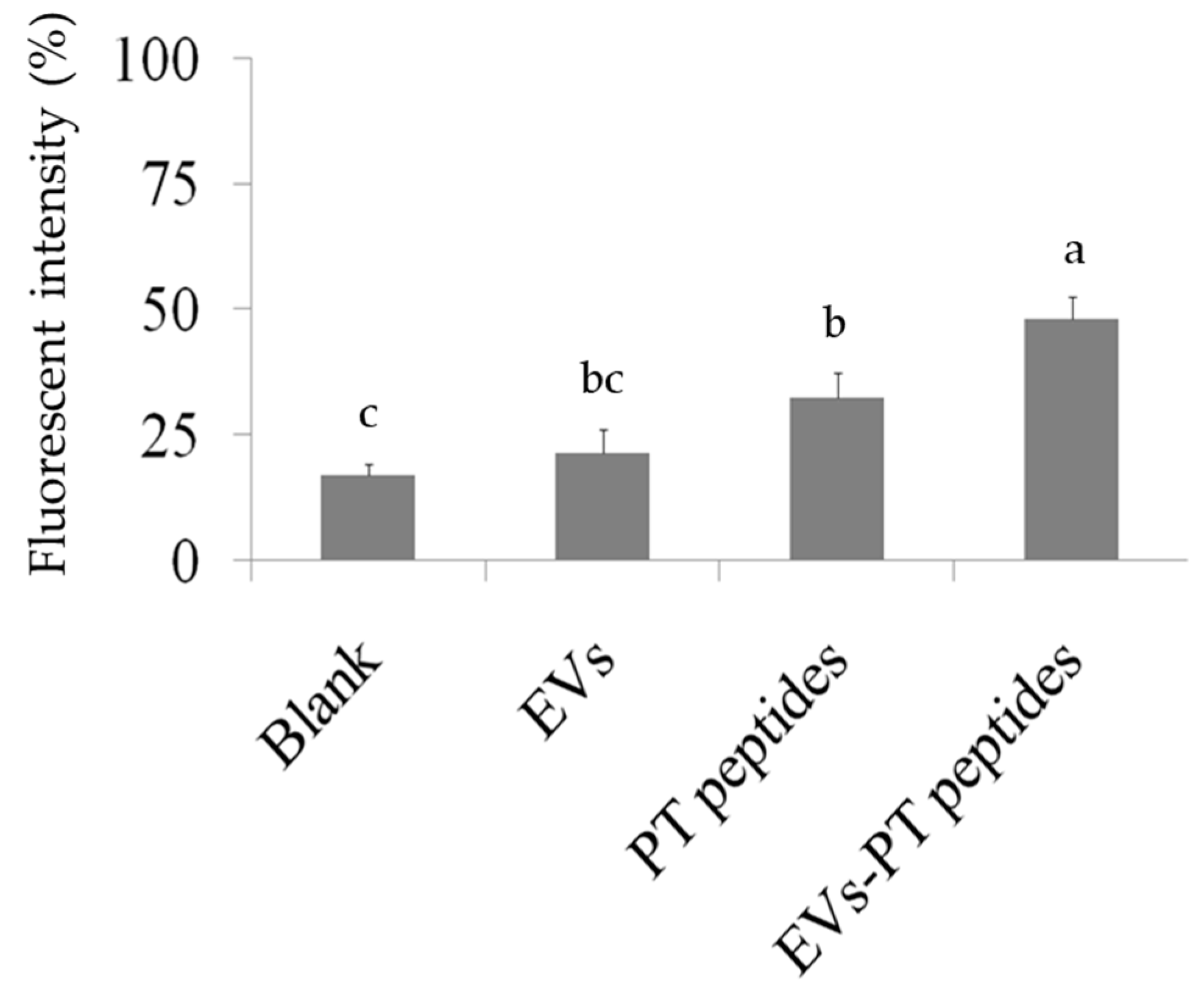
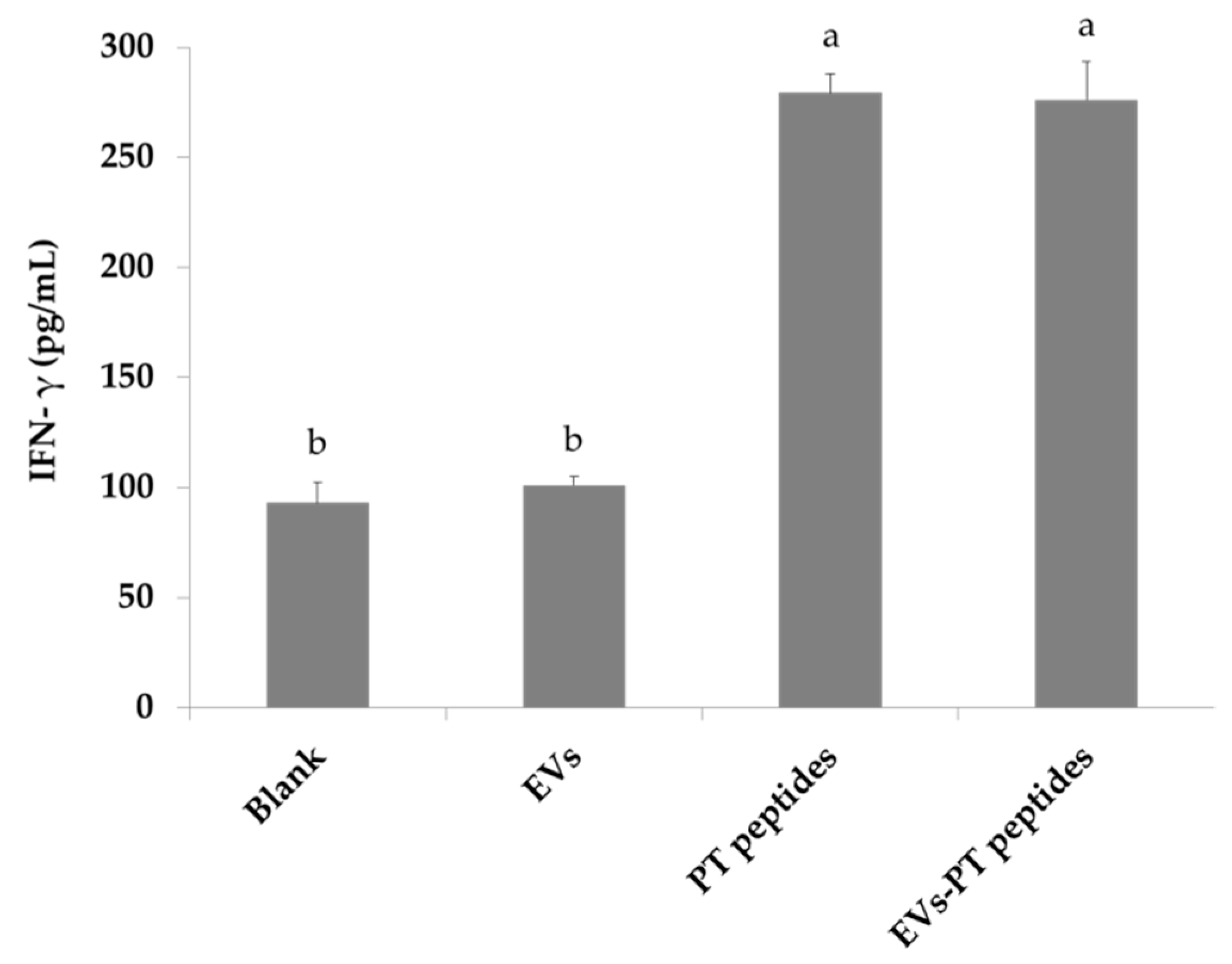
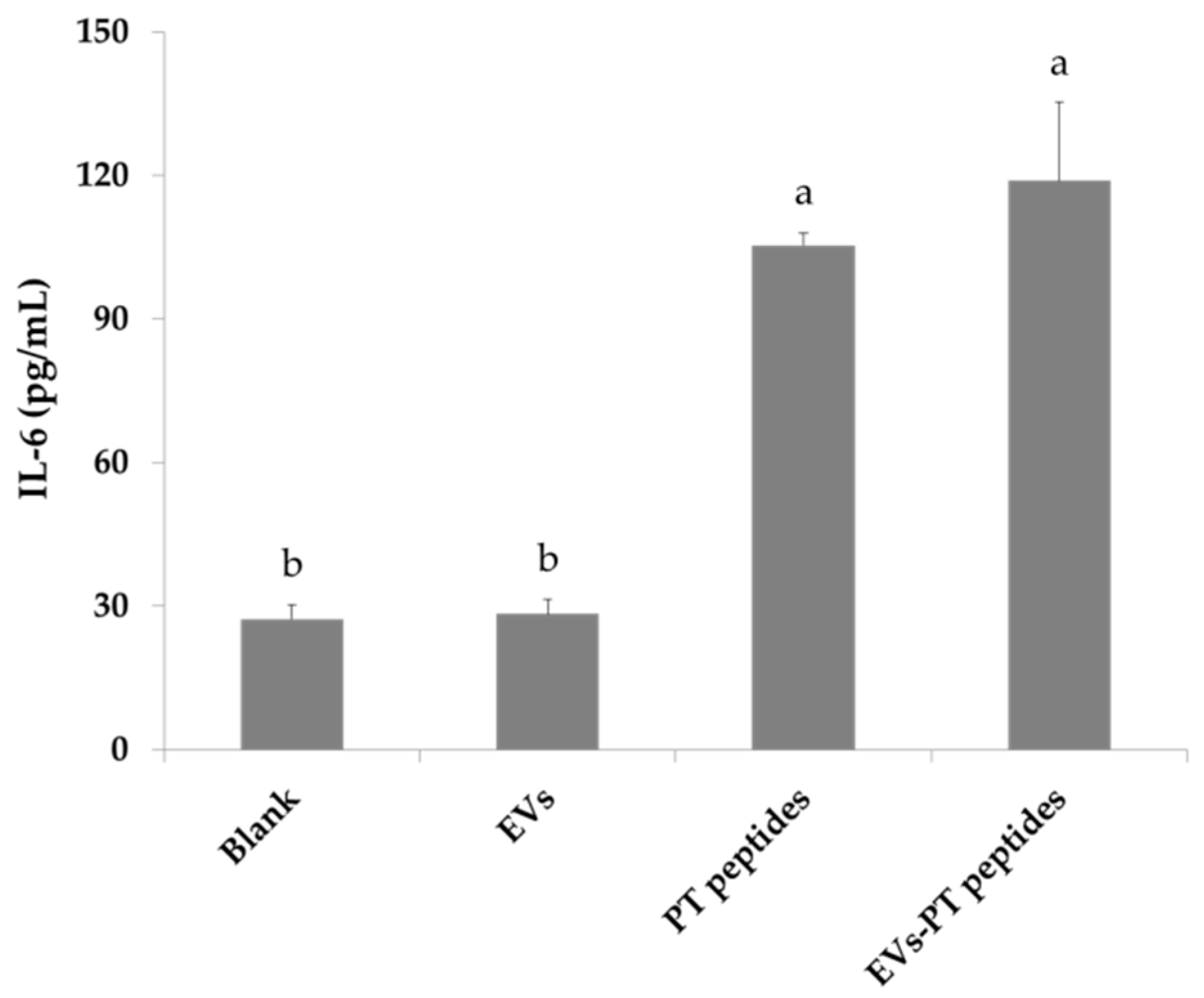
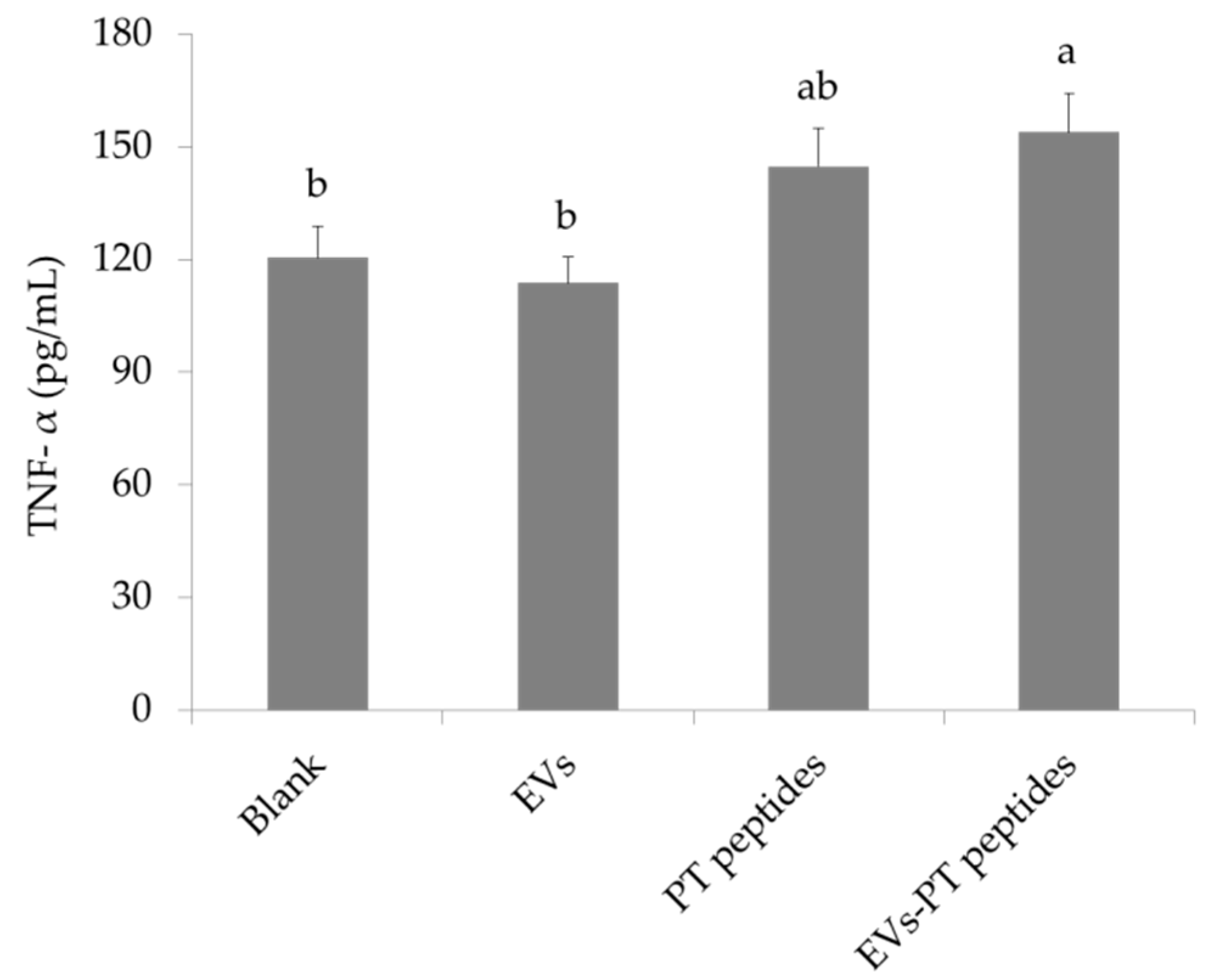
2.3. Immune Stimulation by PT-Peptide and EVs-PT Peptide in THP-1 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Extracellular Vesicles (EVs)
4.3. Encapsulation and Characterization of EVs
4.4. Cell Culture and Cell Viability Assay
4.5. Assay for Cellular Uptake
4.6. Measurement of Cellular and Mitochondrial Superoxide Levels
4.7. Assay for Phagocytosis
4.8. Western Blotting
4.9. Assay for Cytokines
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [PubMed]
- Lin, H.J.; Huang, T.C.; Muthusamy, S.; Lee, J.F.; Duann, Y.F.; Lin, C.H. Piscidin-1, an antimicrobial peptide from fish (hybrid striped bass Morone saxatilis x M. chrysops), induces apoptotic and necrotic activity in HT1080 cells. Zoologic. Sci. 2012, 29, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.P.; Huang, T.C.; Lin, C.C.; Hui, C.F.; Lin, C.H.; Chen, J.Y. Pardaxin, a fish antimicrobial peptide, exhibits antitumor activity toward murine fibrosarcoma in vitro and in vivo. Mar. Drugs 2012, 10, 1852–1872. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Cui, Z.; Li, Y.H.; Hsu, W.H.; Lee, B.H. In vitro and in vivo anticancer activity of pardaxin against proliferation and growth of oral squamous cell carcinoma. Mar. Drugs 2016, 14, 2. [Google Scholar] [CrossRef]
- Hoess, A.; Watson, S.; Siber, G.R.; Liddington, R. Crystal structure of an endotoxin neutralizing protein from the horseshoe crab, Limulus anti-LPS factor, at 1.5 A resolution. EMBO J. 1993, 12, 3351–3356. [Google Scholar] [CrossRef]
