Siderophore (from Synechococcus sp. PCC 7002)-Chelated Iron Promotes Iron Uptake in Caco-2 Cells and Ameliorates Iron Deficiency in Rats
Abstract
1. Introduction
2. Results
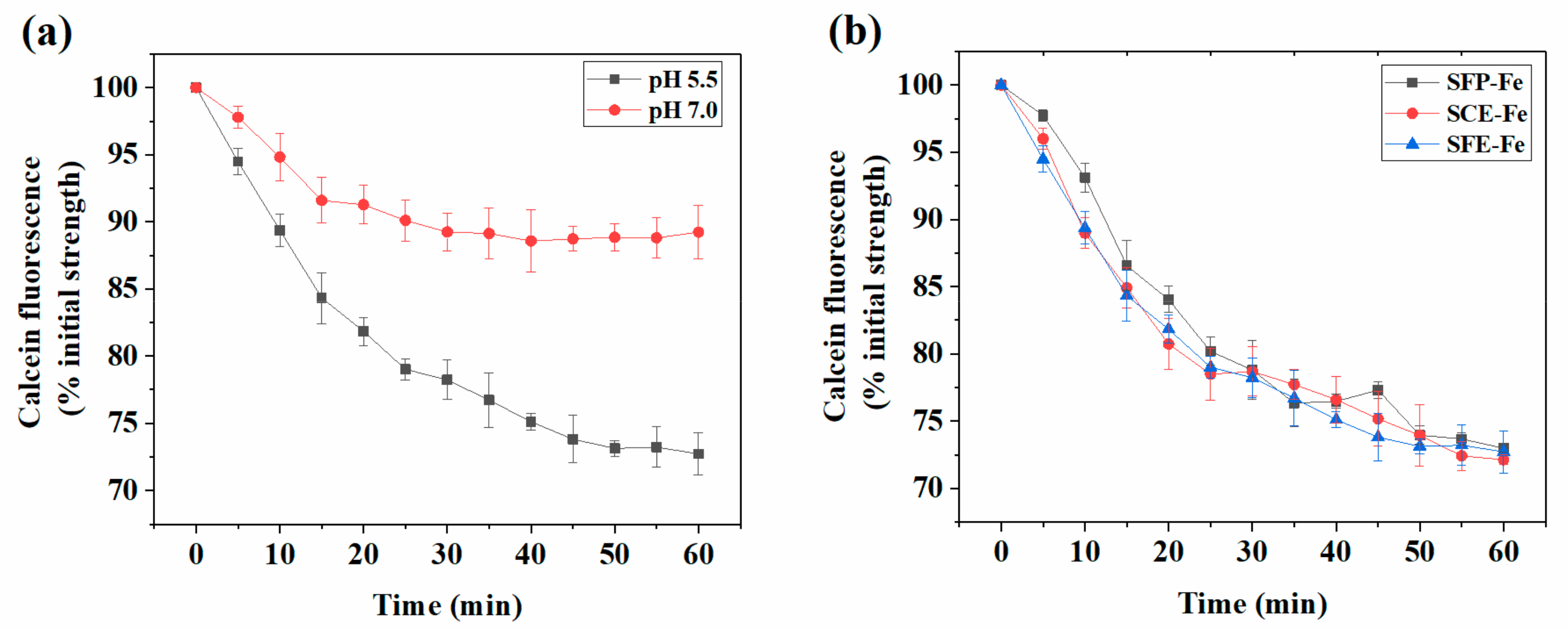
2.1. Analysis of Siderophore Absorption Site in Polarized Human Intestinal Epithelial (Caco-2) Cells
2.2. The Effect of Different Extraction Methods on Iron Absorption
2.3. Analysis of Siderophore Absorption Methods in Polarized Caco-2 Cell Monolayers
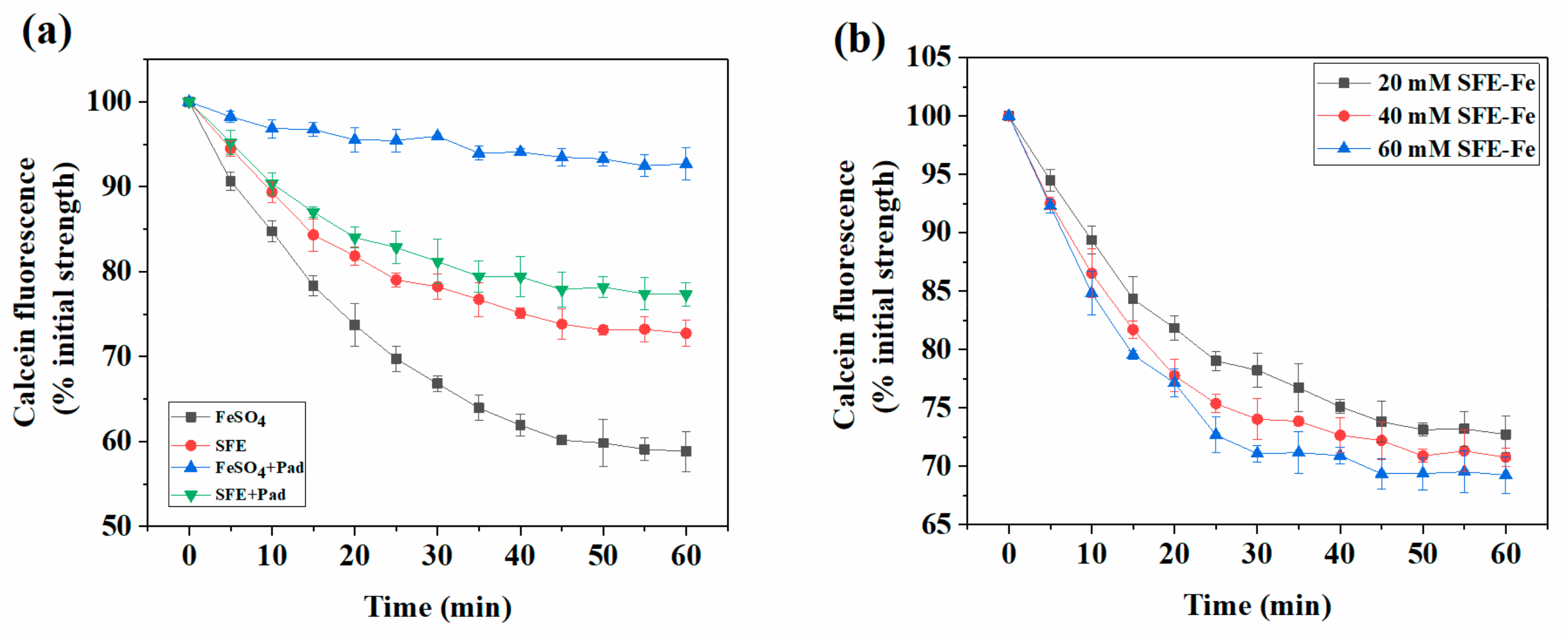
2.4. Analysis of Reduction and Absorption of SFE-Fe in Polarized Caco-2 Cell Monolayers
2.5. Effect of Sodium Phytate on the Absorption of Siderophores-Chelated Iron Intestinal Cells
2.6. Effect of SFE-Fe on the Metabolic Balance in Intestinal Cells
2.7. Measurement of Diet Intake on the Growth and Development of Rats
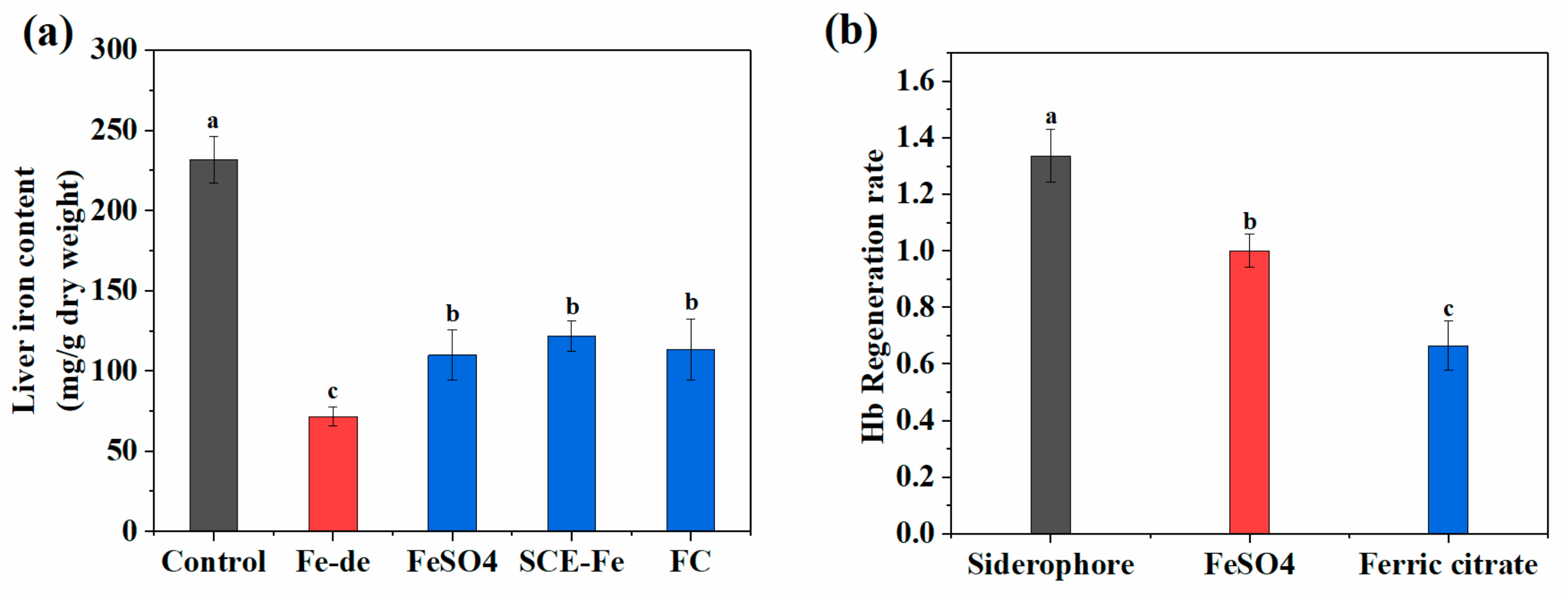
2.8. Measurement of Hb, Hct, and Serum Iron
2.9. Relative Bioavailability of SCE-Fe
3. Discussion
4. Methods and Materials
4.1. Materials
4.2. Preparation of Synechococcus sp. PCC7002 Siderophores
4.3. Construction of the Polarized Caco-2 Cell Monolayers
4.4. Intestinal Iron Absorption in the Polarized Caco-2 Cell Monolayers
4.5. Animal Experiments
4.6. Hb, Hct, and Serum Iron Measurements
4.7. Relative Bioavailability of SCE-FE
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lv, C.Y.; Zhao, G.H.; Lonnerdal, B. Bioavailability of iron from plant and animal ferritins. J. Nutr. Biochem. 2015, 26, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Bresgen, N.; Eckl, P.M. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 2015, 5, 808–847. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.P.; Murray-Kolb, L.E. Iron Nutrition and Premenopausal Women: Effects of Poor Iron Status on Physical and Neuropsychological Performance. In Annual Review of Nutrition; Cousins, R.J., Ed.; Annual Reviews: Palo Alto, CA, USA, 2013; Volume 33, pp. 271–288. [Google Scholar]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef]
- Reddy, M.B.; Armah, S.M.; Stewart, J.W.; O’Brien, K.O. Iron Absorption from Iron-Enriched Aspergillus oryzae Is Similar to Ferrous Sulfate in Healthy Female Subjects. Curr. Dev. Nutr. 2018, 2, nzy004. [Google Scholar] [CrossRef]
- Chen, L.; Dick, W.A.; Streeter, J.G. Production of aerobactin by microorganisms from a compost enrichment culture and soybean utilization. J. Plant Nutr. 2000, 23, 2047–2060. [Google Scholar] [CrossRef]
- Wu, H.H.; Zhu, S.Q.; Zeng, M.Y.; Liu, Z.Y.; Dong, S.Y.; Zhao, Y.H.; Huang, H.; Lo, Y.M. Enhancement of Non-heme Iron Absorption by Anchovy (Engraulis japonicus) Muscle Protein Hydrolysate Involves a Nanoparticle-Mediated Mechanism. J. Agric. Food Chem. 2014, 62, 8632–8639. [Google Scholar] [CrossRef]
- Zhao, G.H. Phytoferritin and its implications for human health and nutrition. Biochim. Biophys. Acta-Gen. Subj. 2010, 1800, 815–823. [Google Scholar] [CrossRef]
- Butler, A.; Theisen, R.M. Iron (III)-siderophore coordination chemistry: Reactivity of marine siderophores. Coord. Chem. Rev. 2010, 254, 288–296. [Google Scholar] [CrossRef]
- Neilands, J.B. Microbial iron compounds. Annu. Rev. Biochem. 1981, 50, 715–731. [Google Scholar] [CrossRef]
- Homann, V.V.; Edwards, K.J.; Webb, E.A.; Butler, A. Siderophores of Marinobacter aquaeolei: Petrobactin and its sulfonated derivatives. Biometals 2009, 22, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Sandy, M.; Butler, A. Microbial Iron Acquisition: Marine and Terrestrial Siderophores. Chem. Rev. 2009, 109, 4580–4595. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Butler, A. Structure of synechobactins, new siderophores of the marine cyanobacterium Synechococcus sp. PCC 7002. Limnol. Oceanogr. 2005, 50, 1918–1923. [Google Scholar] [CrossRef]