- Yue, F.; Pan, L.; Miao, J.; Zhang, L.; Li, J. Molecular cloning, characterization and mRNA expression of two antibacterial peptides: Crustin and anti-lipopolysaccharide factor in swimming crab Portunus trituberculatus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 156, 77–85. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Z.; Li, X.; Song, C.; Li, Q.; Wang, S. A new anti-lipopolysaccharide factor isoform (PtALF4) from the swimming crab Portunus trituberculatus exhibited structural and functional diversity of ALFs. Fish Shellfish Immunol. 2012, 32, 724–731. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, Z.; Li, X.; Song, C.; Li, Q.; Wang, S. Molecular cloning, expression pattern and antimicrobial activity of a new isoform of anti-lipopolysaccharide factor from the swimming crab Portunus trituberculatus. Fish Shellfish Immunol. 2012, 33, 85–91. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Y.; Yu, Z.; Mu, C.; Song, W.; Li, R.; Liu, L.; Ye, Y.; Shi, C.; Wang, C. An MBT domain containing anti-lipopolysaccharide factor (PtALF8) from Portunus trituberculatus is involved in immune response to bacterial challenge. Fish Shellfish Immunol. 2019, 84, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Ohtsubo, S.; Nakamura, T.; Tanaka, S.; Iwanaga, S.; Ohashi, K.; Niwa, M. Isolation and biological activities of Limulus anticoagulant (anti-LPS factor) which interacts with lipopolysaccharide (LPS). J. Biochem. 