- Martinez, J.S.; Carter-Franklin, J.N.; Mann, E.L.; Martin, J.D.; Haygood, M.G.; Butler, A. Structure and membrane affinity of a suite of amphiphilic siderophores produced by a marine bacterium. Proc. Natl. Acad. Sci. USA 2003, 100, 3754–3759. [Google Scholar] [CrossRef]
- Theurl, I.; Aigner, E.; Theurl, M.; Nairz, M.; Seifert, M.; Schroll, A.; Sonnweber, T.; Eberwein, L.; Witcher, D.R.; Murphy, A.T.; et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: Diagnostic and therapeutic implications. Blood 2009, 113, 5277–5286. [Google Scholar]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Dressman, J.B.; Berardi, R.R.; Dermentzoglou, L.C.; Russell, T.L.; Schmaltz, S.; Barnett, J.L.; Jarvenpaa, K.M. Upper Gastrointestinal (GI) pH in Young, Healthy Men and Women. Pharm. Res. 1990, 7, 756–761. [Google Scholar] [CrossRef]
- Fuqua, B.K.; Vulpe, C.D.; Anderson, G.J. Intestinal iron absorption. J. Trace Elem. Med. Biol. 2012, 26, 115–119. [Google Scholar] [CrossRef]
- Wu, L.; Zou, Y.; Miao, Y.; Zhang, J.; Zhu, S.; Zeng, M.; Wu, H. Dietary gelatin enhances non-heme iron absorption possibly via regulation of systemic iron homeostasis in rats. J. Funct. Foods 2019, 59, 272–280. [Google Scholar] [CrossRef]
- Scheers, N.; Sandberg, A. Ascorbic acid uptake affects ferritin, Dcytb and Nramp2 expression in Caco-2 cells. Eur. J. Nutr. 2008, 47, 401–408. [Google Scholar] [CrossRef]
- Sharp, P.A. Intestinal Iron Absorption: Regulation by Dietary a Systemic Factors. Int. J. Vitam. Nutr. Res. 2010, 80, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D. Diagnosis and management of iron-deficiency anaemia. Best Pract. Res. Clin. Haematol. 2005, 18, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F. Fortification: Overcoming technical and practical barriers. J. Nutr. 2002, 132, 806S–812S. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M.; Mendoza, Y.A.; Pereira, D.; Cerami, C.; Wegmuller, R.; Constable, A.; Spieldenner, J. Dietary strategies for improving iron status: Balancing safety and efficacy. Nutr. Rev. 2017, 75, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F.; Griffin, I.; Pressman, E.; McIntyre, A.W.; Cooper, E.; McNanley, T.; Harris, Z.L.; Westerman, M.; O’Brien, K.O. Utilization of Iron from an Animal-Based Iron Source Is Greater Than That of Ferrous Sulfate in Pregnant and Nonpregnant Women. J. Nutr. 2010, 140, 2162–2166. [Google Scholar] [CrossRef]
- Martinez-Torres, C.; Cubeddu, L.; Dillmann, E.; Brengelmann, G.L.; Leets, I.; Layrisse, M.; Johnson, D.G.; Finch, C. Effect of exposure to low temperature on normal and iron-deficient subjects. Am. J. Phys. 1984, 246, R380–R383. [Google Scholar] [CrossRef]
- Soares, M.P.; Weiss, G. The Iron age of host-microbe interactions. EMBO Rep. 2015, 16, 1482–1500. [Google Scholar] [CrossRef]
- Hoffbrand, V.; Moss, P.A.H. Hoffbrand’s Essential Haematology; John Wiley & Sons Inc.: New York, NY, USA, 2015. [Google Scholar]
- Moety, G.A.F.A.; Ali, A.S.; Fouad, R.; Ramadan, W.; Belal, D.S.; Haggag, H. Amino acid chelated iron versus an iron salt in the treatment of iron deficiency anemia with pregnancy: A randomized controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 210, 242–246. [Google Scholar] [CrossRef]
- Lei, X.G.; Weaver, J.D.; Mullaney, E.J.; Ullah, A.H.J.; Azain, M.J. Phytase, a New Life for an “Old” Enzyme. Annu. Rev. Anim. Biosci. 2013, 1, 283–309. [Google Scholar] [CrossRef]
- Trick, C.G.; Wilhelm, S.W. Physiological-Changes in the Coastal Marine Cyanobacterium Synechococcus sp PCC 7002 Exposed to Low Ferric Ion Levels. Mar. Chem. 1995, 50, 207–217. [Google Scholar] [CrossRef]