1985, 97, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Chao, T.T.; Chen, J.C.; Chen, J.Y.; Liu, W.C.; Lin, C.H.; Kuo, C.M. Shrimp (Penaeus monodon) anti-lipopolysaccharide factor reduces the lethality of Pseudomonas aeruginosa sepsis in mice. Int. Immunopharmacol. 2007, 7, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Tharntada, S.; Ponprateep, S.; Somboonwiwat, K.; Liu, H.; Söderhäll, I.; Söderhäll, K.; Tassanakajon, A. Role of anti-lipopolysaccharide factor from the black tiger shrimp, Penaeus monodon, in protection from white spot syndrome virus infection. J. Gen. Virol. 2009, 90, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, S.; Li, F.; Xiang, J. Functional diversity of anti-lipopolysaccharide factor isoforms in shrimp and their charters related to antiviral activity. Mar. Drugs 2015, 13, 2602–2616. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Fr. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Raemdonck, K.; Braeckmans, K.; Demeester, J.; De Smedt, S.C. Merging the best of both worlds: Hybrid lipid-enveloped matrix nano composites in drug delivery. Chem. Soc. Rev. 2014, 43, 444–472. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Zu, Y.J.; Nie, S.F.; Cao, J.; Wang, Q.; Nie, S.P.; Deng, Z.Y.; Xie, M.Y.; Wang, S. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin. J. Nat. Med. 2015, 13, 641–652. [Google Scholar] [CrossRef]
- Aryani, A.; Denecke, B. Exosomes as a nano delivery system: A key to the future of neuro medicine? Mol. Neurobiol. 2016, 53, 818–834. [Google Scholar] [CrossRef]