- Martin, C.D.S.; Garri, C.; Pizarro, F.; Walter, T.; Theil, E.C.; Nunez, M.T. Caco-2 intestinal epithelial cells absorb soybean ferritin by mu(2) (AP2)-dependent endocytosis. J. Nutr. 2008, 138, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Cabantchik, Z.I.; Glickstein, H.; Milgram, P.; Breuer, W. A fluorescence assay for assessing chelation of intracellular iron in a membrane model system and in mammalian cells. Anal. Biochem. 1996, 233, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K.; Porwal, S.K.; Tartakoe, A.; Devireddy, L. Mechanisms of Mammalian Iron Homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef] [PubMed]
- Kalgaonkar, S.; Lonnerdal, B. Receptor-mediated uptake of ferritin-bound iron by human intestinal Caco-2 cells. J. Nutr. Biochem. 2009, 20, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Hilty, F.M.; Arnold, M.; Hilbe, M.; Teleki, A.; Knijnenburg, J.T.N.; Ehrensperger, F.; Hurrell, R.F.; Pratsinis, S.E.; Langhans, W.; Zimmermann, M.B. Iron from nanocompounds containing iron and zinc is highly bioavailable in rats without tissue accumulation. Nat. Nanotechnol. 2010, 5, 374–380. [Google Scholar] [CrossRef]


| Group | Iron Repletion | ||||
|---|---|---|---|---|---|
| Control | Fe-Deficient | FeSO4 | SFC-Fe | Ferric Citrate | |
| N | 8 | 8 | 8 | 8 | 8 |
| Diet iron (mg/Kg) | 36.7 ± 0.12 | 13.08 ± 1.34 | 29.11 ± 0.9 | 29.56 ± 1.3 | 33.32 ± 0.92 |
| Diet intake (g) | 348.64 | 247.36 | 286.77 | 316.01 | 259.78 |
| Initial BW (g) | 254.5 ± 25.66 a | 170.13 ± 28.74 b | 170.33 ± 31.76 b | 171.25 ± 18.99 b | 170.13 ± 19.08 b |
| Final BW (g) | 356.5 ± 32.93 a | 205.38 ± 31.15 b | 245.67 ± 45.46 c | 292.1 ± 34.91 d | 231.63 ± 26.94 c |
| BW changes (g) | 102 ± 9.77 a | 30.25 ± 12.29 b | 75.33 ± 14.83 c | 120.75 ± 17.87 d | 61.5 ± 9.57 e |
| Group | Iron Repletion | ||||
|---|---|---|---|---|---|
| Control | Fe-Deficient | FeSO4 | SFC-Fe | Ferric Citrate | |