- Johnstone, R.M. Revisiting the road to the discovery of exosomes. Blood Cells Mol. Dis. 2005, 34, 214–219. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Agrawal, U.; Sharma, R.; Gupta, M.; Vyas, S.P. Is nanotechnology a boon for oral drug delivery? Drug Disc. Today 2014, 19, 1530–1546. [Google Scholar] [CrossRef]
- Sun, D.M.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Vashisht, M.; Golla, N.; Shandilya, S.; Onteru, S.K.; Singh, D. Milk miRNAs encapsulated in exosomes are stable to human digestion and permeable to intestinal barrier in vitro. J. Funct. Foods 2017, 34, 431–439. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, X.; Yu, J.; Zen, K.; Zhang, C.; Li, L. Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein Cell 2013, 4, 197–210. [Google Scholar] [CrossRef]
- Baier, S.R.; Nguyen, C.; Xie, F.; Wood, J.R.; Zempleni, J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J. Nutr. 2014, 144, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Wolf, T.; Baier, S.R.; Zempleni, J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma Caco-2 cells and rat small intestinal IEC-6 cells. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef]
- Wu, S.C.; Lee, B.H. Buckwheat polysaccharide exerts antiproliferative effects in THP-1 human leukemia cells by inducing differentiation. J. Med. Foods 2011, 14, 26–33. [Google Scholar] [CrossRef]
- Hsu, W.H.; Lee, B.H.; Liao, T.H.; Pan, Z.M. Monascus-fermented metabolite monascin suppresses inflammation via PPAR-γ regulation and JNK inactivation in THP-1 monocytes. Food Chem. Toxicol. 2012, 50, 1178–1186. [Google Scholar] [CrossRef]