| Initial Hb (g/L) | 162.25 ± 20.50 a | 74.5 ± 13.48 b | 73.11 ± 14.24 b | 72.75 ± 12.73 b | 71.75 ± 15.95 b |
| Final Hb (g/L) | 166.25 ± 22.55 a | 71.5 ± 20.97 b | 132.44 ± 16.99 c | 145.75 ± 16.51 c | 113 ± 21.82 d |
| Hb change (g/L) | 4 ± 3.74 a | −3 ± 9.14 a | 59.33 ± 5.27 b | 73 ± 5.89 c | 41.25 ± 7.14 d |
| Initial Hct(%) | 45.65 ± 4.09 a | 15.9 ± 4.43 b | 14 ± 3.32 b | 14.48 ± 3.55 b | 15.35 ± 5.22 b |
| Final Hct (%) | 48.13 ± 6.88 a | 10.5 ± 5.55 b | 36.78 ± 4.27 c | 39.25 ± 9.56 c | 28.15 ± 9.61 d |
| Initial serum iron (μg/g) | 35.46 ± 5.14 a | 10.37 ± 2.21 b | 10.47 ± 3.57 b | 10.6 ± 2.74 b | 10.6 ± 1.99 b |
| Final serum iron (μg/g) | 35.57 ± 7.62 a | 8.72 ± 3.32 b | 28.59 ± 10.76 c | 31.69 ± 8.85 c | 22.26 ± 6.11 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Jiang, S.; Zhang, F.; Wang, R.; Zhao, Y.; Zeng, M. Siderophore (from Synechococcus sp. PCC 7002)-Chelated Iron Promotes Iron Uptake in Caco-2 Cells and Ameliorates Iron Deficiency in Rats. Mar. Drugs 2019, 17, 709. https://doi.org/10.3390/md17120709
Feng X, Jiang S, Zhang F, Wang R, Zhao Y, Zeng M. Siderophore (from Synechococcus sp. PCC 7002)-Chelated Iron Promotes Iron Uptake in Caco-2 Cells and Ameliorates Iron Deficiency in Rats. Marine Drugs. 2019; 17(12):709. https://doi.org/10.3390/md17120709
Chicago/Turabian StyleFeng, Xue, Suisui Jiang, Fan Zhang, Runfang Wang, Yuanhui Zhao, and Mingyong Zeng. 2019. "Siderophore (from Synechococcus sp. PCC 7002)-Chelated Iron Promotes Iron Uptake in Caco-2 Cells and Ameliorates Iron Deficiency in Rats" Marine Drugs 17, no. 12: 709. https://doi.org/10.3390/md17120709
APA StyleFeng, X., Jiang, S., Zhang, F., Wang, R., Zhao, Y., & Zeng, M. (2019). Siderophore (from Synechococcus sp. PCC 7002)-Chelated Iron Promotes Iron Uptake in Caco-2 Cells and Ameliorates Iron Deficiency in Rats. Marine Drugs, 17(12), 709. https://doi.org/10.3390/md17120709