- Papo, N.; Shia, Y. A molecular mechanism for lipopolysaccharide protection of Gram-negative bacteria from antimicrobial peptides. J. Biol. Chem. 2005, 280, 10378–10387. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Liao, Y.; Du, X.; Li, J.; Lonnerdal, B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol. Nutr. Food Res. 2017, 61, 1700082. [Google Scholar] [CrossRef]
- Kusuma, R.J.; Manca, S.; Friemel, T.; Sukreet, S.; Nguyen, C.; Zempleni, J. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis. Am. J. Physiol. Cell Physiol. 2016, 310, C800–C807. [Google Scholar] [CrossRef] [PubMed]
- Kadowak, T.; Inagawa, H.; Kohchi, C.; Nishizawa, T.; Takahashi, Y.; Soma, G. Anti-lipopolysaccharide factor evokes indirect killing of virulent bacteria in kuruma prawn. In Vivo 2011, 25, 741–744. [Google Scholar]
- Yu, S.; Zhao, Z.; Sun, L.; Li, P. Fermentation results in quantitative changes in milk-derived exosomes and different effects on cell growth and survival. J. Agric. Food Chem. 2017, 65, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicle for Parkinson’s disease therapy. J. Cont. Rel. 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hsu, W.H.; Liao, T.H.; Pan, Z.M. Inhibition of leukemia proliferation by a novel polysaccharide identified from Monascus-fermented dioscorea via inducing differentiation. Food Funct. 2012, 3, 758. [Google Scholar] [CrossRef]
- Lin, L.T.; Lai, Y.J.; Wu, S.C.; Hsu, W.H.; Tai, C.J. Optimal conditions for cordycepin production in surface liquid-cultured Cordyceps militaris treated with porcine liver extracts for suppression of oral cancer. J. Food Drug Anal. 2018, 26, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Kalyanaraman, B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radic. Biol. Med. 2010, 48, 983–1001. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-H.; Chen, B.-R.; Huang, C.-T.; Lin, C.-H. The Immune Activity of PT-Peptide Derived from Anti-Lipopolysaccharide Factor of the Swimming Crab Portunus trituberculatus Is Enhanced when Encapsulated in Milk-Derived Extracellular Vesicles. Mar. Drugs 2019, 17, 248. https://doi.org/10.3390/md17050248
Lee B-H, Chen B-R, Huang C-T, Lin C-H. The Immune Activity of PT-Peptide Derived from Anti-Lipopolysaccharide Factor of the Swimming Crab Portunus trituberculatus Is Enhanced when Encapsulated in Milk-Derived Extracellular Vesicles. Marine Drugs. 2019; 17(5):248. https://doi.org/10.3390/md17050248
Chicago/Turabian StyleLee, Bao-Hong, Bo-Rui Chen, Cheng-Ting Huang, and Cheng-Hui Lin. 2019. "The Immune Activity of PT-Peptide Derived from Anti-Lipopolysaccharide Factor of the Swimming Crab Portunus trituberculatus Is Enhanced when Encapsulated in Milk-Derived Extracellular Vesicles" Marine Drugs 17, no. 5: 248. https://doi.org/10.3390/md17050248
APA StyleLee, B.-H., Chen, B.-R., Huang, C.-T., & Lin, C.-H. (2019). The Immune Activity of PT-Peptide Derived from Anti-Lipopolysaccharide Factor of the Swimming Crab Portunus trituberculatus Is Enhanced when Encapsulated in Milk-Derived Extracellular Vesicles. Marine Drugs, 17(5), 248. https://doi.org/10.3390/md17050248





